Abstract
Objective
There are no societal ultrasound (US) guidelines detailing appropriate patient selection for deep vein thrombosis (DVT) imaging in patients with COVID-19, nor are there protocol recommendations aimed at decreasing exposure time for US technologists. We aimed to provide COVID-19-specific protocol optimization recommendations limiting US technologist exposure while optimizing patient selection.
Methods
A novel two-pronged algorithm was implemented to limit the DVT US studies on patients with COVID-19 prospectively, which included direct physician communication with the care team and a COVID-19-specific imaging protocol was instated to reduce US technologist exposure. To assess the pretest risk of DVT, the sensitivity and specificity of serum d-dimer in 500-unit increments from 500 to 8000 ng/mL and a receiver operating characteristic curve to assess performance of serum d-dimer in predicting DVT was generated. Rates of DVT, pulmonary embolism, and scan times were compared using t-test and Fisher's exact test (before and after implementation of the protocol).
Results
Direct physician communication resulted in cancellation or deferral of 72% of requested examinations in COVID-19-positive patients. A serum d-dimer of >4000 ng/mL yielded a sensitivity of 80% and a specificity of 70% (95% confidence interval, 0.54-0.86) for venous thromboembolism. Using the COVID-19-specific protocol, there was a significant (50%) decrease in the scan time (P < .0001) in comparison with the conventional protocol.
Conclusions
A direct physician communication policy between imaging physician and referring physician resulted in deferral or cancellation of a majority of requested DVT US examinations. An abbreviated COVID-19-specific imaging protocol significantly decreased exposure time to the US technologist.
Keywords: COVID, DVT, Technologist exposure
Article Highlights.
-
•
Type of Research: Prospective, observational, cohort study comparing preprotocol and postprotocol optimization to decrease healthcare worker exposure during the coronavirus disease-19 (COVID-19) pandemic
-
•
Key Findings: Direct physician communication resulted in cancellation/deferral of 72% of requested examinations in COVID-19-positive patients. A serum d-dimer of >4000 ng/mL yielded a sensitivity of 80% and a specificity of 70% for deep vein thrombosis. Using the COVID-19-specific protocol, there was a significant (50%) decrease in scan time (P < .0001) in comparison with conventional protocol.
-
•
Take Home Message: Direct physician communication between imaging physicians and referring physicians resulted in deferral/cancellation of the majority of deep vein thrombosis ultrasound examinations and an abbreviated COVID-19 imaging protocol significantly decreased exposure time to technologists.
In the recent global pandemic of coronavirus disease-2019 (COVID-19), a pattern of coagulopathy has been observed in COVID-19-positive hospitalized patients characterized by abnormal coagulation laboratory markers as well as clinical arterial and venous thromboembolic events.1 Accumulating data suggest that the underlying hypercoagulability may be contributing significantly to the morbidity of the disease, leading to recommendations for early interventions including anticoagulation strategies and in rare instances, thrombolysis, especially in critically ill patients based on retrospective cohorts and case series.2 , 3
Determination of hypercoagulability and identification of thrombi in the COVID-19-positive patient cohort remains challenging. Standard clinical symptoms associated with lower or upper extremity thrombus, including swelling or pain, are often difficult to ascertain in intubated patients. This had led to an anticipated surge in ordering of upper and lower extremity vascular ultrasound (US) studies to evaluate for deep venous thrombosis (DVT). The use of US examinations, which involves direct contact between patient and sonographer, incurs risks not fully mitigated by personal protective equipment, and traditional comprehensive protocols often involve prolonged contact. These examinations are often performed at the bedside to justify the initiation of anticoagulation. The Society of Vascular US prepandemic guideline for US venous studies recommend a scan time of ranging from 45 minutes (for CPT code 93971) and 70 minutes (for CPT code 93970), although this duration is variable based on expertise and level of training of the US technologist.4
Given the increase in COVID-19-positive patients in the United States, there has been a sharp increase in bedside imagining requests to evaluate for DVT in hospitals across the country. Imaging technologists perform multiple bedside US on both COVID-19-positive and patients without COVID-19 across hospital systems. Hence, an increase in US examination not only results in significant COVID-19 exposure to vascular technologists, but also creates a simultaneous risk of transmission of COVID-19 to inpatients through the vascular technologists acting as potential asymptomatic carriers.5
There are currently no COVID-19-specific guidelines providing recommendations for or against the use of DVT US examination nor is there a COVID-19-specific DVT scanning protocol that has been modified to decrease the exposure time for vascular technologists. As the number of patients with COVID-19 increased, we aimed to review our experience at a large, tertiary care hospital and provide COVID-19-specific protocol optimization recommendations focused on providing necessary care to patients while limiting US technologist healthcare workers' exposure to COVID-19.
