Abstract
Background and aims
Obesity has been suggested as a possible risk factor for a more severe course of COVID-19; however, conclusive evidence is lacking and few studies have investigated the role of BMI as a risk factor for admission to intensive care unit (ICU) and mortality.
We retrospectively analyzed a COVID-19 cohort recruited during the first 40 days of the epidemic in Italy. We examined the association between obesity and 30-day mortality, admission to ICU, mortality and length of hospital stay in patients with COVID-19.
Methods and results
Demographic, clinical and outcome data were retrospectively analyzed in 331 patients with COVID-19 admitted to hospital between 21 February and 31 March 2020. The predictive effect of obesity on mortality was assessed using a Cox proportional-hazard regression model, its effect on ICU admission and mortality in the ICU using logistic regressions, and its effect on length of hospital stay using a linear regression.
Seventy-four of 331 patients had a BMI ≥30 kg/m2. Among obese patients, 21 (28.4%) required admission in ICU and 25 died (33.8%). After controlling for sex, age, comorbidities and clinical data, obesity was not significantly associated with mortality, mortality in ICU and length of hospital stay. The effect of obesity on ICU admission remained significant after controlling for sex, age, interstitial lung disease, heart disease and serum C-reactive protein.
Conclusions
Obese patients with COVID-19 were more likely to be admitted to ICU than non-obese patients. However, there were no significant differences in mortality between the two groups.
Keywords: COVID-19, SARS-CoV-2, Obesity, Severe disease, BMI, ICU
Highlights
-
•
Obesity has been suggested as a risk factor for a severe course of COVID-19.
-
•
Obesity was assessed as predictor of mortality and admission to intensive care.
-
•
30-day mortality was not higher in obese patients with COVID-19.
-
•
However, obese patients more frequently required admission to intensive care.
Introduction
The Coronavirus disease 2019 (COVID-19) outbreak, sustained by a novel strain of the Coronavirus family identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has been recently declared a pandemic [1]. The global number of cases has now exceeded 4.5 million with a case-fatality rate of approximately 6.7%. Potential risk factors for severe illness and mortality include advanced age and comorbidities such as diabetes, hypertension, cardiovascular diseases and cancer [2]. Obesity is recently emerging as an additional potential risk factor.
According to the recommended classifications for Body Mass Index (BMI) adopted by the World Health Organization (WHO), obesity is defined as a BMI ≥30 kg/m2 [3]. The prevalence of obesity in the world population has nearly tripled in the last forty years, and obesity is now considered a chronic disease which is often associated with a significant increase in morbidity and mortality [4]. Moreover, a BMI ≥30 kg/m2 has been reported to be an independent risk factor for the development of H1N1 pulmonary infection [5] and for Community Acquired Pneumonia [6]. Notably, it has also been shown to correlate with an increased risk of developing severe MERS [7] and to be responsible for a higher hospitalization rate during seasonal influenza epidemics [8].
Although the pathogenesis of COVID-19 is still unclear, a number of patients with a severe disease showed laboratory evidence of systemic inflammation. This is potentially triggered by a cytokine storm [9] and some immunosuppressive drugs targeting specific molecules seemed to be useful to reverse the disproportionate immune activation found in late stages [10]. Interestingly, it has been demonstrated that in overweight and obese patients there are increased circulating levels of adipokines and inflammatory cytokines such as TNFα, IL-6, or C-Reactive Protein (CRP) [[11], [12], [13]] which determine a chronic low-grade inflammation that impairs the adaptive immune responses to viral infections [14] and favor the development of immune mediated diseases [15]. Lifestyle and diet also have a role in inducing inflammation by directly affecting cellular pathways such as STAT3, IKK, MMP9, MAPK, COX2, and NF-Kβ [16,17]. It is debated whether obesity may induce a kind of preconditioning to inflammatory stimuli, making patients more resistant to Acute Respiratory Distress Syndrome (ARDS) [13]. Few studies have investigated the role of BMI as a risk factor for COVID-19-related mortality and speculations have been made on whether the so called obesity paradox [13,18], according to which obesity is associated with a decreased mortality in patients with ARDS [19], would also hold for COVID-19 patients.
The aim of this study is to examine the correlation between obesity, 30-day mortality and admission to Intensive Care Unit (ICU) in patients with a confirmed diagnosis of COVID-19 who had been admitted to a tertiary referral hospital in Northern Italy.
