Abstract
Background: Duchenne Muscular Dystrophy (DMD) is a fatal muscular dystrophy of pediatric population coupled with other secondary comorbidities including mental retardation and neuropsychological impairments. Mutation location in the dystrophin gene, have been associated with neuropsychological functioning in DMD.
Purpose: We investigated temporal changes in the neuropsychological functioning of DMD subjects, hitherto understudied.
Methods: Subjects with suspected DMD were enrolled according to the ethical guidelines. Genetic confirmation by Multiplex Ligation Dependent Probe Amplification was carried out to identify pathogenic deletion or duplication in dystrophin gene. Intellectual and neuropsychological functioning was assessed by using standardized batteries. Investigated neuropsychological domains included visual, verbal and working memory, selective and sustained attention, executive functioning, verbal fluency, and visuo-constructive and visuo-spatial abilities. The assessments were carried out at baseline and followed for one time point in 30 cases.
Result: The follow-up assessment revealed that neuropsychological functioning did not worsen with time. Improvements were seen in block designing task (p = 0.050), serial positioning primacy effect (p = 0.002), Stroop incongruent task (p = 0.006), visual long-term memory (p = 0.003) and attention (p = 0.001). DMD cases with mutation location affecting short dystrophin isoform (Dp140) also showed improvement in these domains.
Conclusion: No temporal alterations were found in DMD subjects, though improvements in few domains were observed. Neuropsychological rehabilitation may be useful in improving the quality of life in DMD subjects.
Keywords: DMD, neuropsychology, cognition, longitudinal, follow-up, dystrophin
Introduction
Duchenne muscular dystrophy (DMD) is a fatal X-linked genetic neuromuscular disorder, characterized clinically by rapidly progressive and disabling muscle weakness, present from birth and exclusively occurring in males. DMD is caused by an X-linked recessive frameshift mutation in the dystrophin gene that ensues absent or non-functional muscle dystrophin protein and resultant muscle fibre degeneration, leading to chronic peripheral inflammation.1 Dystrophin functions as a direct signalling molecule and connects the extracellular matrix to the cytoskeleton. It is a part of the dystrophin-associated glycoprotein complex.2, 3 It is the most common childhood muscular dystrophy with an estimated incidence of 200 per million male live births.4 By the age of 3, patients with DMD exhibit motor inabilities in such as walking, running, climbing, jumping, waddling gait, difficulty in standing, followed by upper limb weakness and pseudohypertrophy by the age of 5. This is followed by progressive worsening of the symptoms and with death due to respiratory failure or cardiac arrhythmia before the third decade of life.5
In addition to skeletal muscle pathology and loss of physical strength, a subset of children with DMD is characterized by global cognitive impairment. Previous works suggest that in DMD patients, intelligence quotient (IQ) distribution is downshifted one standard deviation with a lower verbal IQ than performance IQ. It is reported that DMD patients might also have specific neuropsychological deficits including poor performance in working memory, executive function, attention deficits, and impaired reading and language acquisition skills.1, 6 Previous studies have led to hypothesis that these specific neuropsychological deficits resonate with cerebellar lesions due to similarity in cognitive impairments.7 Even though dystrophin is often characterized in muscles, it is also found in various other tissues including the brain. Multiple studies have shown the association between the loss of dystrophin and cognitive impairments. Multiple studies from both clinical and animal models attribute the lack of dystrophin expression in the brain to the development of the cognitive and behavioural alterations in DMD.8–10 Some patients with DMD also have a higher incidence of neurobehavioral disorders including attention-deficit/hyperactivity disorder (ADHD), anxiety disorder, autism spectrum disorders (ASD), epilepsy and obsessive-compulsive disorder. Experimental studies have found that dystrophin is expressed in neurons within specific brain regions including the cortex, cerebellar Purkinje cells, Cornu Ammonis (CA) region of the hippocampus, retina and the peripheral nerve. These might be responsible for some of the neuropsychological deficits.11
It is important to note that myelination is critical in the central nervous system (CNS) for complex brain processing and therefore the disorders affecting the neuronal myelination, by a process regulated by oligodendrocytes in the CNS, may produce neurological deficits.12 In a recent study, researchers have found that for proper maturation of oligodendrocytes and effective myelination during postnatal brain development, normal expression of dystrophin isoforms is required. Oligodendrocytes express three different forms of dystrophin, Dp427, Dp140 and Dp71, and loss of oligodendroglial dystrophin, particularly Dp427, was found to be contributory to neurodevelopmental deficits in their experimental mdx mouse model of DMD. In this study, in mice without functional Dp427 dystrophin protein had late development of myelination with significantly affecting the cerebral cortex.13 A past review identified lack of Dp427 to be associated with progressive muscle weakness in all DMD patients, likely responsible for both muscle degeneration and brain dysfunction.14
