Dear editor:
More than 25% of colorectal cancer (CRC) have liver metastasis (CRLM), 1 for which the multi‐disciplinary treatment (MDT) has emerged as an alternative of therapeutic strategies in China. 2 In the present study, we reported a long‐term MDT treatment experiences and assessed the advantages of the MDT strategy, and furthermore, we also aimed to define the criteria of the suitable CRLM patients who can be benefited more from MDT strategy.
This study retrospectively enrolled two independent cohorts of consecutive CRLM patients (MDT cohort and No‐MDT cohort). Management of MDT and statistical methods are described in Supporting Information Section 1. From February 9, 2009 to December 28, 2017, a total of 3740 consecutive MDT discussions were studied, and MDT times are shown in Figure S1. The management workflow of two independent cohort is shown in Figure 1A. A total of 1027 CRLM patients received MDT and 401 CRLM patients were treated without MDT. Of 1027 MDT patients, 51% were males and 54% were older than 60 years. The majority of MDT cohort patients with CRLM had more liver metastatic lesions (P < .01) and shorter tumor size (P < .01). More advanced CRLM received MDT (Table S1).
FIGURE 1.
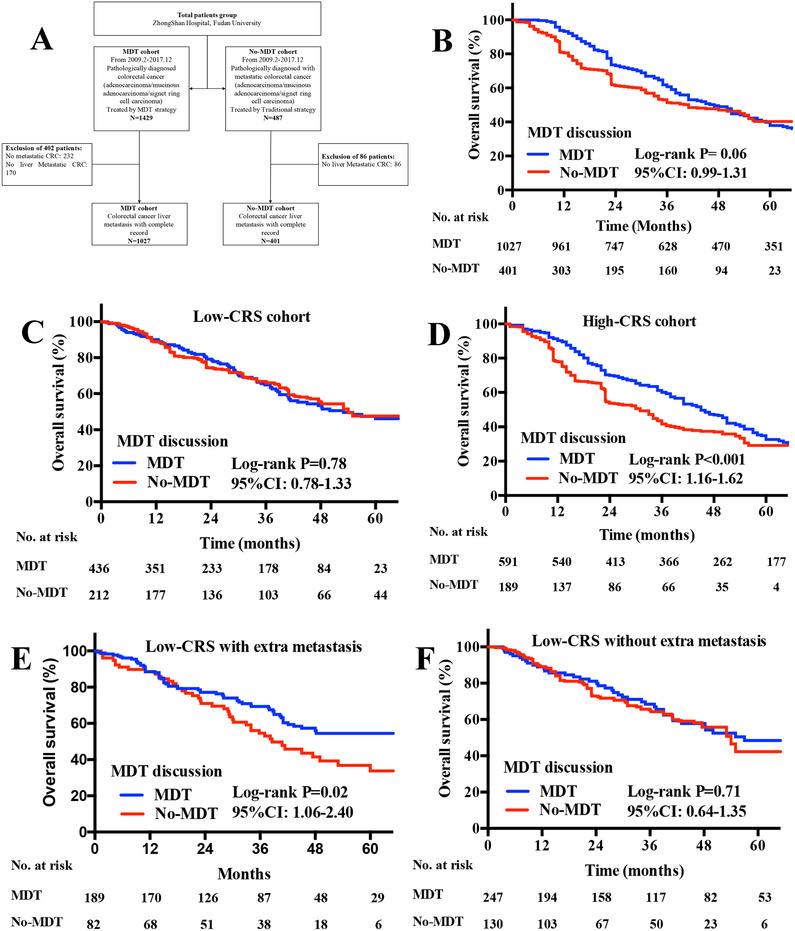
A, Flow diagram of two independent CRLM patients enrolled from Zhongshan Hospital (MDT cohort and No‐MDT cohort); B, Kaplan‐Meier OS curves for CRLM patients of MDT and No‐MDT cohorts; C, Kaplan‐Meier OS curves for low CRS patients of MDT and No‐MDT cohorts; D, Kaplan‐Meier OS curves for high CRS patients MDT and No‐MDT cohorts; E, Kaplan‐Meier OS curves for low CRS patients with extra‐hepatic metastasis of MDT and No‐MDT cohorts; F, Kaplan‐Meier OS curves for low CRS patients without extra‐hepatic metastasis of MDT and No‐MDT cohorts, P‐values were determined by the log‐rank test. Abbreviations: CRLM, colorectal cancer liver metastasis; OS, overall survival; MDT, multi‐disciplinary treatment; CRLM, colorectal cancer liver metastasis; CRS, clinical risk score
Figure S2 demonstrates the compliance rate of surgical and no‐surgical plan in MDT and No‐MDT cohorts. Univariate and multivariate logistics analysis for compliance to treatment suggestion are performed in Table S2, and MDT was regarded as an independent factor for adherence (P < .01). Reasons for changes to treatment plan are shown in Table S3. In addition, thoracic CT scan, CA125/CA724 and pelvic MRI scan, liver MRI scan, RAS/RAF mutation status, and PET/CT of CRLM patients in MDT cohort showed significant difference from No‐MDT controls (Table S4; P < .01). Of 1027 patients in MDT cohort, 264 patients had extra‐hepatic metastasis (25.7%), including 212 lung (21%), 43 bone (4%), and 54 transcelomic metastases (5%). Resectability change in 202 patients (20%) and additional targeted therapies were added in selective 307 patients (30%) (Table S5).
