Abstract
Background: Diabetes-instigated nerve damage is a chronic complication including impaired peripheral nerve function with lowered nerve conduction velocity (NCV), demyelination of nerve fibres and alterations in the behaviour. Many clinical and experimental studies have proved that Phyllanthus amarus and esculetin have potential effects against various diseases.
Purpose: The aim of this study is to assess the neuroprotective role of hydro-ethanolic extract of Phyllanthus amaras (PAE) and esculetin (ESC) on NCV, metabolism, behavioural and structural changes in diabetic rats.
Methods: The extent of protection using PAE and ESC in diabetic rats was determined by checking the HbA1c, NO, myeloperoxidase (MPO), total calcium, protein content, Na+-K+ ATPase activity, acetylcholine content and behavioural alterations using rotarod and maze learning tests on 7, 14 and 21 days. NCV was measured on the 21st day.
Results: The diabetic rats showed increased HbA1c, nitrite, MPO, calcium and decreased protein, Na+-K+ ATPase activity, NCV, acetylcholine, behavioural alterations and morphological changes of sciatic nerve so that diabetic peripheral neuropathy (DPN) is manifested. Continuous treatment for three weeks with Phyllanthus amarus and esculetin significantly minimized the damage to axons and myelin sheath and enhanced the sciatic NCV by reversing all the mentioned parameters.
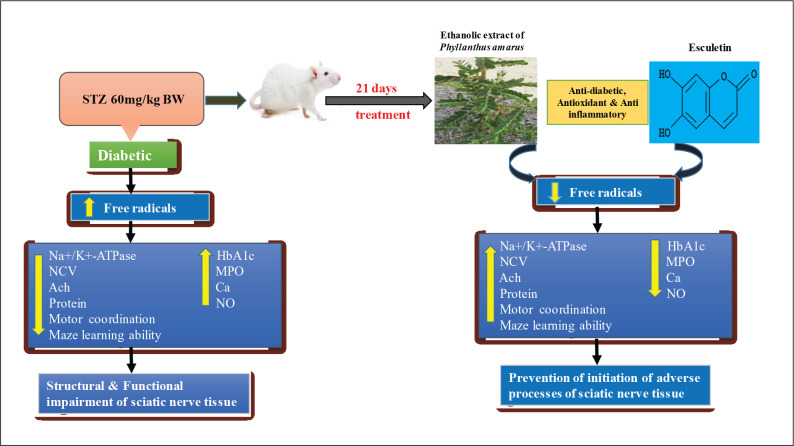
Conclusion: Phyllanthus amarus and esculetin showed the anti-diabetic as well as anti-inflammatory characteristics by prevention of initiation of adverse processes of the sciatic nerve morphology, internal cell functions leading to improved coordination, behavioural and physiological functions in STZ-induced diabetic rats. PAE has shown similar effects with the ESC. However, further studies are essential to confirm their detailed therapeutic effects.
Keywords: Neuropathy, diabetes, myelin, peripheral nerve
Introduction
Diabetic neuropathy is a syndrome consisting of progressive clinical disorders that affect various parts of the peripheral nervous system. It is a common, costly and serious complication of diabetes, and risk factor for ulcers and amputations.1 It occurs in more than 50 per cent of diabetic mellitus (DM) patients but is largely unreported or untreated such that only 8–10 per cent cases may be present at diagnosis level. Etiology of diabetic neuropathy is complex and multi-factorial which is not completely understood. Nerve function impairment is mostly displayed in both experimental and clinical models of diabetes.2 Evidence is that cognitive deficit, dementia and degeneration of neurons prevail in older people of type-2 diabetic patients.3 There is a functional deficit on sensory and motor coordination using rotarod performance test against peripheral sensory neuropathy.4 Chronic hyperglycemia, oxidative and nitrosative stress produce the ischemia and tissue hypoxia which leads to the disruption of microvasculature. This further causes the endothelial dysfunction and finally segmental demyelination of peripheral nerves. Increase in aldose reductase (AR) activity (activation of polyol pathway) indirectly affects the concentration of proteins, HbA1C level, abnormal axonal nerve conduction velocity, cholinergic neurotransmitter synthesis, elevation of ROS, RNS species, microangiopathy and deprivation of fibre density. These reported manifestations are responsible for the loss of balance and coordination, and impairment of cognitive behaviour leading to sensory loss and axonal atrophy of myelinated fibres.5
Currently, the usage of non-synthetic drugs obtained from plant sources has got the attention due to their generous availability and efficacy in decreasing the unfavourable drug reactions.6 In the clinical trials, different protective agents were used from different classes of drugs such as antidepressents, anticonvulsants, AR inhibitors, PKC inhibitors, capsaicin, lidocaine, opiods, α-lipoic acid, benfotiamine (Vit. B1 Analogue), and natural antioxidants such as curcumin, acetyl-L-carnitine, vitamins A, C, and E, melatonin, date extract and α-Lipoic acid which are relatively effective for characteristic relief.7 Hence, a novel approach for slowing down the accurate progression of diabetic peripheral neuropathy (DPN) disease is essential.
Phyllanthus amarus is a commonly available weed in India. Its anti-hyperglycemic,8 anti-nociceptive,9 anti-inflammatory and anti-carcinogenic properties were attributed due to its extensive anti-oxidant effect.10, 11 Besides, it exhibits kinetic inhibition on carbohydrate-metabolizing enzymes due to the presence of polyphenolic content,12 affinity for AR13 and can regenerate the sciatic nerve crush injury.14 Three pure pentacyclic triterpenoids—oleanolic acid, ursolic acid and lupeol—present in aerial parts were able to inhibit the α-amylase activity.15 Polyphenols and vitamin C present in extract of Phyllanthus amarus (PAE) exhibit pronounced systemic antinociceptive effect, particularly in experimental models of neuropathic pain.16 Aerial parts of PAE containing alkaloids, saponins, lignans, flavonoids, galloatinoids, terpinoids and glycosides are known to reduce hyperglycemic levels in streptozotocin (STZ)-induced diabetic rats17 and were responsible for its pleotropic properties against various diseases. Hydro-ethanolic PAE and a coumarin derivative ‘esculetin’ (bioactive compound), able to control hyperglycemia of blood and reversal of anti-oxidant status, and reduce the rate of progression of diabetic neuropathy in STZ-induced diabetic rats, were reported previously in our lab.18
Esculetin, a potent antioxidant is associated with the health benefits by consuming fruits and vegetables.19, 20 Previous study on esculetin has reported the inhibition of BChE, AChE and BACE1 enzymes and showed the protective effect against Alzheimer’s disease.21 It has a systemic inhibitory effect on hyperglycemia by regulating the enzymes of carbohydrate metabolism,22 AR in vitro and cataractogenesis,23 which has a broad range of usage in the emerging drugs. It has an anti-proliferative activity against hepatocellular carcinoma24 and proved its anti-nociceptive effect on inflammatory and non-inflammatory rat models.25 It showed antioxidant effect by impeding 3T3-L1 cellular adipogenesis26 and has neuroprotective activity on degenerated axons or dendrites of brain cells.27 It has shown its extensive therapeutic effect on various diseases. Esculetin can preserve the COX and GSH/GSSG ratio in mice model of stress and unabled contextual memory.28
In the present study, hydro-ethanolic PAE and, its one of the secondary metabolite derivative, esculetin (6, 7-dihydroxy coumarin) were tested further on functional recovery, structural changes and behaviour of rats against diabetic neuropathy to know their neuro-potential benefits.
Methods
Experimental Animals
Male wistar rats (200±50g) were procured from NCLAS, NIN, Hyderabad, Telangana, India. They were maintained at an ambient temperature of 22±2 oC with a 12 h light/dark cycle. Protocols of the study were duly approved by the Animal Ethics Committee (CPCSEA No.: 383/01/a/CPCSEA), Department of Zoology, Osmania University, Hyderabad, Telangana, India, and CPCSEA guidelines were followed for conducting the studies. The animals were given standard pellet diet and normal water ad libitum all through the study.