Methods
Patients
This study is a retrospective analysis of the SMAtteo COvid 19 Registry (SMACORE) cohort. The SMACORE cohort includes patients with confirmed diagnosis of COVID-19 disease referred to our Hospital, Fondazione IRCCS Policlinico San Matteo in Pavia. The SMACORE database includes demographic, clinical (symptoms at admission and comorbidities), laboratory data, treatment and outcome (admission to ICU, death, discharge). In this study, data were extracted from patients hospitalized between 21st February and 31st March 2020. Follow-up included data available until 30th April. The SMACORE study was approved by our Institutional Review Board. Medical records were reviewed and anonymized data abstracted on standardized data collection forms. Demographic data included sex and age. Clinical data included symptoms on admission, CRP, comorbidities (history of cancer, active cancer, heart disease, hypertension, diabetes and obesity) and imaging data (X-ray evidence of interstitial lung disease). Obesity was defined as BMI ≥30 kg/m2. Primary outcomes included 30-day in-hospital mortality rate. Secondary outcomes were ICU admission needing for invasive mechanical ventilation, mortality in the ICU, and hospital length of stay.
RNA assay
SARS COV-2 infection was confirmed by a positive viral RNA from respiratory samples determined by specific real-time reverse transcriptase–polymerase chain reaction (RT-PCR) targeting RNA-dependent RNA polymerase and E genes according to WHO guidelines and Corman et al. protocols [20].
Statistics
Data for continuous variables are presented as means and standard deviation or, in case of non-normality, medians and interquartile ranges (IQR). Data for categorical variables are presented as frequencies and percentages. Comparisons between obese and non-obese patients were performed using chi-square tests for categorical variables, Mann Whitney tests for non-normal continuous data and t-tests for normal continuous data. Incidence, mechanisms and patterns of missing data were then explored. Data were deemed to follow a Missing At Random mechanism and multiple imputation with predictive mean matching (20 datasets, 30 iterations) was employed to account for missing data. This approach was chosen since multiple imputation, contrarily to listwise deletion, reduces bias in parameter estimates and standard errors while maintaining the original relationships among variables [21]. Quality of imputed data was ascertained comparing distributions of imputed datasets with the one with complete data. Multiple imputed datasets were then employed to perform the subsequent regressions. The predictive effect of obesity on mortality was determined using an univariate survival curve analyses and a multivariable Cox proportional-hazard regression model. Results are reported as Hazard Ratios (HR) and 95% Confidence Intervals (CI). The predictive effect of obesity on ICU admission and mortality in the ICU was assessed using multivariable logistic regressions. Results are reported as Odds Ratios (OR) and cutoffs for small, medium and high effects, were 1.68, 3.47, and 6.7 [22]. Finally, to determine the predictive effect of obesity on length of stay we used a multivariable linear regression. In all the multivariable regression models, the effect of obesity was adjusted by including sex, age, presence of interstitial pneumonia at admission, CRP levels and PF ratio at admission, antiviral and antibiotic treatment, and main comorbidities (tumor, heart disease, hypertension, diabetes) as covariates. Respective assumptions of each regression model were checked. Significance threshold was set at α = .05. The analyses were performed using R (version 3.6.0) packages base [23], mice [24] and survival [25].
Results
Four-hundred and twenty-seven patients were included in this analysis.
Demographic features, comorbidities, clinical outcomes are reported in Table 1 . In univariate analyses, obese and non-obese patients were different in terms of CRP >10 mg/l, hypertension, diabetes, length of stay and ICU admission rates, as well as missing data frequencies. The multiple imputation procedure converged and imputed data followed plausible distributions and employed in the following regression models.
Table 1.
