Abstract
The relationship between ABO blood group and the incidence of coronavirus disease 2019 (COVID-19) infection and death has been investigated in several studies. The reported results were controversial, so the objective of the present study was to assess the relationship between different blood groups and the onset and mortality of COVID-19 infection using a meta-analysis method. We searched relevant databases using appropriate MeSH terms. We screened articles on the bases of titles, abstracts and full text, and articles that met the inclusion criteria were selected. Quality assessment was done with the Newcastle-Ottawa scale checklist. The estimated frequency of COVID-19 infection and death in terms of ABO blood group and the overall estimate of the odds ratio between blood group with COVID-19 infection and death was calculated with 95% confidence interval. The pooled frequency of blood groups A, B, O and AB among COVID-19–infected individuals was estimated as 36.22%, 24.99%, 29.67% and 9.29% respectively. The frequency of blood groups A, B, O and AB among patients who died of COVID-19 infection was estimated as 40%, 23%, 29% and 8% respectively. The odds ratio of COVID-19 infection for blood group A versus the other blood groups was estimated as 1.16 (95% confidence interval (CI), 1.02–1.33). The corresponding figures for blood groups O and AB versus other blood groups were estimated as 0.73 (95% CI, 0.60–0.88) and 1.25 (95% CI, 0.84–1.86) respectively. This meta-analysis showed that individuals with blood group A are at higher risk for COVID-19 infection while those with blood group O are at lower risk. Although the odds ratio of death for AB blood group was nonsignificant, it was considerable.
Keywords: Blood group, coronavirus, COVID-19, death, pandemic
Introduction
The Coronaviridae is a family of enveloped, single-stranded, positive-sense RNA viruses with the largest genome among RNA viruses [[1], [2], [3]]. The club-shaped spike (S) proteins on their surface make them look like a crown. Other structural proteins include the haemagglutinin esterase (HE) (only found in some of them), small membrane (E), membrane (M), nucleocapsid (N) and internal (I) protein [3]. They belong to the order of Nidovirales and the suborder of Cornivirineae, and are divided into two subfamilies. One is Ortocoronavirinae, which is divided into four genera: alpha, beta, gamma and delta [4].
Six coronaviruses cause human infections [5], including severe acute respiratory syndrome (SARS), China, 2002 [6], and Middle Eastern respiratory syndrome, Saudi Arabia, 2012 [7]. In late December 2019 the World Health Organization (WHO) reported cases of pneumonia with an unknown cause in Wuhan, China [8]. Further investigation of samples from patients with pneumonia isolated a novel coronavirus, termed 2019-nCoV [9]. The WHO declared a pandemic of 2019-nCoV, or coronavirus disease 2019 (COVID-19), on 11 March 2020 [10]. There have been more than 24 million cases and eight-hundred thousands deaths due to COVID-19 worldwide according to Worldometer until August 27th [11].
There were different risk factors for mortality in COVID-19 patients, including male gender, older age, diabetes, asthma and other medical conditions [12]. Recently some studies found an association between the ABO blood group and COVID-19 morbidity and mortality [[13], [14], [15], [16]]. The ABO blood group has also been reported to be related to different infectious diseases and syndromes. Individuals with blood group O were reported to be more susceptible to Norwalk virus and also had a significantly higher prevalence of Helicobacter pylori infection, but they were less susceptible for SARS [[17], [18], [19]]. In another study blood group A was associated with an increased risk of acute respiratory distress syndrome in trauma and sepsis patients [20]. A study by Lebiush et al. [21] on influenza A (H1N1) suggested a higher seroconversion to a titre of more than 20 in blood groups A and B. B blood group was also reported as a risk factor for prostate and bladder cancer [22], and non-O blood groups were reported to have a higher risk of gastric cancer [23].
Published articles have emphasized the hypothesis of a relationship between ABO blood group and COVID-19. In order to reach more reliable results, we performed a meta-analysis on this subject.
Meta-analysis is one of the study designs that combines the results of preliminary studies and determine a valid estimate. Therefore, our objective was to perform a rapid systematic review and meta-analysis to discover any association between ABO blood group and COVID-19 morbidity and mortality. Because there is not yet much primary evidence regarding the association between blood groups and COVID-19 infection, upcoming relevant studies will be added to the results of the present meta-analysis.