Drugs and Chemicals
STZ and esculetin were purchased from Sigma Aldrich, India. Hydro-ethanolic PAE was prepared in our lab and the dose level of Phyllanthus amarus and esculetin were selected from the previous report and described previously.18 Further chemicals and reagents used were of analytical grade in this experiment.
Induction of Experimental Diabetes
After overnight fasting, the rats were induced diabetic condition with STZ (60 mg/kg b.w, i.p in sodium citrate buffer (0.1 M) at pH 4.5). Diabetic state was confirmed after 48 h by determining glucose levels from tail vein blood using test strips (Dr. Morepen Gluco One). Animals having a glucose level of more than 250 mg/dL in blood were considered for the present study.2
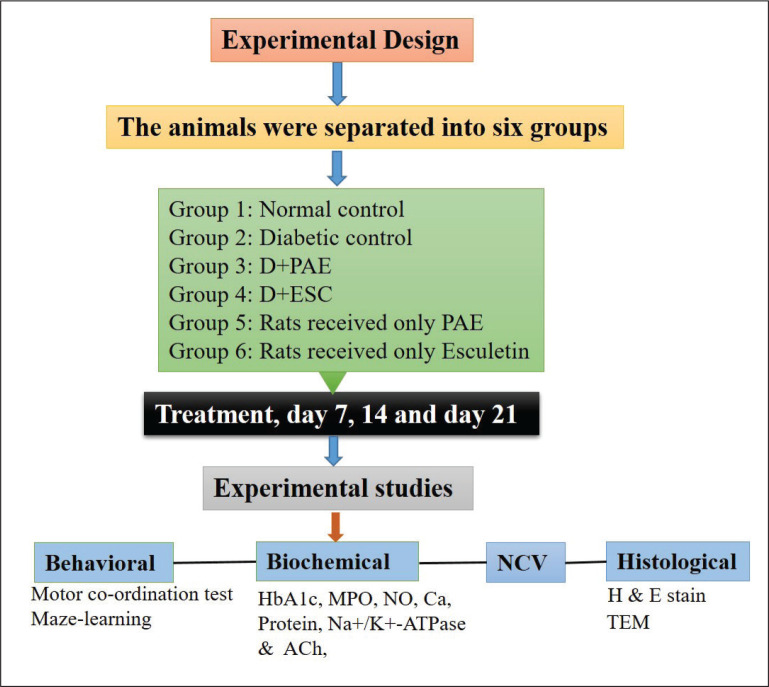
Experimental Design
The animals were separated into six groups, each containing six rats. Group 1: Normal control (NC). Group 2: Diabetic control (DC)—received STZ (60 mg/kg b.w, i.p), observed for 24 days. Group 3: D+PAE-Diabetic rats treated with ethanolic PAE (400 mg/kg b.w/ml/day p.o) for 21 days (day 3–day 24). Group 4: D+ESC-Diabetic rats treated with the esculetin (45 mg/kg b.w/ml/day i.p) for 21 days (day 3–day 24). Group 5 and Group 6: Rats received only PAE and only esculetin, respectively (day 3–day 24) which are non-diabetic rats. After PAE and ESC treatment, day 7, 14 and 21 rats were subjected to motor coordination test and maze learning test to assess the developed neuropathy. Nerve conduction velocity was determined on the 21st day and the blood was collected retro-orbitally for the estimation of glycosylated haemoglobin (HbA1c) level.29 Day 7, 14 and 21 rats were sacrificed and sciatic nerves were taken out30 for further biochemical and histopathological studies.
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Rotarod (Motor Coordination) Test
The rotarod (Dolphin TM instruments) test measures the riding time (in seconds) staying on the rotating rod by avoiding falling on the ground. Animal’s stay time on the rotating rod gives an indication of the level of balance, fore and hind limb coordination, physical condition and motor activity (innate antagonistic reflex activity). Initial time of the instrument was set to 0 seconds and the rotating speed was to 30 rpm (constant mode) prior to the experiment. Before experimental testing, rats were trained for three consecutive days by keeping the animal on the rotating rod for 2 minutes. Performance of the each rat was measured as the maximal time spent on the rod before falling off and endurance time was noted in seconds.31
Maze Learning Test
Maze learning is the practice of learning by rats over a complex branching passage, which appears like a puzzle. The overnight fasting animals trying to acquire the food/target is the primary method of observing spatial learning which is popularly used in behavioural laboratory. Before starting the experimentation, the rats from all groups were trained for two days and the time to reach the food was calculated.32, 33
Estimation of Glycosylated Hb
Procedure given by Nayak and Pattabiraman was followed for the determination of glycosylated HbA1c.34 To the mixture of hemolysate, normal saline and 1 ml of oxalic acid, 1 ml of TCA reagent was added. After centrifugation, to the obtained supernatant, TBA reagent was added and then optical density was measured at 443 nm to calculate the percentage of GHb.
Estimation of Nitrite/In Vivo Antioxidant Activity
Nitrite accumulated in the supernatant is the measure of nitric oxide (NO) produced and was determined according to the method of Green et al.35 Using Greiss reagent, nitrite concentration was studied at 546 nm on spectrophotometer and the amount of nitrite was expressed as µg/mg of protein.
Myeloperoxidase Estimation/Anti-inflammatory Activity
Myeloperoxidase (MPO) was assessed by the method of Bradley et al.36 One unit of MPO activity was expressed in terms of the amount of MPO degrading one micromole of peroxide at 25 oC per 1 min (U/g tissue) by measuring the absorbance at 460 nm.
Estimation of Calcium
Total amount of calcium in sciatic nerve was estimated as detailed by Severinghaus and Ferrebee with modification as suggested (Muthuraman et al.).37, 38, 39 The Ca levels are expressed in ppm/mg of protein.
Protein Estimation
Amount of protein available in the sciatic nerve tissue was estimated as reported by Lowry et al.40
Na+-K+ ATPase
Na+-K+ ATPase enzyme activity in the sciatic nerve was measured according to Kaplay41 and liberated inorganic Pi was determined by Taussky and Shorr.42 The activity was indicated as micromoles of Pi liberated/hr/g of Na+-K+ ATPase enzyme in sciatic nerve tissue of rat.
Nerve Conduction Velocity Measurements
After anesthetizing the rats with sodium pentabarbitone (60 mg/kg b.w. i.p) from each group, sciatic nerve was exposed and bipolar needle electrodes (PowerLab, AD instrument, Model: ML825, Serial: 225-0907, Australia, frequency: 20 Hz, amplitude: 1.5 V and duration: 0.1 ms) were placed at two sides of sciatic nerve. Rectal temperature continued (37 oC) with a warm blanket tissue, drying was avoided by adding mammalian ringer solution. Evoked potential response (m/s) was calculated by dividing the distance (mm) and the difference in time (ms) between two stimulated points.43
Acetyl Choline Estimation
Estimation of acetyl choline was carried according to the Hestrin method.44 The content of acetylcholine was expressed as mg of Ach/gm weight of sciatic nerve tissue.44
Light Microscopy
The rats were subjected to anesthetization using 10 per cent chloral hydrate (350 mg/kg b.w, i.p). After cleaning the skin using 70 per cent alcohol, vertical incision was made along the thigh; sciatic nerves were exposed and excised rapidly. They were transversely sectioned, processed independently, and stained using haematoxylin and eosin (H&E) stain.45 Then the sections were examined under light microscope (NLCD-307B, Lawrence Mayo, China).
Transmission Electron Microscopy (TEM)
The another part of sciatic nerves were fixed using 2.5 per cent glutaraldehyde (0.1 M phosphate buffer, pH 7.2), kept at 4 oC for about 24 hours followed by fixation in 1 per cent osmium tetraoxide, then dehydrated in an increased concentration of a series of graded alcohols. These samples were permeated, embedded with araldite resin and then incubated for 72 hours for proper polymerization at 80 oC. Using a glass knife, 60 nm ultra-thin sections were mould on microtome (Leica Ultra cut UCT-GA-D/E-1/00) and then mounted over the copper grids. Staining was done using saturated aqueous uranyl acetate and counter staining was done using Reynolds lead citrate and then observed under transmission electron microscope (H-7500, HITACHI, Japan).
