Abstract
Objectives
To examine the association between use of second-generation antipsychotics (SGA) and the risk of chronic kidney disease (CKD).
Design
Population-based case-control study.
Setting
Routinely collected laboratory, prescription and diagnostic information on all inhabitants with creatinine measurements residing on the island of Funen, Denmark (2001 to 2015).
Participants
21 434 cases with incident CKD matched with 85 576 CKD-free population controls by risk-set sampling using age, sex and calendar year.
Primary and secondary outcome measures
CKD was defined as an estimated glomerular filtration rate below 60 mL/min/1.73 m2 in a period longer than 3 months. Information on drug exposure and comorbidities were obtained from the Danish National Prescription Register and the Danish National Patient Register. We calculated OR for the association between SGA use and CKD using conditional logistic regression.
Results
Use of SGAs was associated with increased risk of CKD among ever users (OR 1.24, 95% CI: 1.12 to 1.37) and current users (OR 1.26, 95% CI: 1.12 to 1.42). We found no clear evidence of dose-response relationship. Both short duration (one to two antipsychotic prescriptions; OR 1.22, 95% CI: 1.01 to 1.48) as well as long-term use (>30 prescriptions; OR 1.45, 95% CI 1.19 to 1.76) were associated with an increased risk of CKD. Both use of SGAs with mild and high risk of metabolic disturbances was associated with increased risk of CKD (OR 1.21, 95% CI: 1.06 to 1.39 and OR 1.36, 95% CI: 1.11 to 1.68, respectively). Recent use of non-steroidal anti-inflammatory drugs, prior use of lithium, hypertension or prior acute kidney injury were not clearly associated with development of CKD connected to SGA exposure. The highest risk of CKD was found for clozapine (OR 1.81, 95% CI: 1.22 to 2.69).
Conclusion
Use of SGA is associated with a small-to-moderately increased risk of incident CKD. All investigated SGAs, except for aripiprazole, were associated with an increased risk of CKD.
Keywords: clinical pharmacology, epidemiology, nephrology, chronic renal failure, psychiatry
Strengths and limitations of this study.
Improved outcome definition by incorporating creatinine levels to estimate glomerular filtration, which enabled us to include cases of chronic kidney disease who were not treated at hospitals or specialised nephrology departments.
Inclusion of information on comorbidity and prescriptions with high validity from Danish National Health Registers.
Population-based design in a population considered representative for the general Danish population.
Limited number of antipsychotic users among cases, and very few users of second-generation antipsychotics with low risk of metabolic disturbances, such as aripiprazole.
Information on general risk factors for disease as overweight, smoking and lifestyle were not available.
Introduction
Antipsychotics are primarily labelled for maintenance treatment in schizophrenia, bipolar affective disorder and insufficiently responding unipolar depression, but are also used commonly in a number of other psychiatric conditions.1 Maintenance treatment in chronic conditions is often year-long or life-long, which makes tolerability an important concern in choosing and adhering to treatment. Furthermore, the risk of acute adverse events associated with antipsychotics are relevant to both long-term treatment and to episodic treatment. Second-generation antipsychotics (SGA) are associated with a number of adverse effects, including weight gain, metabolic syndrome, diabetes and cardiovascular disease.2 3 Observational studies have linked SGAs to an increased risk of both acute kidney injury (AKI)4 5 and chronic kidney disease (CKD).6 7
CKD can develop in several ways: Following AKI, as a complication to metabolic syndrome and diabetes (diabetic nephropathy), or as a complication to cardiovascular disease, either hypertension (hypertensive nephropathy) or arteriosclerosis.8 Use of SGAs has been associated with all these conditions. For example, case reports have described clozapine, olanzapine and quetiapine to be associated with interstitial nephritis and AKI.9 10 Therefore, maintenance treatment with antipsychotics might contribute to the development of CKD, which is important as the mortality of patients with end-stage renal disease (ESRD) is comparable to patients with coronary heart disease.11
Prior studies on the association of SGAs and CKD have used hospital discharge diagnoses of CKD as outcome definitions.6 7 In advanced stages, CKD will result in hospitalisation, dialysis, kidney transplantation or death, but less severe stages of CKD are usually handled in primary care, which are not recorded in the administrative registers.
