Abstract
Introduction:
Idiopathic pulmonary fibrosis (IPF) is a progressive and irreversible interstitial pulmonary disease that has a poor prognosis. Scutellarein, which is extracted from the traditional Chinese medicine Erigeron breviscapus, is used to treat a variety of diseases; however, the use of scutellarein for the treatment of pulmonary fibrosis and the related mechanisms of action have not been fully explored.
Methods:
This study was conducted using a well-established mouse model of pulmonary fibrosis induced by bleomycin (BLM). The antifibrotic effects of scutellarein on histopathologic manifestations and fibrotic marker expression levels were examined. The effects of scutellarein on fibroblast differentiation, proliferation, and apoptosis and on related signaling pathways were next investigated to demonstrate the underlying mechanisms.
Results:
In the present study, we found that scutellarein alleviated BLM-induced pulmonary fibrosis, as indicated by histopathologic manifestations and the expression levels of fibrotic markers. Further data demonstrated that the ability of fibroblasts to differentiate into myofibroblasts was attenuated in scutellarein-treated mice model. In addition, we obtained in vitro evidence that scutellarein inhibited fibroblast-to-myofibroblast differentiation by repressing TGF-β/Smad signaling, inhibited cellular proliferation by repressing PI3K/Akt signaling, and increased apoptosis of fibroblasts by affecting Bax/Bcl2 signaling.
Discussion:
In general, scutellarein might exert therapeutic effects on pulmonary fibrosis by altering the differentiation, proliferation, and apoptosis of fibroblasts. Although scutellarein has been demonstrated to be safe in mice, further studies are required to investigate the efficacy of scutellarein in patients with IPF.
Keywords: apoptosis, differentiation, fibroblast, proliferation, pulmonary fibrosis, scutellarein
Introduction
Idiopathic pulmonary fibrosis (IPF), an interstitial pulmonary disease, is characterized by progressive and irreversible lung scarring, dyspnea and impaired lung function.1 Worldwide, the incidence of IPF is increasing along with IPF-associated mortality rates and economic health care burdens.2 An abnormal wound healing response in the context of persistent lung injury is viewed as the foremost pathogenic mechanism; this mechanism is attributed to dysregulation of fibroblast differentiation and proliferation, decreased fibroblast apoptosis, excessive extracellular matrix (ECM) deposition and destruction of lung tissue structure.3 Although the targeted antifibrotic agents pirfenidone and nintedanib have been marketed for treatment, the current therapies are limited to patients with mild-to-moderate lung function declines.4 Therefore, discussion of potential therapeutic approaches and discovery of new therapeutic agents for all IPF patients are urgently needed.
Traditional Chinese medicine is attracting increasing interest as a possible alternative strategy for the management of fibrosis. In recent years, research has confirmed that scutellarein, an active flavonoid monomer in Erigeron breviscapus, has therapeutic effects on tumors, Alzheimer’s disease and obesity.5–7 However, its effects on pulmonary fibrosis have not yet been established. Inflammation and oxidative stress are known to play important roles in the development of pulmonary fibrosis, and previous studies have indicated that scutellarein has anti-inflammatory and antioxidant effects.8 Scutellarein is the main metabolite of scutellarin in vivo, and scutellarin inhibits liver fibrosis and myocardial fibrosis.9,10 These findings prompted us to hypothesize that scutellarein may be a good candidate agent for the treatment of pulmonary fibrosis.
To assess its feasibility, we first constructed a mouse model induced by bleomycin (BLM) to evaluate the ameliorative effects of scutellarein on pulmonary fibrosis. Furthermore, we examined the effects of scutellarein on differentiation, proliferation and apoptosis in fibroblasts and explored a series of related signaling pathways. Together, our findings demonstrate that scutellarein can alleviate experimental pulmonary fibrosis and regulate the activation, proliferation and apoptosis of fibroblasts by affecting the TGF–Smad, Pi3k–Akt and Bcl-2/Bax signaling pathways, suggesting that utilization of scutellarein might be an effective approach for amelioration of pulmonary fibrosis in the clinic. We hope to provide a theoretical and experimental basis for the clinical application of scutellarein in IPF treatment.
Material and methods
Reagents and antibodies
Scutellarein [Figure 1(A)] was purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). Recombinant human TGF-β1 was procured from Biolegend, lnc. (CA, USA). BLM was obtained from MedChemExpress, lnc. (NJ, USA). Antibodies against GAPDH, β-actin, α-SMA, collagen I and fibronectin were purchased from Proteintech Co., Ltd (Wuhan, China) and antibodies against p-P85-α, P85-α, p-Akt, Akt, p-Smad2, p-Smad3, Smad2/3, Bax, and Bcl-2 were procured from Cell Signaling Technology, Inc. (MA, USA). A quantitative real-time polymerase chain reaction (qRT-PCR) assay kit was obtained from Takara Co., Ltd (Dalian, China). A Cell Counting Kit-8 (CCK8) was purchased from Dojindo Chemical Technology Co., Ltd (Shanghai, China). A Cell-Light EdU Apollo 488 in vitro Kit was purchased from Guangzhou RIBOBIO Co., Ltd (Guangzhou, China). A FITC Annexin V Apoptosis Detection Kit with PI was procured from Biolegend, Inc. (CA, USA).
Figure 1.
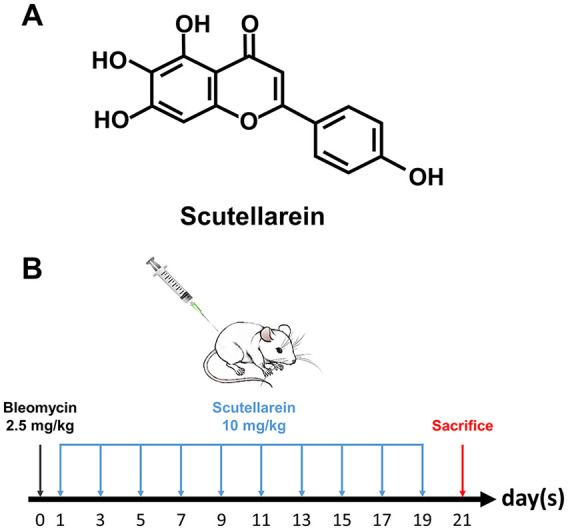
(A) Chemical structure of scutellarein. (B) Bleomycin-induced model of pulmonary fibrosis and the drug-delivery method used for scutellarein in mice.