Characteristics of 427 consecutive patients with laboratory-confirmed COVID-19.
| Total sample |
Stratified by obesity |
||||
|---|---|---|---|---|---|
| BMI ≥ 30 |
BMI< 30 |
||||
| (n = 427) | Missing % | (n = 80) | (n = 252) | p valuea | |
| Demographic features | |||||
| Female sex | 136 (31.8%) | 0% | 31 (39%) | 72 (29%) | 0.12 |
| Age | 67 ± 21 | 0% | 65 ± 12.3 | 66.7 ± 14.4 | 0.31 |
| Clinical manifestations | |||||
| RF ≥ 22 | 131 (51%) | 40% | 32 (40%) | 86 (34%) | 0.39 |
| Fever (BT ≥ 37,5 °C) | 327 (95%) | 19% | 72 (90%) | 214 (85%) | 0.80 |
| Cough | 167 (48%) | 19% | 35 (44%) | 114 (45%) | 0.62 |
| Dyspnea | 212 (62%) | 20% | 57 (71%) | 142 (56%) | 0.09 |
| Dyarrhea | 28 (8%) | 20% | 8 (10%) | 18 (7%) | 0.64 |
| Fatigue | 44 (13%) | 20% | 12 (15%) | 24 (10%) | 0.30 |
| X-ray imaging | |||||
| Interstitial pneumonia | 283 (83%) | 21% | 67 (84%) | 184 (73%) | 0.08 |
| Laboratory data | |||||
| CRP > 10 mg/dl | 233 (56%) | 2% | 55 (69%) | 141 (56%) | 0.05 |
| PF ratio < 260 | 137 (52%) | 38% | 37 (46%) | 90 (36%) | 0.19 |
| Comorbidities | |||||
| Obesity | 80 (24%) | 22% | |||
| Tumor | 22 (6%) | 20% | 3 (4%) | 15 (6%) | 0.65 |
| Heart disease | 98 (28%) | 19% | 18 (23%) | 72 (29%) | 0.25 |
| Hypertension | 174 (50%) | 19% | 49 (61%) | 107 (42%) | 0.01 |
| Diabetes | 66 (19%) | 19% | 24 (30%) | 36 (14%) | <0.01 |
| Treatment data | |||||
| Antibiotic therapy | 297 (96%) | 28% | 66 (83%) | 197 (78%) | 0.28 |
| Antiviral therapy | 263 (87%) | 29% | 65 (81%) | 164 (65%) | 0.12 |
| Outcome data | |||||
| Length of stay (median (IQR)) | 8 (12.5) | 0% | 12 (14.5) | 9 (12) | 0.04 |
| ICU admission | 92 (22%) | 0% | 26 (33%) | 48 (19%) | 0.02 |
| Mortality in patients admitted in ICU (n = 92) | 39 (9%) | 0% | 10 (12%) | 18 (7.1%) | 0.93 |
| Death | 139 (33%) | 0% | 26 (33%) | 89 (35%) | 0.74 |
Note. Percentages are calculated based on the cases without missing data in the corresponding variables.
Abbreviations: RF = respiratory frequency; BT = body temperature; PF ratio = arterial partial pressure of oxygen/fractional inspired oxygen (fiO2).
Comparison between obese and non-obese patients based on chi square tests for categorical data, Mann Whitney tests for non-normal continuous data, or t-tests for normal continuous data.
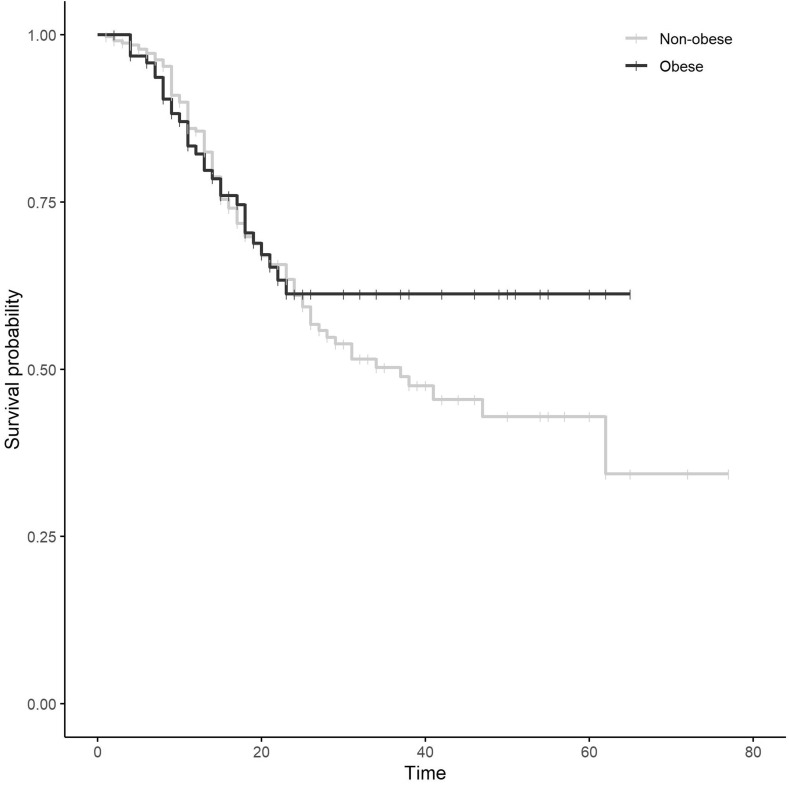
Univariate survival curves showed no significant differences in mortality between obese and non-obese patients (log-rank test p-value = 0.34) (Fig. 1 ). After accounting for demographic, clinical and treatment variables, the multivariable Cox proportional-hazard regression model showed that the effect of obesity was not significant (Table 2 ).
