Abstract
Background
The inflammatory pathology observed in severe COVID-19 disease caused by the 2019 novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is characterized by elevated serum levels of C reactive protein (CRP) and cytokines, including interferon gamma, interleukin 8 (IL-8), and interleukin 6 (IL-6). Initial reports from the outbreak in Italy, China and the USA have provided anecdotal evidence of improved outcomes with the administration of anti-IL-6 agents, and large-scale trials evaluating these therapies are ongoing.
Study description
In this retrospective case series, clinical outcomes and correlates of response to treatment with the IL-6 receptor antagonist sarilumab are described for 15 patients with COVID-19 from a single institution in Southern Italy. Among 10 patients whose symptoms improved after sarilumab treatment, rapid decreases in CRP levels corresponded with clinical improvement. Lower levels of IL-6 at baseline as well as lower neutrophil to lymphocyte ratio as compared with patients whose COVID-19 did not improve with treatment were associated with sarilumab-responsive disease.
Conclusions
This observation may reflect a possible clinical benefit regarding early intervention with IL-6-modulatory therapies for COVID-19 and that CRP could be a potential biomarker of response to treatment.
Keywords: immunomodulation, case reports, inflammation mediators
Introduction
In December 2019, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in Wuhan, China and subsequently spread across the globe to infect as many as four million people worldwide within 5 months.1 The WHO declared the outbreak a pandemic on March 11, 2020,2 and numerous countries imposed stringent social and physical distancing orders in an effort to slow the spread. Despite these measures, the pandemic overwhelmed the capacity of healthcare systems in many areas around the world, with the northern region of Italy experiencing one of the highest mortality rates during the initial months of the crisis.3
Severe COVID-19 is associated with an acute respiratory distress syndrome (ARDS), with observations of interstitial mononuclear inflammatory infiltrates, diffuse alveolar damage, hyaline membrane formation and pulmonary edema in the lungs.4–6 Lung pathology is accompanied by pronounced inflammatory response characterized by very high levels of several cytokines in the serum, especially interleukin 6 (IL-6), IL-1β, IL-8, interferon gamma (IFNγ) and tumor necrosis factor alpha.7–10 The ‘cytokine storm’ seen in patients with severe disease is similar to what has been described in macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH)11 12 or in cytokine release syndrome (CRS) secondary to chimeric antigen receptor (CAR) T cell therapy.13 Administration of IL-6 blocking agents such as tocilizumab and siltuximab has been shown to be effective in reversing CAR T cell therapy-associated CRS,13–15 and tocilizumab was approved by the US Food and Drug Administration for this indication in 2017.16 Although caution is needed in extrapolating the CAR T cell therapy experience, especially because initial data from the COVID-19 pandemic indicate that IL-6 levels are far lower in the context of SARS-CoV-2 infection than seen in CRS,17 18 a 21-patient observational study from China reported that tocilizumab treatment could help prevent clinical deterioration of individuals with severe pneumonitis and pulmonary complications.19 Additionally, in a retrospective, observational cohort study that included 544 adult patients with severe COVID-19 pneumonia who were admitted to tertiary care centers in Bologna and Reggio Emilia, Italy, tocilizumab treatment was associated with a reduced risk of invasive mechanical ventilation or death.20
Modulation of IL-6 has emerged as a potentially promising option for COVID-19-related ARDS.21 IL-6 is a pleiotropic cytokine with nearly ubiquitous expression in stromal and immune cells. Signaling through the IL-6 receptor requires assembly on the cell membrane of a trimeric complex consisting of the cytokine bound to both its 80-kilodalton type 1 a-receptor subunit (IL-6R, also called CD126) and a 130-kilodalton signal-transducing b-receptor glycoprotein (gp130, also called CD130).22 23 The IL-6R exists as both a membrane-bound and soluble form, which can complex with IL-6 to bind gp130, activating the downstream signaling cascade.22 23 Several therapeutics have been developed targeting the IL-6 signaling axis, including tocilizumab and sarilumab, both of which are monoclonal antibodies that antagonize both membrane-bound and soluble IL-6R.24
At the time of manuscript preparation, several large-scale trials evaluating the efficacy of IL-6-modulatory therapies have been initiated, including the international randomized, double-blind, placebo-controlled phase III COVACTA trial for tocilizumab plus standard of care in hospitalized adult patients with severe COVID-19 pneumonia, as well as the Italian TOCIVID-19 (Tocilizumab in COVID-19 Pneumonia) study, an independent phase II trial for tocilizumab in severe cases of COVID-19, which reached accrual of 330 patients within 24 hours of its announcement on March 19, 2020. A global phase II/III trial evaluating sarilumab in hospitalized patients with severe or critical respiratory illness caused by COVID-19 was announced on March 16, 2020.25 Based on the results from the phase II portion of the study, which compared high-dose (400 mg) versus low-dose (200 mg) sarilumab against placebo, the trial protocol was amended so that only critical patients continued to be enrolled to receive sarilumab 400 mg or placebo.26
As ongoing trials of IL-6-modulation in COVID-19 continue to accumulate and report data, it will be important to identify biomarkers of response to treatment as well as determine what populations of patients may derive maximal clinical benefit. In this case series, we report on clinical outcomes and hematological and inflammatory parameters in a single-arm, non-randomized retrospective analysis of 15 patients with COVID-19 from a single hospital in Italy who were treated with sarilumab. Reported here are the final results of the study. We find that all patients presented with serum IL-6 levels at least 10 times the upper limit of normal, and higher levels of IL-6 at baseline are associated with poor clinical outcomes after sarilumab administration. Furthermore, we show that decreases in C reactive protein (CRP) levels correlate with response to treatment. Based on these findings, early intervention with IL-6 blocking agents should be considered to prevent patients with COVID-19 from progressing to requiring intensive care support.
