Abstract
Objectives
To assess the healthcare costs associated with poststroke oropharyngeal dysphagia (OD) and its complications (malnutrition, dehydration, pneumonia and death).
Design
Systematic review following Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations.
Data sources
MEDLINE, Embase and the National Health Service Economic Evaluation Database were searched up to 31 December 2019.
Participants
Patients with poststroke.
Primary outcome measures
The costs associated to poststroke OD and its complications.
Data analysis
Data were synthetised narratively, quality evaluation was done using an adaptation of Drummond’s checklist and Grading of Recommendations Assessment, Development and Evaluation recommendations were used to assess strength of evidence.
Results
A total of 166 articles were identified, of which 10 studies were included. The cost of OD during the hospitalisation was assessed in four studies. One prospective study showed an increase of US$6589 for patients requiring tube feeding. Two retrospective studies found higher costs for those patients who developed OD, (US$7329 vs US$5939) among patients with haemorrhagic stroke transferred to inpatient rehabilitation and an increase of €3000 (US$3950) and SFr14 000 (US$15 300) in hospitalisation costs. One study did not found OD as a predictor for total medical costs in the multivariate analysis. One retrospective study showed an increase of US$4510 during the first year after stroke for those patients with OD. For pneumonia, five retrospective studies showed an increase in hospitalisation costs after stroke of between US$1456 and US$27 633. One prospective study showed an increase in hospitalisation costs during 6 months after stroke in patients at high malnutrition risk. Strength of evidence was considered moderate for OD and pneumonia and low for malnutrition.
Conclusions
This systematic review shows moderate evidence towards higher costs for those patients who developed OD after stroke. The available literature is heterogeneous, and some important aspects have not been studied yet. Further studies are needed to define the specific cost of poststroke OD.
PROSPERO registration number
CRD42018099977.
Keywords: deglutition disorders; deglutition; pneumonia, aspiration; malnutrition; stroke; economics
Strengths and limitations of this study.
This systematic review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations.
The bibliographic research considers MEDLINE, Embase and the National Health Service Economic Evaluation Database databases.
A quality evaluation using an adaptation of the Drummond’s tool was performed.
Strength of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation methodology.
The heterogeneity between the included studies and the variation on costs depending on the context precluded a quantitative data synthesis.
Introduction
Oropharyngeal dysphagia (OD) is a common condition in patients with poststroke, as a result of the brain injuries suffered.1 Incidence is high (37%–78%) in the acute phase2 and, while improvements can be observed in many patients during the first weeks after stroke, OD persists as a chronic condition in nearly 50% and complications arise.3 The latest editions of the International Classification of Diseases (ICD) and Related Health Problems promoted by WHO ICD-9 and ICD-10 classifies poststroke OD with specific codes I-69.391.4
OD can have a high impact on the general health of affected patients and can produce two main types of complications in patients with poststroke: (1) those caused by impaired efficacy of swallow, present in 25%–75% of patients, which leads to malnutrition and dehydration5 and (2) impaired safety of swallow which leads to tracheobronchial aspiration that may cause pneumonia in 50% of cases.6–10 Both OD and aspiration are highly prevalent conditions in patients with stroke.2 6 A recent study on patients with stroke has shown a prevalence of OD of 45.06% on admission, and that OD after stroke was an independent risk factor for prolonged hospital stay and institutionalisation after discharge. Moreover, this study has shown that OD was independently associated with poorer functional capacity and increased mortality 3 months after stroke.6 A significant increase in length of stay and a poor prognosis has also been observed by other authors in patients with poststroke OD.11 The impact of all these complications on the costs of poststroke OD is still unknown.
The state-of-the-art of OD management in patients with poststroke aims for early detection, with swallowing being assessed in the first hours after stroke diagnosis12 in order to prevent the potential complications of OD. Application of specific explorations in the acute setting, therapy development aimed at compensating deficient mechanisms related to this pathology and the recovery of swallowing function in these patients in the long term are key aspects of the management of this condition.13 Poststroke OD is an underdiagnosed and undertreated condition and the most appropriate care is not available for most patients. Significant reductions in rates of pneumonia (9.0% vs 2.8%) and mortality (7.4% vs 4.2%) have been demonstrated through screening and basic OD treatment application and is reflected in antibiotic expenditure with significant savings of around 50%.14 The treatment paradigm for poststroke OD is changing from compensatory strategies, which aim to compensate deficient mechanisms related to OD by using fluid adaptation and postural changes, to the recovery of swallowing function with the promotion of brain plasticity with neurorehabilitation techniques such as transcutaneous or intrapharyngeal electrical stimulation, repetitive transcranial magnetic stimulation and transcranial direct current stimulation. These neurorehabilitation strategies are still in the research phase but can already be perceived as an important progress in poststroke OD management.15
Costs related to poststroke OD comprise acute in-hospital and long-term sanitary costs, direct non-healthcare costs, indirect costs such as productivity losses and intangible costs. In the recent years, more data on the cost of poststroke OD has become available. However, no systematic literature review has been conducted as yet on this topic. The objective of this systematic review is to assess and summarise all the evidence on the cost related to OD and its complications (malnutrition, dehydration, aspiration pneumonia and death) in patients with poststroke. This study is a first step towards establishing the cost benefits of appropriate management of poststroke OD as an aspect to be taken into consideration by healthcare decision-makers.
Methods
This systematic review was carried out using methodology Preferred Reporting Items for Systematic Reviews and Meta-Analyses.16,17 18 In summary, a systematic review of studies related to the cost of OD and its complications (malnutrition, dehydration, aspiration pneumonia and death) in patients with stroke was performed. The main outcome of interest was the additional costs attributable to poststroke OD and its complications during the hospitalisation and follow-up after discharge. Task organisation in this systematic review including those processes performed by two or more authors (selection of studies, data extraction, quality assessment) is explained in the protocol of this systematic review.
Patient and public involvement statement
There was no public or patient involvement in the elaboration of this systematic review.