We aimed to investigate the association between use of SGAs and the subsequent risk of CKD by combining prescription information with laboratory data to substantiate the outcome definition.
Methods
We undertook a population-based case-control study of incident CKD cases among inhabitants residing on the island of Funen, Denmark, who—between 2001 and 2015—had at least two measurements of creatinine performed. We compared the use of SGAs among CKD cases to that of a disease-free control population.
Data sources
We used information from the Funen Laboratory Cohort (FLaC). A more detailed description of FLaC has been published elsewhere.12 In summary, FLaC contains information regarding all biochemistry and laboratory results of all Funen inhabitants who, within the study period, had at least one measurement of plasma creatinine performed. A total of 460 365 patients out of 693 843 Funen inhabitants, had their creatinine measured in this period, comprising a total of 7 742 124 creatinine samples. We linked this information to several nationwide Danish administrative registers: Danish Civil Registration System,13 14 The Danish National Patient Registry,15 Registers in Statistics Denmark recording education level16 and The Danish National Prescription Registry.17 As the Danish National Health Service provides universal tax‐supported healthcare for the entire Danish population, and as all Danish inhabitants are assigned a unique personal 10-digit identifier (Central Personal Register (CPR) number) at birth, it is possible to conduct true population‐based register‐linkage studies covering the entire population.13
Population
All adults with two or more recorded creatinine values and living in Funen and the surrounding islands in the period January 2001 to December 2015 were eligible for inclusion in the study. Funen is a part of the Region of Southern Denmark, and is considered representative for the entire Danish population.18 For each individual, an observation period was defined, starting at the first creatinine measurement during the study period and ending with the last creatinine measurement. Only individuals with normal kidney function were included. In case of emigration from the island of Funen, the observation period ended on the last date of creatinine measurement prior to emigration.
Cases
Cases were defined as individuals with incident CKD during the observation period. We defined CKD according to the Kidney Disease - Improving Global Outcomes (KDIGO) guidelines19 as the first measurement of estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2. The date of this measurement defined the index date. In order to ensure that cases had CKD, the first eGFR measured 3 months after the index date also had to be below 60 mL/min/1.73 m2, as well as all the measurements in the in-between period (from the index date to 3 months after). The eGFR was calculated according to the CKDepi formula.20 Individuals with a discharge diagnosis of renal disease according to the definition of possible CKD, as proposed by Kessing et al 21 prior to the date of biochemical CKD, were excluded. (International Classification of Diseases, 10th revision (ICD-10): N18-N19.9 inclusive plus N00, N01, N03, N04, N05, N06, N8.8 plus N14.1, N14.2, N16.8, N17, N25.1, N26 and N27). Individuals with any eGFR measurement below 60 mL/min/1,73 m2 up to 1 year prior to the study start were also excluded.
Controls
Four population controls were matched on age, sex and calendar time to each case and assigned an index date corresponding to the case’s date of diagnosis. We used risk-set sampling and excluded controls who fulfilled the same exclusion criteria as described for cases. To ensure that controls had not developed CKD since their last creatinine measurement, all controls were required to have at least one creatinine recorded in the year after the index date. This measurement had to be above or equal to 60 mL/min/1.73 m2. Cases could be selected as controls before they became cases, and we allowed the study population to be selected as controls more than once. Because of these criteria, the generated OR is considered an unbiassed estimate of the incidence rate ratio. Please refer to figure 1 for a graphical depiction of the study design.
Figure 1.
