Abstract
Endothelial dysfunction (EnD) occurs with aging and endothelial nitric oxide (NO) production by NO synthase (NOS) can be impaired. Low NO levels have been linked to increased arginase (Ar) activity as Ar competes with NOS for L-arginine. The inhibition of Ar activity can reverse EnD and (−)-epicatechin (Epi) inhibits myocardial Ar activity. In this study, through in silico modeling we demonstrate that Epi interacts with Ar similarly to its inhibitor Norvaline (Norv). Using in vitro and in vivo models of aging, we examined Epi and Norv-inhibition of Ar activity and its endothelium-protective effects. Bovine coronary artery endothelial cells (BCAEC) were treated with Norv (10 μM), Epi (1 μM) or the combination (Epi + Norv) for 48 h. Ar activity increased in aged BCAEC, with decreased NO generation. Treatment decreased Ar activity to levels seen in young cells. Epi and Epi + Norv decreased nitrosylated Ar levels by ~25% in aged cells with lower oxidative stress (~25%) (dihydroethidium) levels. In aged cells, Epi and Epi + Norv restored the eNOS monomer/dimer ratio, protein expression levels and NO production to those of young cells. Furthermore, using 18 month old rats 15 days of treatment with either Epi (1 mg/kg), Norv (10 mg/kg) or combo, decreased hypertension and improved aorta vasorelaxation to acetylcholine, blood NO levels and tetra/dihydribiopterin ratios in cultured rat aortic endothelial cells. In conclusion, results provide evidence that inhibiting Ar with Epi reverses aged-related loss of eNOS function and improves vascular function through the modulation of Ar and eNOS protein levels and activity.
Keywords: Endothelial dysfunction, Aging, Epicatechin, Norvaline, Arginase, Nitric oxide synthase
1. Introduction
Cardiovascular diseases (CVD) are the leading cause of morbidity and mortality worldwide (Benjamin et al., 2019). Aging is one the most important predictors of CVD as major age-related changes occur in the cardiovascular system (Benjamin et al., 2019; Zhou et al., 2018). CVD in aging is associated to endothelial dysfunction (EnD) (O'Rourke and Nichols, 2005; Santhanam et al., 2008; Ungvari et al., 2019) which may be partly reversed through increased bioavailability of nitric oxide (NO). EnD is also a hallmark of diseases such as atherosclerosis, hypertension and diabetes and may be an important pathophysiological element of COVID-19 disease (Chen and Li, 2020; Guzik et al., 2020; Whyte et al., 2020). Preclinical and clinical evidence shows that vasodilation is importantly mediated by endothelium-dependent NO (Crecelius et al., 2011; Diehl et al., 2011; Donato et al., 2011; Manicam et al., 2017). NO is produced by endothelial NO synthase (eNOS), through oxidation of its substrate L-arginine, under the action of receptor agonists or stimulated mechanoreceptors (Iring et al., 2019; Lu and Kassab, 2015). Studies have demonstrated that aging associated oxidative stress (OS) induces abnormal elevation of arginase (Ar) activity (Berkowitz et al., 2003; Sakai et al., 2004), contributes to eNOS uncoupling, impairs expression and/or eNOS activity (Höhn et al., 2017; Passos et al., 2010) therefore promoting EnD (Jae et al., 2009; Shin et al., 2012). Tetrahydrobiopterin (BH4), an essential cofactor for eNOS dimerization, protein stability, and NO synthesis (Alp and Channon, 2004) decreases in the presence of high OS levels, inducing NOS-uncoupling which triggers the switching from NO to superoxide (O2*-) production dropping the intracellular BH4 (Gantzer, 2018). Arginase (isoforms Ar I and Ar II) compete with NOS for their common substrate L-arginine. Ar hydrolyzes L-arginine regulating NOS by substrate depletion (Berkowitz et al., 2003). In addition, there is evidence of increased Ar activity due to OS-induced S-nitrosylation (Ming et al., 2009; Ortiz-vilchis et al., 2018; Pernow and Jung, 2013).
The inhibition of Ar has demonstrated to induce higher L-arginine availability and improved eNOS coupling, leading to increased NO and decreased O2 production (Huynh et al., 2009; Yoon et al., 2014). L-norvaline (Norv), a natural Ar inhibitor increases the endogenous stock of L-arginine, improving NO production, therefore promoting a healthier endothelium (Ming et al., 2009; Pokrovskiy et al., 2011). Furthermore, compounds that increase NO availability (i.e. NO donors, Ar I and Ar II inhibitors) have been shown to protect against EnD (Berkowitz et al., 2003) and ischemia/reperfusion (I/R) injury (Ortiz-vilchis et al., 2018). Flavonoids, particularly (−)-epicatechin (Epi), the most abundant flavanol in cacao, has been reported to inhibit Ar activity in rat kidney, human erythrocyte and in cultured endothelial cells thus, increasing NO production (Schnorr et al., 2008). In the setting of myocardial ischemia/reperfusion injury, we have demonstrated that Epi decreases Ar activity, maintaining eNOS function and reducing infarct size (Ortiz-vilchis et al., 2018). We have also reported on the capacity of Epi to stimulate the production of NO and eNOS protein activation in aged endothelial cells (Ramirez-Sanchez et al., 2010). However, no studies have examined the effects of Epi on Ar activity in aged endothelium and determined its ability to reverse EnD. The purpose of the present study was to evaluate Epi-inhibition of Ar activity, to document its endothelium-protective effects in aged cultured cells and rat aortas and to compare its effects with those evoked from the well known Ar inhibitor Norvaline (Norv).
2. Materials and methods
2.1. Reagents
Primary bovine coronary artery endothelial cells (BCAEC) were obtained from Cell Applications, Inc. Dulbecco's modified Eagle's medium, low glucose cell culture medium, trypsin, antibiotic/antimycotic solution, fetal bovine serum (FBS), and Hank's balanced salt solution (phenol red free) and L-glutamine were from HyClone. Nonessential amino acid solution was from Mediatech Cellgro Inc. Phenol red free medium and low glucose cell culture medium were from Life Technologies, Inc. Bovine serum albumin, EDTA, HEPES, Tween-20, protease and phosphatase inhibitor cocktails, NG-nitro-L-arginine methyl ester hydrochloride (L-NAME), urea, α-isonitrosopropiophenone brain bovine endothelial mitogen, Norv and Epi were from Sigma Aldrich, Inc. Polyvinylidene difluoride (PVDF) transfer membranes were from Millipore Inc. Bradford assay reagent, TGX pre-cast acrylamide gels and free-β-mercaptoethanol Laemli buffer were from Bio-Rad, Inc. An Enhanced Chemiluminescence Plus Western blot detection kit from Amersham. The Nitrate/Nitrite fluorometric assay kit was from Cayman Chemicals Inc. Primary antibodies against phospho-eNOS at Ser-1179, eNOS, GAPDH, normal rabbit IgG control and anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies were from Cell Signaling, Inc. Anti nitrotyrosine Ar 1 and 2 antibodies and protein A/G agarose were from Santa Cruz Biotechnology, Inc. GCH1 antibody and Dihydroethidium (DHE) assay kit were from Abcam. Tetra and Dihydrobiopterine ELISA kits were from Novus Biologicals Inc.
2.2. In silico studies
Docking analysis of interactions between Ar, Norv and Epi was pursued as outlined below. The three-dimensional structure of Ar isoform 1 (pdb code 2AEB) was obtained from www.rcsb.org. The Discovery Studio Visualizer was used to add the Charmm force field. Polar hydrogen atoms were added, followed by Gasteiger charge calculation using Autodock tools (ADT) 1.5.4. The dimensional structures were downloaded from Chem Spider (www.chemspider.com) and saved in protein data bank (pdb) format using Discovery Studio. Polar hydrogen atoms were added, the number of torsions was set and Gasteiger charges were assigned using Autodock tools (ADT) 1.5.4. Docking analysis was performed in AutoDock Vina. A blind docking method was used with the coordinate of origin set at x = 11.946, y = 20.979 and z = 0.033, at the centre of the protein. The box size was set at x = 70, y = 70 and z = 70. Docking simulations to analyze binding affinities and binding sites were run with the number of modes set to 8. The Discovery Studio was used to produce two-dimensional docking representations of the interactions. To evaluate changes in Norv or Epi's free energy (ΔG, kcal/mol), as well as amino acid interactions with Epi, a pdbqt file was created using PyMolwin software. The docking was performed in AutoDock Vina, with the coordinate of origin at x = 2.017, y = − 7.457 and z = − 0.116. The box size was set at x = 70, y = 70 and z = 70 (Ortiz-vilchis et al., 2018).