Search strategy
We searched Medline, Embase and the National Health Service Economic Evaluation Database (NHS EED) up until 31 December 2019. The references of the studies included were further revised to identify possible additional eligible studies. Used search terms are available in the protocol of this systematic review and in the online supplementary appendix.17 18
bmjopen-2019-031629supp001.pdf (325.2KB, pdf)
Eligibility criteria
Studies were included if they were cost studies, studies that provide information on costs in adult (>17 years) patients with stroke with OD and/or its complications or economic evaluation studies in which the cost of the disease was estimated. Studies were excluded if they were not related to OD or if they refer to oesophageal dysphagia or OD caused by causes other than stroke. Full-text assessments were done in order to reject those ones not fulfilling all selection criteria. Two independent reviewers participated in this process. In case of disagreement over one or more studies, a third reviewer revised the study and a final consensus was made. A posterior task was carried out to identify possible duplicated information between the articles.
Data presentation and summary measures
Data were reported in its original format using tables and narrative. A narrative method was used to synthesise this evidence. Results are presented according to the following order: (1) costs related to poststroke OD, (2) costs related to OD complications in the following order: (A) aspiration, (B) pneumonia, (C) malnutrition, (D) dehydration and (E) death. A synthesis of studies separating acute and long-term costs was performed. Whenever feasible, data on cost adjusted for the stroke severity (according to National Institutes of Health Stroke (NIHSS) or Canadian scale) or other confounding factors were considered. Information was presented following recommendations of the Centre for Reviews and Dissemination.19 Results were also discussed as those assessing cost during the hospitalisation compared with cost after discharge and long-term follow-up.
Quality evaluation and strength of the evidence
In this study, we used an adaptation of Drummond’s checklist in order to assess the risk of bias and the reporting quality for each study only using the points in the checklist that were applicable to cost studies.20 Each of these points was rated as: yes, no, partly, not available or not applicable. Two independent reviewers participated in this process. A global score presented as a percentage was calculated for each study dividing the total number of points rated as ‘yes’ (‘partly’ counted as 0.5) between the total points applicable for each study. No study was excluded from this review based on risk of bias results. A higher score indicates a lower risk of bias. Quality assessment of the studies in this systematic review is presented in table 1 and expanded in the online supplementary appendix. In addition, we rated the quality of evidence across studies as high, moderate, low or very low using Grading of Recommendations Assessment, Development and Evaluation methodology.21 The hypothesis of this systematic review is that OD and its main complications are related to high costs in patients with poststroke. If a study demonstrated significantly higher costs for patients with poststroke who developed OD than for those who did not, higher costs for those who develop a complication related to OD or an effect of OD or its complications on total costs of stroke, the study was qualified as a ‘positive study’.
Table 1.
Main design characteristics
| Study ID | Aim | Study population | Design | Time horizon and perspective |
Country, year and currency | Quality assessment* (%) (yes (1)+partly (0.5)/total applicable)x100 |
| Wojner22 AACN Clin Issues 2000 |
Cost of TF | Ischaemic and haemorrhagic stroke ≥18 years |
Prospective | Hospitalisation time Hospital perspective |
USA 1995–1996 USD (year not available) |
50 |
| Katzan27 Neurology 2007 |
Cost of pneumonia | Ischaemic and haemorrhagic stroke | Retrospective | Hospitalisation time Hospital perspective |
USA 1991–1997 2000 USD |
69 |
| Christensen28 Acta Neurol Scand 2009 |
Cost of pneumonia | Ischaemic and haemorrhagic stroke ≥21 years |
Retrospective | Hospitalisation time Hospital perspective |
Argentina 2004–2006 2005 USD |
75 |
| Christensen29 Neuroepidemiology 2009 | Cost of pneumonia | Ischaemic and haemorrhagic stroke ≥21 years |
Retrospective | Hospitalisation time Hospital perspective |
Brazil 2006–2007 2005 USD |
83 |
| Wilson J Stroke Cerebrovasc Dis 201230 |
Cost of pneumonia | Ischaemic and haemorrhagic stroke ≥18 years |
Retrospective | Hospitalisation time Hospital perspective |
USA 2005–2006 2009 USD |
67 |
| Bonilha23 Dysphagia 2014 |
Cost of OD | Ischaemic stroke ≥65 years |
Retrospective | One year poststroke Financer perspective |
USA 2004 2014 USD |
80 |
| Chen24 J Rehabil Med 2015 |
Cost of OD | Ischaemic stroke Transferred to a rehabilitation ward during hospitalisation |
Retrospective | Hospitalisation time Hospital and patient perspective |
Taiwan 2002–2012 2013 USD |
67 |
| Chen25 Top Stroke Rehabil 2016 |
Cost of OD cost of pneumonia |
Haemorrhagic stroke Transferred to a rehabilitation ward during hospitalisation |
Retrospective | Hospitalisation time Hospital and patient perspective |
Taiwan 2002–2012 USD (year not available) |
64 |
| Gomes31 J Stroke Cerebrovasc Dis 2016 |
Cost of malnutrition | Ischaemic and haemorrhagic stroke ≥18 years |
Prospective | 6 months after stroke Hospital perspective |
England 2011–2012 GBP (year not available) |
69 |
| Muehlemann26 PLoS One 2019 |
Cost of OD | Ischaemic stroke | Retrospective | Hospitalisation Hospital perspective |
France and Switzerland 2012 2013 Euros and Swiss Francs |
80 |
*Quality assessment: a higher score indicates a lower risk of bias.
OD, oropharyngeal dysphagia; TF, tube feeding.
Results
In the data base search, 121 articles were identified using the search terms (67 through Medline using PubMed, 12 through Embase using Ovid and 42 through NHS EED) and 45 articles were identified through reference check. A total of 166 studies were assessed in the selection phase. After screening both title and abstract of these articles, 108 articles were excluded because they did not provide information on poststroke OD costs or those of its complications or at least minimal relevant information on this aspect. A second evaluation phase was carried out with the 58 remaining studies. After this second evaluation phase, 48 articles were excluded because they did not meet the criteria for inclusion (4 were duplicated articles, 38 did not provide information on costs and 6 did not refer to OD) and 10 articles were included in this systematic review (figure 1). Included study data and features were summed up and presented in both a narrative presentation and evidence (tables 1–4). A great heterogeneity regarding the economic design among studies was found (mainly among study perspective, type of included costs and follow-up time). Results were, therefore, not comparable and studies were evaluated separately.