Animals and ethical statement
Thirty-two male C57BL/6 mice (8 weeks old, average weight approximately 24.5 g) were purchased from Beijing Huafukang Biology Technology Co. Ltd. (Beijing, China) and housed in a specific pathogen-free animal facility at Tongji Medical College with access to food and water ad libitum. All mice in the study were maintained and used according to the International Association of Veterinary Editors guidelines 2010 and Guide for the Care and Use of Laboratory Animals, 8th edition, 2011, guidelines and the protocols were approved by the Institutional Animal Care and Use Committee at Tongji Hospital, Huazhong University of Science and Technology (TJ-A20190201).
Animal treatment
All animals were randomly divided into four groups: (a) the BLM + scutellarein (BLM+Scu) group, (b) the BLM + DMSO group, (c) the PBS + scutellarein (Scu) group , and (d) the PBS + DMSO group . First, a mouse pulmonary fibrosis model was constructed. All mice were anesthetized with 1% pentobarbital sodium (50 mg/kg) by intraperitoneal injection.11 Sixteen of them were intratracheally administered 2.5 mg/kg BLM (1.25 mg/ml, approximately 50 µl per mouse) (BLM+Scu group and BLM+DMSO group).12 The other 16 mice were treated by intratracheal injection with the same amount of PBS as a control (Scu group and DMSO group). The day of modeling was recorded as day 0. Then, starting on day 1, the mice in the BLM+Scu group and Scu group were treated with scutellarein (10 mg/kg, dissolved in 10% DMSO, approximately 200 µl per mouse) by intraperitoneal injection every other day for 20 consecutive days, while the mice in the BLM+DMSO group and the DMSO group were treated with the same volume of 10% DMSO as a control. All mice were sacrificed on day 21 [Figure 1(B)].
Histological analysis
The left lungs of mice were inflated with 4% neutral buffered paraformaldehyde, removed, and placed in fresh 4% neutral buffered paraformaldehyde for 24 h at room temperature. After paraffin embedding, the tissues were sliced into 5-µm sections and separately subjected to hematoxylin and eosin (H&E), Sirius red and Masson staining according to the method in a previous report.13 The severity of pulmonary fibrosis was assessed by the Ashcroft score,14 and images were obtained with a microscope (Olympus, Shinjuku, Japan).
Cell culture and treatment
Human pulmonary fibroblasts (HPFs) were purchased from ScienCell Research Laboratories, Inc. (CA, USA). The HPFs were cultured in fibroblast medium supplemented with 2% fetal calf serum, 1% fibroblast growth factor (FGF) and a 1% solution of penicillin and streptomycin at 37°C in a humid atmosphere containing 5% CO2. For fibroblast proliferation and apoptosis, the cells were stimulated with scutellarein (25 μM or 50 μM, dissolved in 2‰ DMSO) or an equal amount of 2‰ DMSO for 48 h. For fibroblast differentiation, additional human recombinant TGF-β1 (10 ng/ml, 48 h) was added to stimulate HPF differentiate into myofibroblasts. For signaling pathways detection, the stimulus time was reduced to 1 h.
Proliferation assessment
Proliferation was assessed by CCK8 assay and 5-ethynyl-2′-deoxyuridine (EdU) staining according to previously reported protocols.15 Briefly, cells were seeded in 96-well plates and then treated with one of two different concentrations of scutellarein (25 μM or 50 μM) for 24/48/72 h. For the CCK8 assay, 5 mg/ml CCK8 reagent was added to the wells, and the cells were incubated for an additional 1 h. After thorough mixing, the optical density was measured at 450 nm. For EdU staining, the cells were washed with PBS and then incubated in serum-free fibroblast medium containing 10 μmol/l EdU (RiboBio, China) for 2 h. The cells were fixed and then subjected to Apollo staining and DNA staining according to the manufacturer’s instructions. Images were obtained with a fluorescence microscope (Olympus, Shinjuku, Japan).
Apoptosis assessment
To determine the degree to which fibroblast apoptosis was induced by scutellarein, HPFs were double-stained using an Annexin V/propidium iodide (PI) apoptosis detection kit.16 Briefly, cells were seeded in six-well plates, treated with scutellarein (25 μM or 50 μM) for 48 h, digested by trypsin, collected into tubes, washed with PBS and binding buffer successively, and then resuspended in binding buffer. The cells were stained with a FITC-labeled Annexin V and PI staining solution for 15 min in the dark at room temperature. The fluorescence intensity of Annexin V and PI was detected by a FACSCalibur flow cytometer (BD Bioscience). The extent of apoptosis was quantified as the percentage of Annexin V- or PI-positive cells.
qRT-PCR analysis
Total RNA was extracted from lung tissue and HPFs with TRIzol reagent (Takara).17 The RNA quantity and quality were measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, MA, USA). Complementary DNA synthesis was performed using an M-MLV reverse transcriptase kit (Invitrogen, CA, USA) as previously reported.18 qRT-PCR analysis was performed using SYBR Premix Ex Taq (Takara) as previously reported.19 The primers are listed in Table 1.
Table 1.
Primer name.
| Primer name | Sequence (5′–3′) |
|---|---|
| Mouse Actb forward | AGAAAATCTGGCACCACACCT |
| Mouse Actb reverse | GATAGCACAGCCTGGATAGCA |
| Mouse Acta2 forward | GGACGTACAACTGGTATTGTGC |
| Mouse Acta2 reverse | TCGGCAGTAGTCACGAAGGA |
| Mouse Col1a1 forward | TAAGGGTCCCCAATGGTGAGA |
| Mouse Col1a1 reverse | GGGTCCCTCGACTCCTACAT |
| Mouse Fn forward | GATGTCCGAACAGCTATTTACCA |
| Mouse Fn reverse | CCTTGCGACTTCAGCCACT |
| Human GAPDH forward | CAATGACCCCTTCATTGACC |
| Human GAPDH reverse | ATGACAAGCTTCCCGTTCTC |
| Human ACTA2 forward | GTGTTGCCCCTGAAGAGCAT |
| Human ACTA2 reverse | GCTGGGACATTGAAAGTCTCA |
| Human COL1A1 forward | GAGGGCCAAGACGAAGACATC |
| Human COL1A1 reverse | CAGATCACGTCATCGCACAAC |
| Human FN forward | GAGAATAAGCTGTACCATCGCAA |
| Human FN reverse | CGACCACATAGGAAGTCCCAG |
Western blot analysis
Lung tissues and HPFs were homogenized in RIPA lysis buffer (Biyuntian, China). The proteins were subjected to western blotting with the indicated primary antibodies using established techniques.20 Briefly, the proteins were separated on 10% polyacrylamide gels and transferred onto PVDF membranes. The membranes were blocked with 5% non-fat milk for 1 h and then incubated with primary antibodies overnight at 4°C. After three washes (each for 10 min) the membranes were probed with HRP-conjugated secondary antibodies and then visualized using enhanced chemiluminescence detection (Advansta, CA, USA).