2.3. Cell culture
BCAEC were grown in a complete medium supplemented with 10% FBS, 1% antibiotic/antimycotic solution, and 1% nonessential amino acids. Cells were maintained under a humidified atmosphere at 37°C with 5% CO2 and 95% O2. As per our previous publication (Ramirez-Sanchez et al., 2018), passages 8–13 were used as a model for young endothelial cells (Y) while passages 31–35 were used as a model for aged endothelial cells (A). Cells were used for experiments at 75% confluence.
2.4. Cell treatment
For all experiments 24h before treatment, growth medium was replaced with 1% serum medium, phenol red free, 1% antibiotic/antimycotic solution and 1% nonessential amino acids (starving medium). Treatment was provided as follows: vehicle applied to the cells in the control group (C), Norv (N) 10 μM, Epi (E) 1 μM, or both Epi + Norv (E + N) for 48h. Fresh starving medium and compounds were reapplied every 24 h.
2.5. Total protein extraction
Cells were washed three times with cold buffer (4 ml per plate) and lysed in 80 μl of ice cold lysis buffer (RIPA ThermoFisher Scientific) with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Homogenates were sonicated for 15 min at 4°C, and centrifuged at 13 000 g for 15 min to remove cell debris. The total protein concentration was measured in the supernatant using the Bradford micro method (Bio-Rad) at 595 nm D.O. using a BioQuant 800 spectrophotometer (BioTek Inc.).
2.6. Arginase activity measurements
After treatment, cells were homogenized in 100 μl of solution A (sucrose 2 M, EDTA 0.01 M, HEPES 0.5 M; pH 7.4) and samples centrifuged for 10 min (12,000 g) at 4 °C. The supernatant was collected and protein concentration on it was determined using the Bradford method. To evaluate Ar activity, 100 μg of protein were added to 25 mM Tris-HCl pH 7.4 and 5 mM MnCl2 (100 μl final volume) and activated at 55 °C for 10 min. The enzymatic reaction was started by the addition of 100 μl of 0.5 M L-arginine as substrate (pH 9.7). The mixture reaction was incubated at 37 °C for 60 min and stopped by the addition of 200 μl of acidic mixture containing H2SO4, H3PO4 and H2O (1:3:7) followed by the addition of 25 μl of 9% α isonitrosopropiophenone then heated at 100 °C for 45 min. The urea concentration was quantified by measuring O.D. at 540 nm in a BioQuant spectrophotometer (Biotek, Inc). Ar activity levels were calculated by extrapolating the data onto urea standard curve (Ortiz-vilchis et al., 2018).
2.7. NO measurements
After 48h of treatment with compounds, 100 μl of medium was collected to test NO levels using a fluorescent kit according to the manufacturer's instructions (Ramirez-Sanchez et al., 2018). Briefly, as the final products of NO in vivo are nitrite (NO2 −) and nitrate (NO3 −), the best estimation of total NO production is the sum of both NO2 − and NO3 −. We used a nitrate/nitrite fluorometric assay as an accurate method for the measurement of the total nitrate/nitrite concentration. In the assay, the conversion of nitrate to nitrite utilizes nitrate reductase followed by the addition of an acidic solution of diaminonaphthalene (DAN) and NaOH, which enhances the detection of the fluorescent product, 1(H)-naphthotriazole. Measurement of the fluorescence generated was quantified using a fluorometer (FLx800, Bio-Tek Instruments). Nitrate/nitrite values were normalized against the total protein after the cells were scrapped from the plate and lysed as described previously. Plasma (50 μl) was collected from treated and control animals and NO levels were measured as above.
2.8. Dihydroethidium (DHE) measurement
DHE was evaluated as a surrogate of reactive oxygen species (ROS) production in live BCAEC. DHE was used as a fluorescent probe for the detection of superoxide and hydrogen peroxide. Antimycin A, an inhibitor of complex III of the mitochondrial electron transport chain, was included as a positive control for ROS generation. N-acetyl Cysteine was included as an anti-oxidant control. Breifly, cells were seeded in a 96 well plate at a density of 50 cells/well. After 48 h treatment with compounds cells were washed with PBS and added 150 μl of Cell-Based Assay Buffer and incubated for 10 min at room temp. Cell-Based Assay Buffer, was removed and 20 μl of fresh assay buffer and 130 μl of ROS Staining Buffer per well were added. Then 10 μl of N-acetyl cysteine assay reagent were added to designated negative control wells and incubated for 30 min at 37°C protected from light. Following, we added 10 μl of the Antimycin A reagent to positive control wells and incubated for 1 h at 37°C protected from light. Carefully we aspirated ROS Staining Buffer and added 100 μl of Cell-Based Assay Buffer. Plate was placed on fluorometer (FLx800, Bio-Tek Instruments) plate reader and fluorescence was measured at an excitation wavelength between 480 and 520 nm and an emission wavelength between 570 and 600 nm. ROS generation data was represented as total DHE fluorescence units.
2.9. Tetra and dihydrobiopterine (BH4, BH2) measurements
BH4 and BH2 were measured separately using competitive ELISA kits from Novus Biologicals. The micro ELISA plate provided in the kit is pre-coated with either BH4 or BH2. Breifly, cells were washed with cool PBS and dissociated with trypsin. Cell suspension was collected and centrifuged for 5 min at 1000 g. Cell pellet was washed 3 times with cool PBS and finally resuspended with 250 μl of cool PBS. Cell suspension was sonicate 15 min at 4°C and centrifuged for 10 min at 1500 g at 4°C. The supernatant was collected to carry out the assay according to manufacturer instructions. During the reaction, BH4 or BH2 in the sample or standards competes with a fixed amount of BH4/BH2 on the solid phase of the plate for sites on the Biotinylated Detection Ab specific to BH4/BH2. Excess of conjugate and unbound sample or standard were washed from the plate, and Avidin conjugated to Horseradish Peroxidase (HRP) was added to each microplate well and incubated 30 min at 37°C. Then a substrate solution was added to each well and incubated 15 min in the dark. The enzyme-substrate reaction was terminated by the addition of stop solution and the color change was measured in a BioQuant spectrophotometer (Biotek, Inc) at a wavelength of 450 nm. The concentration of BH4/BH2 in the samples was then determined by extrapolating the O.D. of the samples to the standard curve values.
2.10. Western blots
In order to examine protein levels of Ar I and II, and nitrosylated form, eNOS, phospho (p) eNOS, and related proteins (i.e. GTP cyclohydrolase 1 [GCH1]), immunoblotting was performed. Cells were treated as described above. After treatment, cells were lysed and total protein was extracted. A total of 30 μg of protein was loaded onto a 4–15% gel, electrotransferred, and incubated for 1 h followed by either 1–3 h incubation at room temperature or overnight incubation at 4°C with primary antibodies. Primary antibodies were typically diluted 1:1000 or 1:2000 in buffer plus 5% bovine serum albumin or 2% non-fat milk. Membranes were washed (3× for 5 min) in buffer and incubated 1h at room temperature in the presence of conjugated secondary antibodies diluted 1:5000 in a blocking solution. Membranes were again washed three times in buffer and immunoblots were developed. Band intensities were digitally quantified and normalized against GAPDH.
