Abstract
Since the beginning of the COVID-19 pandemic, healthcare providers worldwide have faced many obstacles in the diagnostic evaluation of patients for severe acute respiratory syndrome coronavirus 2, the causative virus. Even with the application of statistical inference by Bayes’ theorem to estimate the probability of a diagnosis, with and without testing capabilities, some cases may still carry a degree of uncertainty. This has important implications for limiting the spread of disease. The basis for isolation and quarantine is a known diagnosis. This case is an example of a diagnostic conundrum that required more thorough use of testing methods, particularly serological testing, to guide the isolation recommendations for a patient with COVID-19. This will be helpful to other diagnosticians by providing an example of how serological findings may be effectively applied in the course of individual COVID-19 management.
Keywords: general practice / family medicine, public health
Background
The clinical course and diagnostic testing characteristics of COVID-19 have been studied from the beginning of the pandemic.1 Multiple objective studies have described the validation and use of serological testing throughout the COVID-19 pandemic,2 including the utility (or lack thereof) of serology for guiding policy and return-to-work planning. Initially, the possible application of serology was exciting, but many medical groups have recently suggested that serology may have the greatest benefit for epidemiological research and minimal use for disease management in individual patients.3 Here, we describe a case in which serological testing effectively helped guide the clinical care of an individual patient.
Case presentation
A 49-year-old woman sought care for fever, epistaxis and diarrhoea of 5 days’ duration. She had travelled across the USA in mid-March for vacation and had flown home 3 days earlier. Her maximum temperature was 101.5°F. Her history included asthma controlled on bronchodilators. She was treated with azithromycin for community-acquired pneumonia and developing sinusitis.
Two days later, she came to the emergency department for pleuritic chest pain, dry cough, shortness of breath, abdominal pain and diarrhoea. She had wheezing and was using her rescue inhaler. Her temperature was 102.9°F, and heart rate was 113 beats/min. She was admitted because of concern for COVID-19 pneumonia. Because of no benefit from 5 days of oral macrolide, she was started on broad-spectrum antibiotics. For hypoxia, she was given supplemental oxygen by nasal cannula. Blood cultures and urine tests for Legionella and pneumococcus were negative. Initial nasopharyngeal testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by PCR with the cobas 6800 System (Roche; https://www.fda.gov/media/136047/download) was negative. She had mild hyponatraemia and hypokalaemia secondary to diarrhoea and received intravenous fluids with potassium. She reported that her husband had COVID-19 and remained in isolation with minimal symptoms. The next day, a nasopharyngeal swab was taken; full viral respiratory panel was performed (US Food and Drug Administration-cleared Verigene RP Flex Respiratory Panel, Luminex); and SARS-CoV-2 PCR testing was repeated, both of which were negative. Evaluation of diarrhoea was negative for Clostridioides difficile and enteric pathogens. On the following day, she was weaned off oxygen and discharged. She continued levofloxacin empirically for concern for superimposed bacterial infection.
She was seen 12 days later for a 4-day history of calf pain. Ultrasonography showed right lower extremity deep vein thrombosis (DVT). Anticoagulation was started. She had no known personal or family history of coagulopathies. She and her spouse continued to self-isolate away from each other. Serological testing for SARS-CoV-2 IgG was performed because of persistent malaise, cough, runny nose, congestion and nausea, within the setting of twice-negative PCR results and past SARS-CoV-2 exposure from her husband. Serological results were positive, so she was considered to have active COVID-19.