Immunofluorescence analysis
Cells seeded on round coverslips and fixed with 4% paraformaldehyde or frozen tissue sections were permeabilized for 10 min in a 0.2% solution of Triton X-100 and blocked in 1% BSA for 1 h after being washed with PBS. Then, the sections were incubated with 1:100 dilutions of primary antibodies overnight at 4°C and with Alexa 488-conjugated anti-rabbit antibodies (Invitrogen, CA, USA), as previously described.21 The coverslips were mounted with Prolong Gold Antifade Reagent with DAPI (Thermo Fisher Scientific Inc.). The sections were immediately subjected to fluorescence analysis under a fluorescence microscope.
Assessment of liver function, renal function, and heart function
Liver function, renal function and heart function were assessed by measuring blood aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, creatinine (Cr), lactate dehydrogenase (LDH), and creatine kinase (CK) levels at the Department of Clinical Laboratories of Tongji Hospital.
Statistical analysis
The experimental results are expressed as the means ± standard deviations. GraphPad Prism 7.0 (GraphPad Software Inc., CA) was used to analyze differences by one-way analysis of variance and to generate graphs. p < 0.05 was considered to indicate statistical significance.22
Results
Scutellarein treatment ameliorated BLM-induced pulmonary fibrosis in mice
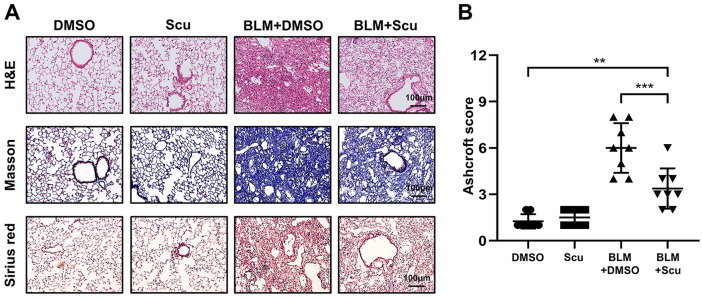
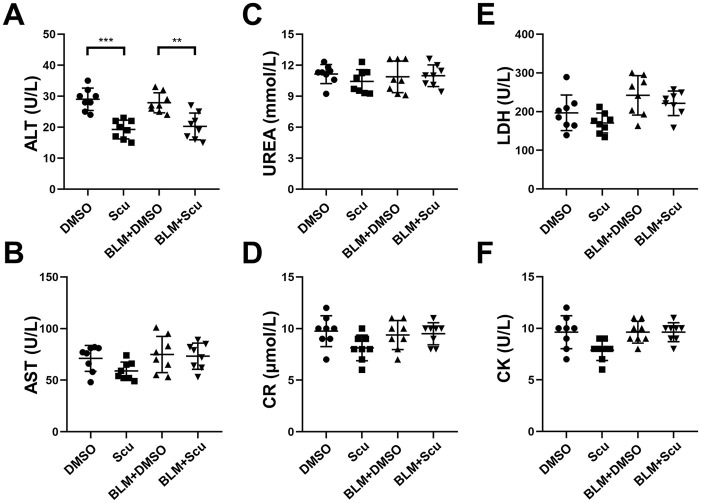
Scutellarein is known to have extensive pharmacological effects on tumors, Alzheimer’s disease and obesity. However, whether it can be used as a feasible treatment agent for pulmonary fibrosis has not been investigated. We first attempted to evaluate the effects of scutellarein on the development of pulmonary fibrosis in a BLM-induced mouse model. For this purpose, mice were intraperitoneally injected with scutellarein every 2 days for 20 days after exposure to BLM. Compared with the BLM+DMSO group, the BLM+Scu group exhibited visible mitigation of lung injury and fibrosis, as evidenced by H&E, Masson and Sirius red staining [Figure 2(A)]. Notably, pulmonary fibrosis was much milder in the BLM+Scu group than in the BLM+DMSO group, as manifested by the Ashcroft scores; in contrast, the mice in the Scu group exhibited fibrotic scores similar to those of the mice in the DMSO group, and neither group had macroscopic pulmonary fibrosis. However, although the application of scutellarein reduced the degree of BLM-induced pulmonary fibrosis, it cannot completely reverse it [Figure 2(B)]. We also investigated whether scutellarein exerted toxicity by testing the liver, renal and cardiac functions of scutellarein-treated mice. As expected, scutellarein had no significant toxic effects on the mice, as indicated by assessment of the plasma concentrations of AST, urea, Cr, LDH, and CK. Surprisingly, the level of ALT decreased to some extent after the intervention of scutellarein, which indicated that scutellarein not only had no toxicity, but also had a protective effect on the liver (Figure 3).
Figure 2.
Effects of scutellarein on pathological changes associated with pulmonary fibrosis in mice. (A) Typical images of H&E-, Masson- and Sirius red-stained lung sections. The images were taken under an original magnification of 200×. (B) Ashcroft scores for the severity of fibrosis. Eight mice were included in each study group. The experimental results are expressed as the means ± standard deviations. The data were analyzed by one-way analysis of variance.
**p < 0.01; ***p < 0.001.
BLM, bleomycin; H&E, hematoxylin and eosin; Scu, scutellarein
Figure 3.
Toxic effects of scutellarein in mice. (A, B) Liver function. (C, D) Renal function. (E, F) Cardiac function. The statistical figures show the data for the eight mice in each group. The experimental results are expressed as the means ± standard deviations. The data were analyzed by one-way analysis of variance.