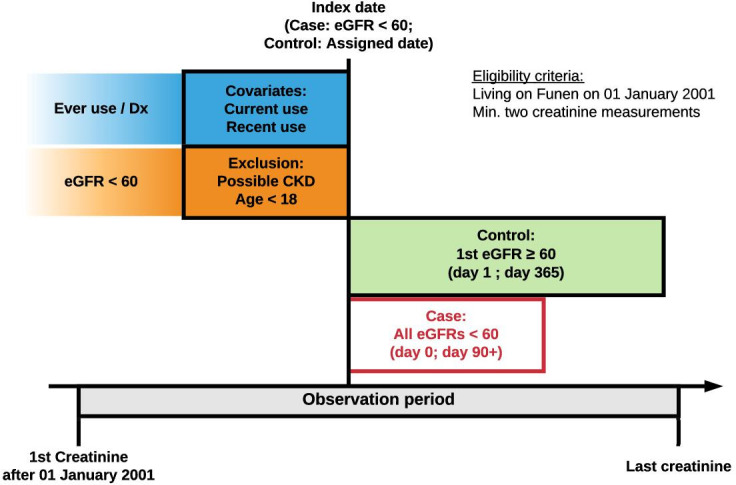
Graphical representation of time periods, case definition, control selection and covariate assessment. CKD, chronic kidney disease; Dx, Diagnosis; eGFR, estimated glomerular filtration rate.
Drug exposure
We obtained information on all filled prescriptions of SGAs and used the defined daily dose (DDD), according to the WHO Collaborating Centre for Drug Statistics methodology.22 We used the DDDs as a surrogate marker of the cumulative exposure but converted them into olanzapine equivalents.23 For an overview of the Anatomical Therapeutic Chemical Classification (ATC) codes and the corresponding DDDs, please refer to online supplementary appendix 1. The DDDs, determined by the WHO, are based on doses in maintenance treatment of schizophrenia. We used the number of filled prescriptions as a surrogate marker of duration of use, as many of the drugs are used off-label in lower doses than for treatment of schizophrenia.
bmjopen-2020-038247supp001.pdf (165.2KB, pdf)
Covariates
We included the following potential confounders in our analysis: (i) Age, sex and calendar time (accounted for by sampling procedure), (ii) use of other drugs known to affect renal function (lithium and non-steroidal anti-inflammatory drugs (NSAIDs)), (iii) history of hypertension and diabetes, and (iv) highest achieved level of education as a proxy for socioeconomic status. Use of lithium was defined as any filling of prescriptions for lithium before the index date. Recent use of NSAIDs was defined as filling of prescriptions within 1 year before the index date. Relevant ICD-10 diagnoses and ATC codes are listed in online supplementary appendix 1.
Statistical analyses
We used conditional logistic regression to estimate OR with 95% CI for the association between SGA use and the risk of CKD. Our primary outcome was risk of CKD in relation to ever use of SGA. Secondary outcomes were risk of CKD in relation to current use, cumulative exposure and cumulative duration. We computed a crude and adjusted ORs (aOR), where the adjusted model included the following predefined clinically relevant potential confounders: prior use of lithium, recent use of NSAIDs, diabetes, hypertension and highest achieved level of education. We conducted subgroup analyses by stratifying on metabolic risk of SGA as proposed by De Hert et al,3 cumulative dose, individual SGAs, diabetes, hypertension and prior AKI. To explore a potential dose response relation, we performed a supplementary analysis, using conditional logistic regression among all users of SGA and restricted cubic splines with knots placed at the value for the 10th, 50th and 90th percentile for cumulative doses among cases. We conducted a sensitivity analysis where eligible controls where not required to have normal eGFR measurement(s) in the year following the index date to assess the potential of selection bias with this criterion. Furthermore, we conducted control analyses to assess the association between CKD and known risk factors (history of diabetes or hypertension, and use of lithium or NSAIDs), and between a negative control exposure and CKD (topical ocular antibiotics—not considered associated with CKD). R V.3.5.1 (R Core Team, Vienna, Austria) was used for all analyses.