2.11. Immunoprecipitation (IP)
After treatment, endothelial cells were lysed with 200 μL of nondenaturing extraction buffer (0.5%, Triton X-100, 50 mmoL/l Tris–HCl, pH: 7.4, 0.15 moL/l NaCl, 0.5 mmoL/l EDTA) and supplemented with protease and phosphatase inhibitor cocktails plus 2 mmoL/l Na3VO4, and 1 mmoL/l NaF. Homogenates were passed through an insulin syringe 3× and incubated on ice with shaking for 25 min and centrifuged at 12,000 g for 15 min at 4°C. A total of 0.5 mg protein was precleared by adding 1 mg of normal rabbit IgG control and 20 μl protein A/G-agarose and mixed for 30 min (4°C) with subsequent centrifugation at 12,000 g for 10 min at 4 °C. The supernatant was recovered and incubated at 4°C under mild agitation for 3 h with 10 μl of IP antibody (rabbit nitrotyrosine). Twenty microliters of protein A/G-agarose was added, and the mixture was incubated overnight at 4°C with shaking. The IP mixture was centrifuged at 12,000 g for 15 min at 4°C, and the supernatant was recovered and stored at 4°C for later analysis. The pellet was washed 3× with extraction buffer under shaking 15 min and centrifuged at 12,000 g for 15 min at 4°C. The IP proteins in the pellet and those remaining in the supernatant were applied to a precast 4–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) for Westerns using Ar I and II antibodies.
2.12. eNOS monomer and dimer Western blotting
Low-temperature SDS-PAGE was performed for detection of the eNOS monomer and dimer (Benson et al., 2013). Briefly, cells lysates were prepared as described above. Protein lysates were resolved using a 7.5% Tris-glycine gel (Bio-Rad) under reducing conditions. All gels and buffers were at 4°C before electrophoresis, and the buffer tank was placed in an ice bath during electrophoresis to maintain the gel temperature below 15°C. Western blots were developed as described above with rabbit anti-eNOS polyclonal antibody.
2.13. Animals
Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee from the National Institute Polytechnic (ESM-CICUAL-01/10-07-2014), and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (National Research Council, 2011, grants. nih. gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf), Mexican federal regulations for animal experimentation and care and the good practices of the Comité Interno para el Cuidado y Uso de los Animales de Laboratorio with regards to research using animals. Male aged Wistar rats (18 months, n = 6/group) were used for in vivo and ex vivo studies. Rats were randomly allocated into 4 groups: (1) Control (vehicle water), (2) Norv (10 mg/kg/day), (3) Epi (1 mg/kg/day) and (4) Epi + Norv and provided with the compounds by oral gavage for 15 days. Dosing of animals was selected based on previous reports by us and others (De et al., 2016; Ramirez-Sanchez et al., 2018). Animals were maintained at room temperature (20–25°C) on a 12 h light/dark cycle, with food and water provided ad libitum.
2.14. Blood pressure
A non-invasive method to measure systolic blood pressure was employed by using a rat tail cuff pressure transducer with values digitally recorded.
2.15. Vascular reactivity
Under anesthesia (pentobarbital 60 mg/kg) animals were decapitated and the thoracic aorta from the diaphragm to the aortic arch isolated. Aortas were immediately submerged in cold Krebs solution to remove all adjacent connective tissue, then cut into ring segments (4–5 mm long), which were then mounted on two stainless steel hooks within an isolated organ chamber. One of the hooks was fixed to the bottom of the chamber and the other to a transducer linked to a Biopac System apparatus for registering changes in tension (force). The isolated organ chamber contained 10 ml of Krebs bicarbonate solution. The chamber was maintained at a constant temperature of 37 °C, pH of 7.4 and a continuous bubbling with a mixture of 95% O2 and 5% CO2. Aorta rings were pre-contracted using phenylephrine [1 × 10−6], and standard concentration–response curves to acetylcholine [1 × 10−7 – 1 × 10−4 M] were constructed to analyze the effect of 15 days of Norv, Epi or both E + N treatment on the vascular reactivity of the pre-contracted aortas.
2.16. Aortic endothelial cell culture
Aged rat aorta endothelial cells were isolated and cultured in order to evaluate arginase activity, BH2 and BH4 assays. Briefly, a large section of thoracic aorta was collected from 6 aged rats/group. Lumen of the vessels was exposed, gently washed with Hank's solution and incubated with 0.05% trypsin for 10 min at 37 °C in a petri dish; the total volume of the trypsin solution was recovered, and a 0.1 vol FBS was added. Cells were harvested and centrifuged at 1200×g/10 min and the pellet was resuspended in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 1% antibiotic-antimycotic mixture, 1% non-essential amino acids and 5 mg/ml brain bovine endothelial mitogen (Ramirez-Sanchez et al., 2010). Endothelial cells were incubated at 37°C and 5% CO2, and four days later used for Ar activity, BH2 and BH4 assays.
2.17. Data analysis
For cell culture experiments, at least 5 independent experiments each in triplicate were performed. For animal studies, 6 rats per group were used. Results are expressed as a mean ± standard error of the mean (S.E.M.). Data analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test using GraphPad Prism (GraphPad Software Inc., San Diego, USA). Data were considered significant when P values were <0.05.
3. Results
3.1. In silico studies
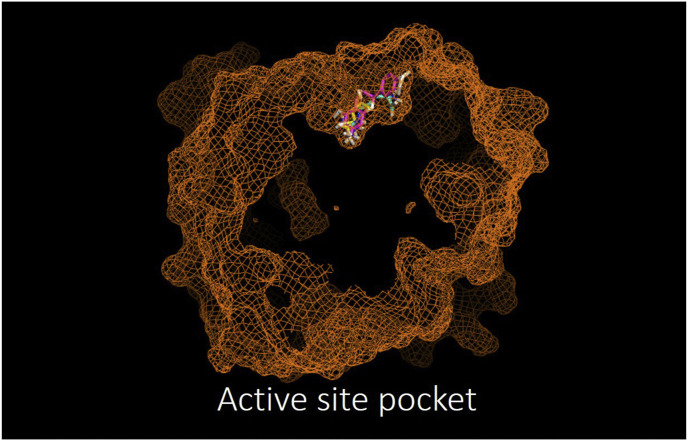
The docking analysis of Norv and Epi interaction with Arg 1 documents interactions which occur at different sites. L-Norv interacts in its active pocket with an affinity of −4.9 kcal/mol and its binding is provided by conventional hydrogen bonds principally (complex 1). Epi was able to interact with the allosteric site with an affinity of −8.1 kcal/mol by van der Waals interactions predominantly (complex 2). (Fig. 1 and Table 1 ). The analysis of Epi binding possibilities when Norv is present (complex 1) shows changes in binding affinity (ΔG = −7.4) while Norv binding analysis when Epi is present (complex 2) shows no changes in binding affinity (ΔG = − 4.4). Additionally, these interactions lead to changes in binding amino acids suggesting conformational changes in Ar.
Fig. 1.
In silico docking analysis of the arginase active site shared by its natural agonist arginine (blue), the antagonist Norvaline (yellow) and (−)-Epicatechin (pink) (as per PyMolwin and AutoDock Vina software). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Gibbs Free Energy comparison between complexes. Data are shown as the mode of a statistical sample (n = 10) of the total number of dockings performed (n = 30).
| ΔG | Conventional Hydrogen Bonds | Pi-Alkyl | Pi-Cation Pi-Anion | Carbon Hydrogen Bond | Pi-Sigma Pi-Pi | vdW | |
|---|---|---|---|---|---|---|---|
| Norvaline-Arginase (Complex 1) | -4.9 | Glu 277 Asp 234 Asp232 Asp128 His141 | His141 His126 | Gly 142 Thr246 His 101 | |||
| Epi-Complex 1 | -7.4 | Phe147 Lys 153 Pro157 | Val 165 Pro 167 | Asp158 | Phe162 | Ile156 | Ser 146 Val 159 Ser163 |
| Epi-Arginase (Complex 2) | -8.1 | Ser 137 His141 Asp232 | His141 Asp 183 | His126 | Asp128 Asn 130 Thr135 Asn 139 Thr246 | ||
| Norvaline-Complex 2 | -4.4 | Asp158 | Ser163 | Phe147 Ile156 Pro157 Phe162 |
3.2. Arginase protein levels and activity
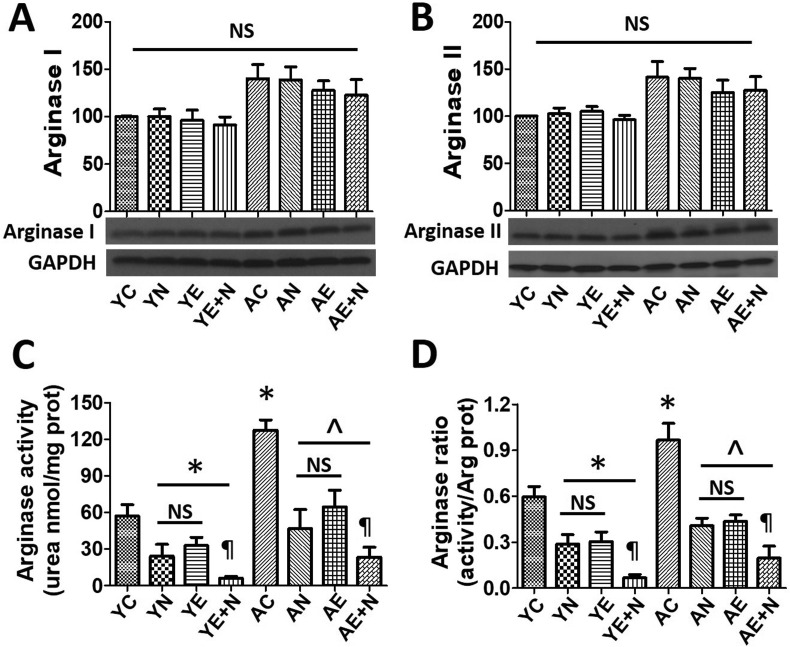
Ar I and Ar II expression and activity were measured in young (Y) and aged (A) BCAEC treated with vehicle (C), Norv (N), Epi (E) or a combination of Norv plus Epi (E + N) (Fig. 2 ).