The larger proportion of MDT cohort patients received chemotherapy plus targeted therapy at the first line or second line treatments (P < .01). FOLFOXIRI regimen and VIC regimen were performed in MDT cohort patients more than in those without MDT (P < .01) (Table S6). Initially unresectable CRC patients received conversion resection demonstrated better median survival duration in both MDT (42.0 vs 37.0 months) and No‐MDT cohorts (36.6 vs 28.4 months) and had liver metastasectomy rate in MDT significantly higher than No‐MDT (P < .01), as shown in Table S7.
The overall survival (OS) rate in recruited patients is shown in Table S8, independent upon clinical T stage (P < .05), N stage (P < .05), metastatic tumor number (P < .05), and tumor size (P < .05), and resection of primary site (P < .05). There was no significant difference of OS rates between patients with and without MDT (Median OS: 47.0 months vs 41.0 months, P = .06) (Figure 1B).
To further identify effective discriminator on survival benefits, risk stratified analysis was performed in Table 1. We found that the OS rate of No‐MDT patients with high clinical risk score (CRS) was significantly worse than those with MDT(Figure 1C, P < .01), rather than No‐MDT patients with low CRS (P > .08) as shown in Figure 1D.
TABLE 1.
stratified analysis for overall survival between different CRLM cohorts
| MDT cohort (N = 1027) | No‐MDT cohort (N = 401) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Median | IQR | Median | IQR | HR (95%CI) | P‐value | |
| Age | ≥60 | 45.0 | 28.0‐54.0 | 44.0 | 26.0‐53.0 | 0.945 (0.768‐1.221) | .87 |
| <60 | 42.0 | 25.0‐57.0 | 43.0 | 23.0‐57.0 | 1.021 (0.452‐1.203) | .68 | |
| Gender | Male | 39.0 | 22.0‐61.0 | 40.0 | 23.0‐52.0 | 1.198 (0.956‐1.324) | .96 |
| Female | 43.0 | 24.0‐58.0 | 42.0 | 25.0‐61.0 | 1.043 (0.867‐1.192) | .88 | |
| Primary site | Left colon | 41.0 | 26.0‐57.0 | 42.0 | 21.0‐57.0 | 1.324 (0.803‐1.672) | .54 |
| Right colon | 40.0 | 20.0‐51.0 | 38.0 | 21.0‐50.0 | 1.254 (0.876‐1.422) | .32 | |
| Rectum | 44.0 | 25.0‐58.0 | 43.0 | 22.0‐57.0 | 0.975 (0.824‐1.306) | .21 | |
| cT stage | T1/T2 | 49.0 | 39.0‐62.0 | 48.0 | 37.0‐60.0 | 0.754 (0.670‐2.432) | .60 |
| T3/T4 | 36.0 | 25.0‐58.0 | 38.0 | 22.0‐57.0 | 1.035 (0.934‐1.216) | .24 | |
| CRS score | low | 53.0 | 45.0‐61.0 | 54.0 | 19.0‐42.0 | 1.019 (0.776‐1.334) | .78 |
| high | 45.0 | 43.0‐64.0 | 31.0 | 23.0‐51.0 | 1.294 (1.157‐1.615) | <.001 | |
| Extra‐hepatic metastasis | Yes | 40.0 | 21.0‐56.0 | 41.0 | 19.0‐56.0 | 1.295 (0.876‐2.323) | .32 |
| No | 44.0 | 25.0‐58.0 | 45.0 | 22.0‐57.0 | 0.785 (0.643‐1.224) | .14 | |
CRLM patients were furthermore divided into low and high CRS subgroups according to CRS system. 3 Clinical T stage, metastatic tumor size, and extra‐hepatic metastasis were regarded as independent risk factors for OS in low CRS patients, while clinical T stage, number of metastasis, metastatic tumor size, and MDT were regarded as independent risk factors for OS in high CRS patients, as shown in Table S9. Among low CRS patients, significant survival benefit of MDT for OS was observed in patients with extra‐hepatic metastasis (Figure 1E, P < .05), while not in patients without extra‐hepatic metastasis (Figure 1F, P > .05).