Statistical Analysis
Data obtained was subjected to one-way analysis of variance (ANOVA) to compare the dissimilarities between and within the groups. The results were presented as Mean ± SEM. The assessment of mean values was done using Student’s t-test at a significance of p < 0.05.
Results
Changes in Motor Coordination, Maze Learning Abilities
The behavioural studies using rotarod and maze learning were conducted on day 7, day 14 and day 21 rats of all the groups. Streprozotocin-affected diabetic rats have produced a significant decrease (34.68%, 47.82% and 53.78% on day 7, 14 and 21, respectively, p < 0.001) in time latency on rotarod at 30 rpm and so affected the motor coordination of rats. Treatment of diabetic rats with Phyllanthus amarus extract (–17.47%, –47.44% and –82.21% on day 7, 14 and 21, respectively, p < 0.01) and esculetin (-22.49%, -51.63%, -91.47% on day 7, 14 and 21 respectively, p<0.005) for three weeks produced a significant redemption on motor coordination (Figure 1). The diabetic rats exhibited much longer time (–67.69%, –108.74% and –203.85% on day 7, 14 and 21, respectively, p<0.001) to reach the goal point in the maze setup than the control rats (Figure 2). Increase of the latency period to achieve the goal and significant decrease of learning ability on maze was observed in diabetic rats compared to the control rats. The diabetic rats treated with PAE (11.26%, 17.18% and 37.86% on day 7, 14 and 21, respectively, p < 0.01) and ESC (14.56%, 23.43% and 44.72% on day 7, 14 and 21, respectively, p < 0.01) showed decrease of the latency period to achieve the goal leading to the recovery of learning ability significantly in contrast to the diabetic rats. The esculetin-treated diabetic rats spent more time on rotarod and reached the goal faster on maze than PAE-treated diabetic rats. The results obtained by rats that received only PAE and ESC groups were similar to those of control group.
Figure 1. Effect of Phyllanthus amarus Extract and Esculetin on Motor-Coordination on Rotarod (Sec) in STZ-Induced Diabetic Rats.
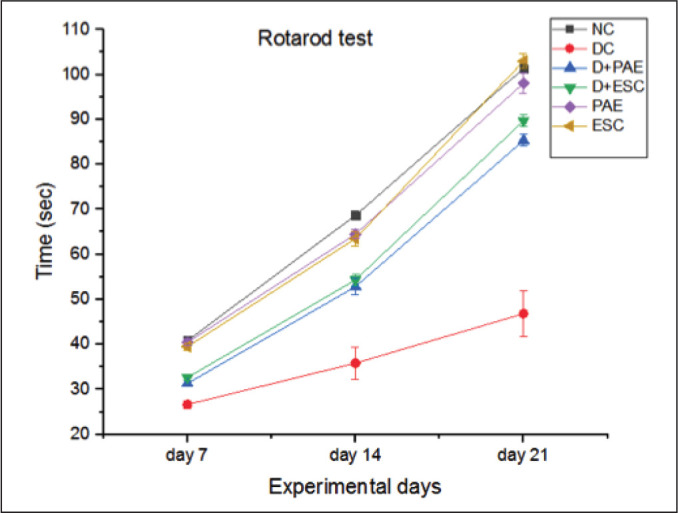
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
#P < 0.001 against normal group and *p < 0.01, **p < 0.005 against diabetic group.
Figure 2. Effect of Phyllanthus amarus Extract and Esculetin on Maze Learning Behaviour in STZ Induced Diabetic Rats (Min).
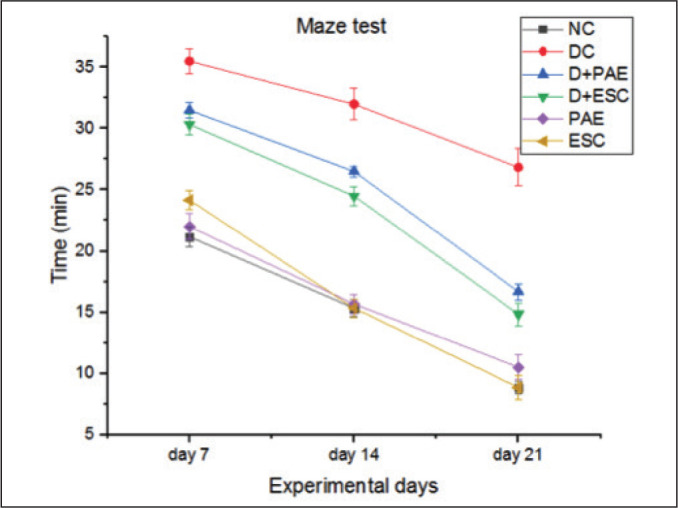
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
Changes in Glycosylated Haemoglobin
Glycosylated haemoglobin (HbA1c) was almost doubled in STZ rats (Table 1). After three days, polydipsia, polyuria and polyphagia were seen in the diabetic rats. HbA1c was decreased significantly when treated with Phyllanthus amarus as well as esculetin and maintained the glycosylated haemoglobin near to the normal range. This proves the role of Phyllanthus amarus and esculetin in controlling the blood glucose.
Table 1. Effects of Phyllanthus amarus Extract and Esculetin on Glycosylated Haemoglobin (%) in STZ-Induced Diabetic Rats.
| Groups | NC | DC | % Change from Control | D+PAE | % Change from Control | D+ESC | % Change from Control | PAE | % Change from Control | ESC |
% Change from
Control |
| HbA1c (%) | 4.78± 0.33 |
8.31± 0.21# |
–73.84 | 5.87± 0.17* |
–22.80 | 6.23± 0.36** |
–0.42 | 4.80± 0.34 |
–0.41 | 4.88± 0.35 |
–0.20 |
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
#P < 0.001 against normal group and *p, **p < 0.001 against diabetic group.
Myeloperoxidase Activity
MPO activity increased significantly in DPN rats when compared to the control rats (Table 2). Treatment with esculetin decreased the MPO levels more significantly. PAE has shown the lowered MPO levels as much as that of esculetin-treated group which maybe due to extensive phytoconstituents present in the plant extract.
Table 2. Effects of Phyllanthus amarus Extract and Esculetin on Myeloperoxidase (MPO) Activity (µmol of Peroxide/min/gm Tissue) in Sciatic Nerve Tissue of STZ-Induced Diabetic Rats.
| Groups | NC | DC | % Change from Control | D+PAE | % Change from Control | D+ESC | % Change from Control | PAE | % Change from Control | ESC | % Change from Control |
| MPO | 17.50± 1.12 |
90.17± 8.31# |
–415.25 | 58±2.67* | –231.42 | 47.33± 1.36** |
–170.45 | 17.83± 1.19 |
–1.88 | 18± 1.06 |
–2.85 |
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
#P < 0.001 against normal group and *p, **p < 0.01 against diabetic group.
Nitrite Content
Total amount of NO was significantly increased in diabetic control rats (–127.20%, –129.92 and –135.29 on day 7, 14 and 21, respectively, p < 0.001). Treatment with PAE (11.97%, 22.53% and 37.18% on day 7, 14 and 21, respectively, p < 0.05) and ESC (14.88%, 34.06% and 44.37% on day 7, 14 and 21, respectively, p < 0.05) attenuated the elevated NO levels (Figure 3).
Figure 3. Effect of Phyllanthus amarus Extract and Esculetin on Nitric Oxide (NO) Level (µg/mg Protein).