Investigations
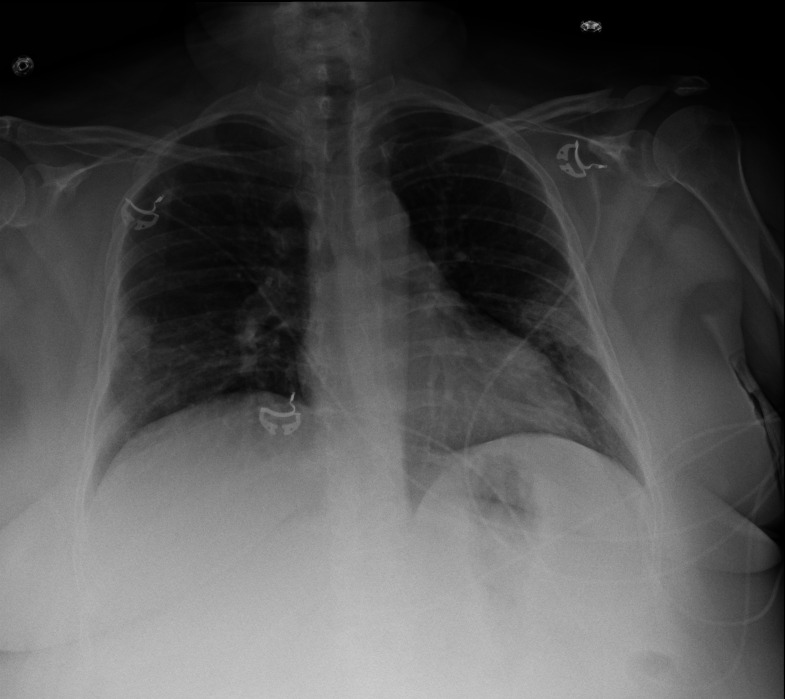
On the patient’s admission to the hospital, a nasopharyngeal swab was taken for SARS-CoV-2 PCR testing, which returned negative results. Her laboratory tests were remarkable for a serum sodium concentration of 134 mmol/L and a potassium concentration of 3.3 mmol/L. Her lactate levels and liver function tests were within normal limits. Her complete blood cell count and further basic metabolic panel were normal. Chest radiography showed patchy, peripheral airspace opacities present in the bilateral mid-lower lungs, suggestive of patchy pneumonitis (figure 1). It was noted that atypical infections, including COVID-19, could have a similar appearance, and correlation with laboratory testing was recommended. Repeated SARS-CoV-2 PCR testing with a nasopharyngeal swab was again negative. By the time of discharge, the patient’s laboratory abnormalities and hypoxia had resolved. Ultrasonography of the right lower extremity postdischarge performed because of concern for DVT showed a non-occlusive DVT in the right popliteal vein and posterior tibial vein in the calf. Shortly thereafter, the Mayo Clinic serological laboratory test for SARS-CoV-2 IgG antibody (anti-SARS-CoV-2 IgG, Ortho-Clinical Diagnostics) returned a positive result (4.63; negative index reference range, <1.01).
Figure 1.
Chest radiography. Anteroposterior images at the time of hospital admission show patchy, peripheral airspace opacities bilaterally in the mid-lower lungs.
Differential diagnosis
Initially, the patient had signs of community-acquired pneumonia: cough, fever and pleuritic chest pain. A course of outpatient oral antibiotics failed. With imaging findings and progressive symptoms, the differential diagnosis included atypical pneumonia of either bacterial or viral origin. With negative PCR test results for SARS-CoV-2, she remained on broad-spectrum antibiotics for atypical bacterial pneumonia. As it became apparent that she had a high likelihood of COVID-19 by exposure to her husband, serological testing was conducted. This confirmed recent, and possibly still active, SARS-CoV-2 infection. From the onset of her symptoms to the timing of serological testing, enough time had passed for IgG antibody formation. However, she still had active symptoms of diarrhoea, malaise and shortness of breath; thus, a strong recommendation was made to treat it as an active infection. Viral culture could have given more precise diagnostic information regarding the infectivity of the patient at the time of serological testing, but this was not an available option, nor would it have been necessary because she was able to isolate together with her husband, who was also being treated for an active infection.
