Abstract
The rising burden of healthcare costs suggests that the healthcare system could benefit from novel methods that allow for continuous learning to provide more data-driven, individualised care at lower costs and with improved outcomes. Here, we present our synergistic Learning approach for Prediction, Interpretation/Inference and Communication (Learning PIC) framework to address the challenges hindering the successful implementation of learning healthcare systems and to enable the effective delivery of evidence-based medicine.
Keywords: health informatics, information technology
Introduction
Medicine is a field in which an individual’s health may depend on making decisions based on imperfect information and incomplete data. Although clinical practice guidelines exist to help physicians care for patients with specific conditions, these guidelines are overall recommendations based on systematic reviews and expert opinion. As a result, it can be difficult to determine how relevant these practice guidelines are to specific individuals. Furthermore, it has long been known that no two patients are exactly alike. In fact, Sir William Osler (often called the ‘Father of Modern Medicine’) stated, ’Variability is the law of life, and as no two faces are the same, […] no two individuals react alike and behave alike under the abnormal conditions which we know as disease’.1
To improve patient outcomes and to reduce unnecessary healthcare costs, individualised and preventive approaches to healthcare are necessary; decision-support tools must be developed to help inform clinical decision-making and to engage the patient as an active participant in the individual’s care. Through the integration of improved statistical frameworks and the development of accompanying software, it is possible to build tools that support individualised health.
Individualised health within learning healthcare systems has the potential to revolutionise the entire field of medicine. Certainly, medicine is not a one-size-fits-all approach, and heterogeneous patient responses to the same intervention must be taken into account. As a result, initiatives have arisen in the areas of individualised; personalised, precision, predictive, preventive, personalised and participatory (P4); or stratified medicine/health to enable data-driven medical decisions tailored to the individual patient.2 Although some make distinctions between these terms to distinguish specific nuances, here we use the terms interchangeably. Overall, these initiatives aim to inform decisions regarding health promotion and disease management based on scientific evidence specific to an individual’s condition and in a manner that acknowledges individual variability in circumstances, preferences, and ideal prevention or treatment strategies for optimal health. To achieve individualised health, clinical decisions require:
improved information from better acquisition, integration and analysis of health data sources.
novel measurements of health/disease states, and
communication tools that more effectively inform patients and clinicians for decision-making that improves health outcomes.3
To answer clinical questions about a particular individual’s health state, disease trajectory and potential response to therapy (eg, What is the patient’s risk for a particular disease?, What is the predicted course of the patient’s disease?, Is the patient likely to respond to a particular treatment?), individualised health draws on data from patients with similar characteristics (ie, ‘subsets’ of patients). With ‘subsetting’, increasingly homogeneous groups of patients against whom to reference the particular patient in question are created in order to draw inferences about the answers to the clinical questions for the individual under consideration. For instance, a patient’s risk of cardiovascular disease can be estimated from a subset of patients with similar age, gender, blood pressure, cholesterol levels, and smoking and diabetes status, among other variables.4
However, it is currently challenging to assess all of the unique characteristics of each individual and to predict whether the patient will benefit from the intervention under consideration. To accelerate the translation of research discoveries into actionable insights in patient care, learning healthcare systems seek to enable the seamless integration of real-time generation and application of new knowledge for the delivery of high-value, evidence-based medicine to improve health outcomes at more affordable costs.5 While the application of statistical models and machine learning to areas such as pharmacogenetics and prognostic modelling has resulted in novel clinical decision-making tools, challenges remain regarding the successful development and implementation of these tools for real-world clinical impact.6 7 Here, we present our Learning approach for Prediction, Interpretation/Inference and Communication (Learning PIC) framework to address the challenges hindering the successful implementation of learning healthcare systems and to enable the effective delivery of evidence-based medicine.