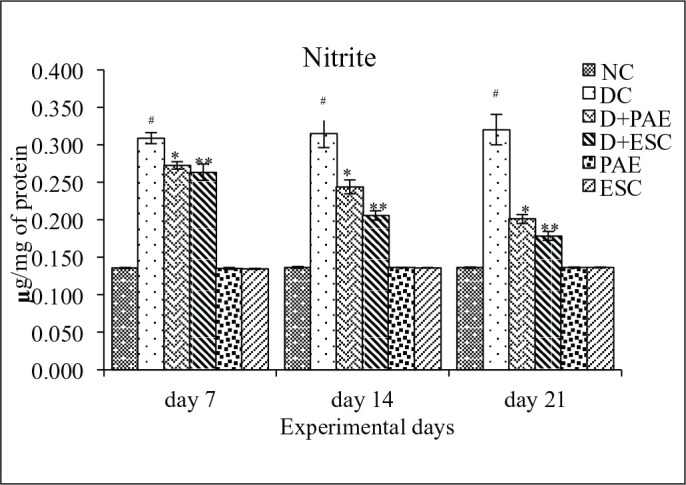
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
#P < 0.001 against normal group and *p < 0.05, **p < 0.05 against diabetic group (n = 6).
Calcium Content
A significant increase in the total calcium level was observed in the sciatic nerve tissue of diabetic rats in contrast to the normal group (Table 3). The diabetic rats received with PAE (400mg/kg, p.o) and ESC (45mg/kg, i.p) attenuated the enhanced levels. However, PAE alone and ESC alone group did not show any alterations in the total calcium level.
Table 3. Effects of Phyllanthus Amarus Extract and Esculetin on Calcium (Ca) Level (ppm/mg of Protein) in Sciatic Nerve Tissue of STZ-Induced Diabetic Rats.
| Groups | NC | DC | % Change from Control | D+PAE | % Change from Control | D+ESC | % Change from Control | PAE | % Change from Control | ESC | % Change from Control |
| Calcium | 4.45± 0.17 |
36.67± 3.43# |
–724.04 | 19.5±0.76* | –338.20 | 16.83± 0.60** |
–278.20 | 4.38± 0.11 |
–8.53 | 4.42± 0.11 |
0.67 |
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
#P < 0.001 against normal group and *p, **p < 0.005 against diabetic group.
Protein Content
The total protein content in control and diabetic rats was represented (Figure 4). The protein level of diabetic rats was lower than the control rats (24.44%, 28.57% and 32.6% lower on day 7, 14 and 21, respectively, p < 0.001). Treatment with PAE (–7.35%, –16.92% and –30.64% lower on day 7, 14 and 21, respectively, p < 0.05) and esculetin (–11.76%, –23.07% and –32.25% lower on day 7, 14 and 21, respectively, p < 0.01) significantly attenuated the mentioned effect.
Figure 4. Effect of Phyllanthus amarus Extract and Esculetin on Protein Content in Sciatic Nerve Tissue (mg/gm Weight of Tissue) of STZ-Induced Diabetic Rats.
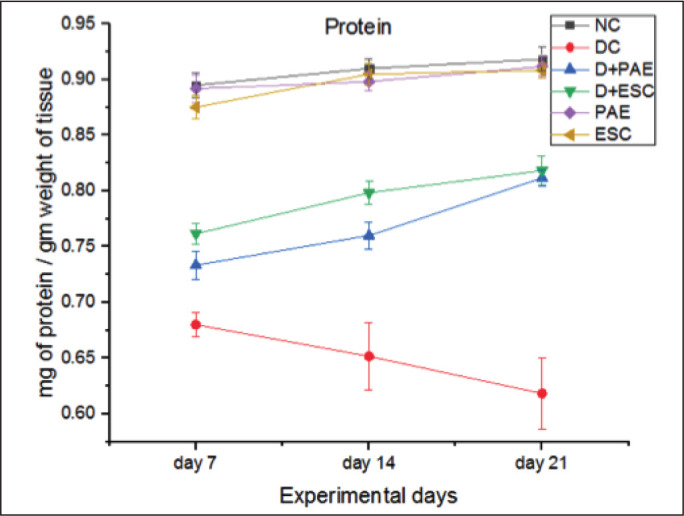
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
#P < 0.001 against normal group and *p < 0.05, **p < 0.01 against diabetic group.
Na+-K+ ATPase Activity
Diabetes resulted in significant decrease in Na+-K+ ATPase activity (44.03%, 50.27% and 57.73% lower on day 7, 14 and 21, respectively, p < 0.001), whereas treating the diabetic group with Phyllanthus amarus (0.48%, –38.8% and –71.82% lower on day 7, 14 and 21, respectively, p < 0.05) and esculetin (–17.48%, –41.82% and –81.67% lower on day 7, 14 and 21, respectively, p < 0.05) significantly improved the Na+-K+ ATPase activity in the sciatic nerve tissue (Figure 5).
Figure 5. Effect of Phyllanthus amarus Extract and Esculetin on Na+-K+ ATPase Activity (µm of Pi Liberated/hr/g) in Sciatic Nerve Tissue of STZ-Induced Diabetic Rats.
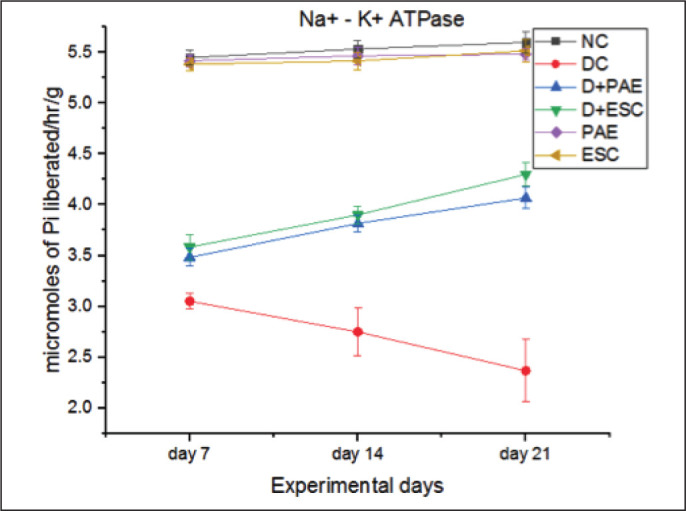
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
#P < 0.001 against normal group and *p, **p < 0.05 against diabetic group.
Nerve Conduction Velocity
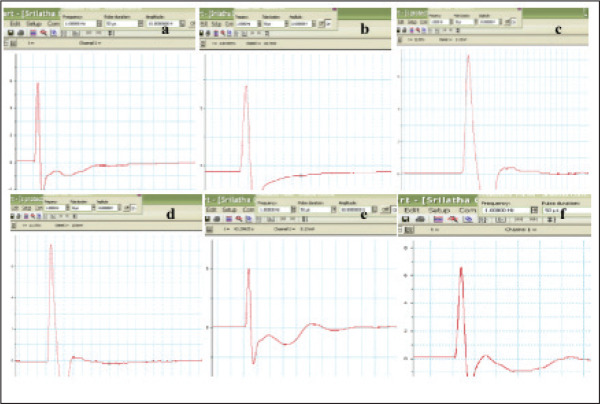
When compared with the control group, diabetic group showed decreased (44.44%, p < 0.001) mean nerve conduction velocity (MNCV). There was a significant increase of MNCV in diabetic groups treated with Phyllanthus amarus plant extract (–32.72%, p < 0.01) and esculetin (–41.82%, p < 0.001) in contrast to the diabetic group. These results show that PAE and esculetin have a protective role by improving the MNCV in diabetic neuropathy rats and also show the efficacy of doses at 400 mg/kg b.w/ml/day p.o and 45 mg/kg b.w/ml/day i.p, respectively (Figures 6a and 6b).
Figure 6a. Samples of NCV Recorded From (a) NC, (b) DC, (c) D+PAE, (d) D+ESC, (e) PAE, (f) ESC (n = 6).
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Figure 6b. Effect of Phyllanthus amarus Extract and Esculetin on Nerve Conduction Velocity in Sciatic Nerve Tissue of STZ-Induced Diabetic Rats (m/s).