Methods
This prospective study included all adult COVID-19-positive patients admitted between March 13, 2020, and April 16, 2020 (a 4-week period), on whom DVT vascular US studies were ordered through both the vascular laboratory and radiology departments (n = 160), where both laboratories offer parallel, accredited services in our hospital (the Intersocietal Accreditation Commission and the American College of Radiology, respectively). This study was reviewed and approved by our institutional review board. Patient consent was not required because the data were deidentified. Each examination request was counted as a single data point regardless of whether the order was for a bilateral or unilateral US examination. Indications for the examination, DVT status, presence or absence of pulmonary embolism (PE), serum d-dimer level, and time from examination begin to examination completion (per the radiology information system) were documented.
A novel algorithm was implemented in an attempt to decrease (a) the number of DVT US studies being performed on COVID-19-positive patients and (b) the amount of time each US technologist spends in COVID-19-positive patient hospital rooms while acquiring images.
At our institution, the vascular laboratory and the radiology department both receive orders for DVT US imaging and perform the studies. Hence, we implemented one protocol aimed at decreasing ordering of studies in our vascular laboratory and another protocol aimed at decreasing scanning time in the radiology department. This study was structured so that each protocol could be individually assessed to determine usefulness.
At our institution, we do not initiate anticoagulation for muscular calf veins (soleal, gastrocnemial) or axial calf veins (posterior tibial, peroneal). Neither the muscular nor the axial calf veins were imaged as part of the curtailed COVID-19 protocol, primarily because it did not result in an actionable, clinical change (ie, anticoagulation). Patients with calf DVT are not routinely treated with therapeutic anticoagulation at our institution; hence, we opted to decrease technologist exposure time by not performing US examinations of the calf veins in these patients.
Decreasing the number of ordered DVT US scans
To guide ordering physicians as to which patients with COVID-19 should undergo DVT US imaging the following algorithm was implemented.
If clinical suspicion for DVT or PE arises regarding a COVID-19-positive patient based on
-
1.
Serum d-dimer of >4000 ng/mL, (reference range <500 ng/mL);
-
2.
Clinical examination noting swelling and/or pain in extremity;
-
3.
Persistent unexplained fevers (>101.5°F);
-
4.
Increased O2 requirements on ventilator settings or new-onset hypoxia; and
-
5.
Late dead space fraction (portion of tidal volume that does not participate in gas exchange consisting of expired gas without carbon dioxide. High levels of dead space are associated with mortality in patients with acute respiratory distress syndrome), and there is no contraindication to therapeutic anticoagulation we recommend initiating therapeutic anticoagulation with low molecular weight heparin over obtaining imaging.
However, if the patient is at increased risk with therapeutic anticoagulation or may not tolerate low-molecular-weight heparin (renal disease) a attending physician level discussion between the vascular laboratory medical director (or cardiovascular or emergency radiology staff of the day) and ordering physician should occur regarding the medical reasoning for the imaging, with the goal of ensuring appropriate benefit/risk analysis, before proceeding with the examination, or, if possible, deferral to a time wherein the patient is less contagious.
