Abstract
Background
We hypothesised that Calabadion 1, an acyclic cucurbit[n]uril molecular container, reverses fentanyl-induced respiratory depression and dysfunction of the CNS.
Methods
Experiments were conducted in male Sprague-Dawley rats. A constant-rate i.v. infusion of fentanyl (12.5 or 25 μg kg−1 over 15 min) was administered followed by an i.v. bolus of Calabadion 1 (0.5–200 mg kg−1) or placebo. The primary outcome was reversal of ventilatory and respiratory depression, assessed by pneumotachography and arterial blood gas analysis, respectively. Key secondary outcomes were effects on fentanyl-induced central nervous dysfunction quantified by righting reflex, balance beam test, and electromyography (EMG).
Results
Calabadion 1 reversed fentanyl-induced respiratory depression across the endpoints minute ventilation, pH, and Paco2 (P=0.001). Compared with placebo, Calabadion 1 dose dependently (P for trend <0.001) reversed fentanyl-induced hypoventilation {81.9 [5.1] (mean [standard error of the mean]) vs 45.5 [12.4] ml min−1; P<0.001}, acidosis (pH 7.43 [0.01] vs 7.28 [0.04]; P=0.005), and hypercarbia (Paco2 43.4 [1.6] vs 63.4 [8.1] mm Hg; P=0.018). The effective Calabadion 1 doses required to reverse respiratory depression by 50% and 90% (ED50Res and ED90Res) were 1.7 and 15.6 mg kg−1, respectively. Higher effective doses were needed for recovery of righting reflex (ED50CNS: 9.6 mg kg−1; ED90CNS: 86.1 mg kg−1), which was accelerated by Calabadion 1 (4.6 [0.3] vs 9.0 [0.7] min; P<0.001). Calabadion 1 also significantly accelerated recovery of full functional mobility and reversal of muscle rigidity.
Conclusions
Calabadion 1 selectively and dose dependently reversed the respiratory system and CNS side-effects of fentanyl.
Keywords: anaesthesia, analgesics, delayed emergence, fentanyl, muscle rigidity, opioid reversal, postoperative complications, respiratory depression
Editor's key points.
-
•
Fentanyl increases the risk of postoperative respiratory depression, sedation, and muscle rigidity, adverse effects that can increase postoperative morbidity.
-
•
In a rat model, Calabadion 1 reversed ventilatory and respiratory depression and central nervous dysfunction in a selective and dose-dependent manner.
-
•
Clinical studies are required to evaluate Calabadion 1 as a potential reversal agent in the perioperative setting to enhance anaesthesia recovery and patient safety.
-
•
Lower effective doses of calabadion 1 were required for recovery of breathing compared with functional mobility.
-
•
Calabadion 1 binds and inactivates fentanyl, and increases its renal elimination.
Fentanyl is one of the most frequently administered intraoperative opioids. High intraoperative doses of fentanyl increase the risk of postoperative respiratory depression, sedation, and muscle rigidity.1, 2, 3, 4 These adverse effects of fentanyl on the respiratory system and CNS can, in part, explain the increased vulnerability for unplanned hospital admission associated with high doses of fentanyl, particularly after ambulatory surgery.5,6 Synthetic opioids, including fentanyl, are also now the most common drugs involved in drug overdose deaths in the USA.7
Whilst opioid receptor antagonists, such as naloxone, are available clinically, they lack specificity for fentanyl and may interfere with postoperative analgesic therapy. Calabadion 1, an acyclic cucurbit[n]uril, binds strongly and selectively to a variety of hydrophobic ammonium cations, including fentanyl,8, 9, 10 thereby providing an opportunity to overcome disadvantages of the current clinical practice of opioid reversal by eliminating bioactive fentanyl from the plasma as opposed to pharmacological antagonism. Other members of this molecular container family have shown effectiveness in reversing the effects of neuromuscular blocking agents, methamphetamine, and i.v. anaesthetics.11, 12, 13
We hypothesised that Calabadion 1 specifically reverses the respiratory system and CNS side-effects of fentanyl. We tested whether Calabadion 1 reverses fentanyl-induced respiratory depression (primary outcome) and central nervous effects, such as impairment of CNS function (key secondary outcome) and muscle rigidity, in a rat model.
Methods
Drugs
Calabadion 1 was synthesised in LI's laboratory (University of Maryland, College Park, MD, USA), as described.9 Fentanyl (Hospira, Inc., Lake Forest, IL, USA), sufentanil (Hospira, Inc.), morphine (McKesson Medical-Surgical, San Francisco, CA, USA), hydromorphone (Fresenius Kabi USA, Lake Zurich, IL, USA), pethidine (Hospira, Inc.), isoflurane (Patterson McKesson Medical-Surgical, San Francisco, CA, USA), and naloxone (Hospira, Inc.) were obtained from clinical suppliers.