Methods
Search strategy
A systematic search was carried out in available relevant databases, including PubMed, Scopus, Cochrane Library and Web of Science, as well as unpublished results in the medRxiv database. We used all MeSH terms and relevant keywords (COVID-19, SARS-CoV-2 infection, COVID-19 virus disease, 2019-nCoV infection, ABO blood group system, ABO factor, blood groups, antigens, blood group). All case–control, cohort and cross-sectional studies until 21 April 2020 were included.
Criteria for study selection
Inclusion criteria were as follows: studies that reported a relationship between ABO blood group and death due to COVID-19; that reported a relationship between ABO blood group and COVID-19 infection; that reported frequency of COVID-19 among different ABO blood groups; and that reported death among COVID-19–infected people.
We excluded case reports and letters to the editor.
Data extraction
Data extracted from the primary studies included first author's name, year of publication, place of conduct, study type, sampling method, number of participants and number of COVID-19 infections and deaths in each blood group, A, B, O and AB. The required data were entered into Microsoft Excel spreadsheets.
Quality assessment
Quality assessment was performed using the Newcastle-Ottawa scale. This checklist has three parts: Selection, Comparability and Exposure. The checklist scores are between 0 and 9. Studies with a score lower than 5 were excluded. The selection criteria for Selection was 4 in maximum, 2 for Comparability and 3 for Exposure [24]. Quality assessment was independently performed by two authors.
Data analysis
Data analysis was performed by Stata 11 software. Heterogeneity between studies was assessed by the Cochrane Q test and the I2 test. Standard error was calculated to assess the frequency of COVID-19 infection and death in each blood group. The overall estimate of the frequency of COVID-19 infection and death with 95% confidence interval (CI) in each ABO blood group was calculated using a random effect model. To assess the relationship between COVID-19 infection and mortality with blood group, the required data were extracted in binary tables. Using the Metan command, the random effect model and reverse variance, the point odds ratio (OR) and 95% CI were illustrated on forest plots. In these plots, the size of the square shows the weight of each study and the lines beside it show the 95% CI. In cases where the CI did not include the number 1, the difference was considered statistically significant.
Results
We found 318 studies using our search criteria. After removing duplicates and checking the titles, abstracts and full text of the remaining articles, 314 irrelevant or duplicate papers were excluded. Quality assessment scores for the four final selected studies [[13], [14], [15], [16]] in the meta-analysis were above 5. The eligible studies included two case–control, one cohort and one cross-sectional study (Fig. 1). Studies included were conducted in China [[13], [14], [15]] and the United States [16]. In total seven datasets were found, from hospitals in Wuhan, Shenzhen, Xi'an, Beijing and New York City. A total of 139 128 participants were enrolled onto these studies, which included 135 940 controls.
Fig. 1.
Flowchart of study selection.
Overall, the frequency of blood group A among COVID-19–infected people had been reported to be between 28.77% and 44.44%. When we combined these results using a random effect model (I2 = 41.5%, Q = 10.25, p 0.115), the pooled frequency of blood group A among COVID-19–infected people was estimated as 36.22% (95% CI, 32.81–39.63) (Fig. 2(A)).
Fig. 2.
Meta-analysis of blood groups A, B, O and AB prevalence within 95% confidence interval (CI) among coronavirus disease 2019 (COVID-19) patients.
The frequency of blood group B among COVID-19–infected people had been reported to be between 17.01% and 30.93%. When we combined these results using a random effect model (I2 = 79.1%, Q = 28.70, p < 0.001), the total frequency of blood group B among all COVID-19–infected people was estimated as 24.99% (95% CI, 20.35–29.62) (Fig. 2(B)).
The frequency of blood group O among COVID-19–infected people had been reported to be between 18.18% and 45.75%. When we combined these results using a random effect model (I2 = 89.6%, Q = 57.77, p < 0.001), the pooled frequency of blood group O among COVID-19–infected people was estimated as 29.67% (95% CI, 22.45–36.89) (Fig. 2(C)).
Of the evidence included in this meta-analysis, five datasets reported that the frequency of blood group AB among COVID-19–infected people varied between 3.08% and 13.68%. When we combined the results using a random effect model (I2 = 93.6%, Q = 62.72, p < 0.001), the frequency of blood group AB among COVID-19–infected people was estimated as 9.29% (95% CI, 4.70–13.88) (Fig. 2(D)).