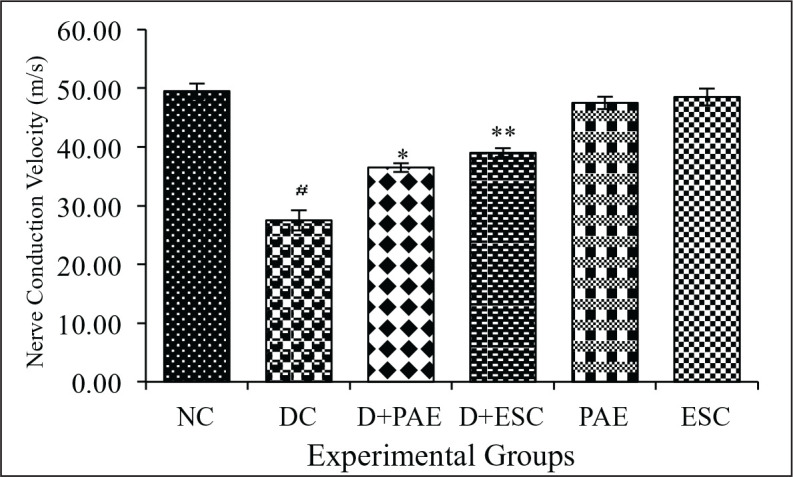
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
*P < 0.001 against normal group and **p < 0.01, ***p < 0.001 against diabetic group.ç
Acetylcholine Content
The acetylcholine content was decreased significantly in sciatic nerve of diabetic rats when compared with the control rats (31.42%, 40.54% and 47.37% lower on day 7, 14 and 21, respectively, p < 0.005). PAE (20.83%, 40.9% and 60% lower on day 7, 14 and 21, respectively, p < 0.05) and ESC (29.17%, 45.45% and 65.00% lower on day 7, 14 and 21, respectively, p < 0.05) treated diabetic rats have significantly attenuated the mentioned effect (Figure 7).
Figure 7. Effect of Phyllanthus amarus Extract and Esculetin on Acetylcholine Content in Sciatic Nerve Tissue (mg/gm Weight of Tissue) of STZ-Induced Diabetic Rats.
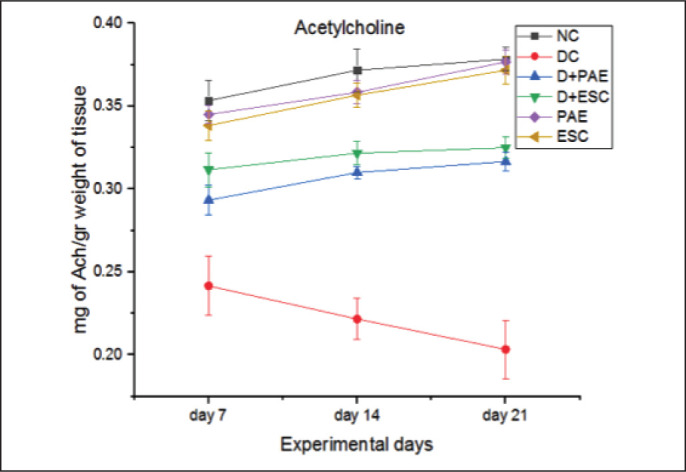
Abbreviations: NC, normal control; DC, diabetic control; D, diabetic; PAE, Phyllanthus amarus extract; ESC, esculetin; STZ, streptozotocin.
Note: The significant differences in between the groups were compared using one-way ANOVA followed by t-test. Data presented as Mean ± SEM (n = 6).
#P < 0.005 against normal group and *p < 0.05, **p < 0.05 against diabetic group.
Light Microscopy
Three weeks after confirmation of diabetes, no pathological changes were observed in control group (Figures 8a, 8b, 8c) rats received with PAE (Figures 8m, 8n, 8o) and ESC (Figures 8p, 8q, 8r). The transverse section of sciatic nerve from the normal control group showed a part of nerve fascicle surrounded by connective tissue, perineurium. It contains axons of various sizes surrounded by myelin sheath and separated by an endoneurial connective tissue. Nuclei of Schwann cells, endoneurium and blood vessels were seen. However, in the diabetic control group, sciatic nerve showed shrinkage, demyelination, necrosis and degenerated nerve fibres surrounded by thin perineurium. Focal loss of endoneurium was also observed due to destructive reactions of STZ three weeks after diabetes induction (Figures 8d, 8e, 8f). Treatment of diabetic rats with Phyllanthus amarus (Figures 8g, 8h, 8i) and esculetin (Figures 8j, 8k, 8l) were able to preserve the normal appearance of axons, myelin spaces and perineurium.
Figure 8. Histological Assessment of T.S of Sciatic Nerve. (a, b, c) Normal Control (NC); (d, e, f) Diabetic Control (DC); (g, h, i) PAE Received Diabetic Group (D+PAE); (j, k, l) ESC Received Diabetic Group; (m, n, o) PAE Received Control; (p, q, r) ESC Received Control.
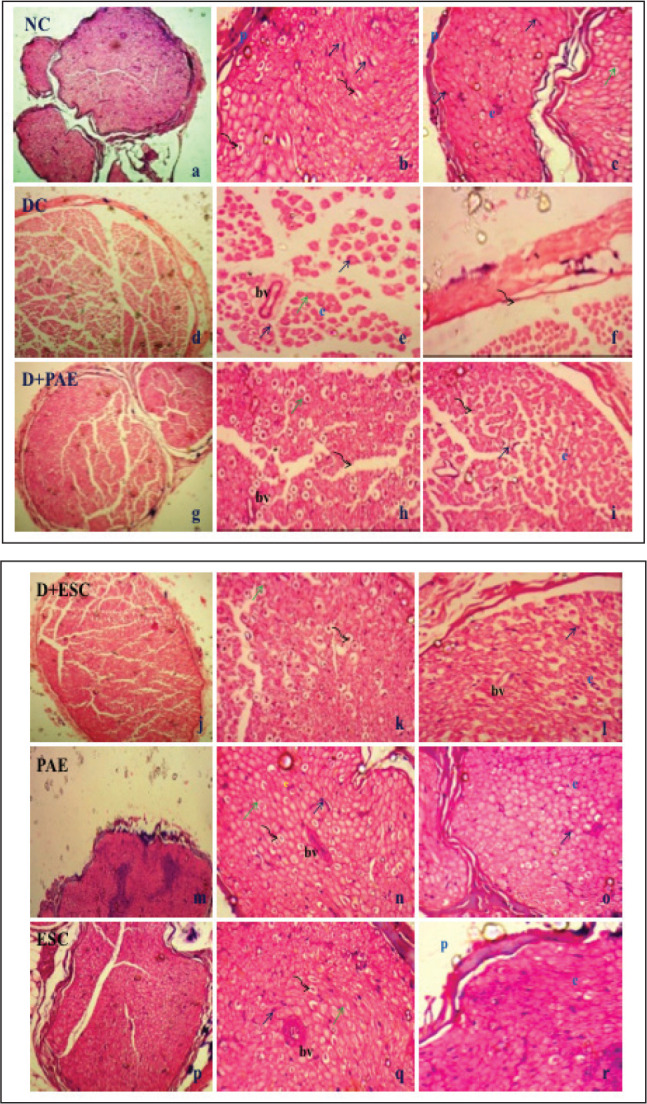
Note: p, perineurium; e, endoneurium; bv, blood vessel; black arrow: Schwann cell nuclei; curved arrow: axon; green arrow: myelin sheath; haematoxylen and eosin staining (10X, 40X, 40X).