Despite involvement of common gene isoforms, Wingeier et al. in their study found no correlation of declining cognitive function with the progression of muscular deterioration.7 Another study reported that cognitive impairment in DMD is non-progressive and unrelated to the severity of muscle disease. Additionally, varying phenotypic expressions of specific neuropsychological impairments is also notable in DMD patients.15 The reason for this divergence is inconclusive, but this might be associated with the timing and localization of human dystrophin isoforms expression.1 In contrast, previous studies reported that intellectual functioning in DMD patients deteriorates as the disease progresses with progressive reduction in all IQ scores.15 As previously noted, varying neuropsychological deficits affect overall cognitive performance of the boys with DMD. For example, boys with DMD often have problems in short-term verbal working memory and increased risk of learning disability resulting from poor phonological awareness/processing. They often encounter problems with reading as discussed in a study, whereby 40% of boys with DMD have been shown to have reading problems. It is also found that they have lower academic achievement scores than expected of their level of cognitive functioning.16 In addition to academic performance, they also face poor health-related global quality of life potentially posing them at risk of depression, anxiety and stress.17, 18 A successful care of DMD patients thus requires comprehensive, multidisciplinary plan including psychosocial care, in addition to a pharmacological approach.
In order to plan clinical trials to establish efficacy of interventions targeting different neuropsychological impairments, longitudinal studies in DMD patients are required. This will help to explore how, over the course of time, neuropsychological function changes with progression of DMD. Additionally, this can help with risk stratification and screening and offering specific neuropsychological rehabilitation. Future studies could include acquisition of longitudinal data in order to examine which cognitive and neuropsychological functions in DMD are non-progressive or progressive. This is important in counselling and future planning. Previous studies suggested that more research is needed about characterizing the features of neuropsychological profile in determining the use and effectiveness of cognitive rehabilitation and retraining for children with DMD.5 In-depth review of the literature has revealed that there are no longitudinal studies that have investigated whether the cognitive and neuropsychological impairment in DMD is progressive. To the best of our knowledge, this is the global first longitudinal study which has described the neuropsychological function in DMD patients. The aim of this longitudinal study was to use a battery of intelligence, learning and memory tests to characterize the neuropsychological profile in boys with DMD by following them up for long-term changes in various domains.
Methods
Subjects: A total of 30 DMD subjects were recruited according to the guidelines of Institutional Ethics Committee (IEC) of Postgraduate Institute of Medical Education and Research, Chandigarh, India. Informed assent and written informed consent was obtained from the participants before enrolment. The study was approved by IEC vide no. INT/IEC/2015/732 dated 19 November 2015. The recruitment guidelines adhered to the Helsinki Declaration. The DMD patients were enrolled with the help of Indian Association of Muscular Dystrophy (IAMD). Cases were also recruited retrospectively with the help of patient support groups. The prevalence-based sample size was derived, that is, 1/3500 males for DMD. For inclusion in the study, cases with characteristic clinical features of the Duchenne phenotype were identified. The cases with BMD or intermediate phenotypes and other myopathies were not considered for inclusion. The entire study was conducted according to the quality assurance protocols of the Neuroscience Research Lab. Genetic diagnosis was carried out by Multiplex Ligation Dependent Probe Amplification (MLPA) as described previously.19, 20
IQ: Malin’s intelligence scale for Indian Children (MISIC), an adaptation to Wechsler intelligence scale for children (WISC), was employed to assess the IQ. Briefly, verbal- and performance-based IQs (VIQ and PIQ) were derived to finally form the IQ. VIQ was derived by six subtests, that is, information, comprehension, arithmetic, digit span, vocabulary and similarity. PIQ was derived from four subtests, that is, picture completion, block designing (BD), coding and maze. The detailed description is provided in the supplementary material.