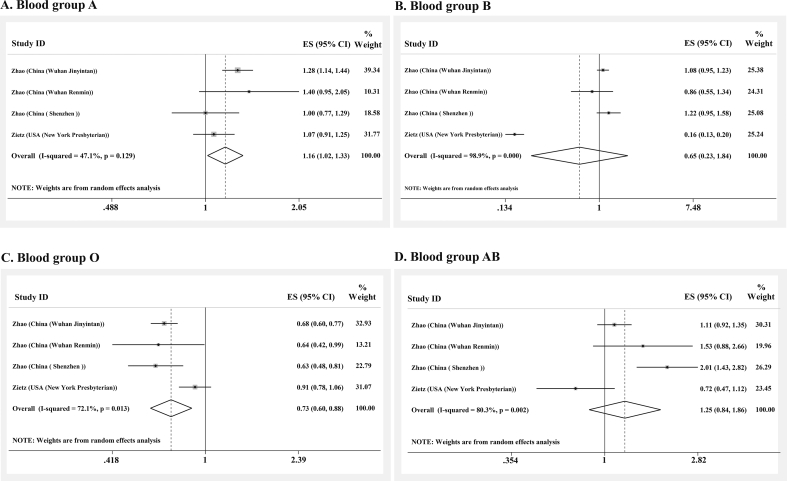
The odds for COVID-19 infection among blood group A versus non-A blood groups was extracted from four of the seven datasets, one of which was statistically significant. Combining the primary ORs using a random effect model (I2 = 47.1%, Q = 5.67, p 0.129), the pooled OR for blood group A was estimated as 1.16 (95% CI, 1.02–1.33) (Fig. 3(A)).
Fig. 3.
Meta-analysis of odds ratios for coronavirus disease 2019 (COVID-19) infection among individuals with blood groups A, B, O and AB.
The odds of COVID-19 infection among blood group B versus non-B blood groups was extracted from four of the seven datasets. It was lower in B blood group than non-B blood groups in two studies, one of which was statistically significant. Combining these results with random effect model (I2 = 98.9%, Q = 266.1, p 0.001), the OR for blood group B was estimated as 0.65 (95% CI, 0.23–1.84) (Fig. 3(B)).
The odds of COVID-19 infection among blood group O versus non-O blood groups had been reported in four datasets, all of which reported lower odds of COVID-19 infection among subjects with blood group O. Three of these associations were statistically significant. By combining these results with a random effect model (I2 = 72.1%, Q = 10.76, p 0.013), the OR for blood group O was estimated as 0.73 (95% CI, 0.60–0.88) (Fig. 3(C)).
The odds of COVID-19 infection among patients with and without blood group AB was reported in four datasets, just one of which was statistically significant. Combining these results using a random effect model (I2 = 80.3%, Q = 15.22, p 0.002), the OR for having blood group AB was estimated as 1.25 (95% CI, 0.84–1.86) (Fig. 3(D)).
The odds for mortality among COVID-19–infected people with blood group A versus non-A blood group was reported by two datasets, one of which reported a higher chance of mortality in patients of blood group A compared to those who were non-A blood group. However, it was not statistically significant. Combining the results of these two pieces of evidence and applying a random effect model (I2 = 0%, Q = 0.41, p 0.522), the OR for death among COVID-19–infected people having blood group A was estimated as 1.12 (95% CI, 0.87–1.45).
Only two studies compared patients with and without blood group B in term of the odds of COVID-19 infection. Both showed lower odds of death among people with blood group B. However, the results were not statistically significant. Combining the results of these two pieces of evidence using a random effect model (I2 = 0%, Q = 0.01, p 0.914), the OR for blood group B versus non-B was estimated as 0.87 (95% CI, 0.65–1.18).
The OR for death of COVID-19–infected people with blood group O was reported by two studies, both of which found a negative association. However, the results were not statistically significant. Combining the results of these two studies and applying a random effect model (I2 = 0%, Q = 0.00, p 0.998), the OR for blood group O was estimated as 0.97 (95% CI, 0.74–1.27).