Fig. 2.
Effects of Norvaline (N) (−)-Epicatechin (E) and N + E on arginase (Ar) I and II protein and activity levels in young (Y) and aged (A) endothelial cells. Panels A and B illustrate representative Western blots images and relative changes in Ar I and Ar II protein levels. Values of Y control (C) set as 100%. Protein levels were normalized using GAPDH values. Panel C depicts Ar activity levels whereas panel D shows Arg activity/protein ratio values. *P < 0.05 vs. YC, ^P < 0.05 vs. AC, ¶P < 0.05 vs. AN or AE.
Ar I (Fig. 2A) and Ar II (Fig. 2B) protein levels did not change in cells treated with Norv, Epi or both vs YC or AC. Ar activity, as urea content, was decreased in YN, YE and YE + N (P < 0.05). Urea content also was increased (~110%) in AC vs. YC (P < 0.05), while AN, AE and AE + N were able to decrease Ar activity almost to YC levels (P < 0.05) (Fig. 2C). Ar activity/Ar protein levels (ratio) was also decreased in Y or A cells treated with Norv, Epi or both compounds (P < 0.05) (Fig. 2D).
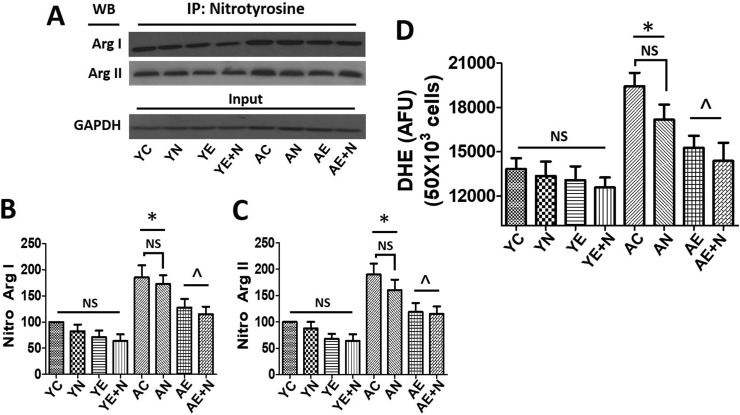
3.3. Ar nitrosylation and oxidative stress
Nitrosylation of Ar I and Ar II as an indicator of enzyme oxidation/activation was measured in young and aged BCAEC treated with Norv, Epi and E + N. In both enzyme isoforms, nitrosylation was increased by ~100% in AC vs YC (P < 0.05) (Fig. 3 A–C). Interestingly, AE and A E + N decreased nitrosylated Ar I and Ar II levels more than 50% vs AC (P < 0.05). Similarly, DHE as a surrogate of ROS production, was significantly increased in AC (~40%) vs YC (P < 0.05) (Fig. 3D). Treatment with Epi and E + N significantly decreased DHE levels in ~25% vs AC (P < 0.05) almost as a YC levels.
Fig. 3.
Effects of Norvaline (N), (−)-Epicatechin (E), and E + N on nitrosylation of arginase (Ar) I and Ar II and oxidative stress levels and in young (Y) and aged (A) endothelial cells. A, Immunoprecipitation (IP) of nitrotyrosine to evaluate Ar I and II nitrosylation by Western blots (WB). Protein levels were normalized using input GAPDH values. B and C, relative nitrosylated (Nitro) Ar I and Ar II levels as compared vs basal conditions (control [C]). Values of YC were set as 100%. D, Reactive oxygen species production levels measured in Y and A endothelial cells using dihydroetidium (DHE) fluorescence levels as surrogate. Data was expressed as arbitrary flourence units (AFU), *P < 0.05 vs. YC, ^P < 0.05 vs. AC.
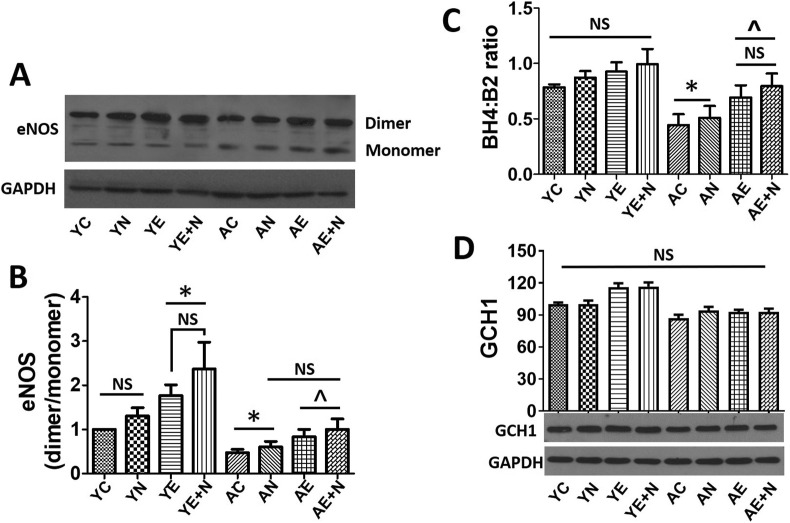
3.4. eNOS dimer/monomer, BH4/BH2 ratio and GCH1 protein levels
To address eNOS proper coupling, dimerization levels were measured (Fig. 4 A and B). Of note is that YE and YE + N groups increased dimerization (~80% and ~120% respectively) vs YC (P < 0.05). AC decreased dimerization of eNOS, while AE and AE + N demonstrated increased dimerization by ~40 and ~50% respectively (P < 0.05). Results demonstrated that Epi and Epi + Norv treatments of aged cells can restore eNOS dimerization levels similar to YC. Additionally, the BH4/BH2 ratio does not shown differences between Y groups (Fig. 4C). However, in AC BH4:BH2 ratio significantly decreased (~40%) vs YC (P < 0.05) (Fig. 4C), and were restored in AE and AE + N almost as YC levels. Norv, per se, was unable to produce any effects on cells. GCH1, the protein related to BH4 biosynthesis, levels did not show any changes between either Y or A groups (Fig. 4D).
Fig. 4.
Effects of Norvaline (N), (−)-Epicatechin (E), and E + N on endothelial nitric oxide synthase (eNOS) protein estability in young (Y) and aged (A) endothelial cells. A, non-denaturing Western blot for the analysis of dimer/monomer eNOS. GAPDH was used to normalize levels and were compared vs. control (C) conditions. B, graphic representation of eNOS dimerization. Values of YC were set as 1. C, tetrahydrobiopterin (BH4): dihydrobiopterin (BH2) ratio was determined. D, illustrates representative Western blots images and relative changes of GCH1 protein levels normalized using GAPDH values. *P < 0.05 vs. YC, ^P < 0.05 vs. AC.