Transmission Electron Microscopy
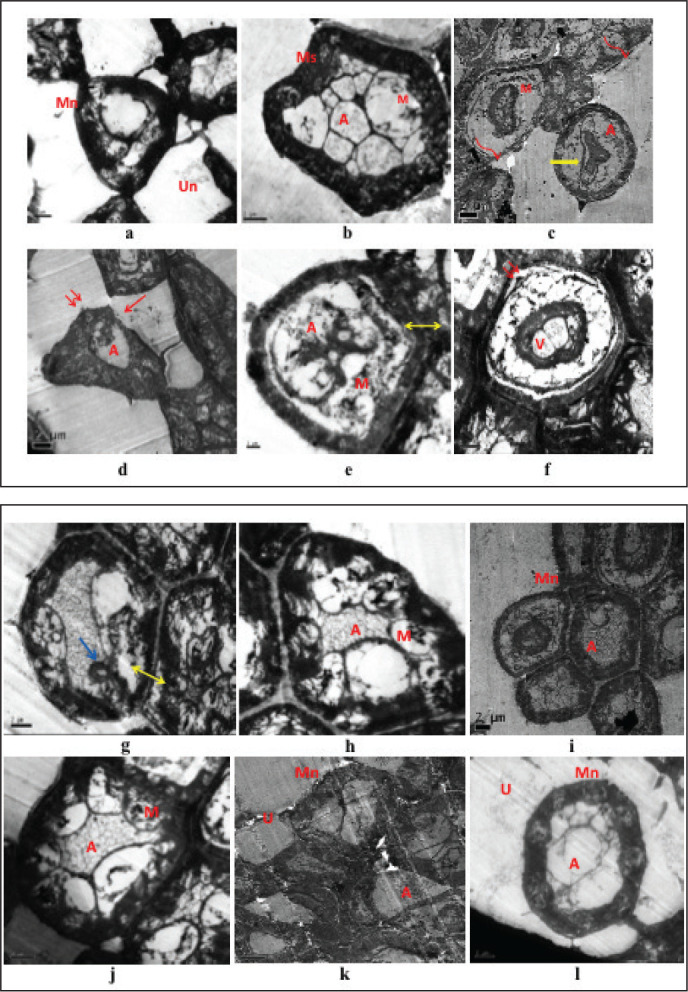
The electronic microscopic structures of the sciatic nerve tissue of normal rats (Figures 9a, 9b) received only PAE (Figures 9i, 9j) and esculetin (Figures 9k, 9l) displayed normal compact structure of nerve fibre with uniform and thick myelin sheath. Lamellae were also arranged with concentric light and dark circles and axonal structures. Sciatic nerve tissue of diabetic rats showed disorganized myelin sheath. It was enlarged and distorted near axonal and stromal sides (Figures 9c, 9d). Fragmentation of lamellae, and demyelination and separation of myelin sheath were clearly observable. Schwann cells were showing the uneven nuclei, vacuolar mitochondria, expanded endoplasmic reticulum and disrupted basement membrane. At a dose of 400mg/kg b.w/ml/day, p.o of Phyllanthus amarus (Figures 9e, 9f) and 45mg/kg b.w/ml/day, i.p of esculetin (Figures 9g, 9h) alleviated the vacuolar deficits, and discrete myelin structure and inhibited the separation of lamellar structures. Increase of Schwann cell number, myelin and axon density was observed.
Figure 9. TEM studies for all groups (a, b) normal control (NC); (i, j) PAE received control; (k, l) ESC received control groups showing normal myelinated (Mn), unmyelinated (Un) nerve fibres, axoplasm (A) containing microtubules and mitochondria (M) wrapped in a thick myelinated sheath with compact lamellar structure and surrounded by Schwann cells. (c, d) Diabetic control (DC) group showing shrinkage of cells, myelin breakdown, discontinuous and disorganised myelin sheath (curved arrow), loss of axoplasm, focal lysis (red arrow) of myelin sheath, irregular nuclei in the blood vessels (yellow arrow), separation of myelin lamellae, loss of compact lamellar structure (double arrow), compressed and distorted axoplasm (A), swollen and destroyed mitochondria (M). (e, f) PAE treated diabetic group (D+PAE); (g, h) ESC treated diabetic (D+ESC) groups showing many axons with normal appearance of myelin sheath and endoneurium in-between, some focal lysis, focal appearance of redundant myelin (blue arrow), separation of myelin sheath from axon (double side arrow), vacuolation of the cytoplasm (V), presence of some microtubules and mitochondria (M). Scale: 2 µm.
Discussion
The protective effects of hydro-ethanolic PAE plant (400 mg/kg b.w/ml/day p.o) and esculetin (45 mg/kg b.w/ml/day i.p) on diabetic neuropathy in STZ -induced diabetic male wistar rats were investigated in the current study through behavioural, biochemical, electrophysiological and sciatic nerve histopathological studies. Evidence indicates that increased glucose levels in the body has toxic effects on peripheral nerves due to increased glucose auto-oxidation, which leads to increase in the synthesis of reactive species. STZ causes elevation of glucose levels in the blood by selectively destructing the cells of islets of pancreas.46 In our previous study, rats with STZ-induced diabetes had significantly elevated the blood glucose levels and water intake levels, and decreased body weights compared with the control rats.18
Behavioural alterations were observed in diabetic rats as soon as diabetes started. Diabetic rats spent less time on rotarod and took more time to reach the goal on maze, compared to the control rats due to synergistic association between metabolic changes related to diabetes and impaired conditions within the peripheral nervous system. These findings are in line with the earlier report.47 The diabetic rats treated with PAE and ESC showed that improved behaviour may be due to consolidated action of learning and eventual memory formation, and coordination between sensory and motor neurons. The organized activity between the complex sensory and motor neurons was deliberated and stimulated by synapses formation, it’s strength, communication48 and by the release of neurotransmitters like acetylcholine.
It is evident from the present investigation using albino rats that STZ administration (60 mg/kg body weight) causes a diabetogenic response significantly. From the results given in Table 1, diabetic rats exhibit increased and almost doubled HbA1c level due to the interaction of excess glucose with haemoglobin to form glycosylated haemoglobin over time in diabetes milletus.49 A significant elevation in glycosylated haemoglobin noticed in diabetic rats was normalized to near normal with the administration of ethanolic PAE and esculetin after three weeks. Presence of flavonoids, lignans, alkaloids, galloatinoids, saponins, terpinoids, flavonoids and glycosides in the aerial parts of Phyllanthus amarus were maybe responsible to reduce the hyperglycemia levels in STZ-induced diabetic rats.17 Esculetin, was able to maintain the normal blood glucose level indicates its anti-diabetic activity role. HbA1c level was decreased significantly when treated with P. amarus and esculetin, maintaining the glycosylated haemoglobin in their normal range, and were quite comparable. This proves that the role of Phyllanthus amarus and esculetin in controlling the blood glucose is possibly mediated via improvement in insulin secretion from the existing β-cells of pancreas through increased expression of insulin receptors and an increased flux of glucose into the glycolytic pathway by avoiding the polyol pathway.
MPO is an inflammatory marker, a specific reductase for neutrophils and is expressed abundantly in polymorphonuclear neutrophils (PMN) in their azurophilic granules. So, estimation of MPO expression in the sciatic nerve tissue can therefore emulate the degree of extravasation of PMN from their microvasculature.50 The increased MPO levels in diabetic rats have shown the expanse of PMN extravasation as well as erratic expansion of neutrophils submitting that there was an in vivo inflammatory reaction caused due to diabetes. The diabetic rats treated with esculetin and PAE rescued the MPO levels thereby lowering the degree of invasion of PMNs from microvasculature tissue thereby reducing the viscosity of blood.
The rise in the total nitrite and calcium levels, and decrease in protein content were observed in the diabetic group in contrast to the control rats. The increased Ca levels will get accumulated in nerve tissue and activate the secondary messengers. Further, they activate the calcium dependent kinases and phosphatases which can change the homeostasis in the nervous system leading to unstability of cytoskeletal proteins of axons which leads to their degeneration.51, 52 Various studies have displayed that calcium, free radical induced oxidative, nitrosative stress and inflammatory mediators play a key role in the progression of many neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases, neuropathic pain, amyotrophic lateral sclerosis, pathophysiology of chronic diabetes and are well reported in chronic constriction injury and axotomy of sciatic nerve.53 Reduced levels of Ca, NO and increased levels of protein content were observed in PAE and ESC treated diabetic rats may be attributed to their anti-oxidative, anti-nitrosative and anti-inflammatory roles.