Methods
Enrollment and informed consent
We report data from a retrospective case series of 15 consecutive patients (12 men, median age 59 (range 53–75)) diagnosed with SARS-COV-2 infection and treated with off-label sarilumab from March 26, 2020 to April 3, 2020 at ‘Azienda Ospedaliera dei Colli – Cotugno Hospital’, Italy. Informed consent for participation in the study was signed by the patients. In cases where the subject was incapable of giving informed consent and an authorized representative was not available without a delay that would, in the opinion of the investigator, compromise the potential life-saving effect of the treatment, sarilumab was administered without consent. Informed consent was obtained for use of clinical data, rendered anonymous, for purposes of clinical research, epidemiology, and study of diseases. All patients were tested for HIV, hepatitis B virus, and hepatitis C virus prior to treatment and were found to be negative.
Enrollment criteria were based on the Italian TOCIVID-19 study, which required patients to be hospitalized due to pneumonia with virological diagnosis of SARS-CoV-2 infection by real-time PCR and oxygen saturation at rest in ambient air ≤93% or requiring oxygen therapy or mechanical ventilation (either non-invasive or invasive (intubated)).
Sarilumab dosing
Sarilumab was administered as a subcutaneous injection of 400 mg. Of the 15 patients treated, 12 received a single injection of sarilumab. Similar to the protocol used in the TOCIVID-19 study, repeat dosing could be permitted if the treating physician considered that there was no improvement after the first administration, and three patients were administered a second dose within 24 hours of the first.
Imaging
Volumetric chest high recolution computed tomography (HRCT) examinations were obtained with both a 128-MDCT Ingenuity scanner (Philips Healthcare) and a 64-MDCT scanner LightSpeed VCT (General Electric Medical System, Milwaukee, Wisconsin), with patients in supine position, only when feasible, at full inspiration. Scanning parameters were 120 kV and 100–200 mAs, pitch 0.75–1.5, and collimation (thickness) 0.625–1.25 mm, applying the smallest field of view according to the patient’s body habitus and with adaptive filtering. Matrix size was 512×512 pixels; images were reconstructed with a 1/1.25 mm slice thickness using high sharp reconstruction algorithm: bone filters (HR filter). In a soft tissue filter was used to better highlight mediastinal space and any other incidental findings (nodules, pleural/pericardial effusion, lymph nodes, aortic/coronary calcifications or other findings). The whole chest volume was processed and stored on a picture archiving and communication system for retrieving images for postprocessing evaluation (PACS-OsiriX). Lung parenchyma was analyzed with a window width of 1.600 Hounsfield units (HU) and at the level of −600 HU.
Sample collection and biomarker measurement
Blood samples were collected on days 0, 1, 2, 3, 5, 7, 10, 11, 12, and 15. Blood counts and CRP were quantified as per institutional protocols. IL-6 was quantified using the Atellica Siemens platform as described previously.
Anthropometric measurements
Weight and height were obtained from patients’ medical records at the time of sarilumab initiation. Body mass index (BMI) was calculated using the formula weight/height2 (kilograms per square meter) and classified according to the WHO categories: underweight, BMI <18.5; normal, 18.5≤BMI≤24.9; overweight, 25≤BMI≤29.9; and obese, BMI ≥30.
Results
Patient characteristics
A total of 15 patients hospitalized for respiratory insufficiency and PCR-confirmed SARS-CoV-2 infections were treated with sarilumab as a single 400 mg subcutaneous injection, with 3 (20%) patients receiving a second dose. The median time from symptom onset to treatment was 11 days (range 6–21). Dates of symptom onset, hospitalization, sarilumab administration and arterial oxygen pressure to fractional inspired oxygen (PaO2:FiO2) values before and after treatment for all patients are shown in table 1. The median PaO2:FiO2 at baseline for all patients was 122 (range 83–240), and 8 of 15 (53.3%) were intubated at the time of sarilumab administration.
Table 1.
Respiratory parameters for all patients before and after sarilumab administration
| Patient | Date of start of symptoms | Date of hospitalization | Initial PaO2:FiO2 | Date(s) of sarilumab administration | PaO2:FiO2 after sarilumab |
| 1 | March 15, 2020 | March 25, 2020 | 83 | March 26, 2020 March 27, 2020 |
135 at d+1 185 at d+3 after second dose |
| 2 | March 15, 2020 | March 25, 2020 | 120 | March 27, 2020 | 296 at d+1 |
| 3 | March 18, 2020 | March 24, 2020 | 180 | March 27, 2020 | 290 at d+1 305 on April 4, 2020 |
| 4 | March 15, 2020 | March 28, 2020 | 192 | March 30, 2020 | 230 at d+1 330 on April 10, 2020 |
| 5 | March 18, 2020 | March 25, 2020 | 200 | March 26, 2020 | 270 at d+1 370 on April 10, 2020 |
| 6 | March 17, 2020 | March 27, 2020 | 240 | March 30, 2020 | 320 at d+1 |
| 7 | March 14, 2020 | March 26, 2020 | 90 | March 27, 2020 March 28, 2020 |
62 at d+1 |
| 8 | March 15, 2020 | March 25, 2020 | 178 | March 30, 2020 March 31, 2020 |
290 at d+1 |
| 9 | March 18, 2020 | March 26, 2020 | 70 | March 27, 2020 | 260 after administration |
| 10 | March 13, 2020 | March 23, 2020 | 137 | April 3, 2020 | 290 at d+1 330 on April 27, 2020 |
| 11 | March 12, 2020 | March 26, 2020 | 120 | 27 March 2020 | 178 at d+1 234 at d+4 382 at d+10 |
| 12 | March 18, 2020 | March 26, 2020 | 60 | March 27, 2020 | 290 after administration |
| 13 | March 15, 2020 | March 25, 2020 | 122 | March 26, 2020 | 72 at d+1 |
| 14 | March 21, 2020 | March 26, 2020 | 88 | March 27, 2020 | 92 at d+1 |
| 15 | March 23, 2020 | March 30, 2020 | 137 | March 31, 2020 | 290 at d+1 |
FiO2, fractional inspired oxygen; PaO2, arterial oxygen pressure.