Animals
All experiments were performed in male Sprague-Dawley rats with an initial weight of approximately 300 g in accordance with the Institutional Animal Care and Use Committee, Subcommittee on Research Animal Care at Massachusetts General Hospital (Boston, MA, USA) (study protocol: 2011N00181; see Supplementary Tables S1 and S2 for an overview of all performed experiments and full specification of utilised instruments). All experiments were performed in a randomised, placebo-controlled setting.
General experimental setup
After induction of general anaesthesia with 5 vol% isoflurane, an i.v. line was placed as described.12, 13, 14 Body temperature was maintained between 36.8 and 37°C using a rectal thermistor and a heat lamp. In experiments with anaesthetised rats, isoflurane anaesthesia (1.5 vol%) was maintained throughout experiments whilst animals were breathing spontaneously on room air. In experiments with conscious animals, rats were allowed sufficient time to recover from isoflurane anaesthesia before experiments (Supplementary section 1 and Table S1).
Primary outcome: reversal of fentanyl-induced respiratory depression
Effects on minute ventilation
The effects of Calabadion 1 on reversal of fentanyl-induced hypopnoea were measured using pneumotachography in six isoflurane-anaesthetised rats in a randomised crossover setting.15,16 The dead space of the respiratory circuit was equivalent to the anatomical dead space (0.3 ml). After baseline measurements, fentanyl was infused i.v. at a constant rate of 0.83 μg kg−1 min−1. After 15 min of fentanyl infusion, a bolus of Calabadion 1 (0.5, 1, 10, or 50 mg kg−1 dissolved in saline 1 ml) or placebo (saline 1 ml) was administered i.v. whilst the infusion of fentanyl was continued for 5 min (Supplementary section 2.1).
Effects on pH and Paco2
Arterial blood gas analyses were performed in isoflurane-anaesthetised (n=6) and conscious (n=6) rats in a randomised crossover setting. After baseline measurements, fentanyl was infused i.v. at a constant rate of 0.83 μg kg−1 min−1 (anaesthetised rats) or 1.67 μg kg−1 min−1 (conscious rats). A bolus of Calabadion 1 (anaesthetised rats: 50 mg kg−1; conscious rats: 100 or 200 mg kg−1) or placebo was administered 15 min after initiation of the fentanyl infusion. The effects of Calabadion 1 on arterial pH and arterial partial pressure of carbon dioxide (Paco2) were measured before fentanyl infusion, 15 min after the start of the infusion, and 5 and 15 min after Calabadion 1 or placebo administration. Fentanyl infusions were continued for the duration of the experiment (Supplementary section 2.2).
Secondary outcomes: effects on fentanyl-induced CNS dysfunction
Acceleration of post-anaesthesia motor recovery and recovery of functional mobility (key secondary outcome)
We examined the effects of Calabadion 1 on motor recovery and recovery of full functional mobility after isoflurane/fentanyl anaesthesia by means of righting reflex and balance beam tests, respectively.12,17 For righting reflex assessment, fentanyl was infused for 15 min (0.83 μg kg−1 min−1) under isoflurane anaesthesia. In a randomised crossover setting, six rats subsequently received either an i.v. bolus of Calabadion 1 (5, 10, or 50 mg kg−1) or placebo, followed by extubation in supine position and termination of isoflurane delivery and fentanyl infusion. Time to recovery of righting reflex was defined as the time between extubation and recovery to standing or sternal recumbent position. Additionally, we examined the effect of Calabadion 1 (50 mg kg−1) or placebo on recovery from isoflurane-only anaesthesia without fentanyl in six rats to examine potential reversal effects of the study drug on isoflurane (Supplementary section 3.1). To assess the time to complete recovery of motor performance by the balance beam test, fentanyl was infused for 15 min (0.83 μg kg−1 min−1) followed by a Calabadion 1 (50 mg kg−1; n=6) or placebo (n=6) bolus. After recovery of righting reflex, rats were placed on a balance beam every 1.5 min to evaluate functional mobility using a score ranging from 0 (none) to 3 (best).17 We repeated the experiment after isoflurane-only anaesthesia (n=6) without administration of fentanyl or study drug (Supplementary section 3.2).