Figure 1.
Univariate survival mortality curves in obese and non-obese patients with COVID-19.
Table 2.
Results of regressions investigating the effects of obesity on mortality, ICU admission, death in ICU, length of hospital stay.
| Mortalitya |
ICU admissionb |
Death in the ICUb |
Length of stayc |
|||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | OR | 95% CI | OR | 95% CI | B | 95% CI | |
| Female sex | 0.73 | 0.44–1.19 | 0.73 | 0.39–1.38 | 0.36 | 0.08–1.68 | 0.68 | −1.96 to 3.32 |
| Age | 1.10 | 1.07–1.12 | 0.99 | 0.97–1.01 | 1.15 | 1.06–1.24 | −0.12 | −0.22 to −0.03 |
| RF ≥ 22 | 1.02 | 0.60–1.73 | 2.18 | 1.02–4.63 | 1.13 | 0.21–5.94 | 3.94 | 0.57 to 7.32 |
| Interstitial pneumonia | 1.41 | 0.70–2.84 | 1.77 | 0.58–5.42 | 0.98 | 0.09–10.72 | 0.76 | −3.18 to 4.71 |
| CRP > 10 mg/dl | 1.24 | 0.81–1.91 | 2.29 | 1.21–4.38 | 1.44 | 0.26–8.02 | 3.51 | 1.03 to 5.99 |
| PF ratio < 260 | 2.55 | 1.52–4.25 | 2.27 | 1.07–4.89 | 2.63 | 0.42–16.44 | 1.25 | −1.77 to 4.28 |
| Tumor | 0.80 | 0.34–1.89 | 0.67 | 0.13–3.53 | 1.16 | 0.06–23.1 | −0.61 | −5.76 to 4.53 |
| Heart disease | 1.11 | 0.71–1.74 | 0.49 | 0.21–1.12 | 2.31 | 0.29–18.54 | −0.23 | −3.54 to 3.07 |
| Hypertension | 0.77 | 0.51–1.17 | 0.75 | 0.39–1.43 | 0.83 | 0.21–3.33 | 1.01 | −1.87 to 3.89 |
| Diabetes | 1.29 | 0.78–2.15 | 0.75 | 0.34–1.68 | 0.73 | 0.12–4.43 | 1.09 | −2.65 to 4.84 |
| Antibiotic therapy | 0.76 | 0.24–2.52 | 0.37 | 0.07–1.93 | 0.45 | 0.01–17.9 | 0.42 | −6.21 to 7.05 |
| Antiviral therapy | 0.52 | 0.27–1.04 | 1.36 | 0.46–4.03 | 1.03 | 0.1–11.03 | 4.67 | 0.38 to 8.97 |
| Obesity | 1.03 | 0.65–1.67 | 1.96 | 1.03–3.75 | 1.65 | 0.38–7.15 | 1.19 | −1.88 to 4.26 |
Notes. The analyses were performed on multiple imputed datasets.
Abbreviations: HR = hazard ratio; OR = odds ratio; RF = respiratory frequency; BT = body temperature; PF ratio = arterial partial pressure of oxygen (PaO2)/fractional inspired oxygen (fiO2).
Cox proportional hazard regression.
Logistic regression.
Linear regression.
In contrast, in the multivariable logistic model performed to assess the effect of obesity on ICU admission, the effect of obesity was significant (Table 2). The size of this effect was small.