Figure 1.
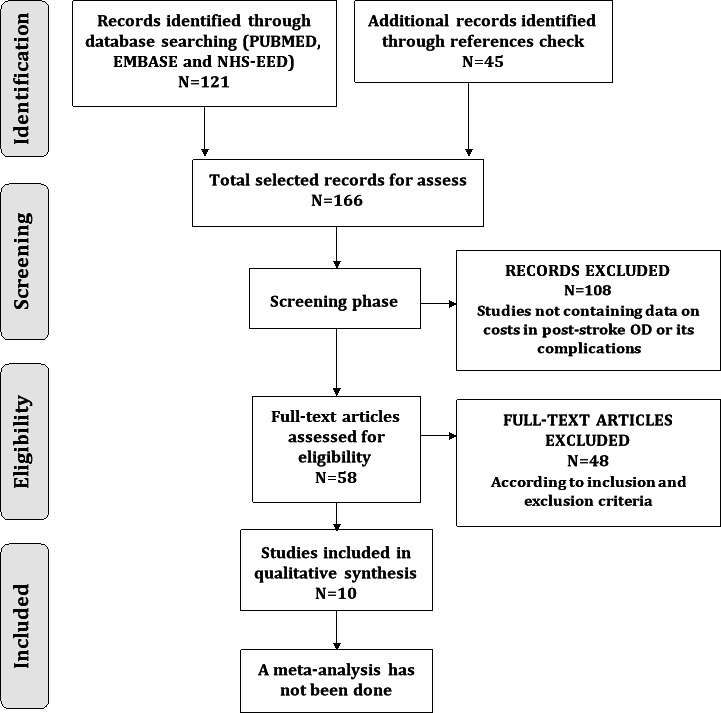
Selection process flow diagram. NHS EED, National Health Service Economic Evaluation Database; OD, oropharyngeal dysphagia.
Table 2.
Specific characteristics of included studies
| Study ID |
Epidemiological design characteristics: (a) Type of study (b) Epidemiological approach (c) Data gathering (d) Time horizon |
Economic design characteristics: (a) Analysis perspective (b) Use of temporary discount rate (c) Sensitivity analysis |
Data source |
Elements of cost considered: (a) Direct healthcare costs (b) Direct non- healthcare costs (c) Indirect costs |
| Wojner22 AACN Clin Issues 2000 |
|
|
Data collected from medical records and the hospital’s cost accounting system. |
|
| Katzan27 Neurology 2007 |
|
|
Data collected from the Cleveland Health Quality Choice Programme from non-federal hospitals in northeast Ohio. Patient charges were obtained from Medicare files. |
|
| Christensen28 Acta Neurol Scand 2009 |
|
|
Medical records. Costs data were obtained from FLENI database. |
|
| Christensen29 Neuroepidemiology 2009 |
|
|
Medical records. Costs data were obtained from Sistema Único de Saúde, 2007 values. |
|
| Wilson J Stroke Cerebrovasc Dis 201230 |
|
|
Data collected from the 2005 and 2006 Nationwide Inpatient Sample from the United States. |
|
| Bonilha23 Dysphagia 2014 |
|
|
Data collected from South Carolina Medicare database. |
|
| Chen24 J Rehabil Med 2015 |
|
|
Medical records and the hospital’s management information system. |
|
| Chen25 Top Stroke Rehabil 2016 |
|
|
Medical records and the hospital’s management information system. |
|
| Gomes31 J Stroke Cerebrovasc Dis 2016 |
|
|
Medical records. Cost data was obtained from the Department of Health Payment by Results Tariff Information Spreadsheet. |
|
| Muehlemann26 PLoS One 2019 |
|
|
Data collected from the French Medical Information System Programme and the Swiss OFS Database ‘Office federal de la statistique: Statistique des couts par cas 2012’. |
|
Table 3.
Specific characteristics of study populations
| Study ID | Age and gender | Patient inclusion or exclusion criteria | Method of OD diagnostic | OD and/or complication incidence | Previous OD or stroke in patients |
| Wojner22 AACN Clin Issues 2000 |
Age: Overall 67.2 (14.3)* TF dependency 72.7 (13.9)* No TF: 64.8 (13.4)* Gender (male): Overall 80 (46.78%)† |
Inclusion criteria:
Exclusion criteria:
|
Dysphagia screening on admission and during hospitalisation. The assessment method was not described. | TF dependence prevalence during hospitalisation: 30 (17.54).† | Not available. |
| Katzan27 Neurology 2007 |
Age: Total: 76.8 (8.14)* Pneumonia: 77.6 (8.24)* No pneumonia: 76.7 (8.13)* Gender (female): Total: 6.405 (56.7)† Pneumonia: 266 (41.9)† No pneumonia: 6.139 (57.6)† |
Inclusion criteria:
Exclusion criteria:
|
Not available. |
Dysphagia: Not available. Pneumonia (%): Hospitalisation: 5.6 |
Not available. |
| Christensen28 Acta Neurol Scand 2009 |
Age: Ischaemic stroke: 64.8 (15.5)* Haemorrhagic stroke: 61.8 (15.5)* Gender (male, %): Ischaemic stroke: 66.7 Haemorrhagic stroke: 58.6 |
Inclusion criteria:
Exclusion criteria:
|
Not available. |
Dysphagia: Not available Pneumonia : Not available |
Only first haemorrhagic or ischaemic stroke patients. Presence of previous dysphagia was not recorded. |
| Christensen29 Neuroepidemiology 2009 |
Age: Ischaemic stroke: 61(14)* Haemorrhagic stroke: 64(13).* Gender (male): Ischaemic stroke: 145(54)† Haemorrhagic stroke: 26(60).† |
Inclusion criteria:
Exclusion criteria:
|
Not available. |
Dysphagia: Not available Pneumonia (%): Hospitalisation: 6 |
Only first haemorrhagic or ischaemic stroke patients. Presence of previous dysphagia was not recorded. |
| Wilson J Stroke Cerebrovasc Dis 201230 |
Age (mean): Pneumonia: 73.3 No pneumonia: 70.6 Gender (female, %): Pneumonia: 49.4 No pneumonia: 54.6 |
Inclusion criteria:
Exclusion criteria:
|
Not available. |
Dysphagia: Hospitalisation: Pneumonia: 0.43 No pneumonia: 0.115 Pneumonia (%): Hospitalisation: 8.1 (95% CI. 7.8 to 8.3) |
Not available. |
| Bonilha23 Dysphagia 2014 |
