Abstract
Retrorectal cysts are cystic lesions located in the retrorectal space and are a distinct subset of retrorectal tumours, which are often misdiagnosed due to their rarity and mimicry of symptoms caused by common diseases. We have described the presentation and management of four patients who were diagnosed with retrorectal cysts from a 10-year retrospective chart review at our institute, a tertiary care centre. In middle-aged women, the following should raise suspicion of retrorectal cyst: gastrointestinal or urinary obstructive features, mass or fullness palpable on the posterior wall on digital rectal examination, presacral dimple, perianal fistula and/or recurrent disease. Such features should prompt an MRI evaluation of the pelvis for definitive diagnosis.
Keywords: gastrointestinal surgery, general surgery
Background
Retrorectal cysts are rare lesions that are often misdiagnosed and managed incorrectly. The difficulty in early diagnosis is due to their rarity and to their protean manifestations that mimic other common clinical conditions. The consequence of such a presentation results in delayed treatment and prolonged suffering. The precise incidence of retrorectal cysts is unknown. Several studies in the literature have estimated that approximately one to six patients are diagnosed and treated annually for retrorectal tumours in tertiary care centres.1–4 The percentage of retrorectal tumours that were reported to be cystic in nature ranged between 29% and 47%.5–8 The objective of this paper is to understand the versatile nature of this rare disease with the help of four cases managed at our institute over the last 10 years and a detailed literature review.
Description of cases
Case 1
A 44-year-old non-Hispanic white woman was referred to surgical oncology department following a futile laparotomy for ovarian cyst management. She had a history of irregular menses 30 months ago. At the beginning of her gynecological evaluation elsewhere 2 years ago, she was diagnosed with an ovarian cyst on transvaginal ultrasound examination. Her symptoms resolved on hormonal therapy. Since the ovarian cyst had not changed in size for 6 monthly follow-up ultrasound examinations, the decision was made by her gynaecologist for a laparoscopy which showed normal bilateral ovaries. Post laparoscopy re-evaluation with transvaginal ultrasound showed the presence of persistent cyst. MRI evaluation of the pelvis with contrast revealed the presence of a retrorectal complex cystic mass measuring 5.5×6.5×7.0 cm with postcontrast enhancement of cystic wall and septations. The differential diagnosis included tailgut cyst and duplication cyst. On re-evaluation at the surgical oncology department, she described a history of constipation of 5-year duration. On vaginal examination, there was firmness over the posterior vaginal wall. Digital rectal examination revealed 3×4 cm palpable mass projecting through the posterior rectal wall. She underwent modified Kraske procedure without postoperative complications. The final histopathological diagnosis was duplication cyst.
Case 2
A 36-year-old non-Hispanic white woman in remission of acute myeloid leukaemia for 2 years presented with low back pain radiating down the right leg for 3 months. Pain worsened over time and was associated with numbness of the calf region. MRI spine evaluation revealed an enhancing expansile mass lesion of the right S1 nerve root with diagnostic features suggestive of Schwannoma. Retrorectal cyst was discovered incidentally on spine MRI, and further evaluation was provided by the surgical oncology department. Physical examination was normal. A contrast MRI of the pelvis revealed a 7.3×8.3×6. 3 cm non-enhancing, simple cystic mass was seen in the retrorectal space with no septations or soft tissue components. Differential diagnoses were tailgut cyst and duplication cyst. Intraoperatively, a 5×8 cm retrorectal cyst was identified, dissected and removed. The content of the cyst was a tan/white, toothpaste-like material. Final histopathological analysis revealed a dermoid cyst with no evidence of malignancy.