The Learning PIC framework to inform evidence-based medicine in learning healthcare systems
A clash of cultures
Although the explosive growth in data, computational power, analytic methods and communication enables the potential for individualised health and learning healthcare systems, thus far, the field has been characterised by a clash of cultures, largely driven by the differences in perspectives that exist between the statistical, computer science/engineering and medical communities. The most basic simplification of the cultural divide can be explained in terms of emphasis on prediction or inference. The statistical community is often characterised by the data modelling culture where long-standing focus has been on inference through creation and fitting of problem-specific models based on prior knowledge and assumptions. In contrast, the machine learning community is often characterised by the algorithmic culture, where emphasis is on high predictive performance through the use of general-purpose learning algorithms that find patterns in data with few user-specified assumptions, often leading to models with impressive predictive ability but minimal interpretability and, thus, ‘black box’ predictions.8 Because of their high performance, there has already been widespread adoption of machine learning methods in areas such as weather forecasting, speech translation, fraud detection, spam filtering and self-driving cars.9 However, in medicine, where both high predictive performance and model transparency are essential for clinical care, challenges remain before these algorithms can reach their full impact to improve human health. With increasing emphasis on individualising healthcare to provide the best care at the right time and at lower costs through learning healthcare systems,10 there is a great need to bring about synergy between the statistical, computer science/machine learning and medical perspectives.
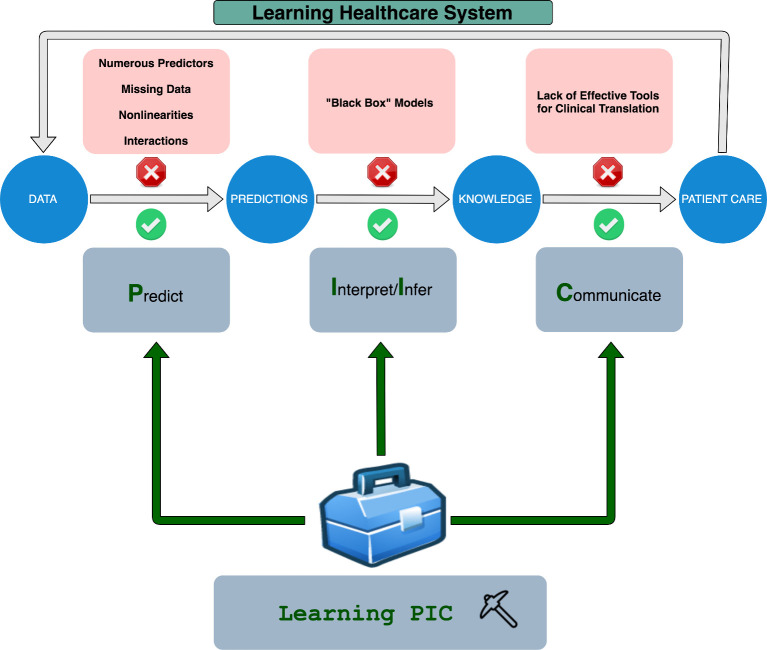
As shown in figure 1, there are currently numerous barriers to the realisation of learning healthcare systems, where data inform clinical practice, which generates additional data and new knowledge, further informing patient care. While data have the potential to inform predictions, difficulties exist when working with missing data and numerous predictors, as well as nonlinearities and interactions. Although sophisticated statistical and machine learning methods have been developed to address these challenges, these predictions are often made in ways that are difficult to interpret. The lack of transparency of ’black boxes’ hinders the generation of knowledge from these predictions and impede effective clinical translation.11–15
Figure 1.
The synergistic Learning PIC framework for evidence-based learning healthcare systems: In a learning healthcare system, data would inform predictions that would allow for the generation of new knowledge to inform patient care. Then, data collected from patient care can be used to inform future decision-making in a cycle of continuous learning. Nevertheless, as shown in the red boxes, there are numerous challenges hindering the realisation of learning healthcare systems. Our synergistic Learning PIC framework is designed to address each of these barriers.
Synergy to enable the common goal of learning
Although statistics and machine learning vary in their history, notation, emphasis and culture, both are essentially ‘the science of learning from data’.16 As the fields advance, there is a growing blurring between the distinctions between the disciplines. To emphasise the need to draw on the strengths of multiple domains and to focus on the common goal of learning from data, we use the term synergistic learning. In synergistic learning, scientific/clinical knowledge imposes structure to the learning models/algorithms in order to draw on the strengths of each discipline and to enable the realisation of individualised health and continuously learning healthcare systems. Specifically, to enable the effective delivery of evidence-based medicine, synergistic learning is learning that:
synergistically incorporates the strengths of traditional statistical approaches, as well as the advantages of machine learning methods,
is structured based on ultimate project objectives, expert domain knowledge and methodological requirements, and
is scientifically/clinically motivated.