**p < 0.01; ***p < 0.001.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BLM, bleomycin; CK, creatine kinase; Cr, creatinine; LDH, lactate dehydrogenase; Scu, scutellarein; UREA, urea.
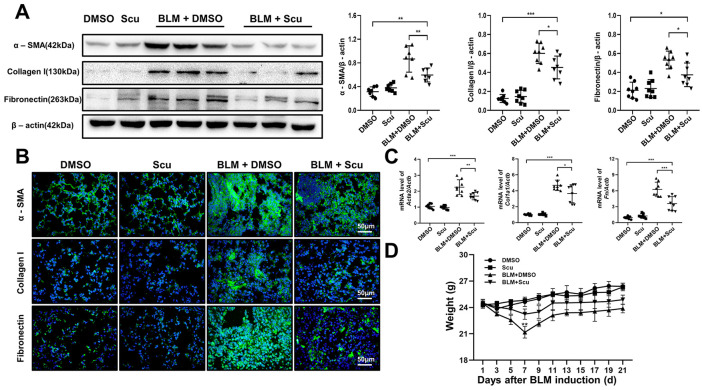
To further evaluate the effects of scutellarein on pulmonary fibrosis, we examined the levels of fibrosis-specific indicators (α-SMA, collagen I and fibronectin) in lung homogenates by western blot analysis. As expected, the expression levels of α-SMA, collagen I and fibronectin were significantly increased after BLM induction and markedly decreased after treatment with scutellarein. The differences in the expression levels of collagen I and fibronectin between the BLM+DMSO group and the BLM+Scu group were more remarkable than those of α-SMA, which may have been due to the influence of the high expression of α-SMA in vascular smooth muscle on the total expression level [Figure 4(A)]. Similarly, qRT-PCR analysis of the fibrotic marker expression levels showed consistent results [Figure 4(C)]. To confirm these observations, we verified the expression of α-SMA, collagen I and fibronectin through immunofluorescence, and the results were consistent with the previously mentioned data. Compared with the BLM+DMSO group, the BLM+Scu group exhibited significantly lower expression levels of the fibrosis-specific indicators [Figure 4(B)]. Changes in mouse body weight can also partially reflect the severity of disease. Scutellarein diminished the effect of BLM on mouse body weight and improved their survival [Figure 4(D)]. In general, based on the histopathological staining results, fibrotic marker expression levels, and weight changes in the mice, we conclude that scutellarein treatment can alleviate pulmonary fibrosis in mice.
Figure 4.
Effects of scutellarein on weight and fibrotic marker levels in mice. (A) Western blot analysis of the fibrotic markers α-SMA, collagen I and fibronectin. Left panel: representative western blot results. Right panel: bar graph showing the mean data for all the mice analyzed in each group. (B) Immunofluorescence of the fibrotic markers α-SMA, collagen I, and fibronectin in lung sections. The images were taken under an original magnification of 400×. (C) Quantitative real-time polymerase chain reaction analysis of the fibrotic markers Acta2, Col1a1, and Fn. (D). Weight changes in the mice. The statistical figures show the data for the eight mice in each group. The experimental results are expressed as the means ± standard deviations. The data were analyzed by one-way analysis of variance.
*p < 0.05; **p < 0.01; ***p < 0.001.
BLM, bleomycin; Scu, scutellarein.
Scutellarein suppressed fibroblast differentiation by inhibiting TGF-β/Smad signaling
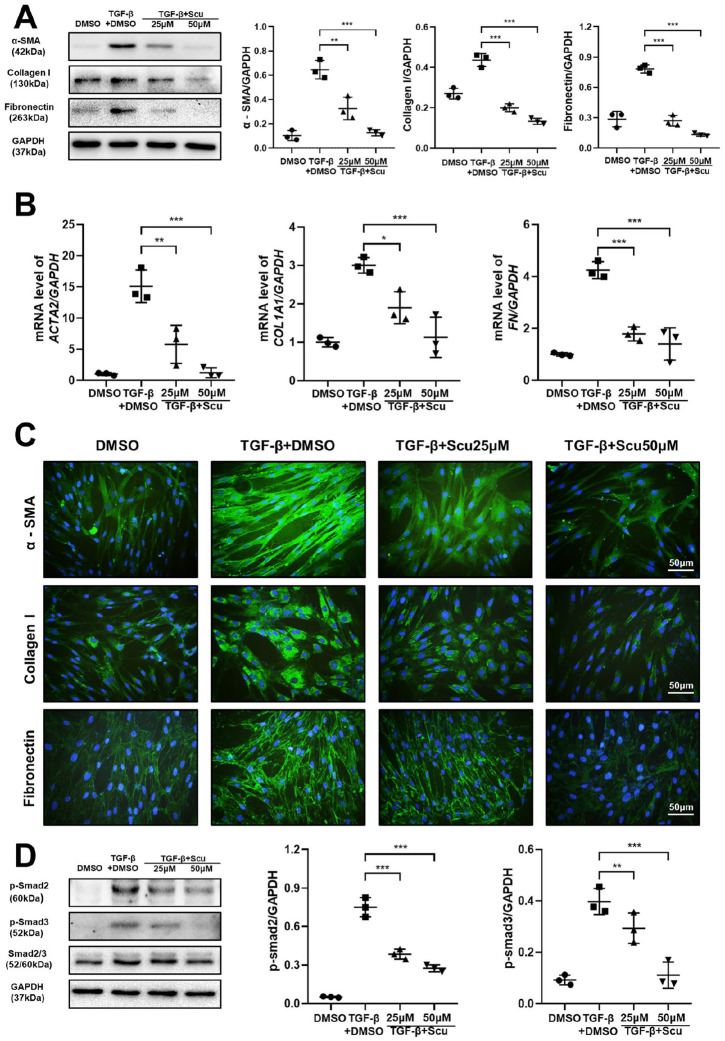
Fibroblasts are involved in the formation of pulmonary fibrosis, and their differentiation into myofibroblasts is a key step during the fibrotic process. Myofibroblasts are typically differentiated from fibroblasts and are essential for wound healing, tissue remodeling and fibrosis formation. When tissue damage occurs, certain cell signals are initiated that activate fibroblasts, thus inducing them to differentiate into myofibroblasts.23 Therefore, we next comprehensively investigated the effect of scutellarein on HPF differentiation. We treated HPFs with TGF-β1 and different doses of scutellarein to observe the expression of typical activation markers. Interestingly, the results showed that compared with the control treatment, administration of TGF-β1 substantially enhanced α-SMA, collagen I, and fibronectin expression; however, application of scutellarein reversed this phenomenon in a dose-dependent manner, as indicated by western blot analysis [Figure 5(A)] and qRT-PCR [Figure 5(B)]. Immunofluorescence analysis gave similar results; compared with that in the TGF-β1-only group, the fluorescence intensity of α-SMA, collagen I and fibronectin was dramatically lower in the group treated with both TGF-β1 and scutellarein. Moreover, scutellarein had a greater apparent therapeutic effect at a concentration of 50 μM than at a concentration of 25 μM [Figure 5(C)]. To further understand the mechanism by which scutellarein hinders fibroblast differentiation, we examined the activity of p-Smad2 and p-Smad3, which are considered to be the most influential signaling molecules affecting fibroblast differentiation. Interestingly, scutellarein markedly inhibited TGF-β1-induced increases in p-Smad2 and p-Smad3 expression levels in a dose-dependent manner [Figure 5(D)]. Collectively, these data provide evidence supporting the hypothesis that scutellarein suppresses the differentiation of fibroblasts by inhibiting TGF-β/Smad signaling.
