The severe acute respiratory syndrome coronavirus-2 pandemic has been unrelenting in disease burden worldwide, with increasing recognition of multisystem inflammatory syndrome in children (MIS-C).1, 2, 3, 4, 5, 6, 7 Although cardiac manifestations are reported in MIS-C,5, 6, 7 the relationship between cardiac biomarkers and echocardiographic abnormalities and its evolution during illness remains unclear. We present our single-institution experience of cardiac manifestations in MIS-C describing changes in cardiac biomarkers and echocardiographic findings across a spectrum of illness severity.
This cohort included all patients meeting the Centers for Disease Control and Prevention or World Health Organization case definitions of MIS-C admitted between May 1, 2020, and June 5, 2020, with follow-up through June 24, 2020. Echocardiography was performed using the Philips iE33 standard machine (Philips Medical Systems, Andover, MA). Two-dimensional left ventricular ejection fraction (LVEF) was calculated using the 5/6 × area × length method. Cardiac magnetic resonance imaging (MRI) evaluation of late gadolinium enhancement was performed using single-shot survey images. Data are presented as median (range) except as otherwise noted. The study was reviewed and approved by our institutional research board (2020P001114).
Twelve children met the inclusion criteria, with a median age of 3.5 years (range, 2 months to 21 years); three (25%) were female. All patients tested positive for severe acute respiratory syndrome coronavirus-2 by either nasopharyngeal polymerase chain reaction or serology.
Laboratory evidence of inflammation was common, as was treatment with immunomodulatory therapies. Serological evidence of myocardial injury was common. Elevated high-sensitivity troponin T (hsTnT) and N-terminal pro B-type natriuretic peptide (NT-proBNP) were seen in nine of 12 (75%) and nine of 11 (82%) patients, respectively (Table 1 ).
Table 1.
Laboratory markers and treatments in MIS-C cohort.
| Laboratory markers | ||
|---|---|---|
| Laboratory study (normal range) | Number abnormal (%) | Median maximum value [range] |
| C-reactive protein (<6.0 mg/L) | 11/12 (92) | 149 mg/L (1.4–300) |
| Erythrocyte sedimentation rate (<13 mm/h) | 9/10 (90) | 50 mm/h (11–133) |
| d-dimer (<500 ng/mL) | 11/12 (92) | 2,523 ng/mL (448–6,154) |
| Ferritin (20–300 μg/L) | 8/12 (67) | 539 μg/L (100–3,729) |
| Lactate dehydrogenase (110–210 U/L) | 9/9 (100) | 359 U/L (250–600) |
| hsTnT (<10 ng/L) | 9/12 (75) | 32 ng/L (<6–2,451) |
| NT-proBNP (<500 pg/mL) | 9/11 (82) | 2,121 pg/mL (248–67,725) |
| Treatments | |
|---|---|
| Treatment | Number receiving (%) |
| Intravenous immunoglobulin | 11/12 (92) |
| Corticosteroids | 5/12 (42) |
| Anakinra | 2/12 (17) |
| Vasoactive medications | 4/12 (33) |
| Mechanical ventilation | 3/12 (25) |
| Venoarterial extracorporeal membrane oxygenation | 2/12 (17) |
Cardiac MRI was performed following the acute phase of illness before discharge in two patients who required mechanical support with extracorporeal membrane oxygenation. One patient with echocardiographic findings of mildly dilated left main and left anterior descending coronary arteries had on MRI subtle midmyocardial late gadolinium enhancement in the septum and inferior left ventricle, normal function (LVEF 60%, right ventricular ejection function 59%), and a small pericardial effusion. The second patient had no coronary abnormalities by echocardiography, with MRI demonstrating no signs of myocarditis, infiltration, or scar, with normal function (LVEF 55%, RVEF 53%) and a small pericardial effusion.
While inpatient, 42 echocardiographic examinations were performed (median, 3 per patient; range, 1–7 per patient), with echocardiographic abnormalities observed in eight of 12 patients (75%). The most common echocardiographic abnormality was reduced LVEF (<55%), noted in seven of 12 patients (58%). The median lowest observed LVEF was 54% (range, 14%–70%), with severely reduced LVEFs (<30%) seen in three of 12 patients (25%) and mildly reduced LVEFs (40%–55%) seen in four of 12 patients (33%). Regional wall motion abnormalities were seen in three of 12 patients (25%), and qualitative right ventricular dysfunction was present in four of 12 patients (33%). Coronary changes were observed in five of 12 patients (42%), including lack of distal tapering of the left anterior descending coronary artery (three of 12 [25%]), left anterior descending coronary artery dilation (one of 12 [8%]), and small saccular aneurysm of the right coronary artery (one of 12 [8%]). All observed effusions were small (four of 12 [33%]), without evidence of tamponade. Mild or moderate mitral regurgitation was seen in three of 12 patients (25%), and two of 12 patients (17%) had mild or moderate tricuspid regurgitation.