The median age of all treated patients was 59 (range 53–75). Consistent with emerging evidence that men are more likely to develop serious disease,27 28 80% (12) of patients in the cohort were men and 20% (3) were women. Of the 10 patients for whom BMI values were available, 2 were of normal weight (18.5<BMI≤24.9), 5 were overweight (25<BMI≤29.9) and 3 were obese (BMI >30), in line with reports that overweight/obesity may be a risk factor for severe respiratory complications in COVID-19.29 30 Comorbidities were present in five patients, including two with hypertension, two with diabetes, one with chronic obstructive pulmonary disease and dilatative cardiomyopathy, and one with opioid addiction. All patients received hydroxychloroquine 200 mg two times per day, lopinavir/ritonavir 800 mg/800 mg two times per day, and heparin 4000–6000 IU two times per day. Eleven patients were also treated with methylprednisolone at 80 mg/day for 5–7 days.
Safety
No injection reactions or dermatological toxicities were reported. Liver toxicity was not detected. No patients reported gastrointestinal or metabolic adverse events. One patient developed grade 2 thrombocytopenia according to the 2020 version of the Common Terminology Criteria for Adverse Events (V.5.0). This patient had established heparin treatment prior to sarilumab administration at the time of thrombocytopenia onset. However it cannot be ruled out as a side effect of sarilumab.
Outcomes
Rapid improvements in respiratory parameters were observed in 10 (67%) patients after sarilumab administration, enabling their eventual hospital discharge or transfer to subintensive care. Three patients required two doses of sarilumab due to an investigator-assessed lack of improvement after the first administration, and all displayed increased PaO2:FiO2 after receiving the second dose. A total of five patients who received sarilumab died. All patients who died were intubated and on ventilators before sarilumab treatment. Among the eight patients in total who required mechanical ventilation prior to sarilumab administration, three displayed sufficient improvement in pulmonary function after treatment to allow for extubation, although one died 5 days after being extubated due to a massive embolism.
The median baseline PaO2:FiO2 among patients with disease that responded to sarilumab was 121, and among patients who died the median PaO2:FiO2 was 178. One day after sarilumab administration, the median PaO2:FiO2 was 270. The median PaO2:FiO2 value on d+1 for patients with sarilumab-responsive disease was 280, whereas the median value at the same time point for patients who died was 178. Respiratory parameters for all patients are summarized in table 1.
Among the 10 patients whose disease responded to sarilumab treatment, 2 were of normal weight (18.5<BMI≤24.9), 4 were overweight (25<BMI≤29.9), 2 were obese (BMI ≥30), and BMI measurements were not available for 2 patients. Of the five patients who died, one was overweight (25<BMI≤29.9), two was obese (BMI ≥30) and BMI measurements were not available for 3 patients (table 2).
Table 2.
Patient demographics
| Patient characteristics | N=15 |
| Gender: female/male, n (%) | 3 (20)/12 (80) |
| Median age | 59 (range 53–75) |
| Median PaO2:FiO2 | 122 (83–240) |
| Median BMI | 28.7 (range 23–45) |
| Normal weight (18.5<BMI≤24.9), n (%) | 2 (13.3) |
| Overweight (25<BMI≤29.9), n (%) | 5 (33.3) |
| Obese (BMI ≥30), n (%) | 3 (20) |
| NA, n (%) | 5 (33.3) |
| Intubated, n (%) | 8 (53.3) |
| Not intubated, n (%) | 7 (46.7) |
| Deaths, n (%) | 5 (33.3) |
BMI, body mass index; FiO2, fractional inspired oxygen; PaO2, arterial oxygen pressure.
Radiological findings
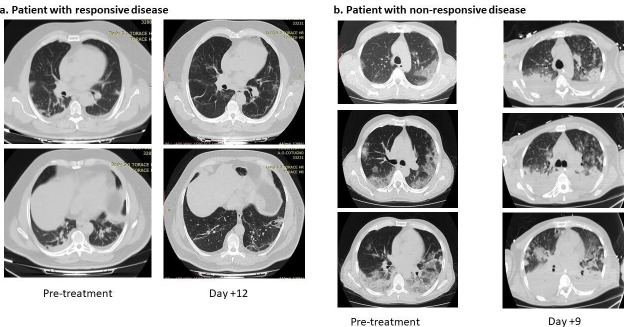
Almost 75% of patients with COVID-19 ARDS present with abnormal findings on chest CT scans, most commonly ground-glass opacities, which are reported in more than half of patients. Other commonly reported findings include patchy infiltrates and interstitial lung disease, although minimal imaging abnormalities are seen in some patients.8 10 31 32 In the treated cohort, abnormal CT imaging features were observed in the lungs of all patients, including ground-glass opacities and crazy paving patterns, which have been described as characteristics of COVID-19.32 33 Pre-sarilumab and post-sarilumab CT scans were available for 11 patients, 9 of whom had disease that responded to treatment while 2 did not. Radiographic improvement was observed in all patients who responded to treatment. Patients repeated a high-resolution chest CT scan after 4 and 8 weeks. Radiological response was assessed by reducing lung consolidation areas and improvement of parenchymal ground-glass. Representative CT scans from one patient whose disease responded to sarilumab and from one patient who died are depicted in figure 1. Radiographs from all patients for whom imaging was available can be found in online supplementary figure 1.
Figure 1.