Besides biochemical and behavioural effects, PAE and esculetin have significantly restored the sciatic nerve conduction velocity and were able to recover the nerve structure in STZ-induced neuropathic rats. Slowing down of nerve conduction velocity is an early and usual sign in neuronal defects and dysfunction as observed in diabetic rats and humans.54 Increase in polyol pathway, AR and sorbitol dehydrogenase catalysis will convert the extra glucose into sorbitol and fructose. As sorbitol is unable to cross the cell membrane, it leads to accumulation, causing hyperosmolarity as well as concomitant release of aurine, myoinositol and adenosine. As a result, ATP synthesis is inhibited resulting in lowered Na+-K+ ATPase, PKC, weakened axonal transport and structural loss of nerves.55 Phyllanthin, a component of Phyllanthus amarus, has affinity towards AR enzyme and is able to decrease the oxidative stress by enhancing the synthesis of NADPH. From the results obtained previously, Phyllanthus amarus and esculetin were able to decrease the AR activity in diabetic rats in vivo.18 Further they can restore the nerve conduction velocity by improving Na+-K+ ATPase activity. As a result, the cholinergic activity was improved by increasing the synthesis of acetylcholine. Thus, PAE and ESC were playing an important role in the deactivation of polyol pathway by enhancing neurotrophic support in the amelioration of acute diabetic neuropathy.
The histological observations in transverse section of sciatic nerve and electron microscopic studies of diabetic rats have shown shrinkage of cells, demyelination, necrosis and degenerated nerve fibres surrounded by thin perineurium and focal loss of endoneurium, disorganized myelin sheath, lamellae, uneven Schwann cells with nuclei, vacuolar mitochondria, expanded endoplasmic reticulum and disrupted basement membrane and these results were parallel with the previous reports.56 After confirmation of diabetes, treatment of diabetic rats for three weeks with Phyllanthus amarus and esculetin were able to retain the normal appearance of axons, myelin spaces and perineurium, normal compact structure of nerve fibre with uniform and thick myelin sheath and lamellae, recovery of Schwann cell number and increase of myelin, axon density were observed. No pathological changes were observed in the control group and control rats received with PAE alone and ESC alone. Sciatic nerve from the normal control group showed a compact structure with perineurium, axons surrounded by myelin sheath and separated by an endoneurial connective tissue.
Esculetin has shown effective activity against diabetes-induced adverse effects in comparison with the Phyllanthus amarus extract. The results indicated the activity of pure compounds against a crude compound even though both PAE and ESC had almost similar effect against STZ-induced diabetes (Figure 10). Coumarins and coumarin-derived compounds possess an extensive pharmaceutical and biological action and have prominent significance in the treatment of various diseases and were clinically very significant. They exhibit notable pharmacokinetic activity due to their rapid absorption and metabolism in the body. In the present study, esculetin (6,7-dihydroxy coumarin) proved a significant effect against diabetic neuropathy by its anti-oxidant,18 anti-diabetic and anti-inflammatory activity.
Figure 10. Possible Mechanism Showed by PAE and ESC in Preventing the Progression of DPN.
The hydro-ethanolic PAE was found to show a sensitive and preventive response on diabetic-induced effects at 400mg/kg b.w concentration. Reduction in the glucose level, which ultimately regulates polyol pathway so that the usual function of the sciatic nerve is attained with proper nerve conduction velocity, was maybe the mechanism involved. The biological activity of Phyllanthus amarus using as herbal medicine appears to be obtained from secondary metabolites having therapeutic activity such as coumarins, lignans, flavonoids, triterpenoids, vitamin C and many alkaloids.57 The obtained results have demonstrated the importance of these metabolites significantly as a therapeutic approach.
Conclusion
On the basis of the results obtained, it can be suggested that Phyllanthus amarus and esculetin produced a protective effect on STZ -induced DPN by the prevention of initiation of adverse processes of sciatic nerve, and the possible recovery of internal and external structures could be accredited to their multifarious effects such as anti-diabetic, anti-oxidative, anti-inflammatory and neuroprotective roles manifested in terms of mitigation of behavioural, biochemical and histopathological changes. PAE and ESC have shown almost similar effects. Further, elaborate studies are required for arrival of therapeutic mechanism of Phyllanthus amarus and esculetin on prevention of diabetic neuropathy.
Acknowledgement
Authors would like to thank UGC-DSA-I SAP-II. Lr. No. F.5-26/2015/DSA-1 (SAP-II) programme for the partial financial assistance.
Author Contributions
Srilatha Kota designed and performed the work and also prepared this article. Pratap Reddy Karnati designed, checked the progression of work, discussed the results and conclusion, and participated in the preparation of this article.
Ethical Statement
Procedures of the present study were duly approved by Animal Ethics Committee, Osmania University, Hyderabad, Telangana, India.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received partial financial support for the research and no financial support for authorship, and/or publication of this article.
References
- 1.Maritim AC, Sanders RA, Watkins JB. 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol 2003; 17(1): 24–38. [DOI] [PubMed] [Google Scholar]
- 2.Erbaş O, Oltulu F, Yılmaz M, Yavaşoğlu A, Taşkıran D. Neuroprotective effects of chronic administration of levetiracetam in a rat model of diabetic neuropathy. Diabetes Res Clin Pract 2016; 114: 106. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer RA, Karter AJ, Yaffe K. Hypoglycaemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009; 301: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry T, Holloway HW, Weerasuriya A. et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol 2007; 203: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int 2015; 515042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar GR, Reddy KP. Reduced nociceptive responses in mice with alloxan induced hyperglycemia after garlic (Alliumsativum Linn.) treatment. Indian J Exp Biol 1999; 37: 662–666. [PubMed] [Google Scholar]
- 7.Cohen K, Shinkazh N, Frank J, Israel L, Fellner C. Pharmacological treatment of diabetic peripheral neuropathy. P and T 2015; 40(6): 372, 375–88. [PMC free article] [PubMed] [Google Scholar]
- 8.Owolabi OA, James DB, Anigo KM, Lormanger GW, Olaiya II. Combined effect of aqueous extracts of phyllathus amarus and vitex doniana sem bark on blood glucose of streptozotocin (STZ) induced diabetes rats and some liver biochemical parameters. Br J Pharmacol Toxicol 2011; 2(3): 143–147. [Google Scholar]
- 9.Patel K, Pate M, Gupta SN. Effect of atibalamula and bhumyamalaki on diabetic neuropathy. AYU 2011; 32(3): 353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calixto JB, Santos ARS, Filho V, Yunes RA. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology, and therapeutic potential. Med Res Rev 1998; 18: 225–258. [DOI] [PubMed] [Google Scholar]
- 11.Devi S, Rashid R, Kumar M. Phytochemistry and pharmacological properties of Phyllanthus amarus Schum: A review. Pharma Innov J 2017; 6(12): 169–172. [Google Scholar]
- 12.Mohamad F, Mahomoodally DD. Kinetic of inhibition of carbohydrate-hydrolysing enzymes, antioxidant activity and polyphenolic content of Phyllanthus amarus Schum. & Thonn. (Phyllanthaceae). J Herbal Med 2014; 208–223. [Google Scholar]
- 13.Masrur H, Ulfa A, Ardiansyah R. Pharmacopore modeling and molecular docking studies on Phyllanthus niruri as a target for diabetes mellitus. Aust J Basic Appl Sci 2015; 9(31): 389–395. [Google Scholar]
- 14.Panakpaporn W. Phyllanthus amarus facilitates the recovery of peripheral nerve after injury. Am J Appl Sci 2012; 9(7): 1000–1007. [Google Scholar]