The ORs for death among COVID-19–infected people with blood group AB were reported by two studies. Both studies reported a greater chance of dying among AB blood group patients. However, neither was statistically significant. Combining the results of these two lines of evidence and applying a random effect model (I2 = 65.5%, Q = 2.90, p 0.088), the OR for blood group AB was estimated as 1.33 (95% CI, 0.51–3.46) (Table 1).
Table 1.
Overall and point ORs with 95% CIs of death due to COVID-19 infection by blood group
| Blood group | N | OR | 95% CI | Heterogeneity index |
||
|---|---|---|---|---|---|---|
| I2 (%) | Q | p | ||||
| A (non-A) | 2 | 1.12 | 0.87–1.45 | 0 | 0.41 | 0.522 |
| B (non-B) | 2 | 0.87 | 0.65–1.18 | 0 | 0.01 | 0.914 |
| O (non-O) | 2 | 0.97 | 0.74–1.27 | 0 | 0.00 | 0.998 |
| AB (non-AB) | 2 | 1.33 | 0.51–3.46 | 65.5 | 2.90 | 0.088 |
CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio.
In three studies, the frequency of death among individuals with COVID-19 infection was reported in terms of different blood groups. Combining the results of these articles, the frequency of death due to COVID-19 among patients with A, B, O and AB groups was estimated as 40% (95% CI, 35–46), 23% (95% CI, 15–30), 29% (95% CI, 16–42) and 8% (95% CI, 5–11) respectively.
Meta-analysis of studies showed no significant association between mortality and different blood groups in COVID-19 patients. However, prevalence of death due to COVID-19 was significantly lower in blood group O compared to other blood groups.
Discussion
In this study, we found that blood group A was a partial risk factor for COVID-19 infection, while blood group O was a protective factor. Moreover, B and AB blood groups were not significantly associated with COVID-19 infection.
In a study investigating ABO blood groups and susceptibility to SARS in 2005, 45 hospital staff members in contact with a patient without any protective clothes were checked. They were tested for SARS-CoV IgG antibody [17]. The results showed that individuals with blood group O were less susceptible to SARS infection; however, the results were not statistically significant for blood group B and were undefined for blood groups A and AB [17]. In our included studies, cases were tested with molecular methods, including reverse transcriptase PCR, or clinical diagnostic criteria, including epidemic history or clinical symptoms and clinical characteristics [[13], [14], [15], [16]]. Our results regarding the cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were consistent with the abovementioned study regarding cases of SARS-CoV in blood group O.
Angiotensin-converting enzyme 2 (ACE2) has been reported to be the SARS-CoV receptor, and the receptor binding domain is presented on the S proteins of the coronaviruses [19]. Guillon et al. [25] investigated whether ABO antibodies could stop the interaction between the SARS-CoV receptor and ACE2. They found that the S protein expressed by A-positive–infected cells shares epitopes of A histo-blood group in vitro, and therefore adhesion of S protein and ACE2 can be inhibited by anti-A natural antibody. The anti-A and anti-B natural antibodies being produced in individuals with blood group O could potentially block viral adhesion to cells, which could explain their lower risk of infection.
Because SARS-CoV and SARS-CoV-2 are from the same genus (Betacoronavirus) [26] and have similarities in the structures of their receptor binding domains, ACE2 has been also suggested to be the receptor for SARS-CoV-2 [15]. Therefore, the same mechanism might explain the lower susceptibility of blood group O to SARS-CoV-2, as we have shown in our study. In explaining the higher risk for blood group A, lack of these antibodies can be expected, although further studies are needed for this to be confirmed [13].
Individuals with blood group O have a lower angiotensin-converting enzyme (ACE) level, while blood group A has positive association within ACE activity [27]. ACE is an enzyme that activates angiotensin; the lower level of this enzyme can thus reduce the risk of hypertension [28], which is a COVID-19 risk factor [29]. This is another proposed mechanism for developing more severe COVID-19 disease in blood group A and less severe disease in blood group O [27]. Although ACE2 is the virus receptor, it can have some benefits. For example, it can attenuate inflammatory response and redox stress; and it can counterbalance the ACE effect, and in the case of lower ACE level it can work even more effectively [27,30].