Age: Overall: 78.1 (6.9)* OD: 79.4 (6.7)* No OD: 78.0 (6.9).* Gender (male): Overall: 1228 (38.4)† OD: 118 (37,2)† No OD: 1110 (38.5)† |
Inclusion criteria: Primary diagnosis of ischaemic stroke. Exclusion criteria:
|
Not available. |
Dysphagia: Hospitalisation: 317 (9.9%) patients.† |
Not available. |
| Chen24 J Rehabil Med 2015 |
Age: Overall: 68.9 (12.2)* Gender (male): Overall: 137 (44.1)† |
Inclusion criteria:
|
Examination or bedside test performed by a physiatrist. |
Dysphagia: Admission: 205 (65.9)† |
Only first ischaemic stroke patients. Presence of previous dysphagia was not recorded. |
| Chen25 Top Stroke Rehabil 2016 |
Age: Overall: 61.3 (13.5)* Gender (male): Overall: 137 (57.8%)† |
Inclusion criteria:
|
Examination or bedside test performed by a clinician. |
Dysphagia: Hospitalisation: 118 (49.8)† |
Only first haemorrhagic stroke patients. Presence of previous dysphagia was not recorded. |
| Gomes31 J Stroke Cerebrovasc Dis 2016 |
Age (mean): Overall: 74.5 Gender (male, %): Overall: 51 |
Inclusion criteria:
|
Screening test (not specified) by nurses on admission. |
Dysphagia (%): Low RoM: 19 Medium RoM: 26 High RoM: 61 RoM: 156/537(29)† were at high RoM |
Presence of previous dysphagia was not recorded. 22% of the patients had a previous stroke. |
| Muehlemann26 PLoS One 2019 |
Age (mean): Not available. Gender (male, %): OD: 47% No OD:52% (France) OD: 51% No OD: 55% (Switzerland) |
Inclusion criteria: -Cerebral infarction or sequelae of cerebral infarction. Exclusion criteria:
|
Not available. |
Dysphagia (%): Hospitalisation: 4.2 (France), 8.4 (Switzerland). |
Not available. |
*Values are mean (SD).
†Values are n (%).
ICD, International Classification of Diseases; OD, oropharyngeal dysphagia; RoM, risk of malnutrition; TF, stands for tube feeding.
Table 4.
Results of individual studies
| Study ID | Aim | Sample size | Crude incremental costs | P value | Adjusted incremental costs | P value |
| Wojner22 AACN Clin Issues 2000 |
Cost of TF | 171 | Mean cost for TF patients: US$12 538±US$6247. Mean cost for non-TF patients: US$5949±US$3428=. |
<0.0001 | – | – |
| Katzan27 Neurology 2007 |
Cost of pneumonia | 11 286 | US$14 901 (95% CI US$14 279 to US$15 524) | – | US$14 836 (95% CI US$14 436 to US$15 236 | – |
| Christense28 Acta Neurol Scand 2009 |
Cost of pneumonia | 167 |
Ischaemic stroke: US$36 149 Haemorrhagic stroke: US$16 893 |
=0.003 =0.003 |
– – |
– |
| Christensen29 Neuroepidemiology 2009 | Cost of pneumonia | 316 |
Ischaemic stroke: Mean cost for non-pneumonia patients: US$1776. Mean cost for patients who developed pneumonia: US$4251. Haemorrhagic stroke: Mean cost for non-pneumonia patients: US$3553. Mean cost for patients who developed pneumonia: US$8485. |
<0.001 =0.015 |
US$1456 – |
<0.001 |
| Wilson J Stroke Cerebrovasc Dis 201230 |
Cost of pneumonia | 183 976 | US$23 102 | – | US$27 633 (95% CI US$27 078 to US$27 988) | – |
| Bonilha23 Dysphagia 2014 |
Cost of OD | 3200 |
Mean total payments for OD patients: US$22 379±US$14 250 Mean total payments for non-OD patients: US$18 560±US$14 429 Crude incremental costs: US$3819 |
<0.0001 |
Mean adjusted total payments for OD patients: US$22 266 (95% CI US$20 839 to US$23 787) Mean adjusted total payments for non-OD patients: US$17 756 (95% CI US$17 372 to US$18 150) Adjusted incremental costs: US$4510 |
<0.0001 |
| Chen24 J Rehabil Med 2015 |
Cost of OD | 311 | Mean cost for OD patients: US$5134.5±US$3064.6 Mean patients cost: US$4606.80±US$2926.1. |
<0.001 | – | – |
| Chen25 Top Stroke Rehabil 2016 |
Cost of OD Cost of pneumonia |
237 | Mean cost for OD patients: US$7329.2±US$3977.2. Mean cost for patients who developed pneumonia: US$9053.7±US$5142.0. Mean patients cost: US$5939.5±US$3578.5D |
<0.001 <0.001 |
– | – |
| Gomes31 J Stroke Cerebrovasc Dis 2016 |
Cost of malnutrition | 543 | Median costs low-risk patients: 4920 (£ 437–£38 200) Median costs medium-risk patients: 6490 (£1050–£19 600) Median costs high-risk patients: 8720 (£552–£31 900) |
<0.001 | – | – |
| Muehlemann26 PLoS One 2019 |
Cost of OD | 62 297 (F) 6037 (S) |
2926 euros (F) 13 959 Swiss Francs (S) |
– | – | – |
F, France; OD, oropharyngeal dysphagia; R, range minimum-maximum; S, Switzerland; TF, tube feeding.