Figure 5.
Scutellarein inhibits fibroblast differentiation. (A) Western blot analysis of the expression of α-SMA, collagen I and fibronectin in fibroblasts. HPF was stimulated by TGF-β1 and scutellarein for 48 h. Left panel: representative western blot results. Right panel: bar graphs of the western blot results. (B) Quantitative real-time polymerase chain reaction analysis of the expression of ACTA2, COL1A1, and FN in fibroblasts. HPF was stimulated by TGF-β1 and scutellarein for 48 h. (C) Immunofluorescence of α-SMA, collagen I. and fibronectin. HPF was stimulated by TGF-β1 and scutellarein for 48 h. (D) Western blot analysis of p-Smad2 and p-Smad3. HPF was stimulated by TGF-β1 and scutellarein for 1 h. Left panel: representative western blot results. Right panel: bar graphs of the western blot results. The statistical figures show the data for three replications. The experimental results are expressed as the means ± standard deviations. The data were analyzed by one-way analysis of variance.
*p < 0.05; **p < 0.01; ***p < 0.001.
HPF, human pulmonary fibroblast; Scu, scutellarein.
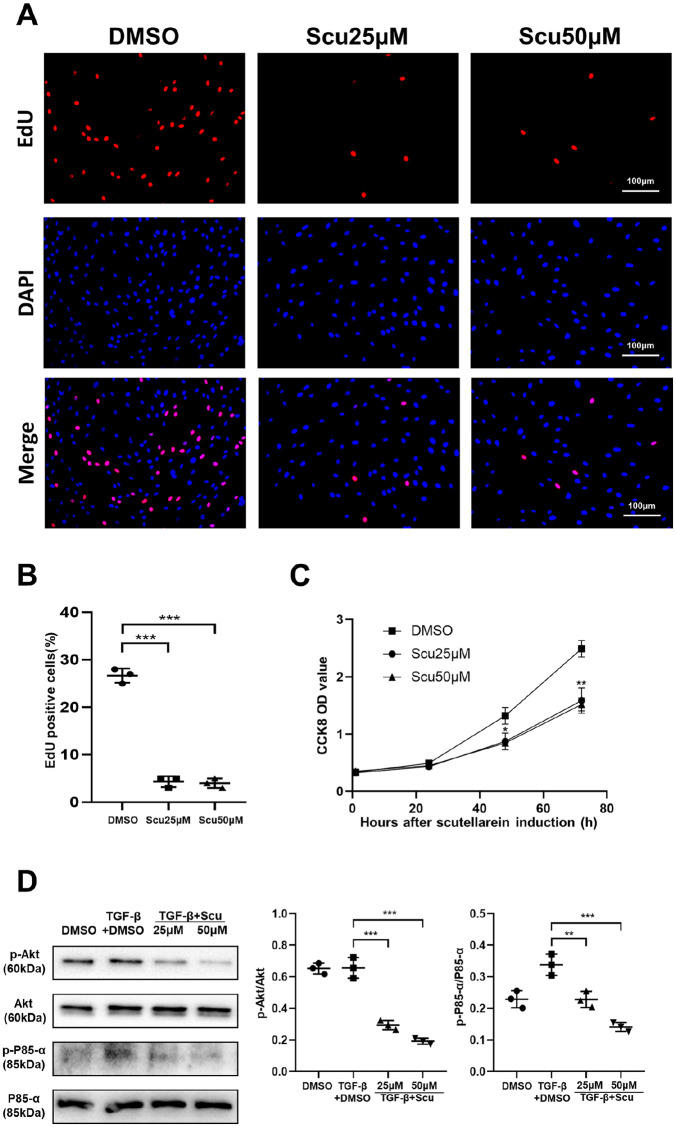
Scutellarein obstructed fibroblast proliferation by repressing Pi3k-Akt signaling
In addition to fibroblast differentiation into myofibroblasts, fibroblast proliferation has been suggested to be a major pathophysiological component of pulmonary fibrosis. We therefore conducted a CCK8 assay to detect the proliferative ability of fibroblasts. With prolonged stimulation time, scutellarein significantly inhibited fibroblast proliferation, regardless of whether the concentration was 25 μM or 50 μM [Figure 6(C)]. To verify this result, we further assessed the effect of scutellarein on fibroblast proliferation through an EdU staining assay. The results of this assay confirmed that scutellarein suppressed the proliferation of fibroblasts, and there was no obvious difference between the 25 μM and 50 μM concentrations [Figure 6(A) and (B)]. Given that the Pi3k–Akt signaling pathway plays significant roles in cell growth and proliferation, we investigated whether scutellarein affects this pathway. Interestingly, scutellarein markedly reduced the expression levels of p-P85-α and p-Akt [Figure 6(D)]. Collectively, these data suggest that scutellarein obstructs the proliferation of fibroblasts by repressing Pi3k-Akt signaling.