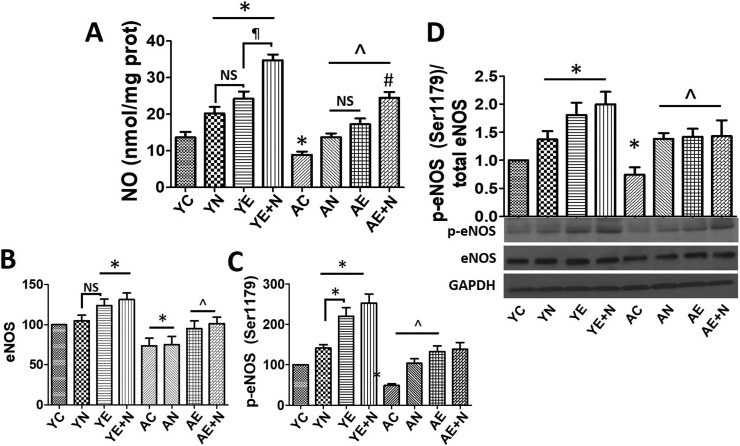
3.5. NO production and eNOS phosphorylation
NO production and eNOS activation/phosphorylation was measured in Y and A cells treated with vehicle, Norv, Epi and E + N (Fig. 5 ). NO levels were increased in YN, YE and Y E + N treatments vs YC (P < 0.05), while AC decreased NO production by 50% vs YC (P < 0.05). NO levels in AN, AE and AE + N were elevated as compared vs. AC (P < 0.05) (Fig. 5A). eNOS protein levels increased in YE and Y E + N (~20% and ~25% respectively) vs. YC (P < 0.05), while AC decreased protein levels by ~25% vs YC (P < 0.05). AE and AE + N groups were able to recover eNOS protein levels as YC (Fig. 5B). eNOS activation (phosphorylation at Serine 1179), was significantly increased in YN (~40%), YE (~120%), YE + N (~155%) as compared vs YC, (P < 0.05) while AC eNOS phosphorylation was decreased by ~55% and increased in AN (~100%), AE (~175%) and AE + N (~185%) (P < 0.05) (Fig. 5B and C). Fig. 5D reports on the phospho eNOS/total eNOS ratio supporting the results in Fig. 5B and C.
Fig. 5.
Effects of Norvaline (N), (−)-Epicatechin (E), and E + N on nitric oxide (NO) production and endothelial nitric oxide synthase (eNOS) phosphorylation in young (Y) and aged (A) endothelial cells. A, nitric oxide, B, eNOS total protein and C, phosphorylation at Serine 1179 levels compared vs Y control (C). D, representative Western blots images for p-eNOS levels (normalized to total eNOS) and total eNOS protein levels were normalized using GAPDH and compared to basal (C) conditions in Y and A endothelial cells. *P < 0.05 vs. YC, ^P < 0.05 vs. AC, ¶P < 0.05 vs. YE + N.
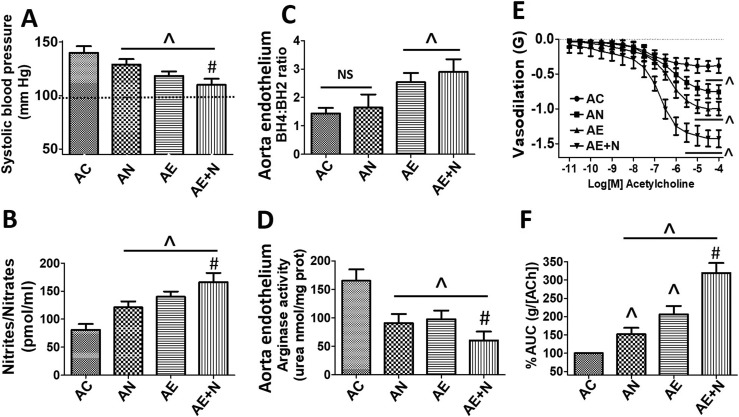
3.6. Blood pressure and NO measurements in aged rats
AC rats demonstrated high blood pressure. However, in AN, AE and AE + N groups blood pressure decreased by ~20%, 30% and 40% respectively (P < 0.05) (Fig. 6 A). NO (Nitrite/Nitrates) production was significantly decreased in AC rats and increased in AN, AE and AE + N groups between 25 and 70% (P < 0.05) (Fig. 6B).
Fig. 6.
In vivo and in vitro analysis of aged rats. A, systolic blood pressure (mmHg) measured in aged (A) controls (C), A Norvaline (N), A (−)-Epicatechin (E) and AE + N, base line = 100 mm Hg represents averaged measures from young rats. B, nitrites/nitrates concentration in plasma of aged rats. Panel C, BH4:BH2 measurements and D depicts Ar activity levels in aortic cultured endothellium. E, show vasodilation measures (force, grams [G]) in aortic rings from aged rats. F, represents area under the curve (AUC in percentage %) from the vasodilation plots. N = 6/group, ^P < 0.05 vs. AC, #P < 0.05 vs. AE.
3.7. BH4, BH2 and Ar activity measurements
BH4, BH2 and Ar activity levels were evaluated in cultured aortic endothelium from aged rats. BH4:BH2 ratio was significantly decreased in AC, while increased in AE and (AE + N ~75%–100%) (P < 0.05) (Fig. 6C). Endothelial cells from AC animals demonstrated increased Ar activity and a significant decrease was observed in AN, AE (~45%) and E + N (~75%) treatments (P < 0.05) (Fig. 6D).
3.8. Vascular reactivity
In aortic rings isolated from AC animals, a vasodilatory effect of acetylcholine was documented at concentrations ranging from 10−7 to 10−4 mol L−1. The calculated ED50 in AC animals was (6.5 × 10−7 mol L−1). An enhanced vasodilatory response was triggered in the AN (ED50 1.9 × 10−7 mol L−1), AE (ED50 2.1 × 10−8 mol L−1) and AE + N (ED50 5.1 × 10−9 mol L−1) (P < 0.05) treated groups; suggesting an increased vasodilatory effect in the aortic rings, reaching a maximal level of relaxation of ~0.4 g in AC vs. ~ 0.75 g in AN group, ~1.0 g in AE group and ~1.45 g in the aortas from AE + N rats (P < 0.05). Results of vascular reactivity measurements analyzed by area under the curve (AUC) calculations demonstrated that AN, AE and AE + N treated groups induced significantly more vasodilation of aortas (~55%, 100% and 220% respectively) vs. AC aortas (P < 0.05) (Fig. 6E and F).
4. Discussion
Unique results from this study demonstrate that Epi has the capacity to bind Ar and inhibit its activity. In aged endothelium, Ar activity is increased, yielding a reduction in NO bioavailability which, is associated with decreased eNOS phosphorylation at activation sites (Ser1179) and higher levels of protein uncoupling (<dimer/monomer ratio). Results document that Epi effectively reverses in vitro and in vivo aging associated EnD which is characterized by increased ROS and Ar activity.
There is greater recognition for the need to identify safe and effective compounds that can be consumed over indefinite periods of time to mitigate EnD and thus, effectively reduce CVD incidence. Such compounds may also serve to mitigate risk factor and/or complications that arise from COVID-19 infection. Inhibiting Ar activity can be a potential therapeutic approach for prevention/treatment of multiple age-related diseases. Published studies have demonstrated that Ar inhibition improves endothelial function reducing cardiovascular risk in diseases such as hypertension and atherosclerosis (El-bassossy et al., 2013; Ming et al., 2004). It has also been shown that blockers of Ar activity decreased the progression of Alzheimer's disease and improve cognition and memory in animal models (Polis et al., 2018). In this study, we compared the effects of Norv a potent Ar inhibitor with well documented biological properties (Gilinsky et al., 2020) and whose consumption is promoted in certain sports supplements. However, Norv consumption has been linked to toxic effects as per in vitro studies. In mammalian cells, Norv decreased cell viability at concentrations as low as 125 μM, causing necrotic cell death and significant changes in mitochondrial morphology (Samardzic and Rodgers, 2019). Thus, Epi emerges as a safe, well tolerated and low cost Ar inhibitor that at the same time can directly stimulate eNOS activity and therefore, be considered as an attractive candidate for the prevention and treatment of age-related EnD.
Our in silico results demonstrate that Epi can strongly bind (−8.1 kcal/mol) an allosteric site whereas Norv binds an orthsoteric site (−4.9 kcal/mol) suggesting a much better affinity for Epi. Indeed, in vitro results confirm that a lower dose of Epi (1 μM) inhibits Ar as efficiently as Norv (10 μM) suggesting a more potent effect.