Patient and public involvement
No patients were involved in designing the study
Approval
This study was approved by the Danish Data Protection Agency (j.nr 2008‐58‐0034) and the Danish Patient Safety Authority (j.nr. 3‐3013‐809/1). According to Danish law, studies based solely on register data do not require approval from an ethics review board.24
Results
We identified 21 434 cases with incident CKD in Funen County between 2001 and 2016, with 48% males and a median age of 71 years (IQR 64 to 78 years). Using risk-set sampling, cases were matched by sex, age and calendar year to 85 576 CKD-free population controls. Hypertension and diabetes were more prevalent among cases than controls at baseline (65 vs 55% and 14 vs 10%, respectively). The most commonly used SGA among cases and controls was risperidone. See table 1 for further details.
Table 1.
Characteristics of cases and controls
| Characteristics | Cases, N (%) | Controls, N (%) |
| All | 21 434 | 85 576 |
| Demographics | ||
| Male sex (%) | 10 277 (48) | 41 010 (48) |
| Age, median (IQR) | 71 (64 to 78) | 71 (64 to 78) |
| History of mental disorders | ||
| Any psychiatric disease (%) | 1549 (7) | 4828 (6) |
| Schizophrenia (%) | 74 (<1) | 217 (<1) |
| Bipolar disease (%) | 120 (1) | 356 (<1) |
| Moderate-to-severe depression (%) | 549 (3) | 1742 (2) |
| Dementia (%) | 122 (1) | 366 (<1) |
| Other comorbidities | ||
| Acute kidney injury (%) | 2436 (11) | 2477 (3) |
| Diabetes (%) | 3057 (14) | 8138 (10) |
| Hypertension (%) | 13 962 (65) | 47 095 (55) |
| Antipsychotic exposure | ||
| Second-generation antipsychotics (%) | 557 (3) | 1731 (2) |
| Quetiapine (%) | 173 (1) | 504 (1) |
| Aripiprazole (%) | 8 (<1) | 41 (<1) |
| Risperidone (%) | 299 (1) | 967 (1) |
| Olanzapine (%) | 220 (1) | 593 (1) |
| Clozapine (%) | 37 (<1) | 82 (<1) |
| Exposure to other medications | ||
| Prior use of lithium (%) | 210 (1) | 563 (1) |
| Recent use of NSAIDs (%) | 5610 (26) | 20 081 (23) |
| Highest achieved level of education (%) | ||
| Level 1 | 10 250 (48) | 38 385 (45) |
| Level 2 | 6785 (32) | 27 778 (32) |
| Level 3 | 2687 (13) | 12 727 (15) |
| Unknown | 1712 (8) | 6686 (8) |
NSAIDs, non-steroidal anti-inflammatory drugs.
Main analysis
Among cases, 557 (2.6%) were ever users of SGAs compared with 1731 (2.0%) of controls, yielding an aOR of 1.24 (95%CI: 1.12 to 1.37). The corresponding aOR for current use was 1.26 (95%CI: 1.12 to 1.42). Control analyses confirmed that each of the assumed risk factors included in the model was positively associated with increased risk of CKD and that a negative control exposure was not associated with increased risk of CKD (online supplementary appendix 2). We did not find evidence of a dose-response relationship, using predefined categories neither in relation to cumulative use nor to duration of use (see table 2). Short duration measured as one to two antipsychotic prescriptions, as well as long-term use (>30 prescriptions) were both associated with increased risk (see table 2).
Table 2.