Reversal of muscle rigidity
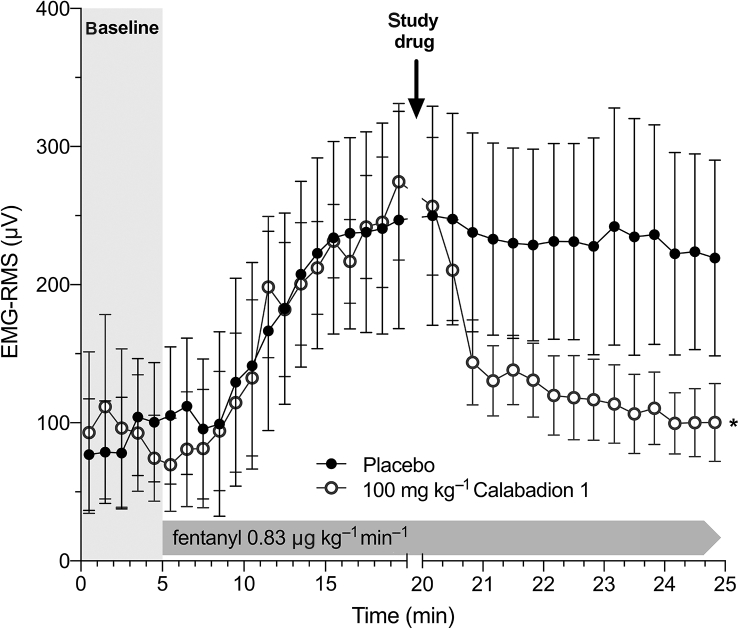
The effects of Calabadion 1 on fentanyl-induced muscle rigidity were evaluated using EMG measurements of the gastrocnemius muscle, as described.18 Fentanyl was infused i.v. at a constant rate of 1.67 μg kg−1 min−1 in conscious rats. After 15 min of fentanyl infusion, a bolus of Calabadion 1 (100 mg kg−1; n=6) or placebo (n=6) was administered i.v. whilst fentanyl infusion was continued for 5 min at the same infusion rate. The magnitude of EMG activity was quantified by calculating the root mean square (RMS) value, a marker of the number of recruited active motor units (Supplementary section 3.3).19
Exploratory analyses
In exploratory analyses, we assessed the effects of Calabadion 1 on respiratory depression induced by other opioids used for postoperative pain management (Supplementary section 4.1), and depth of sedation (Supplementary section 4.2). Additionally, we report the preliminary findings of the effect of Calabadion 1 on the renal excretion of fentanyl (Supplementary section 4.3).
Statistical analyses
Descriptive data are reported as mean (standard error of the mean); change rates are reported with 95% confidence intervals. Using a two-tailed paired t-test, we estimated that a sample size of six animals is necessary to detect a difference (mean [standard deviation]) in minute ventilation of 15 (10) ml min−1 with a power of 80% and a significance level of 0.05. A two-tailed P-value of <0.05 was considered statistically significant.
To assess the effects of Calabadion 1 on outcomes (i.e. minute ventilation, arterial pH and Paco2, EMG activity, time to recovery of righting reflex, and functional mobility), we used linear mixed-effect models with an identity link function for normally distributed probability, as described.12, 13, 14 We analysed the effect of Calabadion 1 across interrelated variables of respiratory depression (i.e. changes in minute ventilation, pH, and Paco2), as measured in percentage change from baseline. Each mixed model included the main effect of the reversal agent (Calabadion 1 vs placebo) as categorical variable, time as continuous variable, and the interaction term of reversal agent and time as fixed effects, whilst allowing intercepts to vary (random-intercept model). Dose dependency was assessed by including dose as a continuous variable in the analyses. EEG frequencies and power spectra were analysed using the median multi-taper spectral estimates between Calabadion 1 and placebo group (Supplementary section 4.2).20 Data analyses were performed using IBM SPSS 23.0 (IBM Corp., Armonk, NY, USA), Stata 13.2 (StataCorp, College Station, TX, USA), LabChart 7 (ADInstruments, Colorado Springs, CO, USA), LabVIEW 2013 (National Instruments, Austin, TX, USA), Prism version 8 (GraphPad Software, La Jolla, CA, USA), and MATLAB 2018 (MathWorks, Inc., Natick, MA, USA).
Results
Primary outcome: reversal of fentanyl-induced respiratory depression
Calabadion 1 reversed fentanyl-induced respiratory depression compared with placebo across the endpoints minute ventilation, pH, and Paco2 (P for interaction=0.001).
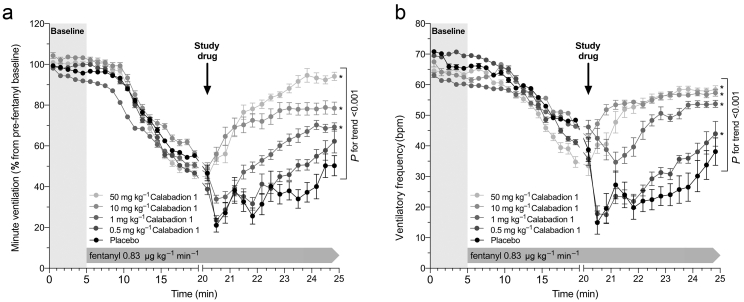
Minute ventilation decreased from 101.5 (0.2) ml min−1 at baseline to 49.6 (2.9) ml min−1 after 15 min of fentanyl infusion (P<0.001). At doses greater or equal to 1 mg kg−1, Calabadion 1 dose dependently (P for trend <0.001) enhanced recovery from fentanyl-induced hypopnoea compared with placebo. At doses of 10 mg kg−1 and higher, Calabadion 1 induced an immediate increase in minute ventilation, reaching its peak effect in 3–4 min. By contrast, with placebo, minute ventilation further decreased slightly during ongoing fentanyl infusion. At a dose of 50 mg kg−1, Calabadion 1 increased minute ventilation to values comparable with pre-fentanyl baseline within 5 min of administering the study drug (Fig. 1a and Supplementary section 2.1). The effective doses required to reverse fentanyl-induced respiratory depression by 50% and 90% within 5 min of administering Calabadion 1 were 1.7 and 15.6 mg kg−1, respectively. Calabadion 1 dose dependently (P for trend <0.001) reversed fentanyl-induced reductions in ventilatory frequency (Fig. 1b), whilst no effects on tidal volume were observed (Supplementary Fig. S1and section 2.1).