After the implementation of this algorithm by the vascular laboratory only, all DVT US on COVID-19-positive patients were reviewed to determine how many US orders were deemed unnecessary by the ordering provider and canceled based on the algorithm. Statistical analysis included descriptive statistics; both sensitivity and specificity of serum d-dimer in 500-unit increments from 500 to 8000 ng/mL were calculated and a receiver operating characteristic curve was generated to determine the serum d-dimer value that was acceptably predictive of DVT. From this, an area under the cure was generated.
Decreasing technologist exposure time while performing DVT US imaging
All COVID-19-positive patients who either underwent DVT US by the radiology department using conventional scanning or underwent DVT US using the COVID-19-specific focused protocol in the radiology department only were included. Demographics, DVT rate, PE rate, serum d-dimer level, and US scanning time in minutes were recorded and compared between patients who had conventional scans versus patients with COVID-19-focused DVT US. The conventional DVT US protocol and the COVID-19-focused scanning protocol are detailed in Table I .
Table I.
Comparison of conventional versus COVID-19-specific ultrasound examination (US) venous imaging protocol for upper and lower extremity in the radiology department
| Veins | Conventional protocol | COVID-19-specific protocol |
|---|---|---|
| Lower extremity veins | ||
| Common femoral vein | TRV: B-mode dual image with and without COMPRESSION calipers SAG: Color Doppler/Spectral Doppler demonstrating distal augmentation |
TRV: B-mode dual image with and without COMPRESSION SAG: Color Doppler/pulse wave demonstrating distal augmentation |
| Proximal femoral vein | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler |
TRV: B-mode dual image with and without COMPRESSION |
| Mid femoral vein | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler |
TRV: B-mode dual image with and without COMPRESSION |
| Distal femoral vein | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler |
TRV: B-mode dual image with and without COMPRESSION |
| Popliteal vein | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler/Spectral Doppler demonstrating distal augmentation |
TRV: B-mode dual image with and without COMPRESSION |
| Posterior tibial vein(s) | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler |
Not imaged |
| Peroneal vein(s) | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler |
Not imaged |
| Upper extremity veins | ||
| Internal jugular vein | TRV: B-mode dual image with and without COMPRESSION calipers SAG: B-mode SAG: Color/Spectral Doppler |
TRV: B-mode dual image with and without COMPRESSION SAG: Color Doppler/pulse wave |
| Subclavian vein | SAG: B-mode SAG: Color/Spectral Doppler |
SAG: Color Doppler/pulse wave |
| Axillary vein | TRV: B-mode dual image with and without COMPRESSION with calipers SAG: B-mode SAG: Color Doppler |
TRV: B-mode dual image with and without COMPRESSION |
| Brachial veins | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler |
TRV: B-mode dual image with and without COMPRESSION |
| Cephalic and basilic veins | TRV: B-mode dual image with and without COMPRESSION/calipers SAG: Color Doppler |
TRV: B-mode dual image with and without COMPRESSION |
SAG, Sagittal; TRV, transverse.
Calipers refer to measuring the diameters.
Continuous outcomes were reported as means and standard deviations or median and interquartile ranges, as appropriate. Dichotomous outcomes were reported as counts and percentages. The protocol groups were compared using the Student t-test or Wilcoxon rank-sum test for continuous outcomes. Fisher's exact test was used for binary outcomes. All tests were two-tailed with an alpha-level of 0.05 indicating statistical significance. Statistical calculations were performed using R (version 3.6.2; The R Foundation, Vienna, Austria).