OR for chronic kidney disease with use of second-generation antipsychotics
| Exposure | Cases, N (%) (n=21 434) |
Controls, N (%) (n=85 576) |
Crude OR (95% CI) | Adjusted OR (95% CI) |
| Never use | 20 877 | 83 845 | 1 (ref) | 1 (ref) |
| Ever use | 557 | 1731 | 1.29 (1.17 to 1.42) | 1.24 (1.12 to 1.37) |
| Current use | 399 | 1206 | 1.32 (1.18 to 1.49) | 1.26 (1.12 to 1.42) |
| Cumulative use (olanzapine eq.) | ||||
| 0 to 899 mg | 380 | 1246 | 1.22 (1.09 to 1.37) | 1.17 (1.04 to 1.32) |
| 900 to 1799 mg | 52 | 124 | 1.70 (1.23 to 2.36) | 1.60 (1.15 to 2.23) |
| 1800 to 3649 mg | 29 | 121 | 0.96 (0.64 to 1.43) | 0.91 (0.60 to 1.38) |
| >3650 mg | 96 | 240 | 1.60 (1.26 to 2.03) | 1.46 (1.14 to 1.86) |
| Number of prescriptions | ||||
| 1 to 2 | 144 | 450 | 1.28 (1.06 to 1.54) | 1.22 (1.01 to 1.48) |
| 3 to 4 | 76 | 236 | 1.28 (0.98 to 1.66) | 1.24 (0.95 to 1.62) |
| 5 to 10 | 68 | 240 | 1.14 (0.87 to 1.50) | 1.06 (0.80 to 1.39) |
| 11 to 30 | 115 | 409 | 1.12 (0.91 to 1.38) | 1.10 (0.89 to 1.36) |
| >30 | 154 | 396 | 1.57 (1.30 to 1.89) | 1.45 (1.19 to 1.76) |
eq, equivalent.
Subgroup analysis
The majority of cases and controls used SGAs with mild risk of metabolic disturbances (eg, risperidone), followed by SGAs with high risk (eg, clozapine and olanzapine) and moderate risk (eg, quetiapine). Use of SGAs with mild and high risk of metabolic disturbances was associated with increased risk of CKD in the adjusted model (ORmildrisk 1.21, 95% CI: 1.06 to 1.39 and ORhighrisk 1.36, 95% CI: 1.11 to 1.68) (see table 3).
Table 3.
Association between exposure to SGA and the risk of chronic kidney disease by subgroups (risk of metabolic disturbances, recent use of NSAIDs, pre-existing diabetes, hypertension, prior AKI and age)
| Exposure | Cases, N | Controls, N | Crude OR (95% CI) | Adjusted OR (95% CI) |
| All | 21 434 | 85 576 | ||
| SGA | ||||
| Low risk | 8 | 41 | 0.85 (0.39 to 1.82) | 0.71 (0.32 to 1.54) |
| Mild risk | 298 | 947 | 1.26 (1.10 to 1.44) | 1.21 (1.06 to 1.39) |
| Moderate risk | 118 | 372 | 1.28 (1.04 to 1.58) | 1.19 (0.96 to 1.48) |
| High risk | 133 | 371 | 1.43 (1.17 to 1.74) | 1.36 (1.11 to 1.68) |
| NSAID | ||||
| No | 15 824 | 947 | 1.29 (1.15 to 1.45) | 1.22 (1.08 to 1.38) |
| Yes | 5610 | 20 081 | 1.13 (0.84 to 1.51) | 1.10 (0.81 to 1.49) |
| Diabetes | ||||
| No | 18 377 | 77 438 | 1.27 (1.14 to 1.41) | 1.24 (1.11 to 1.39) |
| Yes | 3057 | 8138 | 1.56 (0.96 to 2.53) | 1.52 (0.90 to 2.54) |
| Hypertension | ||||
| No | 7472 | 38 481 | 1.43 (1.19 to 1.72) | 1.33 (1.10 to 1.60) |
| Yes | 13 962 | 47 095 | 1.21 (1.05 to 1.39) | 1.14 (0.98 to 1.32) |
| Prior AKI | ||||
| No | 18 998 | 83 099 | 1.31 (1.18 to 1.47) | 1.27 (1.14 to 1.42) |
| Yes | 2436 | 2477 | 1.24 (0.59 to 2.58) | 0.96 (0.45 to 2.07) |
| Age group | ||||
| <65 years | 5847 | 23 423 | 1.66 (1.40 to 1.97) | 1.50 (1.25 to 1.80) |
| 65+ years | 15 587 | 62 153 | 1.15 (1.02 to 1.30) | 1.13 (1.00 to 1.28) |
AKI, acute kidney injury; NSAIDs, non-steroidal anti-inflammatory drugs; SGA, second-generation antipsychotics.