H Representative chest CT scans pre- and post-treatment from patients with sarilumab responsive(a) and non-responsive (b) COVID-19.
jitc-2020-001089supp001.pdf (2.3MB, pdf)
Baseline hematological and inflammatory values predictive of responsive disease
Clinical improvement after sarilumab administration was associated with lower baseline values of several hematological and inflammatory parameters. Baseline values for all patients as well as grouped by response to treatment are summarized in table 3. Small differences in baseline d-dimer levels were noted, with a higher median value in patients who died compared with those with responsive disease (1884 ng/mL, range 464–4250 vs 1127 ng/mL, range 200–2907). The median baseline platelet counts were initially slightly higher in patients who died compared with those whose disease responded to sarilumab (320×109/μL, range 138–535 vs 291×109/μL, range 127–393); however, a rapid-onset thrombocytopenia associated with clinical decline was subsequently observed.
Table 3.
Median baseline hematological and inflammatory values
| Marker | All patients | Response to sarilumab | No response to sarilumab |
| (median values) | N=15 | N=10 | N=5 |
| Baseline IL-6 | 61.9 pg/mL (range 5.2–1000) | 56.6 pg/mL (range 5.2–81.5) | 213 pg/mL (range 60.5–1000) |
| < ULN, n (%) | 0 (0) | 0 (0) | 0 (0) |
| ≥ ULN, n (%) | 11 (73.3) | 7 (70) | 4 (80) |
| > 10× ULN, n (%) | 8 (53.3) | 4 (40) | 4 (80) |
| > 20× ULN, n (%) | 2 (13.3) | 0 (0) | 2 (20) |
| NA, n (%) | 4 (26.7) | 3 (30) | 1 (20) |
| Baseline ALC | 740/μL (range 160–1490) | 755/μL (range 160–1490) | 530/μL (range 460–1100) |
| < 800, n (%) | 11 (73.3) | 7 (70) | 4 (800) |
| ≥ 800, n (%) | 4 (26.7) | 3 (30) | 1 (20) |
| Baseline d-dimer | 1151 ng/ml (range 200–4250) | 1127 ng/ml (range 200–2907) | 1884 ng/ml (range 464–4250) |
| < ULN, n (%) | 2 (13.3) | 2 (20) | 0 (0) |
| ≥ ULN, n (%) | 9 (60) | 6 (60) | 3 (60) |
| > 2× ULN, n (%) | 8 (53.3) | 6 (60) | 2 (40) |
| > 5× ULN, n (%) | 5 (33.3) | 3 (30) | 2 (40) |
| NA, n (%) | 4 (26.7) | 2 (20) | 2 (20) |
| Median Baseline PLT | 306 x109/μL (range127–535) | 291 x109/μL (range127–393) | 320 x109/μL (range 138–535) |
| < ULN, n (%) | 14 (93.3) | 10 (100) | 4 (80) |
| ≥ ULN, n (%) | 1 (6.7) | 0 (0) | 1 (20) |
| Baseline CRP | 15 mg/dl (range 1.1–30.5) | 12.9 mg/dl (range 3.5–27.8) | 29.5 mg/dl (range 1.1–30.5) |
| < ULN, n (%) | 0 (0) | 0 (0) | 0 (0) |
| ≥ ULN, n (%) | 12 (80) | 9 (90) | 3 (60) |
| > 10× ULN, n (%) | 8 (53.3) | 6 (60) | 0 (0) |
| > 20× ULN, n (%) | 4 (26.7) | 2 (20) | 0 (0) |
| NA, n(%) | 3 (20) | 1 (10) | 2 (40) |
| Median NLR | 14.6 (range 1.8–25.1) | 9.5 (range 1.9–22.7) | 19.5 (range 14.6–25.2) |
| NLR < 5, n (%) | 2 (13.3) | 2 (20) | 0 (0) |
| NLR ≥ 5, n (%) | 13 (86.7) | 8 (80) | 5(100) |
| NLR ≥ 10, n (%) | 10 (66.6) | 5 (50) | 5 (100) |
ALC, Absolute Lymphocte Count; CRP, C reactive protein; IL-6, interleukin 6; NA, Not Available; NLR, neutrophil to lymphocyte ratio; PLT, platelet; ULN, upper limit of normal.
In line with published observations of neutrophil to lymphocyte ratio (NLR) as a prognostic factor for poor outcomes in COVID-19,34 35 the median value among patients who died was roughly twofold higher than in those whose symptoms improved after sarilumab (median NLR=19.5, range 14.6–25.2 vs median NLR=9.5, range 1.9–22.7). Dramatic differences in baseline CRP levels were also observed between the two groups, with a median value of 12.9 mg/dL (range 3.5–27.8) among 10 patients whose disease responded to treatment compared with 29.5 (range 1.1–30.5) in 5 patients who died.
Elevated IL-6 levels were observed in all patients, with a median value of 61.9 pg/mL (range 5.2–1000). The measured values were all higher than those seen under normal homeostatic conditions, where serum concentrations of IL-6 are typically lower than picograms per milliliter, but lower than the levels seen in severe CRS, which can reach nanograms per milliliter,17 or fatal sepsis, where up to micrograms of IL-6 per milliliter have been reported.36 Notably, IL-6 levels at baseline were lower overall in patients who displayed clinical improvement after sarilumab. Among the group whose disease responded to sarilumab, the median baseline IL-6 was 56.6 pg/mL (range 5.2–81.5) compared with 213 pg/mL (range 60.5–1000) in those who died. The differences observed between the two groups suggest that the timing of intervention with sarilumab during the clinical course of COVID-19 inflammatory pathology may be an important determinant of efficacy.
