Dear Editor,
BACE1 and GSK3β are the key targets for Aβ production and tau phosphorylation, respectively. Aβ activated the phosphorylation of GSK3β, resulting in the increase of tau phosphorylation. 1 In addition, tau protein stimulated Aβ toxicity, formed tau Fyn‐Aβ pathway, and increased postsynaptic Fyn level and NMDA receptor sensitivity, thus making neuronal dendrites more sensitive to Aβ toxicity. 2 Given the cross‐talk between BACE1 and GSK3β, a well‐designed multitarget‐directed ligand (MTDL) capable of targeting these two enzymes simultaneously may be a valuable and promising therapeutic strategy. 3
So far, only two well‐studied scaffold derivatives, triazinone scaffold and curcumin scaffold, have been reported as potential BACE1 and GSK3β dual inhibitors. 4 , 5 Getting benefit from virtual screening in silico, we identified a furan coumarin (Notopterol) with simultaneously inhibitory activity on BACE1 and GSK3β from Notopterygium incisum (Table S1). This study is the first to systematically elucidate the benefits of BACE1 and GSK3β dual inhibitors on the pathological mechanism of Alzheimer's disease (AD) in vitro and in vivo.
Notopterol was docked into the binding site of the proteins, and the predicted binding modes at the two targets are shown in Figure 1A and D. In BACE1, the hydroxyl group of the Notopterol fatty chain interacted with Asp32, Asp228, and Thr231, and formed a water bridge with Gly230 (Figure S1). For GSK3β, Notopterol bound in the hinge region of the kinase and establishes two strong hydrogen‐bonding interactions with the backbone of Asp133 and Tyr134. To evaluate the binding stability of protein ligand complexes, we have performed molecular dynamics (MD) simulations for 100 ns. The RMSD values of protein skeleton remained stable at about 2–3 Å during the simulation (ligand fit on protein). These results showed that Notopterol can bind to protein in a stable state in the process of molecular dynamics simulation. We also tested the inhibitory effect of Notopterol on BACE1 and GSK3β enzymes: Notopterol showed moderate inhibitory against BACE1 (IC50: 26.01 μM) and strong inhibitory against GSK3β (IC50: 1 μM).
Figure 1.
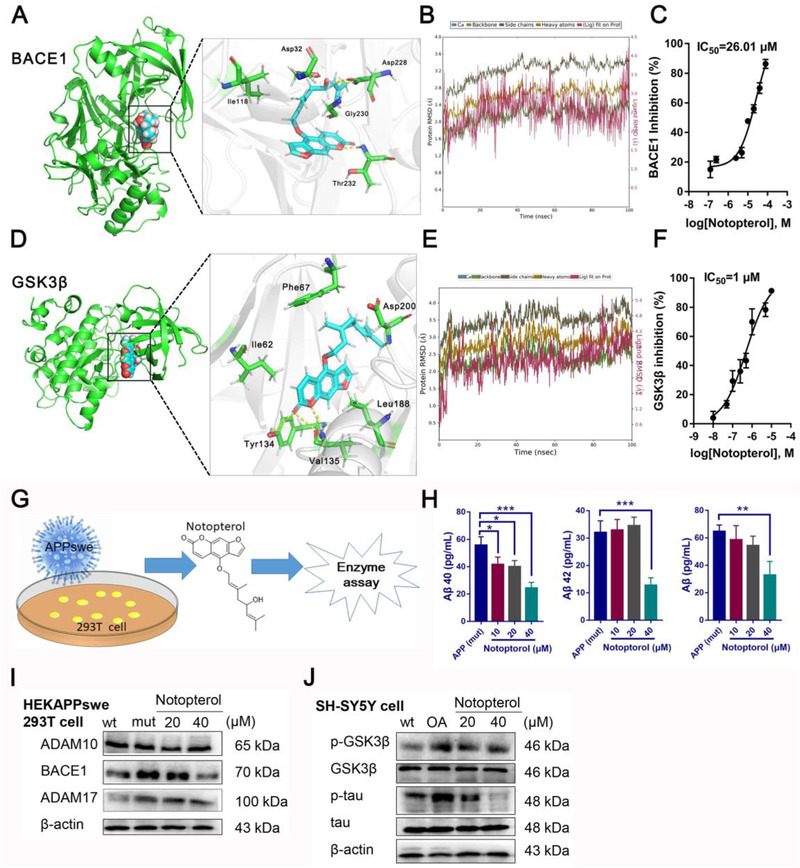
Binding modes of Notopterol with BCAE1 (5CLM) and GSK3β (2OW3) and inhibiting the generation of Aβ and phosphorylated tau. A, Predicted conformation of Notopterol combined with BACE1. B, RMSD curve of Notopterol/BACE1 complex. C, Notopterol inhibited the activities of BACE1 enzyme. D, Predicted conformation of Notopterol combined with GSK3β E, RMSD curve of Notopterol/GSK3β complex. F, Notopterol inhibited the activities of GSK3β enzyme. G, The experimental protocol for measuring Aβ levels by enzyme linked immunosorbent assay (ELISA). H, Aβ40, Aβ42, and total Aβ in HEKAPPswe239T cells with or without Notopterol. I, Western blot of ADAM10, BACE1, and ADAM17 in APPswe239T cells. J, Western blot of p‐GSK3β, GSK3β, p‐tau, and tau in Okadaic acid‐treated SH‐SY5Y cells. The error bars represent the SD. (*P < 0.05, **P < 0.01 and ***P < 0.001, other comparisons were not significant; One‐way analysis of variance followed by Turkey's post hoc multiple‐comparisons test)
HEK APPswe293T cells stably overexpress human APP‐695 harboring the Swedish double mutation (APPswe). 6 The secretion of Aβ in HEK APPswe293T cells is much higher than that of wild type cells, so it is an ideal cell model for screening drugs against AD. 7 Figure 1H demonstrates that Notopterol could inhibit the production of Aβ. Okadaic acid induced tau hyperphosphorylation in SH‐SY5Y cell is usually used as a cell model for tau pathology. 8 Western blot results showed that Notopterol decreased the expression of ADAM17 and BACE1 in HEK APPswe293T cells, as well as the phosphorylation of tau in SH‐SY5Y cell exposed in Okadaic acid (Figure 1I and J).