Fig 1.
Reversal of fentanyl-induced hypopnoea by Calabadion 1. (a) Minute ventilation and (b) ventilatory frequency were measured after increasing bolus dose of i.v. Calabadion 1 compared with placebo during a constant-rate fentanyl infusion in orotracheally intubated rats, spontaneously breathing 1.5 vol% isoflurane in air in a randomised crossover model (n=6). Data points represent mean (standard error of the mean) in percent of pre-fentanyl baseline. Across all experiments, minute ventilation and ventilatory frequency significantly decreased after 15 min of fentanyl infusion (P<0.001). Calabadion 1 dose dependently (P for trend <0.001) enhanced recovery from fentanyl-induced hypopnoea at doses greater or equal to 1 mg kg−1 compared with placebo. Asterisks indicate statistical significance compared with placebo (∗P<0.05).
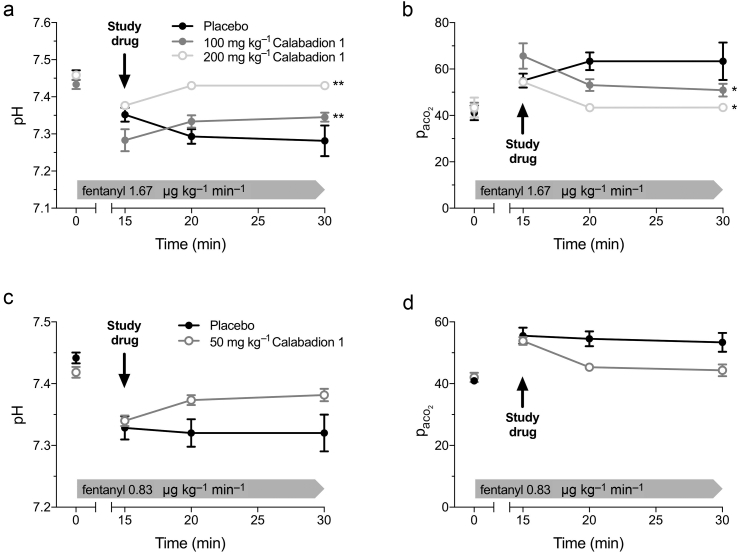
Fentanyl-induced acidosis and hypercarbia were reversed in conscious rats by Calabadion 1 (100 or 200 mg kg−1), but not by placebo (pH: P=0.006 and P=0.005, respectively; Paco2: P=0.012 and P=0.018, respectively; Fig. 2). The effects of Calabadion 1 in conscious rats were dose dependent (pH: P for trend 0.012; Paco2: P for trend=0.023). Whilst improvements in fentanyl-induced acidosis and hypercarbia were also observed in isoflurane-anaesthetised rats after administration of Calabadion 1 (50 mg kg−1) (Fig. 2 and Supplementary section 2.2), these changes were not statistically different from placebo over time (pH: P=0.075; Paco2: P=0.137). In post hoc comparisons between groups at single time points, Paco2 was lower and pH higher 5 min after Calabadion 1 administration (pH: P=0.033; Paco2: P=0.030).
Fig 2.
Reversal of fentanyl-induced acidosis and hypercarbia by Calabadion 1 in conscious and anaesthetised rats. Arterial blood gas analysis was performed at baseline, 15 min after the start of fentanyl infusion, and at 5 and 15 min after Calabadion 1 or placebo bolus administration. Fentanyl administration resulted in decreased pH and increased arterial partial pressure of carbon dioxide (Paco2) in (a and b) conscious and (c and d) anaesthetised rats (all P<0.001). (a and b) Conscious rats were randomised to either Calabadion 1 (100 or 200 mg kg−1) or placebo bolus in a crossover model (n=6 in each group). Both doses of Calabadion 1 reversed fentanyl-induced acidosis (∗∗P<0.01) and hypercarbia (∗P<0.05). In (c and d) isoflurane-anaesthetised rats, fentanyl and Calabadion 1 doses were halved. Fentanyl-induced acidosis and hypercarbia recovered slightly, but this change was not significantly different from the placebo group over time. Data are reported as mean (standard error of the mean).
Secondary outcomes: effects of Calabadion 1 on fentanyl-induced CNS dysfunction
Calabadion 1 reversed fentanyl-induced CNS dysfunction compared with placebo across the endpoints time to recovery of righting reflex, functional mobility, and muscle rigidity.