Polyphenols have shown to improve EnD (Frombaum et al., 2012; Schmitt and Dirsch, 2009; Suganya et al., 2016). In an in vitro study, procyanidin oligomers of cocoa decreased Ar activity thereby improving endothelial function (André et al., 2011). Epi has also been reported to inhibit Ar activity increasing the availability of L-arginine, leading to increased eNOS activity and decreasing the generation of reactive oxygen/nitrogen species formed by uncoupled eNOS (dos Reis et al., 2013; Loke et al., 2008). We previously reported on the deleterious effects of aging on endothelium that is accompanied by a loss of mitochondrial function, decreased NO production and, demonstrated that Epi reversed these effects to those comparable to young cells and animals (Ramirez-Sanchez et al., 2018).
Results from the present study demonstrate that in aged endothelium Ar activity is increased leading, to a reduction in NO production which, is associated with decreased eNOS phosphorylation at the activation site (Ser1179), higher levels of eNOS uncoupling (<dimer/monomer ratio), oxidative stress (DHE), increases in Nitro-Ar I and Nitro-Ar II, effects that were decreased in the presence of Epi, Norv or both when combined.
Other studies using models of aging and CVD have shown that endothelial Ar competes for L-arginine reducing eNOS activity and therefore, decreasing NO bioavailability (Pandya et al., 2019; Shin et al., 2012; Zhu et al., 2017). On the other hand, Ar inhibition improves endothelial function in elderly healthy subjects (Mahdi et al., 2019). Nevertheless, evidence obtained in endothelial cells collected from human and animal arteries demonstrate that decreases in eNOS protein expression cannot by itself, explain the reduction in NO bioavailability seen with aging (Donato et al., 2009). Therefore, increased Ar activity may be co-responsible for the low NO availability effects which is in line with our results. We previously reported on the capacity of Epi to inhibit Ar activity by decreasing nitrosylated form of the enzyme in a myocardial ischemia/reperfusion injury setting, yielding cardioprotection (Ortiz-vilchis et al., 2018). Interestingly, resveratrol a polyphenol present in red wine, is also capable of attenuating EnD through the downregulation of Ar expression (Dal-ros et al., 2011; Dal-Ros et al., 2012) suggesting the modulation of increased Ar activity as a target to improve endothelial/vascular function. Our results agree with reports in endothelial cells from aged rats showing that S-nitrosylation stabilized Ar I thus, upregulating Ar activity which, was associated with decreased NO production and impaired endothelium-dependent vasodilation (Grandvuillemin et al., 2018).
Together with the increased Ar expression and activity, enhanced production of ROS is a common feature in many vascular pathologies which may further stimulate Ar activity (Caldwell et al., 2016). Increased ROS negatively affect the BH4 levels. eNOS activity is also affected by low BH4 levels since it is involved in the proper coupling of the reductase and oxidase domains of eNOS, modulating its activity. In this regard, it has been reported that decreasing the levels of uncoupled eNOS by restoring BH4 improves EnD in older adults (Eskurza et al., 2005). Results of our study also document that Epi and the combination Epi with Norv stimulate eNOS activity, maintain NO availability and upregulate the BH4:BH2 ratio thus, optimizing eNOS coupling in endothelial cells.
Furthermore, to determine if Epi treatment induces improvements in blood vessels from aged rats, we evaluated vascular reactivity of aorta. Our results showed that Epi restores endothelium-dependent vasodilation of the aged rat aorta to a better extent than Norv and to levels comparable to those found in young animals (Ramirez-Sanchez et al., 2018). These effects were associated with decreased levels of Ar activity in aorta endothelium and improved NO levels, as well as in the BH4:BH2 ratio, demonstrating the capability of Epi to mitigate aging related loss of vascular function. Additionally, Epi and Epi + Norv treatments in aged rats decreased significantly blood pressure and improved blood NO levels.
Our results are further supported by results from other research groups whom reported that Epi administration induces both endothelium-dependent and endothelium-independent relaxation in isolated arteries from rats and humans (Aggio et al., 2013; Huang et al., 1999; Novakovic et al., 2015). In contrast to our findings, in a model of hypertensive rats treated with Epi, the final vasodilatory response to ACh was not significantly different vs. controls (Kluknavsky et al., 2016). As MacRae et al., proposed the explanation for these discrepancies may be related to the fact that the vasodilatory effects of Epi require a functional endothelial layer and when the endothelium is severely damaged or absent, Epi-mediated vasodilation is poor (MacRae et al., 2019). Being aging a less aggressive and slower “damaging” process than hypertension it also helps to explain the difference in Epi-mediated effects. Indeed, vasodilatory responses were improved in aged rat aortas which possess a “more” functional endothelium in contrast to hypertensive rat aortas which, have a significantly damaged endothelium (Durante et al., 2007; Jackson et al., 2018; Ming et al., 2004; Nelin et al., 2005; Wei et al., 2000).
The effects of Epi may also be secondary to the activation of the G protein-coupled estrogen receptor (GPER) as we recently documented (Moreno-Ulloa et al., 2016) and other groups have corroborated (Arefin et al., 2014; Fredette et al., 2011; Meyer et al., 2014; Pang and Thomas, 2017).
Altogether, results from this study allow us to propose the following mechanisms by which Epi restores endothelial function: 1) Epi inhibits Ar activity therefore improving L-arginine bioavailability for eNOS and, 2) Epi induces eNOS activation through a membrane receptor (i.e. GPER) increasing NO production leading to vasodilation. These events could occurr independently or in concert and subsequently, also modulate pathways involved in the regulation of Ar activity.
Our results suggest an interdependence between eNOS and Ar however, the ensuing competition between the two enzymes is difficult to visualize. Endothelial cells in culture have intracellular arginine levels ranging from 0.1 to 0.8 mM, which are enough to saturate eNOS activity given eNOS’ high arginine affinity (Km = 1–5 μM). On the other hand, Ar has a rather low affinity for arginine (Km = 1–20 mM) meaning that eNOS will modify at least 1000 times more arginine than Ar. These facts together with a different enzyme cellular location makes explaining the results difficult. More work is therefore necessary to understand this complex interaction.
5. Conclusion
In conclusion, Epi inhibits Ar activity and modulates l-arginine availability. Epi and Epi plus Norv also improve endothelial and vascular function in aging via the modulation of eNOS. Epi can therefore, be proposed as a candidate for the treatment of pathologies where an EnD is prominent such as with aging, cardiometabolic diseases or hypertension. These results warrant the implementation of clinical trials using Epi.
Funding
DoD PR150090, NIH DK98717, AG47326, VA I01BX3230 to Dr. Villarreal, CONACyT 253,769 to Dr. Ceballos, SIP20171348 and CONACYT 283938 to Dr. Ramirez-Sanchez.
CRediT authorship contribution statement
Alejandra Garate-Carrillo: Experiments and, Data curation, Investigation, Writing - original draft, preparation, Writing - review & editing. Viridiana Navarrete-Yañez: Experiments and, Data curation, Investigation, Writing - original draft, preparation. Pilar Ortiz-Vilchis: Experiments and, Data curation. Gustavo Guevara: Experiments and, Data curation. Carmen Castillo: Experiments and, Data curation. Patricia Mendoza-Lorenzo: Experiments and, Data curation. Guillermo Ceballos: Conceptualization, Writing - review & editing. Miguel Ortiz-Flores: Conceptualization, Experiments and, Data curation. Nayelli Najera: Visualization. Moises Muratt Bustamante-Pozo: Visualization. Ivan Rubio-Gayosso: Conceptualization. Francisco Villarreal: Conceptualization, Supervision, Writing - review & editing. Israel Ramirez-Sanchez: Conceptualization, Experiments and, Data curation, Writing - review & editing.
Declaration of competing interest
Drs. Villarreal is a co-founder and stockholder (Dr. Ceballos) of Epirium Therapeutics, Inc.