To assess whether Notopterol treatment had any beneficial effects on the cognition of APP/PS1 mice, we performed Morris water maze assay. Compared with APP/PS1 mice, the escape latency of Notopterol‐treated mice was significantly decreased (Figures 2A and B). The levels of Aβ40, Aβ42, and Aβ in the hippocampus and cortex of were determined by enzyme linked immunosorbent assay (ELISA) assay. As shown in Figure 2C, Notopterol significantly reduced the levels of Aβ40, Aβ42, and Aβ in hippocampus and cortex compared to that of transgenic mice. In addition, we also verified that the Aβ42 level in cortex was positively correlated with the escape latency of mice (Figure 2D), which is consistent with previous studies. 9 Given Notopterol is a BACE1 and GSK3β dual inhibitor, we first investigated the deposition of amyloid plaques and the phosphorylated tau in the hippocampus and cortex. The immunohistochemistry results showed that the deposition of Aβ plaques was significantly decreased in the hippocampus of Notopterol‐treated APP/PS1 transgenic mice (Figure 2E and G), as well as the phosphorylated tau in cortex. Furthermore, we investigated the proteins expression of the Aβ and tau pathways. We found that Notopterol can regulate the protein expression of ADAM10‐BACE1‐ADAM7 and GSK3β‐tau signaling pathway in hippocampus (Figure 2F). These results indicated that the advantage of BACE1 and GSK3β dual inhibitor is reducing both the amyloid plaques and hyperphosphorylatic tau, which can improve the ability of AD mice to learn.
Figure 2.
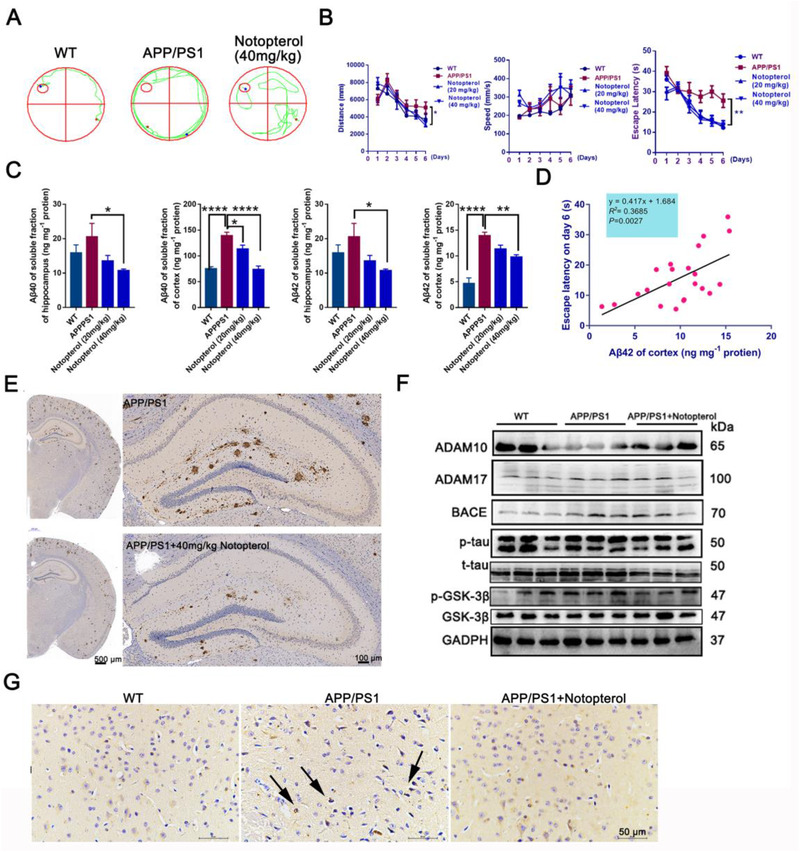
Notopterol improves the cognitive decline in APP/PS1 mice. A, Representative water maze traces of WT mice (left), APP/PS1 mice (middle), and Notopterol‐treated (right) APP/PS1 mice on day 6. B, Analysis of the MWM test of WT, Notpterol‐treated, or without treated APP/PS1 mice. C, The levels of Aβ40 and Aβ42 in hippocampal and cortes in APP/PS1 mice. D, The level of Aβ42 in the cortex of APP/PS1 mice was positively correlated with memory impairment. E, Immunohistochemistry staining of deposited Aβ in APP/PS1 mice and Notopetrol (40 mg/kg) treated APP/PS1 mice; scale bars, 100 μm and 500 μm. F, Western blot of ADAM10, BACE1, ADAM17, p‐GSK3β, GSK3β, p‐tau, and tau in hippocampus of APP/PS1 mice and Notopetrol‐treated (40 mg/kg) APP/PS1 mice. Quantitative data are shown in Figure S4. G, Immunohistochemistry staining of phosphorylated tau in cortex of APP/PS1 mice and Notopetrol‐treated (40 mg/kg) APP/PS1 mice; scale bars, 50 μm. The error bars represent the SD. (*P < 0.05, **P < 0.01 and ***P < 0.001, other comparisons were not significant; One‐way analysis of variance followed by Turkey's post hoc multiple‐comparisons test)