Postanaesthesia recovery of motor activity (key secondary outcome)
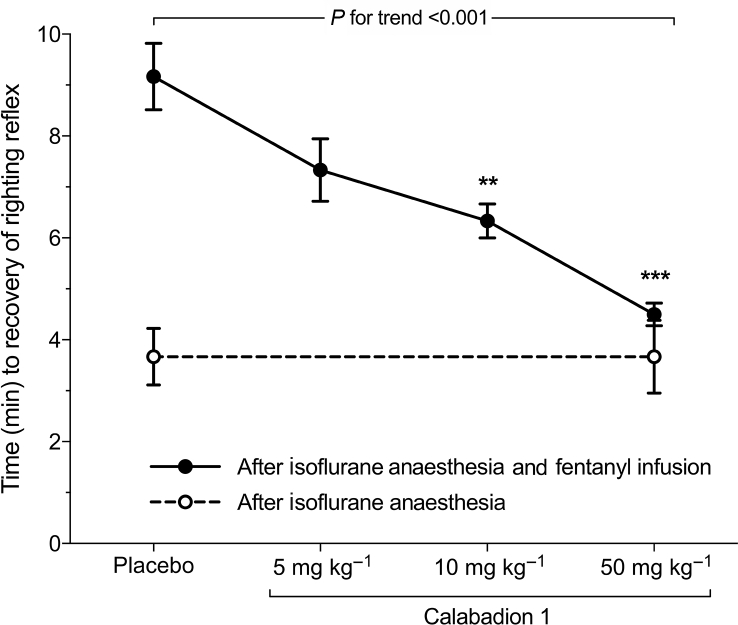
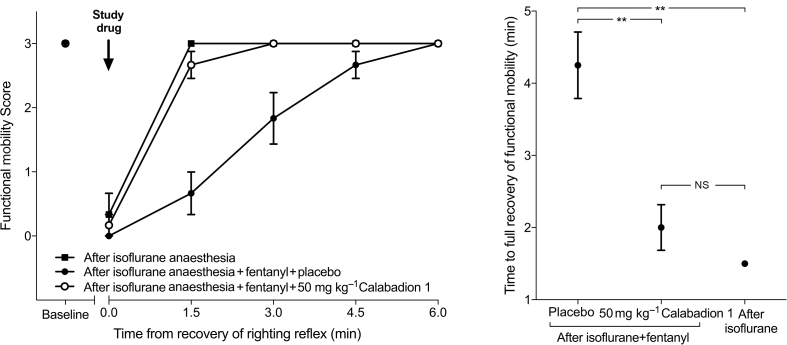
Calabadion 1 dose dependently accelerated recovery of motor function from isoflurane/fentanyl anaesthesia (P for trend <0.001): times to recovery of righting reflex after administration of Calabadion 1 (5, 10, or 50 mg kg−1) were 7.1 (0.7), 6.3 (0.4), and 4.6 (0.3) min, respectively, compared with 9.0 (0.7) min in the placebo group. Calabadion 1 doses of 10 or 50 mg kg−1 accelerated time to recovery of righting reflex compared with placebo (P=0.002 and <0.001, respectively) (Fig. 3 and Supplementary section 3.1). The effective doses for recovery of righting reflex to 50% and 90% were 9.6 and 86.1 mg kg−1, respectively. Time to full recovery of functional mobility after isoflurane/fentanyl anaesthesia was lower after administration of 50 mg kg−1 of Calabadion 1 (2.0 [0.3] min) compared with placebo (4.3 [0.5] min; P=0.002). The effect of Calabadion 1 was comparable with recovery times after fentanyl-free isoflurane-only anaesthesia (1.5 [0.0] min; P=0.175) (Fig. 4 and Supplementary section 3.2).17
Fig 3.
Acceleration of postanaesthesia motor recovery time by Calabadion 1. Time to recovery of righting reflex was assessed after administration of a bolus of either Calabadion 1 (5, 10, or 50 mg kg−1) or placebo in isoflurane/fentanyl-anaesthetised rats (filled circles; n=6). Calabadion 1 dose dependently accelerated time to recovery of righting reflex after isoflurane/fentanyl anaesthesia (P for trend <0.001). Time to recovery of righting reflex was faster in Calabadion 1 (10 mg kg−1 series) compared with placebo (∗∗P=0.002), and in Calabadion 1 (50 mg kg−1 series) (∗∗∗P<0.001). Calabadion 1 did not accelerate recovery of righting reflex after isoflurane-only anaesthesia (open circles; n=6). Data are reported as mean (standard error of the mean).
Fig 4.
Acceleration of postanaesthesia recovery of functional mobility by Calabadion 1. Time to recovery of full functional mobility was assessed by the balance beam test. After recovery of righting reflex, the time rats remained on the balance beam was measured to evaluate balance and body strength on a scale of 0 (inability to maintain grip or balance) to 3 (ability to reach support at the other end of the beam). Animals were randomised to receive either i.v. Calabadion 1 (50 mg kg−1; n=6) or placebo (n=6). Additionally, functional mobility was assessed after isoflurane-only anaesthesia (n=6). Time to full recovery of functional mobility after isoflurane/fentanyl anaesthesia was less after administration of Calabadion 1 (50 mg kg−1) compared with placebo (∗∗P<0.01). The effect of Calabadion 1 was comparable with recovery times after fentanyl-free isoflurane-only anaesthesia. Data are reported as mean (standard error of the mean). NS, not significant.