- 15.Ali H, Houghton PJ, Amala S. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol 2013; 107(3): 449–455. [DOI] [PubMed] [Google Scholar]
- 16.Roengrit T, Wannanon P, Prasertsri P, Kanpetta Y, Sripanidkulchai BO., Leelayuwat N. antioxidant and anti-nociceptive effects of Phyllanthus amarus on improving exercise recovery in sedentary men: A randomized crossover (double-blind) design. J Int Soc Sports Nutr 2014; 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadkar KA, Magdum CS, Patil SS, Naikwade NS. Antidiabetic potential and Indian medicinal plants. J Herbal Med Toxicol 2008; 2: 45–50. [Google Scholar]
- 18.Srilatha K, Pratap RK. Neuroprotective potential of Phyllanthus amarus and Esculetin in STZ-induced neuropathy in rats. Int J Res Pharm Sci 2018; 9(4): 1331–1342. [Google Scholar]
- 19.Gennian M, Shiyun Z, Huihui S. et al. Synthesis, biological activities and therapeutic properties of esculetin and its derivatives. J Chem Pharm Res 2015; 7(4): 122–130. [Google Scholar]
- 20.Cemaluk C. Egbuonu A, E. Ogbu A, I. Ijeh I, US Ezeanyika L. Sub-chronic esculetin (6,7-Dihydroxy-Coumarin)-induced alterationin some haematological and serum parameters in normal male wistar rats. Asian J Biochem 2015; 10(6): 306–311. [Google Scholar]
- 21.Ali MY, Jannat S, Jung HA, Choi RJ, Roy A, Choi JS. Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac J Trop Med 2016; 9: 103–111. [DOI] [PubMed] [Google Scholar]
- 22.Prabakaran D, Ashokkumar N. Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats. J Funct Food 2012; 4: 776–783. [Google Scholar]
- 23.Kim CS, Kim J, Lee YM, Sohn E, Kim JS. Esculetin, a coumarin derivative, inhibits aldose reductase activity in vitro and cataractogenesis in galactose-fed rats. Biomol Ther 2016; 24(2): 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Lu ML, Dai HL, Zhang SP, Wang HX, Wei N. Esculetin, a coumarin derivative, exerts in vitro and in vivo antiproliferative activity against hepatocellular carcinoma by initiating a mitochondrial-dependent apoptosis pathway. Braz J Med Biol Res 2015; 48(3): 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Przemyslaw R, Emilia G, Slawomir M, Magdalena BZ. Antinociceptive properties of esculetin in non-inflammatory and inflammatory models of pain in rats. Clin Exp Pharmacol Physiol 2015; 42: 213–219. [DOI] [PubMed] [Google Scholar]
- 26.Younghwa K, Junsoo L. Esculetin inhibits adipogenesis and increases antioxidant activity during adipocyte differentiation in 3T3-L1 cells. Prev nutr Food Sci 2017; 22(2): 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadakol A, Sharma N, Kulkarni YA, Esculetin: Gaikwad AB. A phytochemical endeavor fortifying effect against non-communicable diseases. Biomed Pharmacother 2016; 84: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 28.Martín Aragón SM, Villar A, Benedí J. Age-dependent effects of esculetin on mood-related behavior and cognition from stressed mice are associated with restoring brain antioxidant status. Prog Neuropsychopharmacol Biol Psychiatry 2016; 65: 1–16. [DOI] [PubMed] [Google Scholar]
- 29.Drabkin DL, Austin JM. Spectrophotometric constants for common hemoglobin derivatives in human, dog and rabbit blood. J Biol Chem 1932; 98: 719–733. [Google Scholar]
- 30.Mizisin A. Schwann cells in diabetic neuropathy. J Mol Cell Biol 2004; 31: 1105–1116. [Google Scholar]
- 31.Hutter-Saunders JA, Gendelman HE, Mosley RL. Murine motor and behavior functional evaluations for acute 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) intoxication. J Neuroimmune Pharmacol 2012; 7(1): 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winocur G, Morris M. Hippocampul and prefrontal cortex contributions to learning and memory; Analysis of learning and aging effects on maze learning in rats. Behav Neurosci 1990; 104(4): 544–551. [DOI] [PubMed] [Google Scholar]
- 33.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp 2011; 53: 2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayak SS, Pattabiraman TN. A newcotodmetric method for the estimation of glucosylated hemoglobin. Clin Chim Acta 1981; 109: 267–274. [DOI] [PubMed] [Google Scholar]
- 35.Green LC, Wagner DA, Glagowski J. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem 1982; 126: 131–138. [DOI] [PubMed] [Google Scholar]
- 36.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982; 78(3): 206–209. [DOI] [PubMed] [Google Scholar]
- 37.Severinghaus JW, Ferrebee JW. Calcium determination by flame photometry; methods for serum, urine, and other fluids. J Biol Chem 1950; 187: 621–630. [PubMed] [Google Scholar]
- 38.Muthuraman A, Jaggi AS, Singh N, D: Singh. Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine-induced painful neuropathy in rats. Eur J Pharmacol 2008; 587: 104–111. [DOI] [PubMed] [Google Scholar]
- 39.Muthuraman A, Diwan V, Jaggi AS, Singh N, Singh D. Ameliorative effects of Ocimum sanctum in sciatic nerve transection-induced neuropathy in rats. J Ethnopharmacol 2008. b; 120(1): 56–62. [DOI] [PubMed] [Google Scholar]
- 40.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265. [PubMed] [Google Scholar]
- 41.Kaplay SS. Erythrocyte membrane Na+-K+ ATPase activated ATPase in protein calorie malnutrition. Am J Clin Nutri 1978; 31: 579. [DOI] [PubMed] [Google Scholar]
- 42.Taussaky HH, Shorr E. A micro calorimetric method for the determination of inorganic phosphorus. J Biol Chem 1953; 202: 675–683. [PubMed] [Google Scholar]
- 43.Shabani M, Nazeri M, Parsania S. et al. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology 2012; 33(5): 1314–1321. [DOI] [PubMed] [Google Scholar]
- 44.Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem 1949; 180: 249–261. [PubMed] [Google Scholar]
- 45.Lillie RD, Pizzolato P, Donaldson PT. Nuclear stains with soluble metachrome mordant lake dyes. The effect of chemical end group blocking reactions and the artificial introduction of acid groups into tissues. Histochemistry 1976; 49: 23–35. [DOI] [PubMed] [Google Scholar]
- 46.Sajeeth CI, Manna PK, Manavalan R, Jolly CI. Antidiabetic activity of a polyherbal formulation, Esf/ay/500 in streptozotocin induced diabetic male Albino rats: A research. Int J Drug Formul Res 2012; 1(1): 311–322. [Google Scholar]
- 47.Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain 2000; 88: 239–248. [DOI] [PubMed] [Google Scholar]
- 48.Yamada H, Inokawa H, Hori Y. et al. Characteristics of fast-spiking neurons in the striatum of behaving monkeys. Neurosci Res 2016; 105: 2–18. [DOI] [PubMed] [Google Scholar]
- 49.Krishnamurti U, Steffes MW. Glycohemoglobin: A primary predictor of the development of reversal of complications of diabetes mellitus. Clin Chem 2001; 47: 1157–1165. [PubMed] [Google Scholar]
- 50.Li M, Huang D, Liu X, Lin L. Tang-Tong-Fang confers protection against experimental diabetic peripheral neuropathy by reducing inflammation. Evid Based Complement Altern Med 2015; 574169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young W. Role of calcium in central nervous system injuries. J Neurotrauma 1992; 9: 9–25. [PubMed] [Google Scholar]
- 52.Glass JD, Culver DG, Levey AI, Nash NR. Very early activation of m-calpain in peripheral nerve during Wallerian degeneration. J Neurol Sci 2002; 196: 9–20. [DOI] [PubMed] [Google Scholar]
- 53.Otto M, Bak S, Bach FW, Jensen TS, Sindrup SH. Pain phenomena and possible mechanisms in patients with painful polyneuropathy. Pain 2003; 101: 187–192. [DOI] [PubMed] [Google Scholar]
- 54.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci 2008; 9: 36–48. [DOI] [PubMed] [Google Scholar]
- 55.Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: Therapeutic perspectives. Oxid Med Cell Longev 2013; 168039: 1–168039: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hariprasad MG, Razdan Rema, Yasha TC, Thyroxine: Tripathy Amrutanand S. A putative neuroprotectant in diabetes induced peripheral neuropathy in rats. J Diabetes Metab 2015; 6: 595. [Google Scholar]
- 57.Unander DW, Webster GL, Blumberg BS. Usage and bioassays in Phyllanthus (Euphorbiaceae): IV. Clustering of antiviral uses and other effect. J. Ethnopharmacol 1995; 45: 1–18. [DOI] [PubMed] [Google Scholar]