Costs of OD after stroke
Five studies have assessed the cost of OD in patients with stroke from different perspectives and using different methodologies. These five studies had a longitudinal design. Studies’ sample size ranged from 171 to 68 334 participants and the mean age of participants ranged from 61.3 to 78.1 years.22–26 Two studies were performed in the USA,22 23 two were performed in Taiwan,24 25 and one was performed in France and Switzerland.26 The US studies and the study performed in France and Switzerland were cost analysis studies22 23 26 and the Taiwanese studies were cost prediction studies.24 25 Incidence of OD ranged from 4.2% to 65.9%. Screening for OD on admission was performed only in the study by Wojner and Alexandrov.22 One study analysed hospitalisation costs in patients depending on tube feeding (TF) from a hospital perspective.22 One study analysed hospital care, nursing home, care provider, home health, outpatient and durable medical equipment costs during the first year after ischaemic stroke and from the perspective of Medicare.23 Two studies analysed hospital costs including diagnoses, ward, laboratory, X-rays, therapeutic and surgical procedures, blood/plasma, anaesthesia, special materials, TF, rehabilitation, drugs, dispensing and injection services, haemodialysis and psychiatric treatment for patients transferred to a rehabilitation ward after ischaemic24 and haemorrhagic25 stroke from the hospital and patient perspectives. One study analysed the cost of hospital stay from the hospital perspective.26
Wojner and Alexandrov found significantly higher costs during the hospitalisation for those patients who depended on a TF. Mean hospitalisation costs were US$12 538±US$6247 for those patients who depended on a TF and US$5949±US$3428 for those who did not (p<0.0001), suggesting a non-adjusted effect of TF of approximately US$6300. The study also showed that those patients depending on a TF were older, with a greater neurological impairment and had longer hospital stays. NIHSS score was found to be an independent risk factor for TF dependency.22 Bonilha et al found an increase of US$4510 (p<0.0001) on the total medical costs during the first year after stroke for patients with ischaemic stroke who developed OD, controlling for age, comorbidities, ethnicity and time alive. Nevertheless, stroke severity was not shown as a significant independent predictor for the cost model.23 Chen et al examined the predictors for total medical costs in first ischaemic stroke patients transferred to a rehabilitation ward in Taiwan. In this study, OD was significantly related with total medical costs during hospitalisation in the univariate analysis but not in the multivariate analysis. Significant predictors for total medical costs after multivariate analysis were: impaired consciousness, hypoalbuminaemia, fever, hypokalaemia and hyponatraemia. Mean total costs for dysphagia patients during the hospitalisation were 5134.5±3064.6, and total medical costs for all patients (including those who already had dysphagia) were US$4606.8±US$2926.1.24 Following the same methodology and perspectives, Chen et al examined the predictors for total medical costs in patients with a first haemorrhagic stroke event. This study shows that OD is related to a significant increase in the total medical costs with a beta coefficient of 1025.8 (95% CI 193.9 to 1857.8; p<0.001). Total cost for OD patients was US$7329.2±US$3977.2 while total medical cost for all patients (including those who already had OD or pneumonia) was US$5935.5±US$3578.5.25 Muehlemann et al found an incremental cost for patients who had OD of €3000 (approx. US$4300, 2019 USD) in France and SFr14 000 (approx. US$16 900, 2019 USD) in Switzerland. OD was associated with a significant increase in hospital costs during admission after adjusting for the presence of motor or/and sensory stroke complications (p<0.0001).26
Cost of poststroke safety of swallow complications: pneumonia
We found five studies that evaluated the cost of pneumonia in patients with stroke,25 27–30 one of which has been previously mentioned for providing information on the cost of OD after stroke.25 These five studies had a longitudinal, retrospective cost analysis design with sample sizes between 167 and 183 976 participants with a mean age of 61.3–76.8 years.25 27–30 Two studies were performed in the USA,27 30 one in Taiwan,25 one in Argentina28 and one in Brazil.29 Presence of OD among analysed patients was reported in two studies.25 30 Chem et al found that 49.8% of the assessed patients suffered OD during hospitalisation25 and Wilson et al found that OD was present in 42.9% of patients who developed pneumonia and in 11.5% of those who did not (p<0.0001).30 Pneumonia incidence ranged from 5.6% to 8.1%25 27–30 The studies, except for the one performed by Chen and Ke,25 analysed hospitalisation costs after stroke from the hospital perspective.27–30
Chen and Ke showed that pneumonia was related to a significant increase in the total medical costs with a beta coefficient of 2330.1 (95% CI 1339.5 to 3320.7; p<0.001). Total cost for patients who developed pneumonia was US$9053.7±US$5142.0 while total medical cost for all patients (including those who already had pneumonia) was US$5939.5±US$3578.5.25 Katzan et al showed an incremental cost for patients who developed pneumonia during the hospitalisation of US$14 863 (95% CI 14 436 to 15 236) adjusting for stroke severity, stroke patients’ propensity for pneumonia and other factors associated with higher hospitalisation costs. Pneumonia was more commonly identified in those patients with a more severe stroke (p<0.001).27 Wilson found an additional adjusted cost of US$27 633 (95% CI 27 078 to 27 988) for a pneumonia episode during hospitalisation after stroke. Costs were adjusted for age, gender, hospital factors (teaching, rural, urban), admission from emergency department, illness severity, propensity for pneumonia and comorbidities.30 Christensen et al estimated that patients who developed pneumonia incurred significantly higher costs in both ischaemic (US$36 149; p=0.003), and haemorrhagic stroke (US$16 893; p=0.003) in Argentina.28 Finally, in the Christensen et al study performed in Sao Paulo, Brazil, an increase in hospitalisation cost for patients who developed pneumonia was observed for haemorrhagic stroke (US$8485 vs US$3553; p=0.015) and for ischaemic stroke (US$4251 vs US$1776; p<0.001). Development of pneumonia during admission was found to be a significant independent predictor of acute treatment costs in the multivariate analysis. An adjusted cost of pneumonia of US$1456 was found when adjusting for all patient and treatment characteristics (p<0.001).29
Cost of poststroke efficacy of swallow complications: malnutrition