Case 3
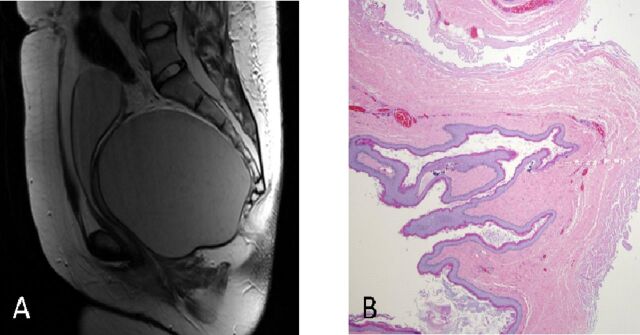
A 25-year-old Hispanic woman presented with acute urinary retention in the emergency department at an outside facility. Clinical examination revealed an enlarged, palpable urinary bladder per abdomen. Transabdominal ultrasound demonstrated a distended urinary bladder and a cystic pelvic mass. Following urinary catheterisation, a 10×10 cm pelvic mass was palpable on bimanual vaginal and digital rectal examinations. History revealed straining at micturition with gradual difficulty in passing urine, intermittent history of urinary frequency, dysuria, of 4 months duration and frequent loose stools for 3 weeks. Further evaluation with a T2-weighted MRI image depicted a 14×11×11 cm unilocular cystic mass without spetations and solid components located in the presacral space. Histopathological image of case 3 demonstrating benign squamous epithelium lined tail gut cyst (figure 1). Differential diagnoses were left ovarian cyst and left adnexal cyst. She was subjected to elective laparoscopy by the gynaecologist for further management. Intraoperatively, bilateral adnexa were normal, and the cyst was observed to be located posterior to the rectum. Patient was referred to the surgical oncology department for further evaluation. Contrast MRI of abdomen and pelvis revealed a unilocular, simple cystic mass with no solid components or septations arising from the presacral space. Differential diagnoses of tailgut cyst and duplication cyst were made. Patient underwent modified Kraske procedure for the cyst excision. The cyst content was sebaceous in nature on gross pathological examination. Final histology supported the diagnosis of duplication cyst.
Figure 1.
(A) T2-weighted MRI image of case 3 depicting a 14×11×11 cm unilocular cystic mass without spetations and solid components located in the presacral space. (B) Histopathological image of case 3 demonstrating benign squamous epithelium lined tail gut cyst.
Case 4
A 60-year-old Hispanic woman with a history of chronic lower back pain underwent MRI of the spine for evaluation. She was incidentally found to have retrorectal cysts associated with degenerative disc disease at the L4–5 level. She had a history of polypectomies for two benign colonic polyps that were diagnosed on colonoscopic evaluation for bleeding per rectum 16 months ago. She also had a history of endometrial cancer that was treated and cured 10 years ago. She was referred to the department of surgical oncology for further management of the retrorectal cyst. Clinical examination was normal and no mass was detected by digital rectal examination. On pelvic MRI evaluation with contrast, two non-enhancing variable intensity cystic masses in the retrorectal space of dimensions 2.2×2.2×1.9 cm and 2.2×1.7×1.3 cm were identified. The differential diagnoses were duplication cyst, tailgut cyst and haematoma of varying ages. A modified Kraske procedure was performed for the removal of the cystic lesions. Her postoperative recovery was uneventful and she reported relief of constipation. Two connected retrorectal masses with solid and cystic components were found intraoperatively. Histopathological evaluation of these lesions revealed the final diagnosis to be tailgut cysts. The cases are summarised in table 1.
Table 1.
Summary of the four cases
| Cases | Age | Sex | Presenting complaints | Physical examination | Radiology findings |
Intraoperative findings | Histopathological diagnosis |
| 1 | 60 | F | Chronic back pain Incidentally detected retrorectal cysts on MRI Spine |
NAD | Contrast MRI pelvis— two simple cysts with non-enhancing, varying intensity, walls of dimensions 2.2×2.2×1.9 cm and 2.2×1.7×1.3 cm | Two connected retrorectal masses of 2×2×2 cm and 2×1×2 cm with solid and cystic components | Tailgut cyst |
| 2 | 44 | F | Chronic constipation Retrorectal cyst misdiagnosed as ovarian cyst during the evaluation of menstrual irregularities |
Mass palpable on both vaginal and digital rectal examinations | Contrast MRI pelvis—5.5×6.5×7 cm complex cystic mass with postcontrast enhancement of cyst wall and septations DD—tailgut cyst, hamartomatous cyst |
6×7×5 cm large multiloculated cystic lesion in the retro rectal space | Duplication cyst |
| 3 | 25 | F | Intermittent episodes of urinary tract infections with gradual history of straining at micturition Frequent bowel movements Retrorectal cyst was misdiagnosed as ovarian cyst on ultrasound and CT scan of the abdomen |
Mass palpable on both vaginal and digital rectal examinations | CECT abdomen and pelvis—simple, cystic, non-enhancing, midpelvic mass measuring 13.3×10.7×10.6 cm, slightly to the left of midline DD—left ovarian cyst, left adnexal cyst. Contrast MRI pelvis—14×11×11 cm simple, retrorectal cyst without wall thickening, solid component or septations |
16×14×12 cm cystic mass in the retrorectal space | Tailgut cyst |
| 4 | 36 | F | Case of acute myeloid leukaemia in remission presented with lower back pain radiating down the right leg for 2 months Spine MRI showed a retrorectal cystic mass in addition to the nerve sheath tumours of S2, S3 on the right |
Mass palpable on digital rectal examination | Contrast MRI pelvis: 7.3×8.3×6.3 cm simple, retrorectal cyst with no soft tissue components and abnormal enhancement | 5×8 cm retrorectal cyst with tan white paste like material | Dermoid cyst |
CECT, contrast-enhanced CT; DD, differential diagnosis; NAD, no abnormalities detected.