In summary, here we reported is the discovery of Notopterol as the natural small molecule capable of simultaneously inhibiting BACE1 and GSK3β. We have demonstrated that inhibition of the activity of BACE1 and GSK3β by Notopterol can effectively repair the pathophysiological and cognitive impairment of AD caused by abnormal Aβ accumulation and tau hyperphosphorylation. Given the complexity of AD pathology, BACE1 and GSK3β dual inhibitor may be a promising treatment strategy.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). This study was approved by the Ethics Committee of the Institutional Animal Care and Use Committee of Shenyang Pharmaceutical University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Qingchun Zhao and Huiyuan Gao designed this study, Xiaowen Jiang performed this research and wrote the paper. Jingda Li and Qiong Wu assisted in isolating animal tissue. Jingda Li and Wenwu Liu performed Morris water maze test and analyzed data. Hongyuan Lu and Zihua Xu performed docking analysis. Qinglong Qiao and Haotian Zhang analyzed data.
Supporting information
Supporting Information
ACKNOWLEDGMENT
The authors thank Prof. Jian Wang (Key Laboratory of Structure‐Based Drug Design & Discovery, Ministry of Education, Shenyang Pharmaceutical University) for his help in the experiment and analysis of molecular docking. This work was supported by the National Natural Science Foundation of China (Nos. 81673328 and 81973209) and Liao Ning Revitalization Talents Program (No. XLYC1905019).
Contributor Information
Huiyuan Gao, Email: gaohuiyuan@syphu.edu.cn.
Qingchun Zhao, Email: zhaoqingchun1967@163.com.
REFERENCES
- 1. Zheng WH, Bastianetto S, Mennicken F, Ma W, Kar S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115(1):201‐211. [DOI] [PubMed] [Google Scholar]
- 2. Polanco JC, Li C, Bodea LG, Martinez‐Marmol R, Meunier FA, Götz J. Amyloid‐β and tau complexity: towards improved biomarkers and targeted therapies. Nat Rev Neurol. 2018;14(1):22‐39. [DOI] [PubMed] [Google Scholar]
- 3. Rampa A, Gobbi S, Concetta Di Martino RM, Belluti F, Bisi A. Dual BACE‐1/GSK‐3beta inhibitors to combat Alzheimer's disease: a focused review. Curr Top Med Chem. 2017;17(31):3361‐3369. [DOI] [PubMed] [Google Scholar]
- 4. Prati F, De Simone A, Bisignano P, et al. Multitarget drug discovery for Alzheimer's disease: triazinones as BACE‐1 and GSK‐3β inhibitors. Angew Chem Int Ed Engl. 2015;54(5):1578‐1582. [DOI] [PubMed] [Google Scholar]
- 5. Martino Di MR, De Simone A, Andrisano V, et al. Versatility of the curcumin scaffold: discovery of potent and balanced dual BACE‐1 and GSK‐3beta inhibitors. J Med Chem. 2016;59(2):531‐544. [DOI] [PubMed] [Google Scholar]
- 6. Lo AC, Haass C, Wagner SL, Teplow DB, Sisodia SS. Metabolism of the “Swedish” amyloid precursor protein variant in Madin‐Darby canine kidney cells. J Biol Chem. 1994;269(49):30966‐30973. [PubMed] [Google Scholar]
- 7. Zhu Z, Yan J, Jiang W, et al. Arctigenin effectively ameliorates memory impairment in Alzheimer's disease model mice targeting both beta‐amyloid production and clearance. J Neurosci. 2013;33(32):13138‐13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. More SV, Kumar H, Cho DY, Yun YS, Choi DK. Toxin‐induced experimental models of learning and memory impairment. Int J Mol Sci. 2016;17(9):1447‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Busche MA, Kekuš M, Adelsberger H, et al. Rescue of long‐range circuit dysfunction in Alzheimer's disease models. Nat Neurosci. 2015;18(11):1623‐1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