Gomes et al aimed to assess the validity of a nutrition-screening tool (Malnutrition Universal Screening Tool) to predict poor outcomes and hospitalisation costs in patients after a stroke episode. A total of 543 patients were enrolled in a longitudinal, prospective study. Costs were measured during 6 months after stroke from a hospital perspective. Mean age of participants was 74.5 years. The study was performed in London in two hyperacute stroke units and compared the hospitalisation costs according to risk of malnutrition. The study showed that an increase in malnutrition risk involved an increase in hospitalisation costs, from £4920 sterling (GBP) 2011 (approx. US$8780, 2019 USD) in low risk patients to £8720 (approx. US$15 560, 2019 USD) in high-risk patients (p<0.001). Risk of malnutrition was identified as an independent predictor for hospitalisation costs. Moreover, the study showed an association between high risk of malnutrition and inadequate swallow on hospital admission.31
Synthesis of the study’s findings
Short-term cost of OD and its complications during hospital admission
In the case of patients admitted to a rehabilitation ward due to haemorrhagic stroke, the median cost for those who developed OD was US$7329.2 while for the entire sample it was US$5939.5.25 In the case of ischaemic stroke patients admitted to a rehabilitation ward, OD was not identified as a predictor for the total medial costs in the multivariate analysis. An additional cost for those patients may be US$528 per case.24 For patients who needed TF, an approximate increase of US$6300 was observed.22 For patients hospitalised in France and Switzerland after stroke an increase of €3000 and SFr14 000 was observed.26 In the five studies reporting data on the cost of pneumonia after stroke, an adjusted effect of pneumonia on costs during hospitalisation after stroke was found to be between US$1456 and US$27 633 depending on the setting and the design of the study.25–30
Long-term cost of OD and it’s complications including post hospital discharge costs
We found only two studies assessing cost related to OD or it’s complications beyond the first hospital stay due to acute stroke. The study performed by Bonilha et al found an increase of US$4510 for those patients who developed OD during the first year after acute stroke.23 In malnutrition, Gomes et al showed an increase in all hospitalisation costs during the first 6 months after stroke. These costs increased from £4920 (approx.US$8780, 2019 USD) in low risk of malnutrition patients to £8720 (approx. US$15 560, 2019 USD) in high-risk patients.31
Quality assessment
Risk of bias was assessed for each study. Total scores for each study are available in table 1 and specific results for each study are available in the online supplementary appendix. Productivity losses secondary to the pathology were not assessed in any of the studies and quantities of consumed resources were only reported by Christensen et al and Muehlemann et al and separately from their unit costs26 28 Methods for the estimation of quantities and units costs were barely described in the studies, the studies by Katzan et al and Christensen et al were the only ones to completely include them.27–29 A sensitivity analysis was only offered in the studies by Christensen et al and Gomes et al.28 29 31 The majority of data for these studies was retrospectively obtained from databases based on diagnosis codes. This can cause major information loss when calculating a disease cost as some conditions are underdiagnosed. We suspect that OD and/or pneumonia underdiagnosis could occur in some studies due to the absence of screenings for OD and low codification of these events in the databases.
Confidence in cumulative evidence
In the case of OD, one prospective and four retrospective studies were assessed. The risk of bias ranged from 50 to 80. High consistency with a large and direct effect was found among the results of four studies. In the study performed by Chen et al, OD was significantly correlated with the total medical costs in univariate analysis but not in multivariate analysis so we have not qualified this study as a positive study.24 We consider the level of evidence regarding higher costs related to poststroke OD to be moderate. In the case of pneumonia, we also consider the level of evidence in favour of higher costs related to this complication to be moderate based on the five retrospective positive studies. The risk of bias punctuation ranged from 64 to 83. Despite the fact that the longitudinal design of the considered studies indicates moderate strength of scientific evidence, a high consistency with a large and direct effect was found among the results of the different studies. In the case of malnutrition, we only found one prospective study and the level of evidence was low.
Discussion
This systematic review shows moderate quality evidence towards higher costs for those patients who developed OD or pneumonia after stroke. Acute in-hospital costs related to OD were analysed in five studies. Despite this, design and results were very different across studies and it is difficult to show definitive conclusions as a quantitative synthesis of the results cannot be made. The results of these economic studies do not match recent clinical studies that clearly show OD after stroke is an independent risk factor for prolonged hospital stay and institutionalisation after discharge; OD has been shown to be an independent risk factor for poorer functional capacity and increased mortality 3 months after stroke.6 A recent study performed in the USA also related OD to longer length of stay, higher inpatient costs and likelihood of being transferred to postacute care facility and inpatient mortality during hospitalisation.32 Monetisation of these poor clinical outcomes specifically caused by OD is urgent and would probably indicate specific incremental costs for OD. Regarding the costs of pneumonia, Christensen et al showed, in Brazil and Argentina, an increase in hospitalisation costs for stroke patients who developed pneumonia of between US$1456 and US$36 149 depending on the type of the stroke and the country.28 29 These higher health costs more closely approximate what we estimate could be the increment in costs from OD and pneumonia from the clinical trials we are performing: US$27 633 to US$36 149 seem reasonable and can be translated to a 2019 USD value of US$32 500 to US$48 000. In the study performed by Gomes et al, an association between OD and malnutrition is observed on admission. However, this study did not directly assess OD costs or deglutition alterations and it cannot be directly extrapolated that malnutrition costs were a consequence of OD although we can assume that malnutrition in OD patients can also lead to higher costs31 It should be noted that the cost associated with complications related to OD accounted for a large part of the total costs of hospitalisation in the included studies and that it is possible that hospitalisation costs do not include all the costs attributable to these complications. Health economic studies on the cost of other complications of stroke are scarce. No article measuring aspiration, dehydration or mortality costs was found.