Neuropsychological Assessments: Neuropsychological assessments were carried out in 30 DMD cases. Memory (visual and verbal), attention (selective and sustained), executive functioning (cognitive flexibility, cognitive control, response inhibition, interference), verbal fluency (semantic and category) and visuo-constructive ability were assessed using standard test batteries including Rey Auditory Verbal Learning Test (RAVLT), Rey–Osterrieth Complex Figure Test (RCFT), Stroop Colour and Word Test (SCWT), Colour Cancellation Test (CCT), Children’s Colour Trail Test (CCTT), Visual Recognition test (VRT), Controlled Oral Word Association (COWA), Animal Naming Test (ANT). Follow-up assessments were carried out at single time point. The detailed description is provided in the supplementary material.
Statistical Analysis
We used SPSS version 21 to analyse the neuropsychological data. Normal distribution was analysed by Kolmogorov–Smirnoff statistics. Normally distributed data was further analysed by paired t test. Level of significance was analysed at p < 0.05.
Results
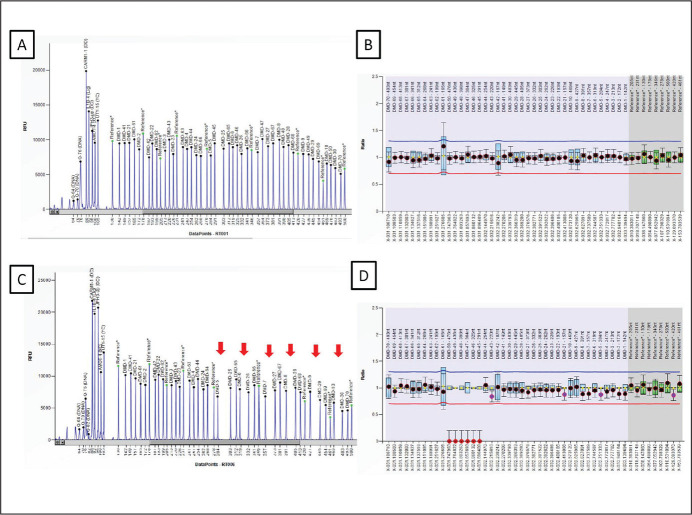
Participants: A total of 30 cases diagnosed with DMD were enrolled. Participant demographic details have been provided in Table 1. Genetic investigations were carried out in all DMD cases. Representative electropherogram is provided in Figure 1.
Table 1. Details of Participants.
| Variables | Mean (SD) |
| Cases | n = 30 |
| Gender | All males |
| Age | 11.54 (2.71) |
| Education | 4.93 (2.87) |
| Age of onset | 3.54 (1.41) |
| Disease duration | 8.31 (3.17) |
| Follow-up duration | 10 months |
| Dp140 isoform alteration | n = 20 |
Source: Authors’ own data.
Figure 1. Electropherogram Obtained after Multiplex Ligation Dependent Probe Amplification (MLPA) PCR Followed by Capillary Electrophoresis of the Amplified Products. (A & B) Electropherogram and Ratio Chart Representing Profile of a Normal Control Sample. (C & D) Electropherogram and Ratio Chart Representing Deletions Between Exon 45–50 (see arrow) in the Patients Clinically Diagnosed for DMD. Ratio Between 0.70 and 1.30 is Considered in the Normal Range While a Ratio of 0.00 is Considered as Deletion (Depicted in Red Dots).
Source: Authors’ own.
Longitudinal Analysis of Cognitive and Neuropsychological Profile in DMD Subjects
Follow up of 30 DMD subjects was carried out to assess the progression of impairment in the general and specific cognitive domains. The mean follow-up duration was 10 months. Among the MISIC subsets, the DMD group showed marginally significant improvement in the block designing task (t = –2.074, p = 0.050). Moreover, the mean levels achieved in the block designing task was improved to two levels with significant improvement in the block designing efficiency (t = –2.706, p = 0.014). However, the mean time in completing the block designing task was significantly increased in the follow-up (t = –2.741, p = 0.013). An improved serial positioning effect of primacy component in trial 1 showed a statistically significant improvement (t = –3.422, p = 0.002). DMD subjects also performed better and took less time in the colour cancellation task in the follow-up (t = 3.929, p = 0.001). Remaining variables were comparable to the pre-follow-up status (Tables 2–5).