Key features of the Learning PIC
Here, we outline the key features of the Learning PIC framework. Table 1 provides example approaches and use cases for each aspect of the Learning PIC.
Table 1.
Key considerations and example use cases for learning healthcare systems
| Key considerations | Example use cases | |
| Prediction |
|
|
| Interpretation/Inference |
|
|
| Communication |
|
|
Prediction
The current advancements in biomedical and information technologies increase the potential to substantially improve clinical decision-making with large amounts of data. Furthermore, methods from machine learning and statistical prediction are increasing our capacity to learn from a wide variety of data sources.17 18 Nevertheless, the majority of approaches to clinical-support tools only employ a small fraction of the available data. Specifically, even when variables are repeatedly measured (eg, multiple laboratory blood tests on the same individual over time), oftentimes the patient’s risk score is based only on the last available measurement rather than the full history of the measurements. Additionally, to date, most risk models are based on regression approaches.19 For example, the Cox regression model was used to develop the Framingham Risk Score and the logistic regression model was used to develop the 30-day mortality risk prediction for patients with ST-elevation myocardial infarction.20 Nevertheless, these regression strategies suffer from a number of limitations. For instance, these methods can typically handle only a small number of predictors, disregard potential interactions with time and assume constant predictor effects throughout their entire range.
The challenges not well handled by typical regression modelling strategies include non-linearities, heterogeneity of effects (interactions) and consideration of many potential predictors. The basic assumption of a regression model is that there is a linear relationship between the risk factor and outcome. Although this can be an appropriate approximation for some risk factors, in many cases, predictors have non-linear relationships with the outcome. For example, the risk of death sharply rises with increasing age. In other cases, values both above and below the normal ranges are indicative of high risk (eg, hypoglycaemia, hyperglycaemia, and body mass index for underweight and obese individuals). Furthermore, a variable’s impact on the prediction can depend on the level of another variable (eg, gene–environment interactions and treatment–race interactions). In standard regression approaches, interactions need to be prespecified, requiring the individual developing the model to know a priori to consider the interaction term in the model. In addition, with a large number of potential predictor variables to consider, it can be challenging to determine which to include in the model, and strategies must also be taken to avoid overfitting.
When clinical decision-making requires the consideration of a large number of predictors, as well as interactions and non-linear predictor effects, moving beyond traditional regression approaches offers the potential to improve predictive performance.19 The increasing emergence of large, heterogeneous data sets, such as electronic health records, for risk prediction requires novel tools to support improved clinical decisions to inform evidence-based medicine. Further development of approaches to learn from large amounts of heterogeneous data has potential to accelerate the progress towards individualised health.19 In contrast to traditional regression strategies or parametric approaches that make assumptions about the underlying model, machine learning approaches allow the data to ‘speak for themselves’ and often result in impressive predictive ability.19 21 However, machine learning approaches are sometimes criticised as ‘data hungry’ (meaning that they require large amounts of data) and for their lack of concern for enabling understanding of the relationships between the inputs and outcomes.22 As a result, effective learning healthcare systems require a synergistic approach that draws on the strengths of traditional statistical approaches, as well as the advantages of machine learning methods combined with expert domain knowledge (table 1).
Interpretation/Inference
Despite their high predictive performance, the ability of machine learning algorithms to ‘learn by themselves’ often results in poor interpretability. Additionally, because these algorithms have traditionally been developed and evaluated primarily on predictive performance, moving beyond prediction may not result in valid inferences. For instance, an illustrative example is the following: ‘algorithms might select discolored teeth as a better predictor of lung cancer than self-reported smoking status, which might be correct in some groups but because it is not a causal factor, may be useless in others, such as populations with different dietary patterns or dental care.’23–25 Despite the promise of novel learning algorithms for improving healthcare, there remains enormous concern regarding the lack of transparency of these algorithms and the risk that their predictions may be based on noise rather than on clinically meaningful relationships (ie, ‘overfitting’; see table 1).11–15
The current high predictive ability of these learning algorithms is not sufficient to facilitate their adoption in healthcare. Overall, clinicians have expressed hesitation in integrating these black boxes into their practice without an understanding of how these algorithms are generating their predictions and how to communicate these predictions to their patients. With the goal of not only achieving high predictive performance but also generating an understanding of how this predictive ability is obtained, new methods for peeking into these black boxes are currently in development. To increase interpretability, methods have been developed to determine feature importance (eg, ranking of the feature’s importance to the prediction or performance of the model) and feature effect (eg, visualisation of how changes in a feature impact the prediction).26–29 Additionally, there is growing interest in incorporating causal inference tools to enable causal reasoning within machine learning to allow cause-and-effect relationships to be examined and questioned.23–25 For instance, a clinician may be interested in determining, prior to the initiation of treatment, what the patient’s expected outcome would be under treatment A versus treatment B. Learning algorithms can help clinicians reason about the best course of action tailored to the specific patient under consideration and provide transparency behind their predictions to help facilitate evidence-based individualised health. However, expanding the ability of these algorithms to enable interpretability and inference must also be considered in the context of clinical communication.