We believe that from our data we can estimate the cost of OD in the acute phase might be up to €15 000 (approx. US$16 900) and the cost of an episode of aspiration pneumonia up to €24 000 (approx. US$27 600). Our systematic review differs from a previously published systematic review because it focused on poststroke OD and explored the costs related to OD complications. This previous systematic review aimed to assess the influence of OD secondary to all aetiologies on length of stay and costs showed an increase of 40.36% in costs of patients with OD. A subgroup evaluation showed a higher and more variable length of stay of 4.73 days (95% CI 2.7 to 7.2) for patients with stroke.33
Two important aspects in this field must be highlighted. First, poststroke OD is a condition for which effective interventions are available that may reduce long-term cost and related complications. Costs related to caring for poststroke OD patients have been little reported. Further research should be done to assess the poststroke OD economic and social burden to better understand and raise awareness about minimal care for this common and severe complication in patients with poststroke. It is becoming increasingly difficult to ignore the need for early screening and basic treatment in poststroke OD patients to reduce their mortality rate and improve their outcomes. Second, in most of the included studies, patients with poststroke were not screened for OD so only the most severe patients may have been diagnosed with OD. This fact could affect the prevalence of OD in these studies and the results of this systematic review and explain the underdiagnosis of OD in some of these studies compared with the literature.2 Furthermore, the high presence of false negatives could have led to a dilution of the effect. A future prospective study on costs related to poststroke OD in a sample of patients screened early for dysphagia could provide more accurate results.
We found only one study providing information on OD cost beyond the first admission due to acute stroke. However, we could not find any economic evaluation regarding other relevant complications of poststroke OD such as: (1) the need for institutionalisation after discharge, (2) loss of functional capacity, (3) costs related to home care of these patients, (4) and the related short-term and long-term mortality and impaired quality of life.6 Furthermore, costs of patient care outside the acute hospital setting, social costs and non-sanitary costs have been barely studied. This systematic review shows the need for future high-quality studies to quantify the acute and chronic cost of poststroke OD and its specific complications.
Conclusions
This systematic review only partially fulfils the proposed objectives. The studies found were conducted in very different contexts and following very different approaches, limiting the analysis to a narrative explanation of what has been investigated to date. In addition, for those studies related to complications associated with OD, the relationship between OD and the complication was not clearly established. Despite this, this systematic review shows increased economic costs during hospitalisation and long-term follow-up in patients who developed poststroke OD or its complications. Future studies on OD after stroke on patients screened and detected early for OD will enable the long-term costs of OD and the true cost of its severe complications to be calculated. Taking into account the chronic nature of this condition, it is necessary to discover the real health and social costs associated with this pathology. In addition, due to the severe complications that patients with poststroke with OD present and their increased associated cost, it would be interesting to assess the cost-effectiveness of the current available treatments for these patients.
Supplementary Material
Acknowledgments
The authors thank Agustí Viladot, member of Mataró Hospital Library Service, for his assistance with literature search development and realization, Jane Lewis for reviewing the English and Maurice Driessen for reviewing the final version of this manuscript and advice on healthcare economics. This work has been conducted within the framework of a doctoral thesis in medicine from the Medicine Department of the Autonomous University of Barcelona.
Footnotes
Contributors: SM is the guarantor. He drafted the first version of this manuscript. He provided expertise on health economics. He contributed to the realisation of the introduction and the conclusions. He reviewed and extracted data of selected studies and contributed with Drummond’s and GRADE application. He contributed with data synthesis. MS-P provided expertise on investigation methodology and health economics. He collaborated in writing the manuscript. He provided important references for the development of the methodology of this work. He reviewed and made contributions on the methodology of all the sections of this protocol. He reviewed the correct use of all the economic terms included in this manuscript. He reviewed and extracted data of selected studies and contributed with Drummond’s and GRADE application. He contributed with data synthesis. He read and approval the final revision of this systematic review. OO provided expertise on poststroke OD treatments and interventions. He collaborated in writing the manuscript. He contributed on the development of the data collection, quality assessment and data synthesis sections. He provided a critical revision of all the sections of this systematic review. He reviewed and extracted data of selected studies and contributed with Drummond’s and GRADE application. He contributed with data synthesis. PC provided expertise on poststroke OD and the design of the study. He reviewed the correct use of all the medical terms included in this manuscript. He contributed on the study design, and the development and writing of the introduction, the methodology and the conclusions of the manuscript. He provided a critical revision of all the sections of this protocol. He contributed to the correct following of the recommendations proposed by PRISMA. He provided a critical revision of all the sections of this systematic review. He reviewed and extracted data of selected studies and contributed with Drummond’s and GRADE application. He contributed with data synthesis. All authors provided a critical revision and read and approved the final revision of this systematic review. All authors contributed with the realisation of the protocol for this systematic review.
Funding: This study is funded by an educational grant from Nutricia Advanced Medical Nutrition. This study is supported by Fundació Salut del Consorci Sanitari del Maresme, Ciberehd (in turn supported by the Instituto de Salud Carlos III, Barcelona, Spain) and Fundació de Recerca en Gastroenterologia (FUREGA).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This systematic review is the first part of a research project. The full extent of this project includes (1) a systematic review on the cost of OD and its complications after stroke; (2) a systematic review on economic evaluations of interventions related to screening, diagnosis, management and treatment of OD after stroke; (3) a cost of illness study with one year follow up in patients with poststroke to assess the acute and chronic cost of OD and its complications; and (4) a cost-effectiveness study of compensatory versus active interventions aimed at recovering swallowing function in patients with poststroke.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No additional data is available.