Results
Decreasing number of ordered DVT US scans
During the study period of 4 weeks, a total of 66 US requests in COVID-19-positive patients were received by the vascular laboratory. The protocol was implemented halfway through the study period so a comparison between before and after the implementation of the algorithm could be performed. Twenty-five studies were ordered after the protocol was implemented and 41 were ordered before algorithm implementation for a total of 66 US requests. Application of the algorithm (including direct conversations with the medical care team component) resulted in 18 of the 25 ordered studies being canceled (72%). This decision was made jointly between the referring faculty and vascular laboratory physician to cancel (or defer) the examination. In all cases where the US examination was canceled, a physician-to-physician conversation did occur; after implementation of the algorithm, only 7 of the 25 (28%) originally ordered US examinations were deemed necessary and performed. None of these seven US examinations were positive for DVT. Overall, in the 48 patients who underwent DVT US imaging during the 4-week study period (41 performed in the preprotocol timeframe + 7 performed in the post protocol timeframe), the primary indication for the study was “swelling or pain” in the limb (Table II ).
Table II.
Table showing ultrasound (US) venous studies performed in the vascular laboratory during the study period
| Examination type | Indication | PE status | DVT | d-dimer |
|---|---|---|---|---|
| RLE | Swelling and pain LE | Negative | Negative | 814 |
| RUE | Concern for thrombus | Negative | Positive | 1145 |
| LUE | Swelling and pain LE | Negative | Negative | 1128 |
| LLE | Swelling and pain LE | Negative | Negative | 1535 |
| BLE | Swelling and pain LE | Negative | Negative | 8040 |
| BLE | Swelling an pain LE | Negative | Negative | 610 |
| BLE | Swelling and pain LE | Positive | Positive | 6687 |
| BLE | Swelling and pain LE | Negative | Positive | 2288 |
| LLE | Swelling and pain LE | Negative | Negative | 1435 |
| BLE | Swelling and pain LE | Negative | Negative | 2640 |
| BLE | Swelling and pain LE | Negative | Negative | 1704 |
| BLE | Swelling and pain LE | Negative | Negative | 2173 |
| BUE | Swelling and pain LE | Negative | Negative | a |
| BLE | Rule out DVT | Negative | Negative | 3429 |
| BLE | Swelling and pain LE | Positive | Negative | 4326 |
| BLE | Swelling and pain LE | Negative | Positive | 8460 |
| RLE | Swelling and pain LE | Negative | Negative | 348 |
| BLE | PE | Positive | Negative | 4237 |
| BLE | Swelling and pain LE | Negative | Negative | 2800 |
| LUE | Swelling and pain LE | Negative | Positive | 10000 |
| BLE | Swelling and pain LE | Negative | Negative | 10000 |
| BUE | Swelling and pain LE | Negative | Negative | a |
| BLE | Swelling and pain LE | Negative | Positive | 10000 |
| BLE | Swelling and pain LE | Negative | Negative | 1216 |
| LUE | Swelling and pain LE | Negative | Positive | 5716 |
| BLE | Swelling and pain LE | Negative | Negative | 1189 |
| BLE | Swelling and pain LE | Negative | Negative | 849 |
| BLE | Swelling and pain LE | Negative | Negative | 3419 |
| BLE | Swelling and pain LE | Negative | Negative | 6884 |
| LLE | Swelling and pain LE | Negative | Negative | 1276 |
| LUE | Swelling and pain LE | Negative | Negative | 5242 |
| BLE | Swelling and pain LE | Negative | Negative | 1771 |
| BLE | Swelling and pain LE | Negative | Negative | 885 |
| BLE | Swelling and pain LE | Negative | Negative | 1396 |
| BLE | Septic thrombophlebitis | Negative | Negative | 10000 |
| BLE | Swelling and pain LE | Negative | Negative | 1317 |
| BLE | Swelling and pain LE | Negative | Negative | 560 |
| BLE | PE | Positive | Negative | 6778 |
| BLE | +COVID/ARDS with ongoing tachycardia/fevers, c/f DVT | Negative | Negative | 1696 |
| BUE | Elevated d-dimer unable to obtain CT-PE or V/Q study | Negative | Negative | 1599 |
| BLE | Concern for DVT, emboli, dislodged femoral line | Negative | Negative | 1896 |
| RLE ART | Concern for DVT, emboli, dislodged femoral line | Negative | Negative | 1896 |
| BLE | Evaluate for DVT | Negative | Negative | 809 |
| BUE | Hypercoagulable, concern for DVT. +COVID | Negative | Negative | 8700 |
| BLE | Fevers c/f DVT | Negative | Negative | a |
| BLE | Persistent tachycardia and sudden worsened hypoxia with d-dimer >4000 | Negative | Negative | 4510 |
| BUE | Eval for DVT | Negative | Negative | 9750 |
| BLE | COVID-19 positive, persistent O2 requirement and pleuritic chest pain | Negative | Negative | 1805 |
ARDS, Acute respiratory distress syndrome; ART, antiretroviral therapy; BLE, bilateral lower extremities; BUE, bilateral upper extremities; c/f, concerning for; DVT, deep vein thrombosis; LE, lower extremity; LLE, left lower extremity; LUE, left upper extremity; PE, pulmonary embolism; RLE, right lower extremity; RUE, right upper extremity.