Effects of Calabadion 1 on muscle rigidity
The average EMG-RMS value of 91 (14) μV across all animals at baseline increased to 260 (42) μV after 15 min of fentanyl infusion (P<0.001), indicating muscle rigidity. After administration of Calabadion 1, muscle rigidity rapidly decreased, such that after 3 min the RMS values had dropped to 117 (31) μV in Calabadion 1-treated animals, whereas they remained elevated at 228 (60) μV in placebo-treated animals (P=0.002 for interaction between time and group [placebo vs Calabadion 1]) (Fig. 5).
Fig 5.
Reversal of fentanyl-induced muscle rigidity by Calabadion 1. The EMG activity of the gastrocnemius muscle was quantified by the root mean square (RMS) value after an i.v. bolus of Calabadion 1 (100 mg kg−1; n=6) or placebo (n=6). Across all experiments, EMG-RMS increased after 15 min of fentanyl infusion, indicating muscle rigidity (P<0.001). Calabadion 1 enhanced recovery from fentanyl-induced muscle rigidity compared with placebo (∗P=0.002). Data are reported as mean (standard error of the mean).
Exploratory analyses
Results from exploratory analyses are reported in the online supplement, including the effects of Calabadion 1 on respiratory depression induced by other opioids (Supplementary section 4.1), depth of sedation (Supplementary section 4.2), and renal excretion of fentanyl (Supplementary section 4.3).
Discussion
This study shows that the acyclic cucurbit[n]uril Calabadion 1 reverses fentanyl-induced respiratory depression and CNS dysfunction in rats. Calabadion 1 did not reverse respiratory depression induced by the long-acting opioids morphine, hydromorphone, or pethidine.
We showed that fentanyl infusion induces progressive respiratory depression characterised by decreased minute ventilation and respiratory acidosis, which corresponds to previous findings in rodents.21 Calabadion 1 dose dependently reversed fentanyl-induced respiratory depression both in conscious and isoflurane-anaesthetised rats, even at doses as low as 1 mg kg−1. We did not observe signs of reoccurrence of fentanyl-induced respiratory depression after Calabadion 1 administration. The main component of Calabadion 1-induced reversal of respiratory depression was driven by recovery of ventilatory frequency, whilst changes in tidal volumes from baseline were minimal. This is in line with the clinically observed respiratory depression induced by i.v. opioids, which is primarily mediated by a decrease in ventilatory frequency. These findings further suggest that alveolar ventilation was maintained, which may, in part, explain the observed discrepancies between large changes in ventilation and relatively minimal changes in respiratory parameters.
Fentanyl has sedative properties that impair motor function.3 We assessed recovery of CNS function using recovery of righting reflex as a marker of arousal from anaesthesia,12 and recovery of motor performance as a marker of functional mobility.17 After administration of Calabadion 1, time to arousal from anaesthesia and motor function recovery were accelerated by about 50%. In contrast, times to recovery of righting reflex after placebo and Calabadion 1 administration were identical in rats exposed to isoflurane-only anaesthesia, as shown previously.12 These findings confirm that the effects of Calabadion 1 are mediated by fentanyl encapsulation rather than an effect on the complex interaction of isoflurane and fentanyl on respiration. The accelerated recovery of motor function may be explained by a reversal of the effects of fentanyl on the cerebral cortex.3 Fentanyl-induced sedation is characterised by an increase in EEG delta slow-wave activity.22, 23, 24 In our study, the EEG power spectrum decreased across the delta, theta, and low gamma sub-bands after Calabadion 1, indicating a transition to a more desynchronised state of cortical oscillations. This is consistent with a lighter state of fentanyl-induced depth of sedation.
In line with other literature,2,18 we observed a more than two-fold increase in compound muscle action potential in the gastrocnemius muscles during opioid infusion resembling fentanyl-induced muscle rigidity, which was completely reversed by Calabadion 1 in all animals. Fentanyl-induced muscle rigidity of the laryngeal, thoracic, and abdominal muscles can adversely affect breathing, mask ventilation, and tracheal intubation.25, 26, 27 If fentanyl-induced muscle rigidity impairs ventilation in the operating theatre, reversal with Calabadion 1 might be desirable, especially when other treatments, such as neuromuscular blocking agents, are not options, for instance, during monitored anaesthesia care or moderate sedation.