Outcome and follow-up
Case 1: Six months follow-up was unremarkable.
Case 2: On 6 months follow-up, the patient had worsening of her back pain. Evaluation revealed recurrence of the leukaemia with lesions on the lower lumbar spine and pelvic bone marrow regions. She received chemotherapy and bone marrow transplant for the recurrence and is on follow-up.
Case 3: Patient’s symptoms resolved following surgery. She is 14 months into follow-up with no evidence of recurrence of symptoms.
Case 4: Patient is 11 years into follow-up with no evidence of recurring symptoms.
Discussion
Anatomy
The retrorectal space also known as presacral space is bounded by rectum anteriorly, sacrum posteriorly, the peritoneal reflection (at the level of S2, S3) superiorly, the levator ani and coccygeus muscles inferiorly and the iliac vessels and ureters laterally. The normal contents of the space include branches of sacral and sympathetic plexuses, middle rectal, iliolumbar and middle sacral vessels and lymphatics.5 6
Embryology
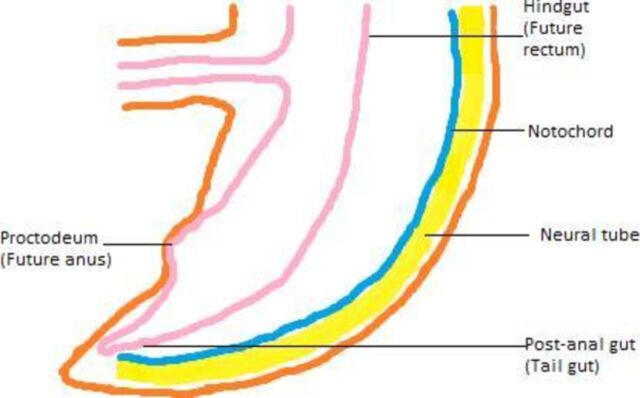
All four of our cases were congenital in origin. A brief discussion on the aetiology of the various types of retrorectal cysts follows. The developing embryo has a true tail which is most prominent during the fifth week of gestation. The constituents of the embryonic tail are tailgut and neureneteric canal. The tailgut also known as the postanal gut is the continuation of the hindgut (derivative of the primitive gut). As it develops into the rectum, the hindgut gets disconnected from the tailgut. The tailgut disappears by the end of eighth week of gestation during the process of regression (figure 2). In the event of failure to regress, the tailgut remnants develops into a tailgut cyst. The neureneteric canal is formed by the fusion of endoderm and mesoderm. It may fail to regress completely, giving rise to tailgut cyst.9 The embryological basis of the epidermoid and dermoid cysts is faulty ectodermal closure. Duplication cyst may arise from the fetal gut diverticula, which might get pinched off later in life. Conversely, a form of hindgut caudal twinning may develop, with one end becoming pinched off, forming retrorectal cyst.10
Figure 2.
Graphics of the longitudinal section of embryo at 7 weeks of gestation depicting the embryonic tail occupied by tailgut, notochord and neural tube. The postanal gut or the tailgut is the continuation of hindgut, the future rectum. It is called postanal gut as it lies caudal to the proctodeum, the future anus. (Figure created by Kirithiga Ramalingam.)