Acknowlegments
A. Garate-Carrillo is a Ph.D. candidate supported by CONACyT #612125.
References
- Aggio A., Grassi D., Onori E., D'Alessandro A., Masedu F., Valenti M., Ferri C. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur. J. Nutr. 2013;52:263–272. doi: 10.1007/s00394-012-0320-x. [DOI] [PubMed] [Google Scholar]
- Alp N.J., Channon K.M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- André C., Herlem G., Gharbi T., Claude Y. A new arginase enzymatic reactor : development and application for the research of plant-derived inhibitors. J. Pharmaceut. Biomed. Anal. 2011;55:48–53. doi: 10.1016/j.jpba.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Arefin S., Simoncini T., Wieland R., Hammarqvist F., Spina S., Goglia L., Kublickiene K. Vasodilatory effects of the selective GPER agonist G-1 is maximal in arteries of postmenopausal women. Maturitas. 2014;78:123–130. doi: 10.1016/j.maturitas.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O'Flaherty M., Pandey A., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Benson M.A., Batchelor H., Chuaiphichai S., Bailey J., Zhu H., Stuehr D.J., Bhattacharya S., Channon K.M., Crabtree M.J. A pivotal role for tryptophan 447 in enzymatic coupling of human endothelial nitric oxide synthase (eNOS): effects on tetrahydrobiopterin- dependent catalysis and eNOS dimerization. J. Biol. Chem. 2013;288:29836–29845. doi: 10.1074/jbc.M113.493023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D.E., White R., Li D., Minhas K.M., Cernetich A., Kim S., Burke S., Shoukas A.A., Nyhan D., Champion H.C., Hare J.M. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Caldwell R.B., Toque H.A., Narayanan S.P., Caldwell R.W., Way O.F. Arginase: an old enzyme with new tricks. Trends Pharmacol. Sci. 2016;36:395–405. doi: 10.1016/j.tips.2015.03.006.Arginase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li X. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius A.R., Kirby B.S., Richards J.C., Garcia L.J., Voyles W.F., Larson D.G. Mechanisms of ATP-mediated vasodilation in humans : modest role for nitric oxide and vasodilating prostaglandins. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1302–H1310. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Ros S., Bronner C., Auger C., Schini-Kerth V.B. Red wine polyphenols improve an established aging-related endothelial dysfunction in the mesenteric artery of middle-aged rats: role of oxidative stress. Biochem. Biophys. Res. Commun. 2012;419:381–387. doi: 10.1016/j.bbrc.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Dal-ros S., Zoll J., Lang A., Auger C., Keller N., Bronner C., Geny B., Schini-kerth V.B. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance : role of NADPH oxidase. Biochem. Biophys. Res. Commun. 2011;404:743–749. doi: 10.1016/j.bbrc.2010.12.060. [DOI] [PubMed] [Google Scholar]
- De A., Singh M.F., Singh V., Ram V., Bisht S. Treatment effect of l-Norvaline on the sexual performance of male rats with streptozotocin induced diabetes. Eur. J. Pharmacol. 2016;771:247–254. doi: 10.1016/j.ejphar.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Diehl K.J., Stauffer B.L., Greiner J.J., Weil B.R., Ph D., Desouza C.A., Ph D. Nitric oxide-mediated endothlium-dependent vasodilation is impaired with borderline high-LDL cholesterol. Clin Transl Sci .. 2011;5:21–26. doi: 10.1111/j.1752-8062.2011.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato A.J., Gano L.B., Eskurza I., Silver A.E., Gates P.E., Jablonski K., Seals D.R. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato A.J., Magerko K.A., Lawson B.R., Durrant J.R., Lesniewski L.A., Seals D.R. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 2011;589(Pt.18):4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis M.B.G., Correa Manjolin L., do Carmo Maquiaveli, Claudia Andrade Santos-Filho O., da Silva E.R. Inhibition of Leishmania (Leishmania) amazonensis and rat arginases by green tea EGCG, (+)-catechin and (-)-epicatechin: a comparative structural analysis of enzyme-inhibitor interactions. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante W., Johnson F.K., Johnson R.A. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007;34:906–911. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-bassossy H.M., El-fawal R., Fahmy A., Watson M.L. Arginase inhibition alleviates hypertension in the metabolic syndrome. Br. J. Pharmacol. 2013;169:693–703. doi: 10.1111/bph.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I., Myerburgh L.A., Kahn Z.D., Seals D.R. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J. Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredette N.C., Meyer M.R., Prossnitz D.E.R. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. Physiol. Behav. 2011;176:139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frombaum M., Le S., Bonnefont-rousselot D., Borderie D. Biochimie Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and NO bioavailability : potential bene fi ts to cardiovascular diseases. Biochimie. 2012;94:269–276. doi: 10.1016/j.biochi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Gantzer J. eNOS and BH4; endothelial function or dysfunction. Importance of tetrahydrobiopterin (BH4) J. Neurol. Clin. Neurosci. 2018;2:1–4. doi: 10.13140/RG.2.2.26142.18246. [DOI] [Google Scholar]
- Gilinsky M.A., Polityko Y.K., Markel A.L., Latysheva T.V., Samson A.O., Polis B., Naumenko S.E. Norvaline reduces blood pressure and induces diuresis in rats with inherited stress-induced arterial hypertension. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/4935386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandvuillemin I., Buffat C., Boubred F., Lamy E., Fromonot J., Charpiot P., Simoncini S., Sabatier F., Dignat-George F., Peyter A.C., Simeoni U., Yzydorczyk C. Arginase upregulation and eNOS uncoupling contribute to impaired endothelium-dependent vasodilation in a rat model of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R509–R520. doi: 10.1152/ajpregu.00354.2017. [DOI] [PubMed] [Google Scholar]
- Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., Madhur M.S., Tomaszewski M., Maffia P., D'Acquisto F., Nicklin S.A., Marian A.J., Nosalski R., Murray E.C., Guzik B., Berry C., Touyz R.M., Kreutz R., Wang D.W., Bhella D., Sagliocco O., Crea F., Thomson E.C., McInnes I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. cvva. 2020;106 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhn A., Weber D., Jung T., Ott C., Hugo M., Kochlik B., Kehm R., König J., Grune T. Redox Biology Happily (n) ever after : aging in the context of oxidative stress , proteostasis loss and cellular senescence. Redox Biol. 2017;11:482–501. doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Chan N.W.K., Lau C.W., Yao X.Q., Chan F.L., Chen Z.Y. Involvement of endothelium/nitric oxide in vasorelaxation induced by purified green tea (-)epicatechin. Biochim. Biophys. Acta Gen. Subj. 1999;1427:322–328. doi: 10.1016/S0304-4165(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Huynh N.N., Harris E.E., Chin-Dusting J.F.P., Andrews K.L. The vascular effects of different arginase inhibitors in rat i/ated aorta and mesenteric arteries. Br. J. Pharmacol. 2009;156:84–93. doi: 10.1111/j.1476-5381.2008.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iring A., Jin Y.J., Albarrán-Juárez J., Siragusa M., Wang S.P., Dancs P.T., Nakayama A., Tonack S., Chen M., Künne C., Sokol A.M., Günther S., Martínez A., Fleming I., Wettschureck N., Graumann J., Weinstein L.S., Offermanns S. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Invest. 2019;129:2775–2791. doi: 10.1172/JCI123825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D., Connolly K., Batacan R., Ryan K., Vella R., Fenning A. Epicatechin reduces blood pressure and improves left ventricular function and compliance in deoxycorticosterone acetate-salt hypertensive rats. Molecules. 2018;23:1–14. doi: 10.3390/molecules23071511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae H.K., Bugaj L.J., Young J.O., Bivalacqua T.J., Ryoo S., Soucy K.G., Santhanam L., Webb A., Camara A., Sikka G., Nyhan D., Shoukas A.A., Ilies M., Christianson D.W., Champion H.C., Berkowitz D.E. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J. Appl. Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluknavsky M., Balis P., Puzserova A., Radosinska J., Berenyiova A., Drobna M., Lukac S., Muchova J., Bernatova I. (-)-Epicatechin prevents blood pressure increase and reduces locomotor hyperactivity in young spontaneously hypertensive rats. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/6949020. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke W.M., Hodgson J.M., Proudfoot J.M., McKinley A.J., Puddey I.B., Croft K.D. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- Lu X., Kassab G.S. Integrins mediate mechanical compression – induced endothelium- dependent vasodilation through endothelial nitric oxide pathway. J. Gen. Physiol. 2015;146:221–232. doi: 10.1085/jgp.201411350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae K., Connolly K., Vella R., Fenning A. Epicatechin's cardiovascular protective effects are mediated via opioid receptors and nitric oxide. Eur. J. Nutr. 2019;58:515–527. doi: 10.1007/s00394-018-1650-0. [DOI] [PubMed] [Google Scholar]