Biomarkers of response to treatment
Hematological and inflammatory parameters were monitored for 15 days after sarilumab administration. Consistent with published observations,34 35 37–39 all patients presented with elevated IL-6, CRP and d-dimer combined with high NLR and thrombocytopenia, signatures that have been described as indicative of disseminated intravascular coagulation and/or cytokine storm.38 40 At baseline, elevated NLR was observed in all patients (median 14.6, range 1.8–25.1), with values ≥10 measured in 10 patients (66.6%). All patients for whom measurements were available displayed elevated levels of CRP (median 15 mg/dL, range 1.1–30.5) as well as IL-6 (median 61.9 pg/mL, range 5.2–1000 pg/mL). Pronounced thrombocytopenia was present at baseline in 14 (93.3%) patients, and the median platelet count was 306×109/μL (range 127–535). In patients who died, platelet counts decreased dramatically in 2 days after sarilumab treatment, whereas levels remained relatively constant throughout the course of the study for patients who experienced clinical improvement. Among the 11 patients for whom d-dimer measurements were available before treatment, levels were greater than two times the upper normal limit in eight patients (53.3%). The median d-dimer value for the entire cohort was 1151 ng/mL (range 200–4250).
In all patients, a decrease in white cell counts and absolute neutrophil counts was observed after sarilumab administration. Reductions in d-dimer after sarilumab were also observed across all patients (online supplementary figure 2). Distinct patterns in the values for several markers did emerge between patients whose disease responded and those who died, most notably for CRP, IL-6, NLR, and eosinophil counts. The average values and box plots for IL-6, CRP, NLR, eosinophils and platelets are presented in figure 2.
Figure 2.
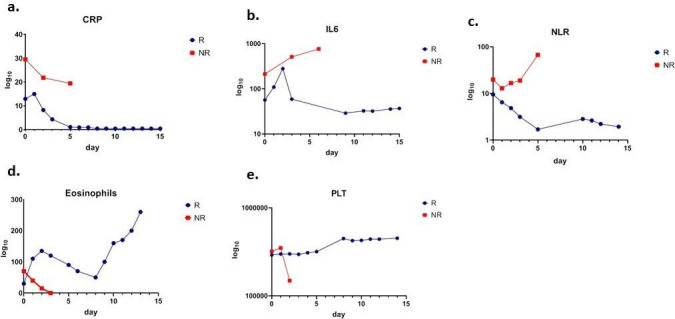
Hematologicand inflammatory parameters in patients with sarilumab responsive (bluemarkers) and non-responsive (red markers) COVID-19. Mean values for c-reactiveprotein (CRP) (panel a), interleukin 6 (IL-6) (panel b), neutrophil-to-lymphocyte ratio (NLR) (panel c), eosinophils (panel d) andplatelets (panel e) at baseline (day 0) as well as days 1, 2, 3, 5, 7, 10, 11,12, and 15 after sarilumab administration are shown.
jitc-2020-001089supp002.pdf (4.1MB, pdf)
Among patients with COVID-19 that improved after treatment, dramatic reductions in serum CRP were observed immediately following sarilumab administration, with levels mostly decreasing to below the upper normal limit within 5 days. Although serum CRP did decrease after sarilumab in patients who died, levels among these patients never declined below the highest values seen in the group with responding disease (figure 2A). The reduction in CRP levels among responding patients corresponded with improvements in respiratory parameters. Notably, serum IL-6 levels increased after sarilumab in both patients whose disease responded to treatment and those who died, a phenomenon that has also been observed in patients receiving the IL-6R antagonist tocilizumab for CRS subsequent to CAR T cell therapy.41 Among the patients whose symptoms improved after treatment, the increase was transient, persisting for no longer than 2 days, with a sharp subsequent decrease and IL-6 levels reaching a steady low point from day +9 onward. By contrast, IL-6 levels continuously increased among patients who died, eventually reaching values almost 100 times the normal ranges (figure 2B). In this cohort, CRP values were more informative than IL-6 measurements as a surrogate for response to treatment.
NLR steadily decreased over 5 days after administration in patients whose disease responded to sarilumab, whereas average values increased continually among patients who died (figure 2C). Similar patterns were observed with the derived NLR (online supplementary figure 2). Total white cell counts slightly decreased with similar kinetics for the first 3 days after sarilumab administration in both groups; however, a modest rebound was observed between days +5 and +15 in patients whose disease responded to treatment. Absolute lymphocyte counts remained relatively stable for 15 days after sarilumab administration among patients with responding disease, with a concomitant steep decrease in absolute neutrophil counts during the first 5 days. In patients who died, lymphocyte counts decreased steadily and only modest reductions in absolute neutrophil counts were observed during the first 3 days after sarilumab treatment.
Although the role that eosinophils play in response to SARS-CoV-2 infection has yet to be definitively characterized, a retrospective review of 85 fatal cases of COVID-19 in Wuhan, China found that 81% of patients were eosinopenic at the time of hospital admission.42 In sarilumab-treated cohort, eosinophilia was associated with improved outcomes after administration (figure 2D). A marked eosinophilia was observed in patients whose disease improved after sarilumab, with sharp escalation in eosinophil counts in the first 2 days after administration followed by a transient decrease between days +3 and +8 and then a subsequent increase throughout the end of the observation period. By contrast, eosinophil counts declined steadily in patients who died.
Discussion
This case series describes 15 patients with COVID-19 who were treated with sarilumab. Rapid improvements in respiratory function as well as normalization of inflammatory markers were observed in 10 of 15 patients. Preliminary analysis of hematological and inflammatory parameters indicates that elevated values of CRP, IL-6 and the NLR were associated with worse outcomes and a lack of response to treatment. Although these results will need to be validated in larger patient cohorts and randomized trials, our study indicates that a subset of patients with COVID-19 may benefit from treatment with sarilumab or other IL-6-modulatory therapies.