Pathology
The majority of retrorectal cysts are congenital. The common cysts that are found in the retrorectal space are tailgut cysts, duplication cysts, epidermoid cysts, dermoid cysts, anterior sacral meningocele. Aetiology, pathology, clinical and radiological findings of these common diagnoses of retrorectal cysts are summarised in the following table (table 2). The other possible rare pathological diagnoses of retrorectal cysts are cystic sacrococcygeal teratoma, anal duct or gland cyst, necrotic rectal leiomyosarcoma, extraperitoneal adenomucinosis, cystic lymphangioma, pyogenic abscess, neurogenic cyst and necrotic sacral chordoma.11 Malignant transformation, though uncommon in retrorectal cysts, has been documented well in the literature. Malignancies that are commonly associated with retrorectal cysts are adenocarcinoma in duplication cysts and carcinoid in tailgut cysts, respectively. It is important to systematically section the cyst to rule out malignancy. None of our patients had a malignant disease on pathological evaluation.
Table 2.
Features of the common retrorectal cysts seen in adults
| Features | Epidermoid and dermoid cysts |
Duplication cysts or enterogenous cysts | Tailgut cysts or cystic hamartomas | Anterior sacral meningocele |
| Aetiology | Failure of closure of the ectoderm. | Sequestration of the developing hindgut due to a diverticulum formation or caudal twinning. | Arise from the remnant portion of embryonic tail, that is, tailgut or neureneteric canal that fails to regress. | Herniation of the dural sac due to a defect in the anterior part of the sacrum. |
| Pathology | Epidermoid cysts— lined by stratified squamous epithelium. Dermoid cysts—lined by stratified squamous epithelium with skin appendages. |
As they arise from the endoderm of the primitive gut, they can be lined by squamous, cuboidal, columnar, or transitional epithelium. In addition, the cyst wall also contain two layers of muscles with myenteric nerve plexus in between them. |
The lining is similar to intestinal lining with squamous, columnar or duplication cysts histologically. They are distinguished from duplication cysts by the lack of well-organised two-layered muscular wall and a myenteric plexus |
Thick fibrous wall, lined by flattened arachnoid cells. |
| Clinical/ radiological findings |
May communicate with the skin and be associated with a post anal dimple or sinus. This finding often misleads to a diagnosis of perianal fistula/sinus or Pilonidal sinus. |
Multiloculated appearance with multiple satellite lesions and a dominant lesion. | Clinical/ radiological features may mimic that of epidermoid/ dermoid cysts or duplication cysts. Tailgut cysts can only be distinguished definitively from the duplication cysts on the basis of histology. |
‘Scimitar sign’ with rounded concave border of the sacrum on the defective side on plain radiograph of the pelvis is pathognomonic for the diagnosis of meningocele. |
Clinical features
Whereas, the four cases were similar with respect to surgical management, however, they differed widely with respect to clinical presentation, radiological features and pathology. The differences in the clinical manifestations of these cases illustrate the importance of recognising various clinical symptoms and signs of retrorectal cysts that lead to prompt diagnosis and appropriate management. Unlike retrorectal tumours, which are common in paediatric age groups, retrorectal cysts are more common in adults. The age range of retrorectal cysts quoted in the literature was wide ranging from 16 to 77 years with mean age of diagnosis being 41. The age range in our study was narrower. However, the mean age at the time of diagnosis was similar to the published reports. In this series, women were accounted for all four cases. Similar female preponderance was noted in the reported literature for retrorectal cysts. Since race was not specified in most of the published reports, it is not possible to generalise about the association of race with the retrorectal cysts. However, it is noteworthy to learn that retrorectal cysts are much more common in whites if we combine our data with the data of Hjermstad and Helwig12 study on tailgut cysts which has information on the race of patients.
The diagnosis of retrorectal cyst was incidental in nearly half of the patients in large case series. In our case series, one of four patients had an incidental diagnosis. Majority of the incidental diagnoses in the literature were made on routine physical and pelvic examinations, and the remainder were found on workup for other pathologies.5 6 In the present report, both cases of incidental diagnoses was discovered on the diagnostic evaluation of chronic back pain. Published reports indicate that the most common symptoms of retrorectal cysts are pain in the pelvis or back. The other common symptoms described are pain during defecation, constipation, chronic proctorrhagia, painless rectal bleeding, change in stool calibre, urinary frequency, urinary retention and fistula or abscess formation.5 7 8 13 In our patients, symptoms were mostly due to compressive effect on the bladder and rectum with features of urinary tract infection, urinary retention and constipation.