Missing data.
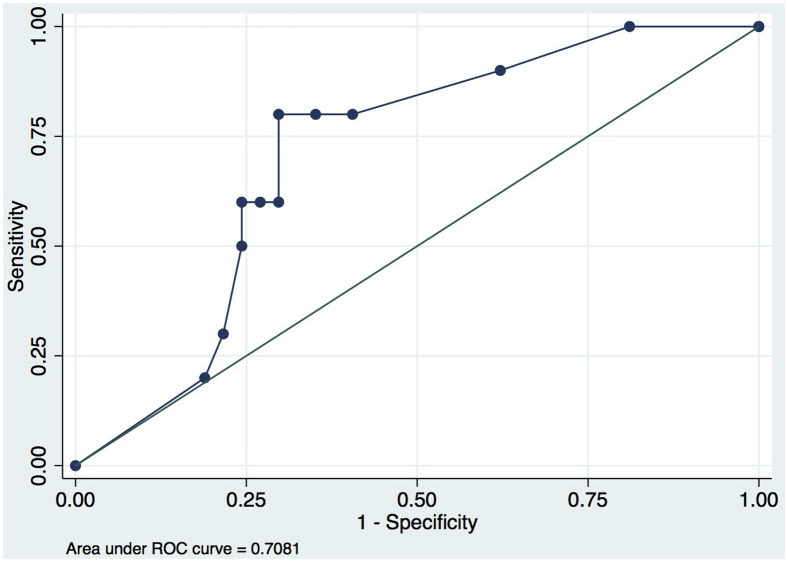
Implementation of the protocol decreased the DVT US volume by 72%; only 28% of the COVID-19-positive patients had an indication for DVT US that would change medical management based on the guidelines implemented by the vascular laboratory. This cohort included patients who had renal disease precluding the use of low-molecular-weight heparin (resulting in increased nursing contact owing to the need for heparin drip titration), patients where anticoagulation may be lethal if a bleed ensued (ie, patients who refuse blood products), or those with recent surgery or hemorrhagic strokes. The patient scanning orders canceled occurred after an attending to attending discussion regarding the usefulness of the scan in the face of a serum d-dimer of >4000 ng/mL and/or clinical suspicion of DVT. Serum d-dimer levels were found to be accurate to predict VTE (area under the cure, 0.71; 95% confidence interval, 0.54-0.86). A serum d-dimer cutoff of >4000 ng/mL yielded a sensitivity of 80% and a specificity of 70% (Fig ).
Fig.
Receiver operating characteristic (ROC) curve of d-dimer values, indicating sensitivity and specificity of d-dimer level for thrombosis (n = 48).
All 18 patients who had US requests canceled or deferred based on the algorithm were administered therapeutic anticoagulation. There were no bleeding events associated with this patient group.