Another clinically meaningful advantage of Calabadion 1 reversal relates to its selectivity towards fentanyl. Calabadion 1 has 100-fold higher binding affinity for fentanyl (1.1×107 M−1) compared with morphine (5.3×105 M−1) or hydromorphone (1.8×105 M−1).11 These results reflect the structural differences between morphine and its derivatives, which are based on a morphinan ring, and the phenethyl piperidine fentanyl.28 Although the cavity of Calabadion 1 is able to flex like a hand when binding larger compounds, it is narrow in the absence of target compound. The empty cavity is complementary in terms of size and shape to a single aromatic ring (e.g. the phenylammonium ion moiety of fentanyl) resulting in high binding affinity. Conversely, Calabadion 1 is able to expand its cavity by flexing its methylene bridged glycoluril tetramer backbone to accommodate the wider and bulkier morphinan ring system, but this conformational change is energetically costly and reduces the binding affinity of Calabadion 1 towards these compounds. We found that Calabadion 1, even at the highest dose of 500 mg kg−1, did not affect long-acting opioids (morphine, hydromorphone, and pethidine) that are frequently used for postoperative analgesia.
In observational studies, high doses of opioids, including fentanyl, were associated with postoperative respiratory complications6 and 30 day readmission, particularly after ambulatory surgery.5 Further studies are required to test whether Calabadion 1 may reduce the increased risk of hospital readmission attributed to high-dose fentanyl administration.
As the elimination half-time of fentanyl is context sensitive, encapsulation (by Calabadion 1) compared with competitive antagonism (by naloxone) may enhance patient safety, particularly after administration of high fentanyl doses. For opioids with longer half-lives, reversal with naloxone carries the risk re-narcotisation after a single dose of naloxone.29 In contrast, we have shown that Calabadion–drug complexes are rapidly eliminated by renal excretion, thereby avoiding this risk of rebound effects.12 Our preliminary analyses confirmed the renal elimination of Calabadion 1–fentanyl complexes, which decreases the amount of circulating fentanyl.
In summary, this study provides proof of principle for dose-dependent reversal of adverse respiratory system and CNS effects of fentanyl by Calabadion 1 in a rat model. Clinical studies are required before Calabadion 1 can be considered as a potential reversal agent in the perioperative setting to enhance anaesthesia recovery and ensure patient safety.
Authors' contributions
Study conception: ME.
Study design: TT, JFC, ME.
Conducting of experiments: TT, JDB, MG, LI, JFC.
Supervision of experiments: OA, JFC, ME.
Data analysis: all authors.
Data interpretation: SDG, PS, PR, OA, JFC, ME.
Manuscript drafting: TT, SDG, PS, PR, LI, ME.
All authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript.
Acknowledgements
The authors would like to acknowledge D. Diaz-Gil and M. Hammer for providing advice on the statistical analyses and manuscript writing, M. Rudolph for help with experiments, and S. Ganapati for Calabadion 1 supply.
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: From breathtaking to encapsulation: a novel approach to reverse respiratory depression from opioid overdosing by Dahan et al., Br J Anaesth 2020:125:e16–e17, doi: 10.1016/j.bja.2020.03.020
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.02.019.
Declarations of interest
ME has received funding for research projects from Merck & Co. and a philanthropic grant from Jeff and Judy Buzen, and receives funding from the National Institutes of Health (UG3HL140177; Bethesda, MD, USA). JFC has received funding from the National Institutes of Health (R01 HL117871). LI has received funding from the National Institutes of Health (CA168365 and GM132345). ME, LI, and JFC are inventors on patents relating to the use of calabadion in substance abuse disorders. OA has received funding from the National Institutes of Health (R01 AG053582). The remaining authors declare that they have no conflicts of interest.