Examination findings of the retrorectal cysts reported in the literature are dimple in the postanal midline, chronic fistula, digital rectal examination findings demonstrating smooth firm mass palpable through the posterior rectal wall with or without a bulge into the rectal lumen.5–9 Some patients may not have any abnormalities at all on clinical examination. Three of four in our case series had palpable lesions on digital rectal examinations, and none had a postanal dimple or a fistula. More than half of the cases reported elsewhere with symptoms were misdiagnosed as abscess, pilonidal sinus, teratoma and ovarian tumour due to the confusing clinical picture, lack of awareness of its existence and rarity.5 8 12 14 Similarly, two patients in this study were misdiagnosed as ovarian cysts and were subjected to unwanted surgical exploration.
Radiological features
Plain radiograph of the pelvis may show bony erosion due to the cyst or the pathognomonic Scimitar sign due to a sacral defect on the ipsilateral side indicative of an anterior sacral meningocele.15 Fistulogram may demonstrate a communication between the cyst and an external fistulous opening.11 Ultrasound of the pelvis often reveals the presence of cyst in the presacral space. Ultrasound evaluation may misguide the diagnosis of retrorectal cyst as an ovarian cyst especially when the size of the cyst is large with an atypical location. Endorectal ultrasound could help characterise the nature of the lesion. CT of the pelvis can demonstrate the nature of the cyst in terms of cyst wall thickness and its contents, contrast enhancement and relationship to the surrounding structures. An MRI evaluation of the pelvis can clarify the nature of the cyst in terms of wall thickness, presence of solid components in the cyst and detailed anatomical relations of the cyst to the surrounding structures.11 16 All four of our patients were evaluated by MRI of the pelvis to confirm the diagnosis of retrorectal cyst and identify the anatomy to guide surgical excision.
Management
Whereas the presence of a solid component in a retrorectal cyst on imaging is more common in malignant lesions, it can also be seen in benign lesions (less common).11 Performing fine-needle biopsy for a retrorectal lesion to assess the pathological features is difficult because of the location. Endorectal biopsy is not advised for the fear of infection and malignant seeding of the track. In asymptomatic patients for whom conservative management is contemplated, a CT-guided transperineal approach is recommended. CT is especially effective for biopsy sampling in patients who may have increased morbidity with surgical resection. Since colorectal cancer can present with obstructive symptoms, a thorough evaluation of the presence of red-flag symptoms and signs of colorectal cancer must be done to rule out this condition especially in the elderly population. Definitive management of retrorectal cyst mandates surgical excision based on the current literature. However, as nearly 50% of the patients with retrorectal cysts are asymptomatic (diagnosed incidentally on imaging done for other reasons), performing a surgical procedure needs justification especially in cases where the lesion is a small, simple cyst lacking malignant features on radiological imaging.
Current practices offer four different approaches for the excision of retrorectal cysts.8 The posterior approach is most commonly used to excise retrorectal cysts. Alternative approaches that may be used are transabdominal or combined transabdominal and posterior. A transabdominal approach is recommended when the cyst is located higher up in the retrorectal space in the supralevator plane or when malignancy is suspected.10 A combined approach is recommended when the cyst is large. In a combined approach, the transabdominal approach is performed first to dissect the cyst off from it adjacent structures and the posterior approach is followed for further dissection and cyst removal. Transabdominal approach can be performed either by a laparotomy or minimally invasive techniques such as laparoscopic or robotic surgery. We were able to excise the cysts successfully in all four patients through the posterior approach using the modified Kraske method. The Kraske procedure involves a midline vertical skin incision over the coccyx. The incision is deepened through the scarpa’s fascia, anorectal ligament and levator muscle. The cyst is circumferentially dissected. In majority, surgical excision is possible without excising the distal coccyx as employed in the classic Kraske method. Intactness of the rectal wall is confirmed by digital rectal examination at the end of the procedure. A fourth approach described for excision is transanal. However, since there is a risk of malignant seeding and contamination resulting in recurrence and infection, this approach is not routinely recommended.
Learning points.
In middle-aged women, the following should raise suspicion of retrorectal cyst: gastrointestinal or urinary obstructive features, mass or fullness palpable on the posterior wall on digital rectal examination, presacral dimple, perianal fistula and/or recurrent disease. Such features should prompt an MRI evaluation of the pelvis for definitive diagnosis.
The pathology of these cysts varies considerably and cannot be predicted accurately by radiological examination or fine-needle biopsy.