This is the first study to complementarily evaluate long‐term experiences of MDT on CRLM cases. The number of Zhongshan CRLM MDT increased gradually from 235 to 564 per year. MDT as an independent method could improve adherence, although there are many risk factors for non‐adherence, such as patient factors (eg, age, sex, and emotional functions), family environment, and therapeutic settings. 4 Although MDT patients received more necessary diagnostic tests and more standardized systematic treatment regimen, no significant difference on prognosis was noticed between patients with or without MDT. However, the stratified analysis on factors for OS among demonstrated that MDT was more effective and improved survival outcome from 31 to 45 months in high CRS subgroup (P < .01) and in patients with low CRS and extra‐hepatic metastasis (P < .05).
These findings were not consistent with previous studies, 5 , 6 which could be explained as follows: (1) Lordan's report 5 evaluated CRLM cases after hepatic resection, which was limited to represent all CRLM; (2) Jen‐Kou Lin 6 reported cases from 2001 to 2010 and there were significant differences for diagnosis and treatment for CRLM such as targeted therapy, which was widely used on CRLM patients after 2009. Besides, our results was also reasonable for, (1) in the new era of MDT that is composed of many doctors, after reviews and experiences of many cases, one specialist doctor could be able to provide appropriate suggestions for selective CRLM cases; (2) after analysis, low CRS CRC liver‐limited metastasis patients had better prognosis and were not hard to be properly dealt with. Considering the high incidence of CRLM and time‐consuming nature of MDT, we suggested that MDT should not be recommended to selective low CRS liver‐limited patients due to the low efficacy on decision making and long‐term prognosis, when patients could be managed according to a standard guidelines. 7 , 8 , 9
In summary, we found that MDT could be helpful to improve the accuracy of diagnosis, efficacy of adherence and standardized treatment, and rate of conversion resection. Benefits of MDT on overall survival were more obvious in high CRS patients and low CRS patients with extra‐hepatic metastasis.
CONFLICT OF INTEREST
The authors declare no conflicts of interest for the publication of this manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Written informed consent was obtained by all the patients. The study protocol followed the ethical guidelines of the Declaration of Helsinki and was approved by the Ethical Committee of Zhongshan Hospital of Fudan University.
AVAILABILITY OF DATA AND MATERIAL
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Supporting information
Supporting Information.
ACKNOWLEDGMENTS
We thank all the doctors and nurses during the treatment process. This work was supported by National Natural Science Foundation of China (Grant No. 81602040 and 81402341), Clinical Science and Technology Innovation Project of Shanghai (SHDC12016104), and Shanghai Science and Technology Committee Project (17411951300). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in the writing of the manuscript.
Contributor Information
Guo‐dong He, Email: angelhgd@163.com.
Jian‐Min Xu, Email: xujmin@aliyun.com.
REFERENCES
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 2. Morris E, Haward RA, Gilthorpe MS, Craigs C, Forman D. The impact of the Calman‐Hine report on the processes and outcomes of care for Yorkshire's colorectal cancer patients. Br J Cancer. 2006;95(8):979‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309‐318. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mitchell AE, Scarcella DL, Rigutto GL, et al. Cancer in adolescents and young adults: treatment and outcome in Victoria. Med J Aust. 2004;180(2):59‐62. [DOI] [PubMed] [Google Scholar]
- 5. Lordan JT, Karanjia ND, Quiney N, Fawcett WJ, Worthington TR. A 10‐year study of outcome following hepatic resection for colorectal liver metastases ‐ The effect of evaluation in a multidisciplinary team setting. Eur J Surg Oncol. 2009;35(3):302‐306. [DOI] [PubMed] [Google Scholar]
- 6. Lan YT, Jiang JK, Chang SC, et al. Improved outcomes of colorectal cancer patients with liver metastases in the era of the multidisciplinary teams. Int J Colorectal Dis. 2016;31(2):403‐411. [DOI] [PubMed] [Google Scholar]
- 7. Xu J, Fan J, Qin X, et al. Chinese guidelines for the diagnosis and comprehensive treatment of colorectal liver metastases (version 2018). J Cancer Res Clin Oncol. 2019;145(3):725‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diagnosis, Treatment Guidelines For Colorectal Cancer Working Group C . Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin J Cancer Res. 2019;31(1):117‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakedis J, Squires MH, Beal EW, et al. Update on current problems in colorectal liver metastasis. Curr Probl Surg. 2017;54(11):554‐602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.