References
- 1.Rofes L, Arreola V, Romea M, et al. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil 2010;22:851–e230. 10.1111/j.1365-2982.2010.01521.x [DOI] [PubMed] [Google Scholar]
- 2.Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–63. 10.1161/01.STR.0000190056.76543.eb [DOI] [PubMed] [Google Scholar]
- 3.Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke 1999;30:744–8. 10.1161/01.str.30.4.744 [DOI] [PubMed] [Google Scholar]
- 4.WHO, International classification of disease [online], 2016. Available: http://www.who.int/classifications/icd/en
- 5.Ortega O, Cabré M, Clavé P. Oropharyngeal Dysphagia: Aetiology & Effects of Ageing. Journal of GHR 2014;3:1049–54. [Google Scholar]
- 6.Rofes L, Muriana D, Palomeras E, et al. Prevalence, risk factors and complications of oropharyngeal dysphagia in stroke patients: a cohort study. Neurogastroenterol Motil 2018;23:e13338. 10.1111/nmo.13338 [DOI] [PubMed] [Google Scholar]
- 7.Ortega O, Martín A, Clavé P. Diagnosis and management of oropharyngeal dysphagia among older persons, state of the art. J Am Med Dir Assoc 2017;18:576–82. 10.1016/j.jamda.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 8.Cabre M, Serra-Prat M, Palomera E, et al. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing 2010;39:39–45. 10.1093/ageing/afp100 [DOI] [PubMed] [Google Scholar]
- 9.Clavé P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther 2006;24:1385–94. 10.1111/j.1365-2036.2006.03118.x [DOI] [PubMed] [Google Scholar]
- 10.Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology 1999;116:455–78. 10.1016/S0016-5085(99)70144-7 [DOI] [PubMed] [Google Scholar]
- 11.Altman KW, Yu G-P, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg 2010;136:784–9. 10.1001/archoto.2010.129 [DOI] [PubMed] [Google Scholar]
- 12.European Society for swallowing disorders (2012) ESSD position statements: oropharyngeal dysphagia in adult patients. Available: www.myessd.org/docs/position_statements/ESSD_Position_Statements_on_OD_in_adult_patients_for_web.pdf [Accessed 10 Feb 2019].
- 13.Cabib C, Ortega O, Kumru H, et al. Neurorehabilitation strategies for poststroke oropharyngeal dysphagia: from compensation to the recovery of swallowing function. Ann N Y Acad Sci 2016;1380:121–38. 10.1111/nyas.13135 [DOI] [PubMed] [Google Scholar]
- 14.Ickenstein GW, Riecker A, Höhlig C, et al. Pneumonia and in-hospital mortality in the context of neurogenic oropharyngeal dysphagia (NOD) in stroke and a new NOD step-wise concept. J Neurol 2010;257:1492–9. 10.1007/s00415-010-5558-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rofes L, Arreola V, Almirall J, et al. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol Res Pract 2011;2011:1–13. 10.1155/2011/818979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;21:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marin S, Serra-Prat M, Ortega O, et al. Cost of oropharyngeal dysphagia after stroke: a systematic review. prospero: International prospective register of systematic reviews. 2018. CRD42018099977. Available: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=99977
- 18.Marin S, Serra-Prat M, Ortega O, et al. Cost of oropharyngeal dysphagia after stroke: protocol for a systematic review. BMJ Open 2018;8:e022775. 10.1136/bmjopen-2018-022775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centre for Reviews and Dissemination Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York, 2009. [Google Scholar]
- 20.Drummond MF, O'Brien B, Torrance GW, et al. Methods for the economic evaluation of healthcare programmes. 2nd ed Oxford: Oxford University Press, 1997. [Google Scholar]
- 21.Guyatt G, Oxman AD, Akl EA, et al. Grade guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 22.Wojner AW, Alexandrov AV. Predictors of tube feeding in acute stroke patients with dysphagia. AACN Clin Issues 2000;11:531–40. 10.1097/00044067-200011000-00006 [DOI] [PubMed] [Google Scholar]
- 23.Bonilha HS, Simpson AN, Ellis C, et al. The one-year attributable cost of post-stroke dysphagia. Dysphagia 2014;29:545–52. 10.1007/s00455-014-9543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C-M, Chang C-H, Hsu H-C, et al. Factors predicting the total medical costs associated with first-ever ischeamic stroke patients transferred to the rehabilitation ward. J Rehabil Med 2015;47:120–5. 10.2340/16501977-1894 [DOI] [PubMed] [Google Scholar]
- 25.Chen C-M, Ke Y-L. Predictors for total medical costs for acute hemorrhagic stroke patients transferred to the rehabilitation ward at a regional hospital in Taiwan. Top Stroke Rehabil 2016;23:59–66. 10.1179/1945511915Y.0000000006 [DOI] [PubMed] [Google Scholar]
- 26.Muehlemann N, Jouaneton B, de Léotoing L, et al. Hospital costs impact of post ischemic stroke dysphagia: database analyses of hospital discharges in France and Switzerland. PLoS One 2019;14:e0210313. 10.1371/journal.pone.0210313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katzan IL, Dawson NV, Thomas CL, et al. The cost of pneumonia after acute stroke. Neurology 2007;68:1938–43. 10.1212/01.wnl.0000263187.08969.45 [DOI] [PubMed] [Google Scholar]
- 28.Christensen MC, Previgliano I, Capparelli FJ, et al. Acute treatment costs of intracerebral hemorrhage and ischemic stroke in Argentina. Acta Neurol Scand 2009;119:246–53. 10.1111/j.1600-0404.2008.01094.x [DOI] [PubMed] [Google Scholar]
- 29.Christensen MC, Valiente R, Sampaio Silva G, et al. Acute treatment costs of stroke in Brazil. Neuroepidemiology 2009;32:142–9. 10.1159/000184747 [DOI] [PubMed] [Google Scholar]
- 30.Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis 2012;21:61–7. 10.1016/j.jstrokecerebrovasdis.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes F, Emery PW, Weekes CE. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J Stroke Cerebrovasc Dis 2016;25:799–806. 10.1016/j.jstrokecerebrovasdis.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 32.Patel DA, Krishnaswami S, Steger E, et al. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus 2018;31:1–7. 10.1093/dote/dox131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attrill S, White S, Murray J, et al. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC Health Serv Res 2018;18:594. 10.1186/s12913-018-3376-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031629supp001.pdf (325.2KB, pdf)