Communication
For learning algorithms to gain clinical utility, strategies must be developed not only for interpretability/inference but also for communication of these results to clinicians and patients. It is essential to provide communication tools that allow clinicians to gain understanding and trust in these predictions, as well as to gain confidence in their ability to explain these predictions to their patients. Developing visualisations that are easy to interpret and based on familiar ways clinicians understand algorithms or results can help in translating these predictive tools into clinical practice. Focus groups with clinicians and patients can provide important feedback on how these tools can be optimised to help inform clinical decision-making in ways that are clinician and patient friendly and tailored to the unique needs/preferences of the end user. Furthermore, discussions regarding the ethical considerations of integrating research and clinical practice are necessary (table 1). A possible guiding ethical framework is one that is founded on a ‘moral priority (of) learning’, specifically with regard to the obligation of health professionals, health care institutions and patients to engage in ‘ongoing learning that is integrated with … health care’.30 In order to achieve effective learning healthcare systems with novel learning algorithms, multidisciplinary teams, ranging from engineers and computer scientists to clinicians and patients, will be increasingly important to develop impactful tools that empower evidence-based medicine to learn from data and to continuously improve.
Conclusion
Here, we present the Learning PIC framework, an approach for synergistic learning that draws on the strengths of machine learning as well as statistical approaches and clinical knowledge and is scientifically/clinically motivated and is structured based on expert domain knowledge, methodological requirements and ultimate project objectives. To address the challenges hindering the successful realisation of an evidence-based learning healthcare system, we emphasise the need to move beyond historic cultural divides that have existed between the statistical, computer science/engineering and medical communities due to their distinct histories and specific goals. To deliver evidence-based individualised health within learning healthcare systems, we highlight the importance of embracing a synergistic learning approach with the key focus areas of prediction, interpretation/inference and communication. Overall, the Learning PIC framework represents a cohesive approach for prediction, interpretation/inference and communication to enable learning from data in a clinically meaningful way to inform evidence-based medicine.
Footnotes
Twitter: @wshannon_w, @ScottZeger
Contributors: Concept and design: SW and SLZ. Drafting of the manuscript: SW. Critical revision of the manuscript for important intellectual content: SW and SLZ.
Funding: National Institutes of Health: 5T32GM007309-42, F30 HL142131-01, 1P30AR070254-01; PCORI ME 1408-20318; Scleroderma Research Foundation 90077243
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Silverman M, Murray T, Bryan C. The Quotable Osler. Philadelphia: American College of Physicians, 2008. [Google Scholar]
- 2. Fröhlich H, Balling R, Beerenwinkel N, et al. From hype to reality: data science enabling personalized medicine. BMC Med 2018;16:150 10.1186/s12916-018-1122-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johns Hopkins inHealth. Johns Hopkins medicine [Internet]. https://www.hopkinsmedicine.org/inhealth/index.html (Cited 17 Jan 2019).