Figure 6.
Scutellarein inhibits fibroblast proliferation. (A) Typical image of EdU-stained HPFs following scutellarein stimulation. HPF was stimulated by scutellarein for 48 h. (B) Bar graph of the EdU staining results. (C) CCK8 proliferation curve. HPF was stimulated by scutellarein for 0h, 24 h, 48 h and 72 h. (D) Western blot analysis of p-Akt and p-P85-α. HPF was stimulated by TGF-β1 and scutellarein for 1 h. Left panel: representative western blot results. Right panel: bar graphs of the western blot results. The statistical figures show the data for three replications. The experimental results are expressed as the means ± standard deviations. The data were analyzed by one-way analysis of variance.
**p < 0.01; ***p < 0.001.
CCK8, Cell Counting Kit-8; EdU, 5-ethynyl-2′-deoxyuridine; HPF, human pulmonary fibroblast; Scu, scutellarein.
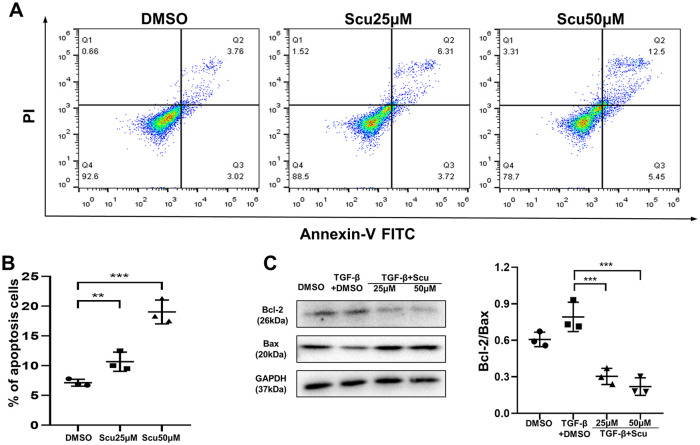
Scutellarein promoted fibroblast apoptosis by regulating Bcl-2/Bax signaling
Along with increased proliferation, decreased apoptosis of fibroblasts promotes pulmonary fibrosis. Therefore, we investigated the effect of scutellarein on fibroblast apoptosis. The results of flow cytometry showed that scutellarein was capable of facilitating fibroblast apoptosis after 48 h of stimulation. Moreover, it was visibly better able to promote apoptosis at a concentration of 50 μM than at a concentration of 25 μM [Figure 7(A) and (B)]. Furthermore, we investigated pathways that may affect fibroblast apoptosis. Surprisingly, in HPFs, the Bcl-2/Bax signaling pathway was obviously affected by scutellarein; scutellarein inhibited the expression of the antiapoptotic gene Bcl-2 and increased the expression of the proapoptotic gene Bax [Figure 7(C)]. Therefore, we speculate that scutellarein may induce apoptosis of fibroblasts by regulating the Bcl-2/Bax signaling pathway.
Figure 7.
Scutellarein promotes apoptosis of fibroblasts. (A) Annexin-V/PI flow cytometry analysis of HPF apoptosis following scutellarein stimulation. HPF was stimulated by scutellarein for 48 h. (B) Bar graph of the flow cytometry analysis results. (C) Western blot analysis of Bcl-2 and Bax. HPF was stimulated by TGF-β1 and scutellarein for 48 h. Left panel: representative western blot results. Right panel: bar graph of the western blot results. The statistical figures show the data for three replications. The experimental results are expressed as the means ± standard deviations. The data were analyzed by one-way analysis of variance.
**p < 0.01; ***p < 0.001.
HPF, human pulmonary fibroblast; PI, propidium iodide; Scu, scutellarein.
Discussion
In our current study, we investigated the curative effects of scutellarein on BLM-induced pulmonary fibrosis and the comprehensive influences of scutellarein on the differentiation, proliferation, and apoptosis of HPFs. We ultimately concluded that scutellarein could inhibit the development of pulmonary fibrosis in mice. The possible mechanisms of the protective effects of scutellarein might be attributable to suppression of fibroblast differentiation into myofibroblasts, inhibition of fibroblast proliferation and promotion of fibroblast apoptosis. In general, we believe that scutellarein may hold promise for use in the clinical treatment of IPF.
IPF is a refractory disease of unknown origin, and there is no particularly effective treatment at present. Scutellarein is a kind of traditional Chinese medicine monomer extracted from E. breviscapus. Many studies have proven its pharmaceutical value, and it has been widely used as an antitumor agent and in other applications. However, the effects of scutellarein on IPF and pulmonary fibroblasts have never been reported. Considering the anti-inflammatory effects of scutellarein and the crucial role of inflammation in pulmonary fibrosis,24 we hypothesized that scutellarein could inhibit the development of pulmonary fibrosis.
Establishing an appropriate IPF model is a core step in deciphering the pathogenesis of IPF. Thus, to test our hypothesis, we first established a mouse model of pulmonary fibrosis. BLM has been verified to be appropriate for the construction of stable animal fibrosis models. Therefore, we used BLM to establish our pulmonary fibrosis model. First, BLM (2.5 mg/kg) was injected into the airways of mice, and then the mice were treated with scutellarein (10 mg/kg, dissolved in 10% DMSO) intraperitoneally every other day for 20 consecutive days. Considering the toxicity of DMSO itself and the other reactions it may cause, we set up a DMSO group (10% DMSO) as a control. First, we verified the inhibitory effect of scutellarein on pulmonary fibrosis in mice via H&E, Masson, and Sirius red staining and Ashcroft score assessment. α-SMA, collagen I and fibronectin are the three most specific fibrotic markers, and their levels can reflect the severity of pulmonary fibrosis and the degree of fibroblast differentiation. Through detection of these fibrotic markers, we demonstrated that scutellarein could suppress the development of pulmonary fibrosis. Both the mRNA and protein levels of the markers were consistently and significantly decreased by scutellarein treatment in the mice with BLM-induced pulmonary fibrosis. The safety of any drug is a prerequisite for its clinical application.25 Based on this principle, we tested liver function, kidney function and cardiac function in scutellarein-treated mice. The results were in line with our expectations that the liver, kidney, and cardiac functions of the mice would not be damaged. Surprisingly, ALT levels were decreased to some extent by scutellarein treatment, suggesting that scutellarein may also have a protective effect on the liver. However, testing this possibility was not within the scope of our study, so we did not conduct further experiments.