Table 2. Comparison of General Intelligence on Pre and Post Follow-Up in DMD Subjects (n = 30) Using Paired t Test.
| Cognitive Domain and Neuropsychological Battery | Neuropsychological Battery Variables | DMD-Pre Mean ± SD | DMD-F Mean ± SD | t Value | P Value |
| Verbal intelligence Performance intelligence General intelligence |
Information | 93 ± 14.63 | 94 ± 11.99 | –0.576 | 0.570 |
| Comprehension | 84 ± 21.71 | 88 ± 12.43 | –1.192 | 0.245 | |
| Arithmetic | 85 ± 14.28 | 86 ± 11.60 | –0.438 | 0.665 | |
| Digit span | 88 ± 14.43 | 86 ± 14.97 | 0.974 | 0.340 | |
| Vocabulary | 78 ± 11.57 | 75 ± 14.17 | 1.022 | 0.334 | |
| Similarity | 94 ± 35.14 | 102 ± 33.26 | –1.063 | 0.303 | |
| VIQ | 89 ± 11.87 | 92 ± 13.36 | –1.464 | 0.154 | |
| Picture completion | 79 ± 21.14 | 83 ± 12.62 | –1.078 | 0.293 | |
| Block designing | 93 ± 30.23 | 103 ± 19.97 | –2.074 | 0.050 | |
| Coding | 84 ± 35.94 | 95 ± 23.60 | –1.569 | 0.132 | |
| Maze | 107 ± 39.00 | 110 ± 12.53 | –0.414 | 0.683 | |
| PIQ | 66 ± 10.80 | 63 ± 6.78 | 0.559 | 0.591 | |
| IQ | 97 ± 14.00 | 100 ± 15.62 | –1.419 | 0.167 |
Source: Authors’ own data.
Note: Bold values represent significant p values.
Table 3. Comparison of Neuropsychological Variables in DMD Subjects on Follow-Up (n = 30) Using Paired t Test for RAVLT Variables.
|
Cognitive Domain
and Neuropsychological Battery |
Neuropsychological Battery
Variables |
DMD-Pre
Mean (SD) |
DMD-F
Mean (SD) |
t Value | P Value |
|
RAVLT Verbal learning Working memory Short-term verbal memory Long-term verbal memory |
RAVLT-trial 1 | 6.68 (2.58) | 7.50 (3.27) | –1.856 | 0.074 |
| RAVLT-trial 5 | 12.00 (3.09) | 12.61 (2.45) | –1.030 | 0.312 | |
| RAVLT-learning capacity | 50.11 (12.44) | 52.39 (12.86) | –1.156 | 0.258 | |
| RAVLT-IR | 11.04 (3.12) | 11.71 (3.02) | –1.565 | 0.129 | |
| RAVLT-DR | 10.61 (3.00) | 11.36 (3.65) | –1.446 | 0.160 | |
| LTPR | 90.02 (23.39) | 90.50 (30.53) | –0.066 | 0.948 | |
|
RAVLT Serial positioning effect Working memory |
Primacy T1 | 2.53 (1.23) | 3.39 (1.34) | –3.422 | 0.002 |
| Middle-T1 | 1.96 (1.07) | 2.17 (1.33) | –0.691 | 0.495 | |
| Recency-T1 | 1.86 (1.09) | 1.93 (1.65) | –0.232 | 0.818 | |
| Primacy-total | 18.60 (4.05) | 19.92 (4.31) | –1.655 | 0.110 | |
| Middle-total | 15.53 (4.24) | 16.35 (4.89) | –1.107 | 0.278 | |
| Recency-total | 15.10 (4.66) | 16.60 (5.3) | –1.499 | 0.145 | |
|
RAVLT Susceptibility to interferences |
Proactive interference | 0.93 (0.34) | 0.94 (0.68) | –0.113 | 0.911 |
| Retroactive interference | 0.93 (0.19) | 0.94 (0.26) | –0.258 | 0.799 | |
| Forgetting speed | 0.97 (0.18) | 0.90 (0.29) | 1.164 | 0.254 | |
| RAVLT efficiency | 1.96 (0.28) | 2.03 (0.34) | –1.287 | 0.209 |
Source: Authors’ own data.
Note: Bold values represent significant p values.