Limitations of this study include the relatively small number of patients treated and the lack of randomization. In this study, 8 of 15 (53%) patients were intubated at the time of sarilumab administration, and the mortality rate among patients requiring mechanical ventilation was 62.5%. The proportion of intubated patients in this cohort is lower than the 88% rate published in a retrospective analysis of 1591 patients with confirmed SARS-CoV-2 infections who were admitted to ICUs in Lombardy, Italy between February 20 and March 18, 2020.43 The rates in this cohort as well as in Italy overall are higher than those reported in other countries, as illustrated by an analysis of 5700 patients in New York City, where 20.2% (1151) required mechanical ventilation and of these 24.5% (282) died.44 Several factors have been proposed to account for the disproportionately high fatality rate observed thus far in Italy compared with other countries, including the older age of the population and testing and case reporting disparities.
Comedication with other COVID-19 therapies is another potential confounding factor, as all patients in this cohort received hydroxychloroquine, lopinavir/ritonavir, and heparin. Additionally, 11 patients were also treated with methylprednisolone. Hydroxychloroquine likely had minimal effects on the results. The initial study purporting improved outcomes with hydroxychloroquine for the treatment of COVID-19 was retracted,45 and subsequent analysis from the UK’s RECOVERY trial found no benefit for the drug in hospitalized patients when compared with standard of care.46 Similarly, a randomized, controlled, open-label trial of lopanivir/ritonavir for hospitalized adult patients with confirmed SARS-CoV-2 infection found no differences in time to clinical improvement with the antiviral compared with standard of care and overall similar mortality rates at 28 days (19.2% vs 25.0%; difference, −5.8 percentage points; 95% CI −17.3 to 5.7).47 Steroid use has reported more encouraging results for COVID-19. In the RECOVERY trial, which compared outcomes for 2104 patients randomly allocated to receive dexamethasone with 4321 patients concurrently allocated to usual care, dexamethasone reduced deaths by roughly one-third in patients receiving invasive mechanical ventilation (29.0% vs 40.7%, rate ratio (RR) 0.65 (95% CI 0.51 to 0.82); p<0.001) and by approximately one-fifth in patients receiving oxygen without invasive mechanical ventilation (21.5% vs 25.0%, RR 0.80 (95% CI 0.70 to 0.92); p=0.002), although it did not reduce mortality in patients not receiving respiratory support at randomization (17.0% vs 13.2%, RR 1.22 (95% CI 0.93 to 1.61); p=0.14).48 Methylprednisolone has also been reported to reduce risk for mechanical ventilation. In a case series of 15 severe and critical patients with COVID-19 in China who received pulse single dosage of 40–500 mg methylprednisolone, oxygenation improved significantly, no deaths occurred and only one needed mechanical ventilation.49
Although caution must be exercised when comparing across trials, the outcomes observed in this case series roughly correspond to the topline results that were announced for the global randomized phase II/III trial of sarilumab for critically ill patients with COVID-19. In the trial, of the 88 patients in the critical group who received high-dose sarilumab (400 mg), 52 (59%) achieved clinical improvement after treatment and 20 (23%) died.26
Modulation of the severe inflammatory state associated with severe COVID-19 may offer a potentially important strategy to limit pulmonary complications of the disease, reducing the necessity for intensive care unit support and ultimately mortality. It is evident, however, that the clinical course of SARS-CoV-2 infection is highly heterogeneous, with presentations ranging from mild respiratory symptoms to irreversible respiratory failure.4 32 50 Furthermore, the ultimate etiology of acute respiratory distress in COVID-19 remains a matter of some controversy, with the relative contributions of unrestrained viral replication versus hyperinflammation still incompletely understood.51 52 The timing of intervention may be important, as treatment with anti-IL-6 therapies too early in the course of infection could hinder the eventual development of a protective adaptive immune response.53 Conversely, the benefit of IL-6 modulation may be limited in cases where the hyperinflammatory cascade is too far advanced.54 55 Multiple strategies may be needed due to the overlapping regulation of proinflammatory cytokines. Ongoing trials are investigating agents directed against granulocyte-macrophage colony-stimulating factor (GM-CSF), IFNγ and IL-1, among others.24 Encouraging results have been reported in a small-scale study of the IL-1 receptor antagonist anakinra in hospitalized patients with COVID-19, where a reduced risk of invasive mechanical ventilation or death was observed with treatment compared with historical controls (HR 0.22, 95% CI 0.11 to 0.41; p<0.0001).56 Identifying biomarkers of response to treatment with sarilumab or other IL-6-modulatory therapies will be important for the ultimate incorporation of these strategies into widespread use. Among patients studied here, the most reliable marker of clinical response was CRP, which declined steeply immediately after sarilumab administration among patients with responsive disease. Although IL-6 levels also eventually decreased, the initial kinetics (characterized by a transient increase after treatment) make measurements of the cytokine non-ideal for clinical decision making. Furthermore, given that same-day access to IL-6 levels may be limited in some hospitals, CRP is a more useful marker of real-time evaluation of the effectiveness of treatment.
One striking difference between sarilumab-responsive and non-responsive disease was the dramatic eosinopenia observed in patients who died after treatment and the biphasic pattern of eosinophilia seen in patients who recovered. Eosinophils are protective against respiratory tract infections, although they also play a role in inflammatory airway pathology such as asthma.57 Eosinophils have been conspicuously absent from lung samples in published analyses from autopsies both early in the disease and postmortem,58 and the characteristic pattern of immune infiltration associated with infection is typically dominated by monocytes and mononuclear cells.5 59 Although type 1 immunity is likely important early on in the course of infection, dysregulated inflammatory responses may contribute to pathology, and severe disease has been associated with a characteristic proinflammatory T helper 17 signature for both SARS-CoV-2 infections and the Middle East respiratory syndrome (MERS) coronavirus.60 61 Given the importance of type 2 immunity for both the generation of high antibody titers and resolution of inflammation,62 the eosinophilia observed after sarilumab administration in patients who recovered may represent an important hallmark of infection control.