Finally, the effect of obesity was not significant on both death in the ICU and length of stay (Table 2).
Discussion
The role of obesity in the development of severe COVID-19 and mortality has not yet been thoroughly addressed, even though existing studies point to a correlation between COVID-19 and some major consequences of obesity, such as arterial hypertension, cardiovascular disease and diabetes [2]. Our study offers some perspective on this matter among those recently conducted in Italy. Although obesity is considered to be a global pandemic, outcome data from obese COVID-19 patients are lacking. For instance, a recent report describing the epidemiology and risk factors in 5700 patients hospitalized for SARS-CoV-2 infection, reported a 41% prevalence of obesity, omitting to report the outcome of this specific subgroup [26].
The main finding of the present paper is that obese patients with COVID-19 are more likely to be admitted to ICU than people with a BMI <30 kg/m2. In contrast, obesity does not influence overall survival, survival in ICU and length of hospital stay. Our data are in agreement with previously reported evidence of obesity being a hitherto unrecognized risk factor for need for critical care [27], more severe disease [28], and use of invasive mechanical ventilation [29]. In line with this, two French retrospective studies found a higher proportion of obese patients admitted to ICU for COVID-19-related ARDS compared with subjects hospitalized for severe acute pulmonary conditions due to other causes [30,31].
Although the pathogenesis of COVID-19 is still unclear, increasing evidence suggests that systemic hyperinflammation, similar to cytokine release syndrome (CRS), might be related to a more severe disease. Since adipose tissue increases the release of pro-inflammatory cytokines, obesity is associated with low-grade chronic inflammation and a systemic acute-phase response with an elevation of acute-phase protein levels such as CRP [32]. However, assuming that CRP values might properly reflect the inflammatory status of our patients, no significant differences were found in CRP values between obese and non-obese patients in this cohort. This finding suggests that additional factors may contribute to the aforementioned COVID-19 severity in obese individuals.
Of note, similarly to observations in pulmonary complications during influenza virus infections, obese patients tend to have a better outcome [19] even though obesity is classified as a risk factor for severe diseases [33]. This phenomenon is known as obesity paradox and might be explained by a potentially protective role of excessive fat accumulation which might result in a more advantageous environment to withstand a significant breakdown in patients' caloric intake frequently occurring in intensive-care settings, such as sepsis or ventilator-induced lung injury [18].
However, it must be conceded that a blind BMI classification has limitations, being unable to discriminate muscular from adipose tissue.
Another possible explanation of the paradox is that clinicians tend to consider patients with obesity at higher risk of worse outcome, resulting in their earlier admission and a more aggressive and timely management [34].
It is controversial whether mild obesity (BMI between 30 and 35) should be considered as a risk factor for higher mortality, as according to some reports, mortality trends of obese patients increased proportionally to grade of obesity [[35], [36], [37]]. On the other hand, a meta-analyses of 239 prospective studies showed a strong association between all-grade obesity and mortality in never-smokers patients without pre-existing diseases [38]. In the present study, since actual BMI values were unavailable, we could not stratify patients according to BMI ranges and perform comparisons between different grades of obesity. This study has also further limitations, including the relatively small sample size and the retrospective design.
In conclusion, the obesity paradox may also apply to the COVID-19 context, despite the difficult management of obese patients in the ICU setting, in terms of ventilation and nursing. Thus, obesity should not be considered a determinant factor in the unsuccessful outcome of intensive care.
Author contribution
SB, MC, SL, ES, TCP, PV, IG, VZ collected, curated and analyzed data, cared for patients and wrote 1st draft; EG and CC ran statistical analysis and contributed to methodology and writing of the manuscript; MUM designed the study, critically discussed the data, acquired funding and wrote the manuscript.
Funding statement
This study was supported by funds of the Italian Ministry of Health (Ricerca Corrente).
Statement
The authors have nothing to disclose.
Handling Editor: A. Siani
References
- 1.WHO director-general's opening remarks at the media briefing on COVID-19. 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Obesity: preventing and managing the global epidemic. World Health Organization: technical report series. WHO Tech Rep Ser No 894 2000:252. ISBN: 92 4 120894 5. [PubMed]
- 4.Obesity and overweight. n.d. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Accessed 30 April 2020].