Table 4. Comparison of Neuropsychological Variables in DMD Subjects on Follow-Up (n = 30) Using Paired t Test.
|
Cognitive Domain
and Neuropsychological Battery |
Neuropsychological Battery Variables |
DMD-Pre
Mean (SD) |
DMD-F
Mean (SD) |
t Value | P Value |
| COWA and ANT Executive Functioning Semantic Fluency Category Fluency |
COWA-K | 6.04 (3.65) | 6.07 (3.31) | –0.082 | 0.935 |
| COWA-M | 5.18 (3.76) | 5.57 (3.26) | –0.763 | 0.452 | |
| COWA-P | 4.57 (3.61) | 5.03 (3.15) | –1.045 | 0.305 | |
| COWA-Avg | 5.18 (3.48) | 5.45 (3.03) | –0.813 | 0.423 | |
| ANT | 9.60 (3.67) | 8.80 (3.26) | 1.046 | 0.304 | |
| Executive Functioning Cognitive Flexibility Cognitive Control Response Inhibition Interference |
Stroop-w | 52.14 (20.11) | 60.91 (20.70) | –2.523 | 0.020 |
| Stroop-C | 38.05 (13.78) | 47.50 (15.87) | –3.059 | 0.006 | |
| Stroop-CW | 23.79 (9.14) | 26.58 (14.98) | –1.138 | 0.267 | |
| Stroop effect 1 | 14.18 (9.70) | 18.50 (11.20) | –1.617 | 0.121 | |
| Stroop effect 2 | 0.48 (0.16) | 0.51 (0.26) | –0.650 | 0.522 | |
| Stroop effect 3 | 0.65 (0.20) | 0.61 (0.18) | 0.876 | 0.391 | |
| RCFT Visuo-constructive ability Visual short and long-term memory |
RCFT-Copy | 31.05 (6.70) | 32.52 (3.53) | –1.068 | 0.298 |
| RCFT-IR | 21.39 (8.87) | 23.98 (8.84) | –1.661 | 0.111 | |
| RCFT-DR | 21.05 (8.25) | 25.09 (6.11) | –3.417 | 0.003 |
Source: Authors’ own data.
Note: Bold values represent significant p values.
Table 5. Comparison of Neuropsychological Variables in DMD Subjects on Follow-Up (n = 30) Using Paired t Test.
|
Cognitive Domain
and Neuropsychological Battery |
Neuropsychological Battery Variables |
DMD-Pre
Mean ± SD |
DMD-F
Mean± SD |
t Value | P Value |
| DIGIT span test Short term memory Working memory |
DSF | 5.17 (1.03) | 5.30 (1.06) | –0.680 | 0.503 |
| DSB | 3.17 (1.70) | 3.04 (1.55) | 0.680 | 0.503 | |
| Maze Visuo-spatial planning |
MAZE-TT | 158.87 (93.58) | 165.73 (81.84) | –0.272 | 0.790 |
| MAZE-E | 6.69 (8.31) | 6.13 (8.66) | 0.872 | 0.397 | |
| Block design test | BD-TT | 147.19 (104.72) | 215.86 (105.21) | –2.741 | 0.013 |
| BD-levels | 4.95 (2.73) | 6.33 (2.73) | –3.512 | 0.002 | |
| BD-EFFIC | 0.22 (0.24) | 0.35 (0.26) | –2.706 | 0.014 | |
| CCTT and CCT attention Divided attention Focused attention Interference |
CCTT1 | 46.06 (21.76) | 47.17 (24.67) | –0.190 | 0.851 |
| CCTT2 | 83.00 (39.65) | 75.17 (34.82) | 1.677 | 0.112 | |
| CCT | 137.15 (60.20) | 93.45 (40.47) | 3.929 | 0.001 | |
| CCTT interference | 0.89 (0.53) | 0.79 (0.80) | 0.466 | 0.647 | |
| CCT error | 1.22 (2.02) | 2.28 (2.08) | –1.679 | 0.111 | |
| VRT Visual agnosia |
VRT | 8.05 (1.50) | 8.48 (1.36) | –1.441 | 0.165 |
Source: Authors’ own data.
Note: Bold values represent significant p values.
Effect of Mutation Location on Temporal Change in Neuropsychological Functioning
We analysed the trends of neuropsychological functioning in cases with distal mutation location affecting Dp140 isoform. Among 30 DMD subjects, 20 had mutations in the DMD gene affecting Dp140 isoform, that is, exon 44 or upstream. No changes in the cognitive and neuropsychological functioning were observed over time in majority of parameters except primacy, Stroop colour and word task-colour component, and RCFT-delayed recall, which showed improvement from baseline assessment as shown in Table 6.