Users of SGAs, who also had diabetes, had a 50% increased risk of developing CKD compared with controls, but due to the low number of exposed diabetics, the CI overlapped unity (aORdiabetes 1.52, 95% CI: 0.90 to 2.54). Antipsychotic users in the low age category had an increased risk of CKD compared with the higher age category (aOR<65 years 1.50, 95% CI: 1.25 to 1.80). None of the other known risk factors for CKD (use of NSAIDs, hypertension and prior AKI) were clearly associated with development of CKD in connection to SGA exposure (see table 3). The absolute risk of CKD in this population was 3.4% for individuals <65 years and 16% for individuals ≥65 years. For individuals with prior AKI, the absolute risk was 40.8% vs 4.6% for individuals without prior AKI.
Specific SGAs
All SGAs, except for aripiprazole, were associated with increased risk of CKD (see figure 2). The risk was most pronounced for clozapine (aOR 1.81, 95% CI: 1.22 to 2.69) followed by olanzapine (aOR 1.41, 95% CI: 1.19 to 1.65) and quetiapine (aOR 1.28, 95% CI: 1.17 to 1.42).
Figure 2.
Association between exposure to SGAs and the risk of chronic kidney disease by individual drugs. aOR, adjusted OR; SGA, second-generation antipsychotics.
Supplementary and sensitivity analyses
Additional analysis of the association between cumulative dose of SGAs and the risk of CKD, yielded a somewhat uniform dose-response relationship, with risk of CKD increasing slightly with increasing cumulative dose of SGA until approximately 900 to 1000 mg olanzapine equivalents (online supplementary appendix 3).
The risk of CKD in relation to SGA exposure was largely unchanged, when including controls who did not require normal eGFR measurements in the year following their assigned index date (online supplementary appendix 4).
Discussion
In this large population-based study using routinely collected eGFR to define CKD, we found that ever users of SGAs had a higher risk of developing CKD compared with never users. However, there was no clear evidence of a dose-response relationship, and several known risk factors for CKD did not substantially increase the risk of developing CKD (eg, NSAID use, prior lithium use and prior AKI).8 We found a further increased risk of developing CKD among individuals with diabetes, and among those below 65 years of age at the time of CKD diagnosis, although the risk among diabetics was not significant. For individual antipsychotics, the use of clozapine or olanzapine was associated with the highest risk of developing CKD.
Regarding the overall risk of developing CKD in connection to treatment with SGAs, our main findings are in line with previous studies: Tzeng and colleagues6 found a similar increased risk of CKD among individuals with schizophrenia during 3 years of follow-up. (HR 1.36, 95% CI: 1.13 to 1.63), and Wang and colleagues substantiated this finding by observing an increased risk of CKD among individuals with more than 90 and 1000 days of SGA exposure (OR 1.42 and 1.30, respectively).7
In our current study, we observed a 52% increased risk of developing CKD for antipsychotic users who also had diabetes compared with non-diabetics, although not statistically significant. Development of CKD and later potentially ESRD is a well-established complication of diabetes,25 and our finding might underscore the importance of regular monitoring of kidney function in this population. CKD prevalence is related to age, as nephron loss and the prevalence of medical conditions generally increases with age.8 Our finding of the highest risk among the younger age group (table 3) might be explained by the low absolute risk observed in this age group, resulting in greater increases in relative risk, when exposed to SGAs. Another potential explanation for this finding might be a higher proportion of long-term antipsychotic use for severe mental illness in this age category, whereas antipsychotic use in the older age category might represent short-term and/or low-dose use in conditions as dementia and delirium. Furthermore, the observed increase in risk associated with use of few prescriptions is suggestive of some degree of residual confounding. Analysis of the individual SGAs in connection to CKD (see figure 2) found the highest risk associated with olanzapine and clozapine, which was expected as these SGAs are associated with the highest risk of metabolic disturbances and diabetes.2