- 5.Van Kerkhove M.D., Vandemaele K.A.H., Shinde V., Jaramillo-Gutierrez G., Koukounari A., Donnelly C.A. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baik I., Curhan G.C., Rimm E.B., Bendich A., Willett W.C., Fawzi W.W. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 7.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwong J.C., Campitelli M.A., Rosella L.C. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis. 2011;53:413–421. doi: 10.1093/cid/cir442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitulescu G.M., Paunescu H., Moschos S.A., Petrakis D., Nitulescu G., Ion G.N.D. Comprehensive analysis of drugs to treat SARS-CoV-2 infection: mechanistic insights into current COVID-19 therapies (review) Int J Mol Med. 2020;46:467–488. doi: 10.3892/ijmm.2020.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulló M., García-Lorda P., Megias I., Salas-Salvadó J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 12.Festa A., D'Agostino R., Williams K., Karter A.J., Mayer-Davis E.J., Tracy R.P. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes. 2001;25:1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 13.Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D. Obesity – a risk factor for increased COVID-19 prevalence, severity and lethality (review) Mol Med Rep. 2020;22:9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green W.D., Beck M.A. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14:S406–S409. doi: 10.1513/AnnalsATS.201706-447AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budu-Aggrey A., Brumpton B., Tyrrell J., Watkins S., Modalsli E.H., Celis-Morales C. Evidence of a causal relationship between body mass index and psoriasis: a mendelian randomization study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margină D., Ungurianu A., Purdel C., Tsoukalas D., Sarandi E., Thanasoula M. Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. Int J Environ Res Public Health. 2020;17:1–30. doi: 10.3390/ijerph17114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margină D., Ungurianu A., Purdel C., Nițulescu G.M., Tsoukalas D., Sarandi E. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem Toxicol. 2020;143 doi: 10.1016/j.fct.2020.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jose R.J., Manuel A. Does COVID-19 disprove the obesity paradox in ARDS? Obesity. 2020;28:1007. doi: 10.1002/oby.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni Y.N., Luo J., Yu H., Wang Y.W., Hu Y.H., Liu D. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21:36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ginkel J.R., Linting M., Rippe R.C.A., van der Voort A. Rebutting existing misconceptions about multiple imputation as a method for handling missing data. J Pers Assess. 2020;102:297–308. doi: 10.1080/00223891.2018.1530680. [DOI] [PubMed] [Google Scholar]
- 22.Chen H., Cohen P., Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput. 2010;39:860–864. doi: 10.1080/03610911003650383. [DOI] [Google Scholar]
- 23.R Core Team . 2019. R: a language and environment for statistical computing. [Google Scholar]
- 24.van Buuren S., Groothuis-Oudshoorn K. {mice}: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 25.Therneau T.M. 2020. A package for survival analysis in R. [Google Scholar]
- 26.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288:128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 29.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A. Association of obesity with disease severity among patients with COVID-19. Obesity. 2020;28:1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro A.M., Macedo-de la Concha L.E., Pantoja-Meléndez C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev Médica Del Hosp Gen México. 2017;80:101–105. doi: 10.1016/j.hgmx.2016.06.011. [DOI] [Google Scholar]
- 33.Karki S., Muscatello D.J., Banks E., MacIntyre C.R., McIntyre P., Liu B. Association between body mass index and laboratory-confirmed influenza in middle aged and older adults: a prospective cohort study. Int J Obes. 2018;42:1480–1488. doi: 10.1038/s41366-018-0029-x. [DOI] [PubMed] [Google Scholar]
- 34.Schetz M., De Jong A., Deane A.M., Druml W., Hemelaar P., Pelosi P. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45:757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 35.Flegal K.M., Kit B.K., Orpana H., Graubard B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories a systematic review and meta-analysis. J Am Med Assoc. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lainscak M., von Haehling S., Doehner W., Anker S.D. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2012;3:1–4. doi: 10.1007/s13539-012-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carnethon M.R., De Chavez P.J.D., Biggs M.L., Lewis C.E., Pankow J.S., Bertoni A.G. Association of weight status with mortality in adults with incident diabetes. J Am Med Assoc. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Angelantonio E., Bhupathiraju S.N., Wormser D., Gao P., Kaptoge S., de Gonzalez A.B. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]