At discharge, echocardiographic abnormalities persisted in six of eight patients (75%), including small saccular aneurysm of the right coronary artery (one of eight [13%]), small pericardial effusion (one of eight [13%]), mild left ventricular dilation with normal LVEF (two of eight [25%]), mild right ventricular dilation and dysfunction (one of eight [13%]), mild mitral regurgitation (two of eight [25%]), and mild tricuspid regurgitation (one of eight [13%]). All patients had normal LVEF at discharge.
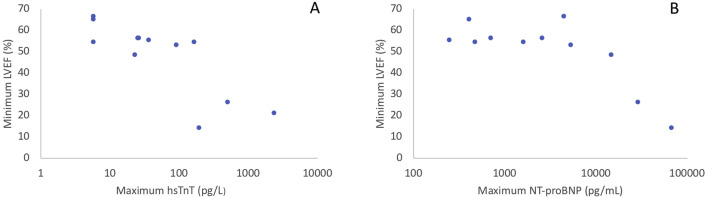
Marked cardiac biomarker elevations were seen, and all patients with LVEFs < 55% had either a peak hsTnT value of >100 pg/L or a peak NT-proBNP value of >1,000 ng/mL (Figure 1 ). Of the children with no abnormalities seen on echocardiography, none had a peak hsTnT > 100 (26.5 pg/mL; range, <6–37 pg/mL) and one of four (25%) had a peak NT-proBNP level > 1,000 (565 pg/mL; range, 248–2,613 pg/mL).
Figure 1.
Association of LVEF with elevations in cardiac biomarkers. (A) The relationship of the minimum LVEF recorded for a single patient against the maximal hsTnT recorded for that same patient. hsTnT is displayed on a logarithmic scale. (B) The relationship of the minimum LVEF recorded for a single patient against the maximal NT-proBNP recorded for that same patient. NT-proBNP is displayed on a logarithmic scale.
Follow-up visits and echocardiograms were available for 10 of 12 patients (83%) (median, 16.5 days from discharge; range, 8–26 days), as one patient transferred care to another institution and the other is awaiting cardiology follow-up. All other patients were asymptomatic at follow-up, with normalization of cardiac biomarkers. All echocardiographic abnormalities resolved, and no new abnormalities were detected.
We describe the evolution of cardiac biomarker elevation and echocardiographic findings in patients with MIS-C. The spectrum of cardiac involvement seen in our cohort is similar to large MIS-C case series published in the United States,5 , 6 with further detail in this report on specific findings during both the acute phase and recovery. In comparing children with less severe and those with more severe echocardiographic involvement, elevated cardiac biomarkers were observed to parallel increased severity of echocardiographic abnormalities. On the basis of our observation, although myocardial injury appears common in MIS-C, significant cardiac biomarker elevation (hsTnT > 100 pg/L and/or NT-proBNP > 5,000 ng/mL) may be more indicative of concerning echocardiographic findings associated with illness severity, including reduced LVEF. Reduced LVEF without significant biomarker elevation was not seen in our cohort, which may play a role in determining triage and disease trajectory in this emerging process. We report the observed findings in our institutional cohort, with a limitation being the need for larger data cohorts with statistical analysis to determine true correlation.
Our experience supports previous reports that children with MIS-C across a spectrum of illness severity appear to recover quickly, with normalization of cardiac function. Further investigation studying the correlation among cardiac biomarkers, clinical outcomes, and echocardiographic data remains urgent to better understand the comprehensive cardiac involvement in patients with MIS-C.
Footnotes
Drs. Valle and Kelly contributed equally to this work.
Conflicts of interest: None.
Wyman W. Lai, MD, MPH, served as guest editor for this report.
References
- 1.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 3.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldstein L.R., Rose E.B., Horwitz S.M., Colling J.P., Newhams M.W., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufort E., Koumans E., Chow E., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. https://link.springer.com/article/10.1007/s00246-020-02391-2 Available at: [DOI] [PMC free article] [PubMed]