Although the primary receptor for SARS-CoV-2 is ACE2 [31], like many pathogens that bind to specific terminal carbohydrates [32], SARS-CoV-2 binds to the carbohydrates that determine the ABO blood groups, which are extensively expressed in mucous membrane of respiratory tract [31,33]. Therefore, blood group AB has the most contact and blood group O the least with the pathogen [31]. In addition, a study by Dai [27] hypothesized that blood group A was considered to have more attachment molecules on the vascular wall by protecting P-selectin and intercellular cell adhesion molecule 1 (ICAM-1) from cleavage which increases adhesion and inflammation and can cause more severe COVID-19 disease [27].
People with blood group O have also higher interleukin 6 (IL-6) levels [34]. IL-6 is a proinflammatory cytokine which can be produced by many cells and which plays an important role in cell defence in the acute phase [35]. However, studies showed that IL-6 is associated with COVID-19 severity, as it can be part of a cytokine storm [[36], [37], [38]]. IL-6 could both play a protective role with its involvement in lung repair responses and exacerbate its role in COVID-19 infection [39]. It should be noted that all of these mechanisms need to be investigated further.
In our study there was no statistically significant association between blood group A and COVID-19 mortality, but the prevalence of blood group A was significantly higher than blood groups B and AB in COVID-19 patients. The prevalence of mortality in COVID-19 patients was also significantly higher in blood group A than groups B and AB. Menter et al. [40] have suggested that blood group A might be associated with coagulopathies and pulmonary circulation disorder in COVID-19 patients because they genetically has higher von Willebrand factor activity and consequently are more susceptible to thrombosis.
Among COVID-19–infected individuals and those who died, blood groups A and AB had the highest and lowest prevalence respectively. However, because blood group A is the most common blood group and AB has the lowest prevalence among other blood groups in China [41] and the United States [42], the countries where the included studies were conducted, it could have potentially affected our results.
No statistically significant association was found between blood group and mortality from COVID-19 in our study. There are many patient factors involved in mortality due to COVID-19, including gender and age, as well as diabetes, asthma and other medical conditions [12]. However, blood group has no significant effect on mortality outcome.
One main limitation of our study is that some of the primary studies entered into the present meta-analysis have not yet been peer reviewed. Confounding factors including diabetes, hypertension and cardiovascular diseases were not adjusted in the study because of incomplete data. More observational studies including large-scale samples and considering confounding factors and blood group distribution in society are needed to address the association more robustly. Studies are also needed to understand the mechanisms involving the protective or deteriorating effect of blood groups on COVID-19 infection.
Conclusion
In this meta-analysis we found that blood group A is a partial risk factor for COVID-19 infection and blood group O is a protective factor. Blood groups B and AB were not significantly associated with COVID-19 infection. There were no significant associations found between blood groups and COVID-19 patients' mortality. Further studies are needed considering distribution of different blood groups in society and other confounding factors concerning COVID-19 patient outcome to reach more robust results.
Conflict of Interest
None declared.
References
- 1.Malik Y. Properties of coronavirus and SARS-CoV-2. Malays J Pathol. 2020;42:3–11. [PubMed] [Google Scholar]
- 2.Pal M., Berhanu G., Desalegn C., Kandi V. Cureus; 2020. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International committee on taxonomy of viruses (ICTV). Taxonomy. Available at: https://talk.ictvonline.org/.
- 5.Su S., Wong G., Shi W., Liu J., Lai A.C., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris J., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO). Disease outbreak news. Pneumonia of unknown cause—China. Available at: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/.
- 9.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO). WHO Director-General’s opening remarks at the media briefing on COVID-19. 11 March 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 11.Worldometer. COVID-19 coronavirus pandemic. Available at: https://www.worldometers.info/coronavirus/..