Elevated levels of CRP, IL-6, and NLR have previously been described as predictive of poor outcomes in COVID-19.4 18 34 35 In the cohort described in this paper, response to sarilumab was associated with lower values at baseline for these markers. Experience from the use of the IL-6R antagonist tocilizumab in China19 has indicated that early administration of cytokine-modulatory therapy is critical, ideally before symptoms start to rapidly deteriorate at the onset of the cytokine storm.63 Taken with the results of this case series, the optimal time for intervention with anti-IL-6 agents may be early in the disease course. Patients with oxygen saturation at rest and without supply ≤93% should be closely monitored and anti-IL-6 should be strongly considered at the first signs of increases in CRP.
Although the SARS-CoV-2 outbreak appears to have peaked in some regions around the world with new cases and fatalities beginning to decline week by week, the healthcare system remains stretched thin. Supplies of personal protective equipment are running low in multiple areas and shortages of several crucial drugs are predicted.64 Effective interventions to reduce the number of patients requiring intensive care are still needed, especially as some areas relax physical distancing mandates and cases rebound. In all likelihood, effective management of COVID-19 will require a strategy encompassing antivirals, multiple anticytokine agents and/or passive immunization using convalescent plasma. Examples of such approaches include the ongoing REMDACTA trial of tocilizumab in combination with remdesivir. Although initial results indicating clinical benefit with the use of sarilumab and other cytokine-modulatory therapies have begun to be reported, further definitive prospective randomized trials should also be conducted with all due haste. Additionally, future studies should further characterize immune and inflammatory responses throughout the course of SARS-CoV-2 infection as well as after treatment. A collaborative and multidisciplinary approach will be needed in order to reduce the human toll of this unprecedented and devastating pandemic.
Acknowledgments
The authors thank the patients and their families. The authors also thank the management of the hospitals for the strong support in their daily activities during the COVID-19 emergency: Dr Maurizio Di Mauro, General Manager of Azienda dei Colli of Napoli, Italy; Dr Attilio Bianchi, General Manager of Istituto Nazionale Tumori IRCCS Fondazione Pascale, Napoli, Italy; and Dr Gerardo Botti, Scientific Director of Istituto Nazionale Tumori IRCCS Fondazione Pascale, Napoli, Italy.
Footnotes
Twitter: @Vincenzo Montesarchio, @millionweaver
VM and RP contributed equally.
Correction notice: This paper has been corrected since it was published online. The author name Roberto Parrella was incorrectly spelt as Roberto Parella.
Contributors: PAA conceptualized the study and provided leadership in determining manuscript structure and content, as well as collection and interpretation of clinical data. Collected and interpreted the data, and provided key insights throughout the duration of the study. CI, AB, EM, FF, CP, GR, PM, RDR, LA, MDA, MC, DM, EC, AMG, MP, CT, and MGV assisted with data collection and interpretation and provided valuable discussions assisting in the preparation of the paper. SMW wrote the paper and made the figures. All authors reviewed and edited the drafts of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AMG: advisory and consultant role for BMS, MSD and Novartis; and travel grants from BMS, Merck Serono, Pierre Fabre, Roche and Novartis. PAA: consultant/advisory role for Bristol-Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Array, Novartis, Merck Serono, Pierre Fabre, Incyte, NewLink Genetics, Genmab, MedImmune, AstraZeneca, Syndax, SunPharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, and Nektar; research funds from Bristol-Myers Squibb, Roche-Genentech, and Array; and travel support from MSD.
Patient consent for publication: Not required.
Ethics approval: The study was conducted under the Declaration of Helsinki and was approved by the departmental review board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.@OurWorldInData Total confirmed COVID-19 deaths: how rapidly are they increasing? : @OurWorldInData, 2020. Available: https://ourworldindata.org/grapher/covid-confirmed-deaths-since-5th-death
- 2.Fauci AS, Lane HC, Redfield RR. Covid-19 — Navigating the Uncharted. N Engl J Med Overseas Ed 2020;382:1268–9. 10.1056/NEJMe2002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020;323:1335 10.1001/jama.2020.4344 [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Wu D, Guo W, et al. . Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian S, Hu W, Niu L, et al. . Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020;15:700–4. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z, Shi L, Wang Y, et al. . Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti P, Ronconi G, Caraffa A, et al. . Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020;34. 10.23812/CONTI-E. [Epub ahead of print: 14 Mar 2020]. [DOI] [PubMed] [Google Scholar]
- 8.Mo P, Xing Y, Xiao Y, et al. . Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis 2020. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C, Zhou L, Hu Z, et al. . Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020. 10.2139/ssrn.3541136. [Epub ahead of print: 12 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Yang B, Li Q, et al. . Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020;367 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Rosée P. Treatment of hemophagocytic lymphohistiocytosis in adults. Hematology Am Soc Hematol Educ Program 2015;2015:190–6. 10.1182/asheducation-2015.1.190 [DOI] [PubMed] [Google Scholar]
- 12.Tamamyan GN, Kantarjian HM, Ning J, et al. . Malignancy-Associated hemophagocytic lymphohistiocytosis in adults: relation to hemophagocytosis, characteristics, and outcomes. Cancer 2016;122:2857–66. 10.1002/cncr.30084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neelapu SS. Managing the toxicities of car T-cell therapy. Hematol Oncol 2019;37 Suppl 1:48–52. 10.1002/hon.2595 [DOI] [PubMed] [Google Scholar]
- 15.Santomasso B, Bachier C, Westin J, et al. . The other side of car T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book 2019;39:433–44. 10.1200/EDBK_238691 [DOI] [PubMed] [Google Scholar]
- 16.Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol 2019;15:813–22. 10.1080/1744666X.2019.1629904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teachey DT, Lacey SF, Shaw PA, et al. . Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016;6:664–79. 10.1158/2159-8290.CD-16-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan Q, Yang K, Wang W, et al. . Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xea X. Effective treatment of severe COVID19 patients with tocilizumab. Chinaxiv. 2020. [Google Scholar]
- 20.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. . Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ascierto PA ea SITC Statement on anti-IL-6/IL-6R for COVID-19 - Society for Immunotherapy of Cancer (SITC), 2020. Available: https://www.sitcancer.org/research/covid-19-resources/il-6-editorial
- 22.Tanaka T, Narazaki M, Kishimoto T. Il-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uciechowski P, Dempke WCM. Interleukin-6: a Masterplayer in the cytokine network. Oncology 2020;98:131–7. 10.1159/000505099 [DOI] [PubMed] [Google Scholar]
- 24.Arnaldez FI, O'Day SJ, Drake CG, et al. . The Society for immunotherapy of cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. J Immunother Cancer 2020;8:e000930. 10.1136/jitc-2020-000930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regeneron and Sanofi Begin Global Kevzara® (sarilumab) Clinical Trial Program in Patients with Severe COVID-19 | Regeneron Pharmaceuticals Inc. [press release] 2020.