Table 6. Representing Temporal Changes in Neuropychological Functioning Due to DMD Gene Mutation Affecting Dp140 Isoform.
| Scale | Variable | Pre (SD) | Post (SD) | t Value | P Value |
| RAVLT | Primacy effect | 2.50 (1.46) | 3.22 (1.43) | –2.060 | 0.050 |
| SCWT | SCWT-colour | 37.46 (13.96) | 46.00 (17.43) | –2.504 | 0.028 |
| RCFT | RCFT-delayed recall | 20.17 (9.21) | 25.32 (6.15) | –3.457 | 0.004 |
| CCT | Colour cancellation | 155.86 (60.01) | 105.21 (42.38) | 3.317 | 0.006 |
Source: Authors’ own data.
Discussion
We provide a comprehensive longitudinal analysis of cognitive and neuropsychological profile in DMD subjects. The detailed analysis of neuropsychological domains and their progressive nature in boys with DMD provide better understanding of the use and effectiveness of specific rehabilitation regime required for retraining these patients. Additionally, this will enable future interventional studies targeting specifically impaired neuropsychological function.
When investigating cognitive process, analysing different aspects of the function is critical. In the present study, 30 boys with DMD were assessed for the progression of impairment in the general and specific cognitive domains over a mean follow-up duration of 10 months. The findings of this study showed that after a mean follow-up of 10 months, boys with DMD had no change in their general, verbal and performance intelligence. Data regarding non-progressive nature of intelligence was consistent with previous findings. DMDs have lower verbal IQ score than performance IQ score, and all IQ scores progressively reduce as the disease progresses.15, 21 The risk of cognitive deficit is determined by the location of mutation in the DMD gene that ensues specific functional dystrophin isoforms as described earlier. For example, patients who get lower IQ score were found to have a mutation in the distal region of the gene, whereas those with full-length mutation had highest scores.22 However, our study confirmed superior cognitive performance on block design task, designed to assess visuospatial ability, with significant improvement in the designing efficiency.
The study also undertook the neuropsychological assessment of boys with DMD for the RAVLT. We found a significant improvement in serial positioning effect of primacy component. In this effect, the person is assessed for the tendency to better recall the first items in a list than those in the middle or last. The finding that DMD patients had improvement in primacy component reflects their ability to improve the long-term memory after repeated exposures. However, there is a paucity of evidence that showed this effect in DMD patients. A previous study investigating serial positioning memory of boys with DMD found their inability to sustain attention to the task; however, temporal changes were not investigated.23
Furthermore, executive function and information processing speed were assessed with Stroop Colour Test (SCT), Stroop Colour and Word Test (SCWT), COWA test. Stroop test is used to measure cognitive flexibility and selective attention.24 Examination was performed at baseline and during follow-up rounds. Our study found significant improvement in the SCT during follow-up, suggesting improvement in the executive function of this population. The improved performance on tests assessing executive functions such as cognitive flexibility is in contrast to a past study which showed poor performance on tests for executive function among DMD patients.25 Chamova et al. reported poor performance on all neuropsychological tests (general cognitive abilities, verbal memory, attention and executive functions) in patients with non-functional Dp140 isoforms.9 Remmelink et al. examined the effect of an absent full-length dystrophins (Dp427) on behavioural consequences in DMD patients and found a deficit in cognitive flexibility.26
In our study, all other neuropsychological functions remained unchanged over the period. However, improvement in colour cancellation task, block design task, visual long-term memory and primacy effect indicate possibilities of improvement in cognitive domains. The domains that remained unchanged can be further analysed in future studies, by profiling the expression of dystrophin isoforms in post-mortem brain samples of the DMD patients. This will help elucidate underlying genetic basis for the observed variable phenotypic changes in the specific neuropsychological function. Additionally, interventional studies can enhance characterization of clinical and genetic variability and develop newer interventions specific to neuropsychological deficits. This may also serve to explore genotype-phenotype relationship in subsets of DMD patients with other coexisting neurodevelopmental disorders such as ADHD and ASD.