The primary strength of the present study is the improved outcome definition. By using creatinine levels to estimate glomerular filtration, we can include CKD cases that are not treated at hospitals and specialised nephrology departments. A considerable proportion of CKD cases might be handled in general practice until severe or ESRD presents. These cases would be missed if our outcome definition only relied on hospital diagnoses. Second, the linkage to Danish registers allowed us to obtain high quality information on comorbidity and prescriptions. Finally, the population of Funen is considered representative for the general Danish population.18
However, several limitations must be acknowledged: First, the number of antipsychotic users among CKD cases was generally low, and most users had very short duration of antipsychotic use (ie, ≤2 prescriptions). Our population included few users with high cumulative doses (ie, >3650 mg olanzapine equivalents). This means that our dose-response analysis is likely to underestimate the associated risk among this subpopulation with high cumulative doses. The population also includes very few users of SGAs with low risk of metabolic disturbances, such as aripiprazole, which makes us unable to conclude if this group is associated with increased risk of CKD or not. Second, we were not able to adjust for use of other potentially nephrotoxic drugs26 (besides lithium and NSAIDs), as these are primarily used in hospitals (ie, aminoglycosides, chemotherapy or X-ray contrast) or dispensed from outpatient clinics (ie, antiretrovirals or calcineurin inhibitors), and thus not captured in our data sources. Third, information on general risk factors for disease such as being overweight, smoking and lifestyle are not included in our data sources.
Our finding of modest increases in risk of CKD with SGAs, does not suggest any clear association between these. Furthermore, the presence of an increased risk with few antipsychotic prescriptions is indicative of some degree of residual confounding. Therefore, we do not believe that SGAs by themselves increases the risk of CKD, but rather contribute to metabolic disturbances, which in the end result in kidney damage. The increased risk among SGA users with diabetes adds to this interpretation. This underscores the importance of frequent monitoring of metabolic status in patients treated with antipsychotics, which could include monitoring of kidney function as standard practice.
In conclusion, we found a small-to-moderately increased risk of incident CKD among individuals using SGAs. All investigated SGAs, except for aripiprazole, were associated with an increased risk of CKD.
Supplementary Material
Footnotes
Twitter: @JlundMikkel, @dphdk
Contributors: MH, LCL, JLEH, PD and DPH initiated and designed the study. LCL analysed the data. MH, LCL, MBH, PD and DPH interpreted the results. MH, PD and DPH drafted the manuscript. All authors critically revised the manuscript and approved the final version for submission.
Funding: This study was partially supported by two grants: one from the Beckett Foundation (Copenhagen, Denmark) and from Hede-Nielsen Family Foundation (Horsens, Denmark).
Disclaimer: The founders had no role in designing the study or deciding to submit the manuscript.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. No additional data available.