Treatment
For the concern of community-acquired pneumonia, the patient was given a course of oral azithromycin. As symptoms progressed, requiring hospitalisation, broad-spectrum antibiotics were added to include ceftriaxone plus levofloxacin. She was given intravenous potassium 40 mEq and 1-litre normal saline for metabolic derangements, which most likely were a result of diarrhoea worsened by azithromycin. She was given oxygen, 2 litres by nasal cannula, with montelukast, budesonide/formoterol and albuterol as needed for her history of asthma and recent hypoxia. She was discharged from the hospital with oral levofloxacin and recommendations to isolate from her COVID-19-positive husband. The DVT was treated with apixaban. After the diagnosis of COVID-19 was established, she was advised about isolation precautions and monitoring in a COVID-19 virtual clinic. She was encouraged to follow through with a test-based strategy to discontinue isolation.
Outcome and follow-up
After her COVID-19 diagnosis, the patient was advised that it was no longer necessary to isolate from her husband. This improved her anxiety about contracting his infection and eased their home situation (eg, use of resources, cleaning practices and hygiene precautions). She remained in isolation and followed a test-based strategy to discontinue isolation 4 days later.4 She had two further consecutive negative PCR tests more than 24 hours apart to end her isolation, 3 days after her husband.
Discussion
To our knowledge, this is the first reported case of a person with two negative SARS-CoV-2 PCR tests and positive IgG serology. These findings affected management decisions specific to isolation precautions in the home with infected family members. In this situation, IgM testing would have been more advantageous and would have allowed management decisions to be made sooner.5 Although this case is unusual (multiple negative PCR tests but positive serology with active symptoms), it is common to see positive serological and negative PCR findings at certain points throughout the course of infection. Sethuraman et al5 show the estimated variation over time in diagnostic test results for detection of SARS-CoV-2 infection relative to symptom onset. After the second week from symptom onset, the probability of detecting IgG antibody would be higher than that of detecting viral RNA. There may be an overlap during convalescence in the timing of discontinuing isolation and seroprevalence of antibodies to SARS-CoV-2.6 If so, positive serological titres can aid in the management of COVID-19. Some reported cases have described concern for an extended period of infection, prevalent false-negative PCR results and still-uncertain duration of convalescence.7 We recommend considering all possible resources as necessary, including serological testing, in the diagnosis of COVID-19.
Patient’s perspective.
The entire time I was in the hospital, I was concerned that I had COVID-19. I was confused and anxious when my tests came back negative for coronavirus. I was certain I had this disease, since my husband had it too, but my swab tests were negative, even though my symptoms were worse than his. When I was discharged from the hospital, my husband and I were not sure how we could comfortably use our small living area to accommodate isolating from each other. Once I had the diagnosis of COVID-19 by the blood tests, it was helpful for us to know. We could again share resources and help support each other without fear of infecting me while we recovered.
Learning points.
Serological testing can change the management strategy for individual patients with COVID-19.
Even with negative results of PCR, clinical judgement must be used to ascertain whether the patient has a high pretest probability of COVID-19.
Serological findings are best used on an individual basis when results of PCR testing are persistently negative and the patient has active symptoms.
Serological findings will most likely be positive if at least 1 week has passed since a patient’s onset of symptoms and the patient has a known exposure to severe acute respiratory syndrome coronavirus 2.
Footnotes
Twitter: @knidac
Contributors: DK: planning, conduct, reporting, conception, design and acquisition of data. JI-A: analysis and interpretation of data
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465–9. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 2.Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA 2020. 10.1001/jama.2020.6170. [Epub ahead of print: 17 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 3.Winter AK, Hegde ST. The important role of serology for COVID-19 control. Lancet Infect Dis 2020;20:758–9. 10.1016/S1473-3099(20)30322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention , 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html
- 5.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020;323:2249 10.1001/jama.2020.8259 [DOI] [PubMed] [Google Scholar]
- 6.Lou B, Li T-D, Zheng S-F, et al. . Serology characteristics of SARS-CoV-2 infection since exposure and post symptom onset. Eur Respir J 2020. 10.1183/13993003.00763-2020. [Epub ahead of print: 19 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodruff A, Walsh KL, Knight D, et al. . COVID-19 infection: strategies on when to Discontinue isolation, a retrospective study. Am J Infect Control 2020. 10.1016/j.ajic.2020.06.220. [Epub ahead of print: 04 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]