Fibroblasts play a crucial role in the formation of fibrosis.26 We demonstrated that scutellarein inhibits the progression of pulmonary fibrosis and fibroblast differentiation in vivo. Next, we attempted to explore the effects of scutellarein on fibroblasts in vitro to further elucidate the mechanism of its action. HPFs purchased from ScienCell Research Laboratories were cultured for study. We verified the effects of scutellarein on the differentiation, proliferation, and apoptosis of the HPFs. Surprisingly, scutellarein not only inhibited the differentiation of HPFs into myofibroblasts but also suppressed HPF proliferation and promoted HPF apoptosis.
By this point, we had elucidated the effects of scutellarein on the phenotypes of fibroblasts. However, the mechanism by which scutellarein inhibits fibroblast differentiation and proliferation and promotes fibroblast apoptosis remained obscure. One of the most vital mechanisms of fibroblast differentiation into myofibroblasts is Smad2 and Smad3 phosphorylation mediated by TGF-β1.27 The Pi3k–Akt pathway is a general pathway related to cell proliferation,28,29 and Bcl-2/Bax is mainly responsible for regulating apoptosis.30 We further explored a series of common signaling pathways, including the previously mentioned pathways, and ultimately found that scutellarein had the most significant effects on the TGF–Smad pathway, the Pi3k–Akt pathway, and the Bcl-2/Bax pathway.
The occurrence and development of pulmonary fibrosis are mediated by complex factors. In addition to fibroblast-related processes, pulmonary inflammation and oxidative stress also play indispensable roles in promoting the formation of pulmonary fibrosis.31 ECM degradation products resulting from oxidative stress may promote fibrogenesis by influencing epithelial, mesenchymal, and inflammatory cell activity.32 The inhibitory effect of scutellarein on oxidative stress has been previously confirmed.5 In terms of inflammation, scutellarein can inhibit IKKα/β and IκBα to suppress cox-2 and iNOS expression, thereby suppressing the inflammatory response.33 In future work, we will further investigate whether scutellarein affects oxidative stress and inflammation in the context of BLM-induced pulmonary fibrosis.
Conclusion
In conclusion, our findings reveal that scutellarein can alleviate the progression of pulmonary fibrosis by inhibiting TGF–Smad pathway-mediated fibroblast differentiation, inhibiting Pi3k–Akt pathway-mediated fibroblast proliferation and promoting Bcl-2/Bax balance-regulated fibroblast apoptosis. However, our current experiment only screened for changes in relevant signaling pathways; it did not identify the direct links between the pathways and their corresponding phenotypic changes. Further research is needed to clarify these links and/or to explore other potential mechanisms in order to provide a more comprehensive perspective of the pharmacological effects of scutellarein. Nevertheless, we hope that our work provides a sufficient theoretical basis for the clinical application of scutellarein and the development of novel therapeutics for IPF.
Research Data
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_1 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_2 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_3 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_4 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_5 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_1 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_2 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_3 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_4 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_5 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the present study was supported by the National Natural Science Foundation of China (81800068, 81500055 and 81770064).
ORCID iD: Yi Wang  https://orcid.org/0000-0002-2109-2157
https://orcid.org/0000-0002-2109-2157
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kang Miao, Department of Respiratory and Critical Care Medicine, Key Laboratory of Pulmonary Diseases of Health Ministry, Key Site of National Clinical Research Center for Respiratory Disease, Wuhan Clinical Medical Research Center for Chronic Airway Diseases, Wuhan, China.
Ting Pan, Department of Respiratory and Critical Care Medicine, Key Laboratory of Pulmonary Diseases of Health Ministry, Key Site of National Clinical Research Center for Respiratory Disease, Wuhan Clinical Medical Research Center for Chronic Airway Diseases, Wuhan, China.
Yong Mou, Department of Respiratory and Critical Care Medicine, Key Laboratory of Pulmonary Diseases of Health Ministry, Key Site of National Clinical Research Center for Respiratory Disease, Wuhan Clinical Medical Research Center for Chronic Airway Diseases, Wuhan, China.
Lei Zhang, Department of Respiratory and Critical Care Medicine, Key Laboratory of Pulmonary Diseases of Health Ministry, Key Site of National Clinical Research Center for Respiratory Disease, Wuhan Clinical Medical Research Center for Chronic Airway Diseases, Wuhan, China.
Weining Xiong, Department of Respiratory and Critical Care Medicine, Key Laboratory of Pulmonary Diseases of Health Ministry, Key Site of National Clinical Research Center for Respiratory Disease, Wuhan Clinical Medical Research Center for Chronic Airway Diseases, Wuhan, China Department of Respiratory Medicine, Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Yongjian Xu, Department of Respiratory and Critical Care Medicine, Key Laboratory of Pulmonary Diseases of Health Ministry, Key Site of National Clinical Research Center for Respiratory Disease, Wuhan Clinical Medical Research Center for Chronic Airway Diseases, Wuhan, China.
Jun Yu, Department of Thoracic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Sciences and Technology, Wuhan, China.
Yi Wang, Department of Respiratory and Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1095 Jiefang Ave., Wuhan, 430030, China.