References
- 1. Marston L, Nazareth I, Petersen I, et al. . Prescribing of antipsychotics in UK primary care: a cohort study. BMJ Open 2014;4:e006135. 10.1136/bmjopen-2014-006135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Correll CU, Detraux J, De Lepeleire J, et al. . Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015;14:119–36. 10.1002/wps.20204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Hert M, Detraux J, van Winkel R, et al. . Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011;8:114–26. 10.1038/nrendo.2011.156 [DOI] [PubMed] [Google Scholar]
- 4. Hwang YJ, Dixon SN, Reiss JP, et al. . Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med 2014;161:242–8. 10.7326/M13-2796 [DOI] [PubMed] [Google Scholar]
- 5. Jiang Y, McCombs JS, Park SH. A retrospective cohort study of acute kidney injury risk associated with antipsychotics. CNS Drugs 2017;31:319–26. 10.1007/s40263-017-0421-4 [DOI] [PubMed] [Google Scholar]
- 6. Tzeng N-S, Hsu Y-H, Ho S-Y, et al. . Is schizophrenia associated with an increased risk of chronic kidney disease? A nationwide matched-cohort study. BMJ Open 2015;5:e006777. 10.1136/bmjopen-2014-006777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H-Y, Huang CL-C, Feng IJ, et al. . Second-generation antipsychotic medications and risk of chronic kidney disease in schizophrenia: population-based nested case-control study. BMJ Open 2018;8:e019868. 10.1136/bmjopen-2017-019868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romagnani P, Remuzzi G, Glassock R, et al. . Chronic kidney disease. Nat Rev Dis Primers 2017;3:17088. 10.1038/nrdp.2017.88 [DOI] [PubMed] [Google Scholar]
- 9. McLoughlin C, Cooney C, Mullaney R. Clozapine-induced interstitial nephritis in a patient with schizoaffective disorder in the forensic setting: a case report and review of the literature. Ir J Psychol Med 2019;2014:1–6. 10.1017/ipm.2019.24 [DOI] [PubMed] [Google Scholar]
- 10. He L, Peng Y, Fu X, et al. . Dibenzodiazepine derivative quetiapine- and olanzapine-induced chronic interstitial nephritis. Ren Fail 2013;35:657–9. 10.3109/0886022X.2013.780615 [DOI] [PubMed] [Google Scholar]
- 11. de Jager DJ, Grootendorst DC, Jager KJ, et al. . Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009;302:1782–9. 10.1001/jama.2009.1488 [DOI] [PubMed] [Google Scholar]
- 12. Henriksen DP, Damkier P, Hallas J, et al. . Sixteen years of creatinine measurements among 460 000 individuals-The Funen Laboratory Cohort (FLaC), a population-based pharmacoepidemiological resource to study drug-induced kidney disease. Basic Clin Pharmacol Toxicol 2019;124:582–90. 10.1111/bcpt.13167 [DOI] [PubMed] [Google Scholar]
- 13. Pedersen CB. The Danish civil registration system. Scand J Public Health 2011;39:22–5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. . The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health 2011;39:91–4. 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 17. Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. . Data resource profile: the Danish national prescription registry. Int J Epidemiol 2017;46:798–798f. 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henriksen DP, Rasmussen L, Hansen MR, et al. . Comparison of the Five Danish Regions Regarding Demographic Characteristics, Healthcare Utilization, and Medication Use--A Descriptive Cross-Sectional Study. PLoS One 2015;10:e0140197. 10.1371/journal.pone.0140197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 20. Stevens LA, Schmid CH, Greene T, et al. . Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 M2. Am J Kidney Dis 2010;56:486–95. 10.1053/j.ajkd.2010.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kessing LV, Gerds TA, Feldt-Rasmussen B, et al. . Use of lithium and anticonvulsants and the rate of chronic kidney disease: a nationwide population-based study. JAMA Psychiatry 2015;72:1182–91. 10.1001/jamapsychiatry.2015.1834 [DOI] [PubMed] [Google Scholar]
- 22. WHOCC - ATC/DDD Index. Available: https://www.whocc.no/atc_ddd_index/ [Accessed 29 Apr 2019].
- 23. Leucht S, Samara M, Heres S, et al. . Dose equivalents for antipsychotic drugs: the DDD method. Schizophr Bull 2016;42(Suppl 1):S90–4. 10.1093/schbul/sbv167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thygesen LC, Daasnes C, Thaulow I, et al. . Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health 2011;39:12–16. 10.1177/1403494811399956 [DOI] [PubMed] [Google Scholar]
- 25. Anders H-J, Huber TB, Isermann B, et al. . CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018;14:361–77. 10.1038/s41581-018-0001-y [DOI] [PubMed] [Google Scholar]
- 26. Awdishu L, Mehta RL. The 6R's of drug induced nephrotoxicity. BMC Nephrol 2017;18:124. 10.1186/s12882-017-0536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038247supp001.pdf (165.2KB, pdf)