- 26.Regeneron and Sanofi Provide Update on U.S. Phase 2/3 Adaptive-Designed Trial of Kevzara® (sarilumab) in Hospitalized COVID-19 Patients | Regeneron Pharmaceuticals Inc. [press release] 2020.
- 27.Jin J-M, Bai P, He W, et al. . Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020;8:152. 10.3389/fpubh.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 2020;8:e20–e. 10.1016/S2213-2600(20)30117-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalligeros M, Shehadeh F, Mylona EK, et al. . Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity 2020;28:1200-1204. 10.1002/oby.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simonnet A, Chetboun M, Poissy J, et al. . High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020;28:1195–9. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian G-Q, Yang N-B, Ding F, et al. . Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM 2020;113:474–81. 10.1093/qjmed/hcaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. . Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020;101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi H, Han X, Jiang N, et al. . Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425–34. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Du X, Chen J, et al. . Neutrophil-To-Lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang A-P, Liu J-P, Tao W-Q, et al. . The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 2020;84:106504. 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda S, Hirasawa H, Shiga H, et al. . Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine 2005;29:169–75. 10.1016/j.cyto.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 37.Daniel OG, Alexandra J, Mushmoom K, et al. . Pulmonary embolism and increased levels of D-dimer in patients with coronavirus disease. Emerging Infectious Disease journal 2020;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. . Hematological findings and complications of COVID-19. Am J Hematol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Yan X, Fan Q, et al. . D-Dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 2020;18:1324–9. 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Zhao Y, Zhang F, et al. . The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 2020;214:108393. 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen F, Teachey DT, Pequignot E, et al. . Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods 2016;434:1–8. 10.1016/j.jim.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du Y, Tu L, Zhu P, et al. . Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med 2020;201:1372–9. 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grasselli G, Zangrillo A, Zanella A, et al. . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson S, Hirsch JS, Narasimhan M, et al. . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020;323:2052. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehra MR, Desai SS, Ruschitzka F, et al. . Retracted: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet 2020. 10.1016/S0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46., 2020. Available: https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19
- 47.Cao B, Wang Y, Wen D, et al. . A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med Overseas Ed 2020;382:1787–99. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horby P, Lim WS, Emberson J, et al. . Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv 2020. [Google Scholar]
- 49.Liu J, Zheng X, Huang Y, et al. . Successful use of methylprednisolone for treating severe COVID-19. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.05.021. [Epub ahead of print: 29 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai C-C, Liu YH, Wang C-Y, et al. . Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect 2020;53:404–12. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felsenstein S, Herbert JA, McNamara PS, et al. . COVID-19: immunology and treatment options. Clin Immunol 2020;215:108448. 10.1016/j.clim.2020.108448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tay MZ, Poh CM, Rénia L, et al. . The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–74. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter CA, Jones SA. Il-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 54.Pedersen SF, Ho Y-C. SARS-CoV-2: a storm is raging. J Clin Invest 2020;130:2202–5. 10.1172/JCI137647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tisoncik JR, Korth MJ, Simmons CP, et al. . Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012;76:16–32. 10.1128/MMBR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huet T, Beaussier H, Voisin O, et al. . Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2020;2:e393–400. 10.1016/S2665-9913(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eng SS, DeFelice ML. The role and immunobiology of eosinophils in the respiratory system: a comprehensive review. Clin Rev Allergy Immunol 2016;50:140–58. 10.1007/s12016-015-8526-3 [DOI] [PubMed] [Google Scholar]
- 58.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol 2020;146:1–7. 10.1016/j.jaci.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Zhou P, Wei Y, et al. . Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med 2020;172:629–32. 10.7326/M20-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahallawi WH, Khabour OF, Zhang Q, et al. . Mers-Cov infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018;104:8–13. 10.1016/j.cyto.2018.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu D, Yang XO. Th17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect 2020;53:368–70. 10.1016/j.jmii.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spellberg B, Edwards JE. Type 1/type 2 immunity in infectious diseases. Clin Infect Dis 2001;32:76–102. 10.1086/317537 [DOI] [PubMed] [Google Scholar]
- 63.Fu B. Personal communication 2020.
- 64.Ranney ML, Griffeth V, Jha AK. Critical Supply Shortages - The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N Engl J Med 2020;382:e41. 10.1056/NEJMp2006141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001089supp001.pdf (2.3MB, pdf)
jitc-2020-001089supp002.pdf (4.1MB, pdf)