- 12.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020 doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J., Fan H., Zhang L., Huang B., Zhu M., Zhou Y. 16 March 2020. Retrospective analysis of clinical features in 101 death cases with COVID-19. medRxiv. [DOI] [Google Scholar]
- 14.Zeng X., Fan H., Lu D., Huang F., Meng X., Li Z. medRxiv; 16 April 2020. Association between ABO blood groups and clinical outcome of coronavirus disease 2019: evidence from two cohorts. [DOI] [Google Scholar]
- 15.Zhao J., Yang Y., Huang H.P., Li D., Gu D.F., Lu X.F. 26 March 2020. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv. [DOI] [Google Scholar]
- 16.Zietz M., Tatonetti N.P. medRxiv; 21 July 2020. Testing the association between blood type and COVID-19 infection, intubation, and death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y., Cheng G., Chui C., Lau F., Chan P.K., Ng M.H. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1447–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 18.Lin C.W., Chang Y.S., Wu S.C., Cheng K.S. Helicobacter pylori in gastric biopsies of Taiwanese patients with gastroduodenal diseases. Jpn J Med Sci Biol. 1998;51:13–23. doi: 10.7883/yoken1952.51.13. [DOI] [PubMed] [Google Scholar]
- 19.Lindesmith L., Moe C., Marionneau S., Ruvoen N., Jiang X., Lindblad L. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 20.Reilly J., Meyer N., Shashaty M., Feng R., Lanken P., Gallop R. ABO blood type A is associated with increased risk of acute respiratory distress syndrome in Caucasians following both major trauma and severe sepsis. Chest. 2014;145:753–761. doi: 10.1378/chest.13-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebiush M., Rannon L., Kark J. The relationship between epidemic influenza A (H1N1) and ABO blood groups. Epidemiol Infect. 1981;87:139–146. doi: 10.1017/s002217240006931x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stakišaitis D., Juknevičienė M., Ulys A., Žaliūnienė D., Stanislovaitienė D., Šepetienė R. ABO blood group polymorphism has an impact on prostate, kidney and bladder cancer in association with longevity. Oncol Lett. 2018;16:1321–1331. doi: 10.3892/ol.2018.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etemadi A., Kamangar F., Islami F., Poustchi H., Pourshams A., Brennan P. Mortality and cancer in relation to ABO blood group phenotypes in the Golestan Cohort Study. BMC Med. 2015;13:8. doi: 10.1186/s12916-014-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Guillon P., Clément M., Sébille V., Rivain J.G., Chou C.F., Ruvoën-Clouet N. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zainol Rashid Z., Othman S.N., Abdul Samat M.N., Ali U.K., Wong K.K. Diagnostic performance of COVID-19 serology assays. Malays J Pathol. 2020;42:13–21. [PubMed] [Google Scholar]
- 27.Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320922370. 2047487320922370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Vark L.C., Bertrand M., Akkerhuis K.M., Brugts J.J., Fox K., Mourad J.J. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158 998 patients. Eur Heart J. 2012;33:2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessami A., Shamshirian A., Heydari K., Pourali F., Alizadeh-Navaei R., Moosazadeh M. medRxiv; 10 July 2020. Cardiovascular diseases burden in COVID-19: systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arend P. 10 August 2020. How blood group A might be a risk and blood group O be protected from coronavirus (COVID-19) infections (how the virus invades the human body via ABO(H) blood group–determining carbohydrates)https://figshare.com/articles/preprint/How_blood_group_O_could_be_protected_from_Coronavirus_Covid-19_infections/12019035 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preece A.F., Strahan K.M., Devitt J., Yamamoto F.I., Gustafsson K. Expression of ABO or related antigenic carbohydrates on viral envelopes leads to neutralization in the presence of serum containing specific natural antibodies and complement. Blood. 2002;99:2477–2482. doi: 10.1182/blood.v99.7.2477. [DOI] [PubMed] [Google Scholar]
- 33.Mattos LCd, Moreira H.W. Genetic of the ABO blood system and its link with the immune system. Rev Brasil Hematol Hemoter. 2004;26:60–63. [Google Scholar]
- 34.Naitza S., Porcu E., Steri M., Taub D.D., Mulas A., Xiao X. A genome-wide association scan on the levels of markers of inflammation in Sardinians reveals associations that underpin its complex regulation. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome–like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J., Zhang S., Wang Q., Shen H., Zhang Y., Liu M. Frequencies and ethnic distribution of ABO and RhD blood groups in China: a population-based cross-sectional study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-018476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garratty G., Glynn S.A., McEntire R. Retrovirus Epidemiology Donor Study. ABO and Rh (D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44:703–706. doi: 10.1111/j.1537-2995.2004.03338.x. [DOI] [PubMed] [Google Scholar]