- Mahdi A., Pernow J., Kövamees O. Arginase inhibition improves endothelial function in an age-dependent manner in healthy elderly humans. Rejuvenation Res. 2019;22:385–389. doi: 10.1089/rej.2018.2135. [DOI] [PubMed] [Google Scholar]
- Manicam C., Ginter N., Li H., Xia N., Goloborodko E., Zadeh J.K., Musayeva A., Pfeiffer N., Gericke A. Compensatory vasodilator mechanisms in the ophthalmic artery of endothelial nitric oxide synthase gene knockout mice. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-07768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.R., Fredette N.C., Howard T.A., Hu C., Ramesh C., Daniel C., Amann K., Arterburn J.B., Barton M., Prossnitz E.R. G protein-coupled estrogen receptor protects from atherosclerosis. Sci. Rep. 2014;4:7564. doi: 10.1038/srep07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X.-F., Barandier C., Viswambharan H., Kwak B.R., Mach F., Mazzolai L., Hayoz D., Ruffieux J., Rusconi S., Montani J.-P., Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- Ming X.F., Rajapakse A.G., Carvas J.M., Ruffieux J., Yang Z. Inhibition of S6K1 accounts partially for the anti-inflammatory effects of the arginase inhibitor L-norvaline. BMC Cardiovasc. Disord. 2009;9:1–7. doi: 10.1186/1471-2261-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Ulloa A., Mendez-Luna D., Beltran-Partida E., Castillo C., Guevara G., Ramirez-Sanchez I., Correa-Basurto J., Ceballos G., Villarreal F. The effects of (−)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER) Physiol. Behav. 2016;176:139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelin L.D., Chicoine L.C., Reber K.M., English B.K., Young T.L., Liu Y. Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am. J. Respir. Cell Mol. Biol. 2005;33:394–401. doi: 10.1165/rcmb.2005-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic A., Marinko M., Vranic A., Jankovic G., Milojevic P., Stojanovic I., Nenezic D., Ugresic N., Kanjuh V., Yang Q., He G.-W. Mechanisms underlying the vasorelaxation of human internal mammary artery induced by (-)-epicatechin. Eur. J. Pharmacol. 2015;762:306–312. doi: 10.1016/J.EJPHAR.2015.05.066. [DOI] [PubMed] [Google Scholar]
- O'Rourke M.F., Nichols W.W. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- Ortiz-vilchis P., Ortiz-flores M., Pacheco M., Ramirez-sanchez I., Moreno-ulloa A., Vega L., Ortiz A., Villarreal F., Rubio-gayosso I., Najera N., Meaney E., Ceballos G. The cardioprotective effects of (-)-Epicatechin are mediated through arginase activity inhibition in a murine model of ischemia/reperfusion. Eur. J. Pharmacol. 2018;818:335–342. doi: 10.1016/j.ejphar.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Pandya C.D., Lee B., Toque H.A., Mendhe B., Bragg R.T., Pandya B., Atawia R.T., Isales C., Hamrick M., Caldwell R.W., Fulzele S. Age-dependent oxidative stress elevates arginase 1 and uncoupled nitric oxide synthesis in skeletal muscle of aged mice. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1704650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Thomas P. Additive effects of low concentrations of estradiol-17β and progesterone on nitric oxide production by human vascular endothelial cells through shared signaling pathways. J. Steroid Biochem. Mol. Biol. 2017;165:258–267. doi: 10.1016/j.jsbmb.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J., Miwa S., Olijslagers S., Hallinan J., Wipat A., Saretzki G., Rudolph K.L., Kirkwood T.B.L., Von Zglinicki T. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:1–14. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernow J., Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc. Res. 2013;98:334–343. doi: 10.1093/cvr/cvt036. [DOI] [PubMed] [Google Scholar]
- Pokrovskiy M.V., Korokin M.V., Tsepeleva S.A., Pokrovskaya T.G., Gureev V.V., Konovalova E.A., Gudyrev O.S., Kochkarov V.I., Korokina L.V., Dudina E.N., Babko A.V., Terehova E.G. Arginase inhibitor in the pharmacological correction of endothelial dysfunction. Int. J. Hypertens. 2011 doi: 10.4061/2011/515047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polis B., Srikanth K.D., Elliott E., Gil-Henn H., Samson A.O. L-norvaline reverses cognitive decline and synaptic loss in a murine model of Alzheimer's disease. Neurotherapeutics. 2018;15:1036–1054. doi: 10.1007/s13311-018-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Sanchez I., Mansour C., Navarrete-Yañez V., Ayala-Hernandez M., Guevara G., Castillo C., Loredo M., Bustamante M., Ceballos G., Villarreal F.J. (−)-Epicatechin induced reversal of endothelial cell aging and improved vascular function: underlying mechanisms. Food Funct. 2018;9:4802–4813. doi: 10.1039/c8fo00483h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Sanchez I., Maya L., Ceballos G., Villarreal F. (-)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Masuda H., Kihara K., Kurosaki E., Yamauchi Y., Azuma H. Involvement of increased arginase activity in impaired cavernous relaxation with aging in the rabbit. J. Urol. 2004;172:369–373. doi: 10.1097/01.ju.0000121691.06417.40. [DOI] [PubMed] [Google Scholar]
- Samardzic K., Rodgers K.J. Cytotoxicity and mitochondrial dysfunction caused by the dietary supplement l-norvaline. Toxicol. Vitro. 2019;56:163–171. doi: 10.1016/j.tiv.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Santhanam L., Christianson D.W., Nyhan D., Berkowitz D.E. Arginase and vascular aging. J. Appl. Physiol. 2008;105:1632–1642. doi: 10.1152/japplphysiol.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C.A., Dirsch V.M. Nitric Oxide Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Schnorr O., Brossette T., Momma T.Y., Kleinbongard P., Keen C.L., Schroeter H., Sies H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch. Biochem. Biophys. 2008;476:211–215. doi: 10.1016/j.abb.2008.02.040. [DOI] [PubMed] [Google Scholar]
- Shin W., Berkowitz D.E., Ryoo S. Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice. Exp. Mol. Med. 2012;44:594–602. doi: 10.3858/emm.2012.44.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganya N., Bhakkiyalakshmi E., Sarada D.V.L., Ramkumar K.M. Reversibility of endothelial dysfunction in diabetes : role of polyphenols. Br. J. Nutr. 2016;116:223–246. doi: 10.1017/S0007114516001884. [DOI] [PubMed] [Google Scholar]
- Ungvari Z., Tarantini S., Kiss T., Wren J.D., Cory B., Griffin C.T., Murfee W.L., Pacher P., Csiszar A. ageing vasculature. 2019;15:555–565. doi: 10.1038/s41569-018-0030-z.Endothelial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.H., Jacobs A.T., Morris S.M., Ignarro L.J. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2000;279:C248–C256. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- Whyte M.B., Vas P., Heiss C., Feher M.D. The contribution of diabetic micro-angiopathy to adverse outcomes in COVID-19. Diabetes Res. Clin. Pract. 2020 doi: 10.1016/j.diabres.2020.108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Park M., Sun B., Ryoo S. Endothelial nitric oxide synthase activation through obacunone-dependent arginase inhibition restored impaired endothelial function in ApoE-null mice. Vasc. Pharmacol. 2014;60:102–109. doi: 10.1016/j.vph.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Zhou L., Zhao L., Wu Yong, Wu Yangfeng, Gao X., Li Y., Mai J., Nie Z., Ou Y., Guo M., Liu X. Ideal cardiovascular health metrics and its association with 20-year cardiovascular morbidity and mortality in a Chinese population. J. Epidemiol. Community Health. 2018;72:752–758. doi: 10.1136/jech-2017-210396. [DOI] [PubMed] [Google Scholar]
- Zhu C., Yu Y., Montani J.P., Ming X.F., Yang Z. Arginase-I enhances vascular endothelial inflammation and senescence through eNOS-uncoupling. BMC Res. Notes. 2017;10:1–8. doi: 10.1186/s13104-017-2399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]