Consequently, surgical excision remains the standard of care. Retrorectal cysts can often be successfully removed by a posterior approach alone.
Acknowledgments
The authors thank Dr Robert Glew for editing and Miss Patricia J Young for assisting with manuscript submission.
Footnotes
Contributors: KR, CF and QS contributed to data collection, drafting of the work and literature review described in the article. AR has contributed to the planning of the study and revising the draft critically for important intellectual content of the work described in the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hobson KG, Ghaemmaghami V, Roe JP, et al. Tumors of the retrorectal space. Dis Colon Rectum 2005;48:1964–74. 10.1007/s10350-005-0122-9 [DOI] [PubMed] [Google Scholar]
- 2.Johnson WR. Postrectal neoplasms and cysts. Aust N Z J Surg 1980;50:163–6. 10.1111/j.1445-2197.1980.tb06658.x [DOI] [PubMed] [Google Scholar]
- 3.Cody HS, Marcove RC, Quan SH. Malignant retrorectal tumors: 28 years' experience at Memorial Sloan-Kettering cancer center. Dis Colon Rectum 1981;24:501–6. 10.1007/bf02604308 [DOI] [PubMed] [Google Scholar]
- 4.Uhlig BE, Johnson RL. Presacral tumors and cysts in adults. Dis Colon Rectum 1975;18:581–96. 10.1007/BF02587141 [DOI] [PubMed] [Google Scholar]
- 5.Jao SW, Beart RW, Spencer RJ, et al. Retrorectal tumors. Mayo clinic experience, 1960-1979. Dis Colon Rectum 1985;28:644–52. 10.1007/bf02553440 [DOI] [PubMed] [Google Scholar]
- 6.Lev-Chelouche D, Gutman M, Goldman G, et al. Presacral tumors: a practical classification and treatment of a unique and heterogenous group of diseases. Surgery 2003;133:473–8. 10.1067/msy.2003.118 [DOI] [PubMed] [Google Scholar]
- 7.Glasgow S, Dietz D, Tumors R. Retrorectal tumors. Clin Colon Rectal Surg 2006;19:061–8. 10.1055/s-2006-942346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodfield JC, Chalmers AG, Phillips N, et al. Algorithms for the surgical management of retrorectal tumours. Br J Surg 2008;95:214–21. 10.1002/bjs.5931 [DOI] [PubMed] [Google Scholar]
- 9.Currarino G, Coln D, Votteler T. Triad of anorectal, sacral, and presacral anomalies. AJR Am J Roentgenol 1981;137:395–8. 10.2214/ajr.137.2.395 [DOI] [PubMed] [Google Scholar]
- 10.Killingsworth C, Gadacz TR. Tailgut cyst (retrorectal cystic hamartoma): report of a case and review of the literature. Am Surg 2005;71:666–73. [PubMed] [Google Scholar]
- 11.Dahan H, Arrivé L, Wendum D, et al. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics 2001;21:575–84. 10.1148/radiographics.21.3.g01ma13575 [DOI] [PubMed] [Google Scholar]
- 12.Hjermstad BM, Helwig EB. Tailgut cysts. Report of 53 cases. Am J Clin Pathol 1988;89:139–47. 10.1093/ajcp/89.2.139 [DOI] [PubMed] [Google Scholar]
- 13.Lev-Chelouche D, Gutman M, Goldman G, et al. Presacral tumors: a practical classification and treatment of a unique and heterogeneous group of diseases. Surgery 2003;133:473–8. 10.1067/msy.2003.118 [DOI] [PubMed] [Google Scholar]
- 14.Prasad AR, Amin MB, Randolph TL, et al. Retrorectal cystic hamartoma: report of 5 cases with malignancy arising in 2.. Arch Pathol Lab Med 2000;124:725–9. [DOI] [PubMed] [Google Scholar]
- 15.Singer MA, Cintron JR, Martz JE, et al. Retrorectal cyst: a rare tumor frequently misdiagnosed. J Am Coll Surg 2003;196:880–6. 10.1016/S1072-7515(03)00133-9 [DOI] [PubMed] [Google Scholar]
- 16.Grandjean J-P, Mantion G-A, Guinier D, et al. Vestigial retrorectal cystic tumors in adults: a review of 30 cases. Gastroenterol Clin Biol 2008;32:769–78. 10.1016/j.gcb.2008.03.011 [DOI] [PubMed] [Google Scholar]