Decreasing technologist exposure time while performing DVT US imaging
The radiology department performed 97 DVT US examinations on COVID-19-positive patients in the study period, of which 53 (55%) were performed conventionally and 44 (45%) were performed using the COVID-19-focused scanning protocol. Although there was no difference between groups in demographics, serum d-dimer values, and DVT or PE positivity rates, the time to perform the COVID-19-focused US examination was significantly less (Table III ) than the standard protocol. On average, the COVID-19-focused examination took 50% less time to complete than the conventional examination (median conventional examination time 13 minutes [interquartile range, 9-15] vs 6 minutes [interquartile range, 5-8] for the COVID-19-specific protocol [P < .001]).
Table III.
Comparison of demographics, serum d-dimer, rates of deep vein thrombosis (DVT), pulmonary embolism (PE), and scan times with conventional versus COVID-19-specific ultrasound (US) protocol
| Variable | DVT US conventional protocol (n = 53) | COVID-19-specific protocol (n = 44) | P value |
|---|---|---|---|
| Age, years | 55 ± 17 | 58 ± 16 | .31 |
| Male sex | 23 (43.3) | 25 (56.8) | .22 |
| d-Dimer, ng/mL | 1704 (1119-6457) | 2636 (1306-5186) | .51 |
| DVT | 14 (24.5) | 5 (15.9) | .08 |
| PE | 6 (11.3) | 4 (9.1) | 1.00 |
| Scan time, minutes | 13 (9-15) | 6 (5-8) | <.0001 |
Values are mean ± standard deviation, median (interquartile range), or number (%).
Boldface entries indicate statistical significance.
Discussion
As of June 21, 2020, the number of COVID-19 cases in the United States was 3,964,361. Hospitalized patients infected with the severe acute respiratory distress coronavirus disease-19 virus have been noted to have clinical characteristics of severe pneumonia, as well as particular patterns in hematologic testing including lymphopenia.5 , 6 In addition, these patients have been noted to have abnormal coagulation testing, including elevated serum d-dimer levels, elevated fibrinogen, mildly prolonged prothrombin time, and, rarely, thrombocytopenia.1 , 7 , 8 Markedly elevated serum d-dimer has been identified as a consistent marker for mortality; moreover, disseminated intravascular coagulation as per criteria representing fulminant activation of coagulation and consumption of factors has been shown to develop in as high as 71.4% of patients that succumbed to COVID-19 pneumonia.1
In addition to laboratory derangements, patients with COVID-19 have been shown to have variable rates of thrombotic complications, as low as 7.7% to as high as 25.0% in critically ill patients.9 , 10 Moreover, an autopsy series of four patients who died of COVID-19 in New Orleans revealed that there was evidence of extensive microthrombi in the pulmonary capillaries and venules, but also evidence of diffuse alveolar hemorrhage, providing support that even in patients without evidence of VTE on imaging studies, microthrombi may be prevalent.11 The evidence for anticoagulation is limited thus far although an area of active clinical investigation. It has been more apparent through the COVID-19 experience globally, that even in the face of a negative DVT US examination, full anticoagulation may be appropriate, given that microthrombi form in these patients causing significant end-organ damage.10
A case series of 449 patients with severe COVID-19 from China—of whom 22% received heparin prophylaxis—showed a difference in mortality for patients receiving heparin (40.0% vs 64.2%; P = .029) with a sepsis induced coagulopathy score of ≥4 (40.0% vs 64.2%; P = .029), or serum d-dimer >6-fold of the upper limit of normal (32.8% vs 52.4%; P = .017).2 It is unknown at this time if anticoagulant doses beyond prophylaxis improves outcomes or decreases risk of VTE; however, some societal and hospital guidelines and protocols are currently recommending low-molecular-weight heparin prophylaxis (unless contraindicated) for all hospitalized patients with COVID-19.8 Our recommendation for patients is therapeutic anticoagulation for all hospitalized COVID-19-positive patients, barring any contraindications as determined by the physician to physician communication. Although there are data to suggest that patients with COVID-19 may be at a higher bleeding risk, we did not have any bleeding events in the patient cohort who were therapeutically anticoagulated based on our algorithm.12 We recognize that our study had a smaller number of patients; however, given the current literature supporting the hypercoagulable state of patients with COVID-19 and the associated d-dimer increase even with no discernable DVT, we support full anticoagulation in this patient group, especially given the lack of bleeding events observed in our study.