The significant improvement of executive functions in our study suggests that genetic prediction models can be developed to facilitate risk assessment, early detection and targeted treatment in such patient populations. Bailey et al. have recently developed a bioinformatics tool, called DMD Open access Variant Explorer (DOVE), to facilitate effective analysis of pathologic DMD gene variants, resulting in scope of precision medicine treatment for DMD.27
The functional improvement observed during the follow-up period shows that boys with DMD may be more amenable to neurocognitive rehabilitation. The substantial economic burden of physical and neuro-developmental disability makes DMD patients vulnerable. Several studies have shown such economic burden of DMD on patients and their family.28, 29 Since the advent and progress in multidisciplinary management for DMD, the functional outcome, quality of life and longevity of the patients have significantly been improved.
Conclusion
The neuropsychological profiling of DMD patients provides a well-recognized pattern of cognitive strengths and weaknesses among DMD patients. This opens new vistas to explore other comorbid neurodevelopmental and neuropsychiatric disorders. The variation in phenotypic manifestation of neuropsychological deficits was found to vary with location of the DMD gene and effect of the mutation on CNS-expressed isoforms. Further research with larger sample size and multi time point analysis will be required to understand the involvement of various domains. The neuropsychological domains that remained unchanged need to be explored in future interventional studies with increased sample size in order to explore the changes on such domains and develop newer targeted neurocognitive interventions. Additionally, improved executive function in our study population reflects their receptibility to neurocognitive interventions. Future longitudinal studies with increased sample size and long-term follow-up are imperative.
Acknowledgements
We acknowledge Dr Mitali Mukerjee and their team at Institute of Genomics and Integrated Biology for providing resources and assistance. We thank Ms Sanjana Goyal, President, IAMD, for providing patient resources.
Author Contributions
Akshay Anand: Conceptualization, management of the study, editing and final approval the manuscript.
Rahul Tyagi: Co-conceptualization under supervision, genetic and neuropsychological data acquisition, experiments and analysis, statistical analysis, drafting and editing the manuscript.
Vivek Podder: Drafting the manuscript.
Harshia Arvind: Neuropsychological data acquisition.
Manju Mohanty: Supervision in neuropsychological assessment, analysis and validation of data.
Ethical Statement
The study was approved by Institute Ethics Committee of PGIMER, Chandigarh vide no. INT/IEC/2015/732 dated 19 November 2015.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Funding support was provided by Department of Atomic Energy, Mumbai, Government of India [Sanction No: 37(1)/14/53/2014-BRNS]. Fellowship support was provided by Indian Council of Medical Research (ICMR). We thank American Society for Human Genetics for travel award to first author.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Doorenweerd N, Mahfouz A, van Putten Met al. Timing and localization of human dystrophin isoform expression provide insights into the cognitive phenotype of Duchenne muscular dystrophy. Sci Rep 2017; 7(1): 12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allikian MJ, McNally EM. Processing and assembly of the dystrophin glycoprotein complex. Traffic 2007; 8(3): 177–183. [DOI] [PubMed] [Google Scholar]
- 3.Constantin B. Dystrophin complex functions as a scaffold for signalling proteins. Biochim Biophys Acta 2014; 1838(2): 635–142. [DOI] [PubMed] [Google Scholar]
- 4.Stark AE. Determinants of the incidence of Duchenne muscular dystrophy. Ann Transl Med 2015; 3(19): 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perumal AR, Rajeswaran J, Nalini A. Neuropsychological profile of Duchenne muscular dystrophy. Appl Neuropsychol Child 2015; 4(1): 49–57. [DOI] [PubMed] [Google Scholar]
- 6.Anand A, Tyagi R, Mohanty M, Goyal M, Silva KR, Wijekoon N. Dystrophin induced cognitive impairment: mechanisms, models and therapeutic strategies. Ann Neurosci 2015; 22(2): 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingeier K GE, Strozzi S, Kreis Ret al. Neuropsychological impairments and the impact of dystrophin mutations on general cognitive functioning of patients with Duchenne muscular dystrophy. J Clin Neurosci 2011; 18(1): 90–95. [DOI] [PubMed] [Google Scholar]