- 4. Ruwanpathirana T, Owen A, Reid CM. Review on cardiovascular risk prediction. Cardiovasc Ther 2015;33:62–70. 10.1111/1755-5922.12110 [DOI] [PubMed] [Google Scholar]
- 5. Institute of Medicine. Best care at lower cost: the path to continuously learning health care in America. Washington, D.C.: National Academies Press, 2013. [PubMed] [Google Scholar]
- 6. Steyerberg EW, Moons KG, van der Windt DA, et al. Prognosis research strategy (PROGRESS) 3: prognostic model research. PLoS Med 2013;10:e1001381 10.1371/journal.pmed.1001381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson AE, Ghassemi MM, Nemati S, et al. Machine learning and decision support in critical care. Proc IEEE Inst Electr Electron Eng 2016;104:444–66. 10.1109/JPROC.2015.2501978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breiman L. Statistical modeling: the two cultures (with comments and a rejoinder by the author). Statistical Science 2001;16:199–231. 10.1214/ss/1009213726 [DOI] [Google Scholar]
- 9. Horvitz E. AI, people, and society. Science 2017;357:7 10.1126/science.aao2466 [DOI] [PubMed] [Google Scholar]
- 10. Budrionis A, Bellika JG. The learning healthcare system: where are we now? A systematic review. J Biomed Inform 2016;64:87–92. 10.1016/j.jbi.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 11. Wang F, Casalino LP, Khullar D. Deep learning in medicine-promise, progress, and challenges. JAMA Intern Med 2018. 10.1001/jamainternmed.2018.7117 [DOI] [PubMed] [Google Scholar]
- 12. Shortliffe EH, Sepúlveda MJ. Clinical decision support in the era of artificial intelligence. JAMA 2018;320:2199 10.1001/jama.2018.17163 [DOI] [PubMed] [Google Scholar]
- 13. Maddox TM, Rumsfeld JS, Payne PRO. Questions for artificial intelligence in health care. JAMA 2019;321:31 10.1001/jama.2018.18932 [DOI] [PubMed] [Google Scholar]
- 14. Cabitza F, Rasoini R, Gensini GF. Unintended consequences of machine learning in medicine. JAMA 2017;318:517 10.1001/jama.2017.7797 [DOI] [PubMed] [Google Scholar]
- 15. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA 2018;319:1317 10.1001/jama.2017.18391 [DOI] [PubMed] [Google Scholar]
- 16. Wasserman L. Rise of the machines.
- 17. Kruppa J, Schwarz A, Arminger G, et al. Consumer credit risk: individual probability estimates using machine learning. Expert Syst Appl 2013;40:5125–31. 10.1016/j.eswa.2013.03.019 [DOI] [Google Scholar]
- 18. Malley JD, Kruppa J, Dasgupta A, et al. Probability machines: consistent probability estimation using nonparametric learning machines. Methods Inf Med 2012;51:74–81. 10.3414/ME00-01-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J 2017;38:ehw302 10.1093/eurheartj/ehw302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrow DA, Antman EM, Charlesworth A, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102:2031–7. [DOI] [PubMed] [Google Scholar]
- 21. Bzdok D. Classical statistics and statistical learning in imaging neuroscience. Front Neurosci 2017;11:543 10.3389/fnins.2017.00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Ploeg T, Austin PC, Steyerberg EW. Modern modelling techniques are data hungry: a simulation study for predicting dichotomous endpoints. BMC Med Res Methodol 2014;14:137 10.1186/1471-2288-14-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pearl J. Theoretical impediments to machine learning with seven sparks from the causal revolution, 2018. [Google Scholar]
- 24. Goodman SN, Goel S, Cullen MR. Machine learning, health disparities, and causal reasoning. Ann Intern Med 2018;169:883 10.7326/M18-3297 [DOI] [PubMed] [Google Scholar]
- 25. Pearl J, Mackenzie D. The book of why : the new science of cause and effect:418. [Google Scholar]
- 26. Greenwell BM, Boehmke BC, McCarthy AJ. A simple and effective model-based variable importance measure, 2018. [Google Scholar]
- 27. Ishwaran H. Variable importance in binary regression trees and forests. Electron J Stat 2007;1:519–37. 10.1214/07-EJS039 [DOI] [Google Scholar]
- 28. Goldstein A, Kapelner A, Bleich J, et al. Peeking inside the black box: visualizing statistical learning with plots of individual conditional expectation, 2013. [Google Scholar]
- 29. Apley DW. Visualizing the effects of predictor variables in black box supervised learning models.
- 30. Faden RR, Kass NE, Goodman SN, et al. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. Hastings Cent Rep 2013;Spec No(s1):S16–S27. 10.1002/hast.134 [DOI] [PubMed] [Google Scholar]