US examinations require direct vascular technologist contact and may result not only in exposure for the technologist, but also in the spread of COVID-19 through inpatients if technologists become asymptomatic carriers.
Our vascular and radiology group has now collaborated to develop an algorithm to curtail the ordering of DVT US. This algorithm included a serum d-dimer level of >4000, which correlated with an acceptable sensitivity of 80% in our cohort. Chinese investigators early in the pandemic reviewed the prevalence of venous thromboembolism (VTE) in patients with COVID-19 and concluded that serum d-dimer was indeed a good index for predicting VTE in patients with severe disease. In their study of 88 patients with COVID-19, they identified 1.5 μg/mL as a good cut-off parameter with a sensitivity of 85% and specificity of 88.5% with a positive predictive value of 70.8% and negative predictive value of 94.7% of VTE identification.9 Hence, if clinical suspicion with supported laboratory values can yield a high likelihood of thrombus and anticoagulation is not contraindicated, treatment should be initiated with therapeutic anticoagulation after a physician-to-physician discussion about patient risk with therapeutic anticoagulation. It is important to acknowledge that, even with a negative US finding, a patient with a high d-dimer may have microthrombi, which is best managed with therapeutic anticoagulation.
We have now implemented an algorithm throughout our institution that should decrease the number of DVT US ordered by 72% and decreases technologists' time per examination by 50% based on our data.
The scanning protocol modifications are detailed in Table I. Most notably, (1) for lower extremities, infrapopliteal (calf) veins were not included for lower extremity veins and (2) the use of color/spectral Doppler US imaging was confined to proximal veins, for example, the common femoral vein in the lower extremity and the internal jugular vein and subclavian veins in the upper extremity. The Society of Radiologist in the Ultrasound Consensus recommendation from pre-COVID-19 era acknowledges that a limited point-of-care US examinations from thigh to knee is appropriate if a complete duplex US examination, including the calf veins, is not available.13 The reported rate of PE was 2.4% in a metanalysis by Franco et al14 that reviewed lower extremity VTE and, with anticoagulation, the rate decreased to 1.4% although this study was not in COVID-19-positive patients. Grillet et al15 reported a PE incidence of 23% in 100 COVID-19-positive patients with oxygen saturations of <92%, a respiratory rate of >25 breaths per minute, a temperature of >40°C, increasing oxygen requirements, and those needing mechanical ventilator or with comorbidities, including malignancy, immunosuppression, and immunocompromise.
There is no uniform consensus on anticoagulation in patients with isolated calf DVT.16 The CACTUS study randomized 259 patients (without COVID-19) into treatment and placebo arms and showed no significant difference in the primary outcomes such as proximal propagation of the thrombus or PE.17 The incidence of PE resulting from upper extremity is low in absence of an intravenous device. Ploton et al18 found pulmonary emboli in 4% of patients with upper extremity thrombosis. Regardless of the PE or DVT risk, anticoagulation in these patients may be the appropriate course of action especially in the face of an increasing d-dimer as this may be the only indication of the microthrombi that may be the cause of end-organ damage.