- 8.Ricotti V, Mandy WP, Scoto Met al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev Med Child Neurol 2016; 58(1): 77–84. [DOI] [PubMed] [Google Scholar]
- 9.Chamova T, Guergueltcheva V, Raycheva Met al. Association between loss of dp140 and cognitive impairment in Duchenne and Becker dystrophies. Balkan J Med Genet 2013; 16(1): 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doorenweerd N, Straathof CS, Dumas EMet al. Reduced cerebral gray matter and altered white matter in boys with Duchenne muscular dystrophy. Ann Neurol 2014; 76(3): 403–411. [DOI] [PubMed] [Google Scholar]
- 11.Rae MG, O’Malley D. Cognitive dysfunction in Duchenne muscular dystrophy: a possible role for neuromodulatory immune molecules. J Neurophysiol 2016; 116(3): 1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 2001; 81(2): 871–927. [DOI] [PubMed] [Google Scholar]
- 13.Aranmolate A, Tse N, Colognato H. Myelination is delayed during postnatal brain development in the mdx mouse model of Duchenne muscular dystrophy. BMC Neurosci 2017; 18(1): 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perronnet C, Vaillend C. Dystrophins, utrophins, and associated scaffolding complexes: role in mammalian brain and implications for therapeutic strategies. J Biomed Biotechnol 2010; 2010: 849426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotton SM VN, Greenwood KM. Association between intellectual functioning and age in children and young adults with Duchenne muscular dystrophy: further results from a meta-analysis. Dev Med Child Neurol 2005; 47(4): 257–265. [DOI] [PubMed] [Google Scholar]
- 16.Banihani R, Smile S, Yoon Get al. Cognitive and Neurobehavioral Profile in Boys With Duchenne Muscular Dystrophy. J Child Neurol 2015; 30(11): 1472–1482. [DOI] [PubMed] [Google Scholar]
- 17.Filippo TD, Parisi L, Roccella M. Psychological aspects in children affected by Duchenne de Boulogne muscular dystrophy. Ment Illn 2012; 4(1): e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abi Daoud MS, Dooley JM, Gordon KE. Depression in parents of children with Duchenne muscular dystrophy. Pediatr Neurol 2004; 31(1): 16–19. [DOI] [PubMed] [Google Scholar]
- 19.Tyagi R KS, Dalal A, Mohammed Fet al. Repurposing Pathogenic Variants of DMD Gene and its Isoforms for DMD Exon Skipping Intervention. Curr Genomics 2019; 20(1): 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma K, Tyagi R, Singh R, Sharma SK, Anand A. Serum Levels of TIMP-3, LIPC, IER3, and SLC16A8 in CFH-Negative AMD Cases. J Cell Biochem 2017; 118(8): 2087–2095. [DOI] [PubMed] [Google Scholar]
- 21.Cotton S VN, Greenwood KM. Intelligence and Duchenne muscular dystrophy: full-scale, verbal, and performance intelligence quotients. Dev Med Child Neurol 2001; 43(7): 497–501. [DOI] [PubMed] [Google Scholar]
- 22.Taylor PJ, Betts GA, Maroulis Set al. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS One 2010; 5(1): e8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson SW RD, Ionasescu VV. Serial position memory of boys with Duchenne muscular dystrophy. Dev Med Child Neurol 1988; 30(3): 328–333. [DOI] [PubMed] [Google Scholar]
- 24.Homack S RC. A meta-analysis of the sensitivity and specificity of the Stroop Color and Word Test with children. Arch Clin Neuropsychol 2004; 19(6): 725–743. [DOI] [PubMed] [Google Scholar]
- 25.Wicksell RK, Kihlgren M, Melin L, Eeg-Olofsson O. Specific cognitive deficits are common in children with Duchenne muscular dystrophy. Dev Med Child Neurol 2004; 46(3): 154–159. [DOI] [PubMed] [Google Scholar]
- 26.Remmelink E, Aartsma-Rus A, Smit AB, Verhage M, Loos M, van Putten M. Cognitive flexibility deficits in a mouse model for the absence of full-length dystrophin. Genes Brain Behav 2016; 15(6): 558–567. [DOI] [PubMed] [Google Scholar]
- 27.Bailey M, Miller N. DMD Open-access Variant Explorer (DOVE): A scalable, open-access, web-based tool to aid in clinical interpretation of genetic variants in the DMD gene. Mol Genet Genomic Med 2019; 7(1): e00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landfeldt E, Lindgren P, Bell CFet al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology 2014; 83(6): 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryder S, Leadley RM, Armstrong Net al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis 2017; 12(1): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]