Funding
Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dahan A., Aarts L., Smith T.W. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 2.Lee T.Y., Fu M.J., Kuo T.B., Lui P.W., Chan S.H. Power spectral analysis of electromyographic and systemic arterial pressure signals during fentanyl-induced muscular rigidity in the rat. Br J Anaesth. 1994;72:328–334. doi: 10.1093/bja/72.3.328. [DOI] [PubMed] [Google Scholar]
- 3.Montandon G., Horner R.L. Electrocortical changes associating sedation and respiratory depression by the opioid analgesic fentanyl. Sci Rep. 2019;9:14122. doi: 10.1038/s41598-019-50613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee L.A., Caplan R.A., Stephens L.S. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122:659–665. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 5.Long D.R., Lihn A.L., Friedrich S. Association between intraoperative opioid administration and 30-day readmission: a pre-specified analysis of registry data from a healthcare network in New England. Br J Anaesth. 2018;120:1090–1102. doi: 10.1016/j.bja.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich S., Raub D., Teja B.J. Effects of low-dose intraoperative fentanyl on postoperative respiratory complication rate: a pre-specified, retrospective analysis. Br J Anaesth. 2019;122:e180–e188. doi: 10.1016/j.bja.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Jones C.M., Einstein E.B., Compton W.M. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010-2016. JAMA. 2018;319:1819–1821. doi: 10.1001/jama.2018.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S., Ruspic C., Mukhopadhyay P., Chakrabarti S., Zavalij P.Y., Isaacs L. The cucurbit[n]uril family: prime components for self-sorting systems. J Am Chem Soc. 2005;127:15959–15967. doi: 10.1021/ja055013x. [DOI] [PubMed] [Google Scholar]
- 9.Ma D., Hettiarachchi G., Nguyen D. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat Chem. 2012;4:503–510. doi: 10.1038/nchem.1326. [DOI] [PubMed] [Google Scholar]
- 10.Ganapati S., Zavalij P.Y., Eikermann M., Isaacs L. In vitro selectivity of an acyclic cucurbit[n]uril molecular container towards neuromuscular blocking agents relative to commonly used drugs. Org Biomol Chem. 2016;14:1277–1287. doi: 10.1039/c5ob02356d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganapati S., Grabitz S.D., Murkli S. Molecular containers bind drugs of abuse in vitro and reverse the hyperlocomotive effect of methamphetamine in rats. Chembiochem. 2017;18:1583–1588. doi: 10.1002/cbic.201700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Gil D., Haerter F., Falcinelli S. A novel strategy to reverse general anesthesia by scavenging with the acyclic cucurbit[n]uril-type molecular container calabadion 2. Anesthesiology. 2016;125:333–345. doi: 10.1097/ALN.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haerter F., Simons J.C.P., Foerster U. Comparative effectiveness of calabadion and sugammadex to reverse non-depolarizing neuromuscular-blocking agents. Anesthesiology. 2015;123:1337–1349. doi: 10.1097/ALN.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann U., Grosse-Sundrup M., Eikermann-Haerter K. Calabadion: a new agent to reverse the effects of benzylisoquinoline and steroidal neuromuscular-blocking agents. Anesthesiology. 2013;119:317–325. doi: 10.1097/ALN.0b013e3182910213. [DOI] [PubMed] [Google Scholar]
- 15.Boghosian J.D., Luethy A., Cotten J.F. Intravenous and intratracheal thyrotropin releasing hormone and its analog taltirelin reverse opioid-induced respiratory depression in isoflurane anesthetized rats. J Pharmacol Exp Ther. 2018;366 doi: 10.1124/jpet.118.248377. jpet.118.248377–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golder F.J., Dax S., Baby S.M. Identification and characterization of GAL-021 as a novel breathing control modulator. Anesthesiology. 2015;123:1093–1104. doi: 10.1097/ALN.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 17.Combs D.J., D’Alecy L.G. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke. 1987;18:503–511. doi: 10.1161/01.str.18.2.503. [DOI] [PubMed] [Google Scholar]
- 18.Weinger M.B., Segal I.S., Maze M. Dexmedetomidine, acting through central alpha-2 adrenoceptors, prevents opiate-induced muscle rigidity in the rat. Anesthesiology. 1989;71:242–249. doi: 10.1097/00000542-198908000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Merletti R., Knaflitz M., DeLuca C.J. Electrically evoked myoelectric signals. Crit Rev Biomed Eng. 1992;19:293–340. [PubMed] [Google Scholar]
- 20.Akeju O., Hamilos A.E., Song A.H., Pavone K.J., Purdon P.L., Brown E.N. GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol. 2016;127:2472–2481. doi: 10.1016/j.clinph.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahan A., Yassen A., Bijl H. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94:825–834. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 22.Scott J.C., Cooke J.E., Stanski D.R. Electroencephalographic quantitation of opioid effect: comparative pharmacodynamics of fentanyl and sufentanil. Anesthesiology. 1991;74:34–42. doi: 10.1097/00000542-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Brown E.N., Purdon P.L., Van Dort C.J. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebel P.S., Bovill J.G., Wauquier A., Rog P. Effects of high-dose fentanyl anesthesia on the electroencephalogram. Anesthesiology. 1981;55:203–211. doi: 10.1097/00000542-198109000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Çoruh B., Tonelli M.R., Park D.R. Fentanyl-induced chest wall rigidity. Chest. 2013;143:1145–1146. doi: 10.1378/chest.12-2131. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad M., Raza T. ‘Jaws of steel’ after very low dose of fentanyl during prebronchoscopy sedation. J Bronchol Interv Pulmonol. 2017;24 doi: 10.1097/LBR.0000000000000329. e9–10. [DOI] [PubMed] [Google Scholar]
- 27.Burns G., DeRienz R.T., Baker D.D., Casavant M., Spiller H.A. Could chest wall rigidity be a factor in rapid death from illicit fentanyl abuse? Clin Toxicol (Phila) 2016;54:420–423. doi: 10.3109/15563650.2016.1157722. [DOI] [PubMed] [Google Scholar]
- 28.Drewes A.M., Jensen R.D., Nielsen L.M. Differences between opioids: pharmacological, experimental, clinical and economical perspectives. Br J Clin Pharmacol. 2013;75:60–78. doi: 10.1111/j.1365-2125.2012.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rzasa Lynn R., Galinkin J.L. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63–88. doi: 10.1177/2042098617744161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.