Limitations
This study is limited by the small number of patients and single-institution design. Our algorithm for patients with clinical suspicion for DVT or PE did not include other variables like history of immobility, days spent in the hospital, or other serum markers like platelets, prothrombin time, or fibrinogen. All direct physician communications with the care teams were performed by a single point person, limiting the evaluation of variation in cancelations or deferrals across multiple physicians. The scan time for US examination was not compared among the technologists based on their years of experience, limiting our assessment of variation in scan time. However, these results are novel and do have merit, given that no protocol currently exists to triage patients with COVID-19 in such a way that to decrease unnecessary exposure to US technologists. Another limitation may be that the modifications of venous US examination recommended in this study were evaluated in isolation so we cannot state that combining the two modalities would be more, less, or equally efficacious.
Conclusions
Across the country, an increase in patients with COVID-19 is resulting in an increase in US testing requests for DVT, which in turn results in significant exposure for vascular technologists. No protocol currently exists to triage patients to ensure that those who would benefit from US examinations would receive them and to minimize healthcare worker exposure in those who may not need it. We have implemented a novel algorithm throughout our institution that decreases the number of DVT US scans by 72%, as well as decreases technologist time per examination by 50%. We have implemented these protocols in both the vascular laboratory and the radiology department. We recommend implementing both protocols as a joint effort to decrease technologist exposure time and volume of DVT US at institutions caring for patients with COVID-19.
Author contributions
Conception and design: AD, VT, RR, DH, SM, RP, JR, BG, ME, JB, SH
Analysis and interpretation: AD, VT, CL SW, RP, SH
Data collection: AD, VT, RR, DH, SM, JR, SH
Writing the article: AD, SH
Critical revision of the article: AD, VT, RR, DH, CL SW, SM, RP, JR, BG, ME, JB,SH
Final approval of the article: AD, VT, RR, DH, CL SW, SM, RP, JR, BG, ME, JB, SH
Statistical analysis: AD, RP, SH
Obtained funding: Not applicable
Overall responsibility: SH
Acknowledgments
We would like to thank Melanie L Orlowski and Janice Write for their support.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upper extremity venous duplex evaluation. https://higherlogicdownload.s3.amazonaws.com/SVUNET/c9a8d83b-2044-4a4e-b3ec-cd4b2f542939/UploadedImages/PPG_Docs/9__Upper_Extremity_Venous_Duplex_Evaluation__Updated_2019_.pdf Available at:
- 5.Black J.R.M., Bailey C., Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Zhou L., Yang Y., Peng W., Wang W., Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thacil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in COVID-19: the first autopsy series from New Orleans. medRxiv. 2020 doi: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T., Chen R., Liu C., Wenhua L., Weijie G., Tang R. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Needleman L., Cronan J.J., Lilly M.P., Merli G., Adhikari S., Hertzberg B.S. Ultrasound for lower extremity deep venous thrombosis: multidisciplinary recommendations from the Society of Radiologists in Ultrasound Consensus Conference. Circulation. 2018;137:1505–1515. doi: 10.1161/CIRCULATIONAHA.117.030687. [DOI] [PubMed] [Google Scholar]
- 14.Franco L., Giustozzi M., Agnelli G., Becattini C. Anticoagulation in patients with isolated distal deep vein thrombosis: a meta-analysis. J Thromb Haemost. 2017;15:1142–1154. doi: 10.1111/jth.13677. [DOI] [PubMed] [Google Scholar]
- 15.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palareti G. Do isolated calf deep vein thrombosis need anticoagulant treatment? J Thorac Dis. 2016;8:E1691–E1693. doi: 10.21037/jtd.2016.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Righini M., Galanaud J.P., Guenneguez H., Brisot D., Diard A., Faisse P. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. Lancet Haematol. 2016;3:e556–e562. doi: 10.1016/S2352-3026(16)30131-4. [DOI] [PubMed] [Google Scholar]
- 18.Ploton G., Raimbeau A., Le Denis, Seve J., Bergère G., Ngohou C. A STROBE cohort study of 755 deep and superficial upper-extremity vein thrombosis. Medicine (Baltimore) 2020;99:e18996. doi: 10.1097/MD.0000000000018996. [DOI] [PMC free article] [PubMed] [Google Scholar]