Reference
- 1. Deng Y, Li Z, Liu J, et al. Targeted resequencing reveals genetic risks in patients with sporadic idiopathic pulmonary fibrosis. Hum Mutat 2018; 39: 1238–1245. [DOI] [PubMed] [Google Scholar]
- 2. Barratt SL, Creamer A, Hayton C, et al. Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med 2018; 7: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014; 9: 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asmani M, Velumani S, Li Y, et al. Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat Commun 2018; 9: 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thirusangu P, Vigneshwaran V, Vijay Avin BR, et al. Scutellarein antagonizes the tumorigenesis by modulating cytokine VEGF mediated neoangiogenesis and DFF-40 actuated nucleosomal degradation. Biochem Biophys Res Commun 2017; 484: 85–92. [DOI] [PubMed] [Google Scholar]
- 6. Sang ZP, Qiang XM, Li Y, et al. Design, synthesis, and biological evaluation of scutellarein carbamate derivatives as potential multifunctional agents for the treatment of Alzheimer’s disease. Chem Biol Drug Des 2015; 86: 1168–1177. [DOI] [PubMed] [Google Scholar]
- 7. Lin Y, Ren N, Li S, et al. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed Pharmacother 2019; 117: 109042. [DOI] [PubMed] [Google Scholar]
- 8. Huang XW, Xu Y, Sui X, et al. Scutellarein suppresses Aβ-induced memory impairment via inhibition of the NF-κB pathway in vivo and in vitro. Oncol Lett 2019; 17: 5581–5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding D, Cai X, Zheng H, et al. Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis. Reprod Sci. Epub ahead of print 17 December 2018. DOI: 10.1177/1933719118817661. [DOI] [PubMed] [Google Scholar]
- 10. Nie J, Yang HM, Sun CY, et al. Scutellarin enhances antitumor effects and attenuates the toxicity of bleomycin in H22 ascites tumor-bearing mice. Front Pharmacol 2018; 9: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang L, Wang Y, Wu G, et al. Blockade of JAK2 protects mice against hypoxia-induced pulmonary arterial hypertension by repressing pulmonary arterial smooth muscle cell proliferation. Cell Prolif 2020; 53: e12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao Y, Wang Y, Zhang Z, et al. Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol Ther 2016; 24: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Fu T, Wang J, et al. Hepatoprotection of yangonin against hepatic fibrosis in mice via farnesoid X receptor activation. Int Immunopharmacol 2019; 75: 105833. [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Xu X, Cheng S, et al. Small interfering RNA directed against microRNA-155 delivered by a lentiviral vector attenuates asthmatic features in a mouse model of allergic asthma. Exp Ther Med 2017; 14: 4391–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira PD, Serra-Caetano A, Cabrita M, et al. Quantification of cell cycle kinetics by EdU (5-ethynyl-2’-deoxyuridine)-coupled-fluorescence-intensity analysis. Oncotarget 2017; 8: 40514–40532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang W, Liang Z. Comparison between annexin V-FITC/PI and Hoechst33342/PI double stainings in the detection of apoptosis by flow cytometry. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014; 30: 1209–1212. [PubMed] [Google Scholar]
- 17. Qi X, Chen H, Huang Z, et al. Aberrantly expressed lncRNAs identified by microarray analysis in CD4+ T cells in asthmatic patients. Biochem Biophys Res Commun 2018; 503: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 18. Qi X, Chen H, Fu B, et al. LncRNAs NR-026690 and ENST00000447867 are upregulated in CD4+ T cells in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis 2019; 14: 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang Z, Cao Y, Zhou M, et al. Hsa_circ_0005519 increases IL-13/IL-6 by regulating hsa-let-7a-5p in CD4+ T cells to affect asthma. Clin Exp Allergy 2019; 49: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Zhu J, Zhang L, et al. Role of C/EBP homologous protein and endoplasmic reticulum stress in asthma exacerbation by regulating the IL-4/signal transducer and activator of transcription 6/transcription factor EC/IL-4 receptor alpha positive feedback loop in M2 macrophages. J Allergy Clin Immunol 2017; 140: 1550–1561e8. [DOI] [PubMed] [Google Scholar]
- 21. Calvani J, Terada M, Lesaffre C, et al. In situ multiplex immunofluorescence analysis of the inflammatory burden in kidney allograft rejection: a new tool to characterize the alloimmune response. Am J Transplant. Epub ahead of print 11 December 2019. DOI: 10.1111/ajt.15699. [DOI] [PubMed] [Google Scholar]
- 22. Chen X, Wang Q, Hu Y, et al. A nomogram for predicting severe exacerbations in stable COPD patients. Int J Chron Obstruct Pulmon Dis 2020; 15: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watsky MA, Weber KT, Sun Y, et al. New insights into the mechanism of fibroblast to myofibroblast transformation and associated pathologies. Int Rev Cell Mol Biol 2010; 282: 165–192. [DOI] [PubMed] [Google Scholar]
- 24. Saghir SAM, Al-Gabri NA, Khafaga AF, et al. Thymoquinone-PLGA-PVA nanoparticles ameliorate bleomycin-induced pulmonary fibrosis in rats via regulation of inflammatory cytokines and iNOS signaling. Animals (Basel) 2019; 9: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu P, Miao K, Zhang L, et al. Curdione ameliorates bleomycin-induced pulmonary fibrosis by repressing TGF- β-induced fibroblast to myofibroblast differentiation. Respir Res 2020; 21: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malsin ES, Kamp DW. The mitochondria in lung fibrosis: friend or foe? Transl Res 2018; 202: 1–23. [DOI] [PubMed] [Google Scholar]
- 27. Shi K, Wang F, Xia J, et al. Pirfenidone inhibits epidural scar fibroblast proliferation and differentiation by regulating TGF- β1-induced Smad-dependent and -independent pathways. Am J Transl Res 2019; 11: 1593–1604. [PMC free article] [PubMed] [Google Scholar]
- 28. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. Epub ahead of print 4 November 2019. DOI: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu Y, Yu J, Wang Q, et al. Tartrate-resistant acid phosphatase 5/ACP5 interacts with p53 to control the expression of SMAD3 in lung adenocarcinoma. Mol Ther Oncolytics 2020; 16: 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohamed MS, Abdelhamid AO, Almutairi FM, et al. Induction of apoptosis by pyrazolo[3,4-d]pyridazine derivative in lung cancer cells via disruption of Bcl-2/Bax expression balance. Bioorg Med Chem 2018; 26: 623–629. [DOI] [PubMed] [Google Scholar]
- 31. Bringardner BD, Baran CP, Eubank TD, et al. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 2008; 10: 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 2010; 49: 707–717. [DOI] [PubMed] [Google Scholar]
- 33. Pandith H, Zhang X, Thongpraditchote S, et al. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-κB pathway. J Ethnopharmacol 2013; 147: 434–441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_1 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_2 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_3 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_4 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
all_original_western_blot_image_5 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_1 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_2 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_3 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_4 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
The_original_western_blot_image_used_in_the_article_5 for Scutellarein inhibits BLM-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis by Kang Miao, Ting Pan, Yong Mou, Lei Zhang, Weining Xiong, Yongjian Xu, Jun Yu and Yi Wang in Therapeutic Advances in Chronic Disease