Abstract
Background
Although most patients with asthma have mild disease, data on how mild asthma is defined, and how frequently exacerbations occur in this patient population are scarce, so we aimed to redress this.
Methods
We searched Medline and Medline In-Process (PubMed), and Embase in OVID for English-language publications containing “mild asthma” plus at least one relevant therapy and outcome/keyword, limited to randomised controlled trials (RCTs) and observational studies published between January 1990 and February 2019. Publications were filtered to ensure appropriate data extraction. The main outcomes were the definitions of mild asthma and exacerbations, baseline exacerbation rates and exacerbation data for placebo recipients in prospective studies. Meta-analysis of exacerbation rates was planned.
Findings
Of 4064 articles identified, 64 were included in our review (49 743 subjects); 54 RCTs and 10 observational/other studies. Six main types of definitions of mild asthma were identified. While care was taken to ensure inclusion only of patients with mild asthma, marked heterogeneity was revealed in the definitions of mild asthma and hence the study populations. Reporting of exacerbations also varied widely between studies, precluding meta-analysis. Between 0–22% of patients were hospitalised for asthma or had a severe exacerbation in the previous year, according to baseline data from prospective studies. In RCTs, severe exacerbation rates in placebo recipients taking only short-acting β2-agonist therapy ranged from 0.20–2.88 per year.
Conclusions
These data provide new evidence of the burden of exacerbations in mild asthma and highlight the need for standardised definitions of mild asthma and of exacerbations to progress further research.
Short abstract
This comprehensive literature review highlights the risk of exacerbations for patients with mild asthma https://bit.ly/3cauSb3
Introduction
Asthma is a common chronic respiratory disease [1, 2]. Among adults, the prevalence of doctor-diagnosed asthma ranges from 0.2% to 21.0% of the population in different countries [3]. The spectrum of asthma severity is highly skewed, such that the majority of patients (approximately 50–75%) are said to have mild disease and only a minority (≈3.6–8%) have severe disease [4–8]. However, the definitions of mild and severe asthma, and hence their reported prevalence, vary from study to study. In addition, these definitions have varied over time [9]. Historically, classification of asthma severity was often based on symptoms, lung function and exacerbation frequency prior to the commencement of maintenance treatment, a classification that remains in current US guidelines [10]. In 2004, the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force on asthma control, severity and exacerbations recommended a classification of asthma severity for clinical trials based on the level of treatment required to maintain good asthma control (i.e. a retrospective label based on treatment response) [11]. Since 2014, a similar approach has been recommended for clinical practice by the Global Initiative for Asthma (GINA). For epidemiological studies, GINA suggests classification of patients by their current treatment step, without inferring severity [1]. By contrast with the approach in publications like these, the term “mild asthma” is widely used by clinicians and patients for those with infrequent, mild, or easily relieved symptoms or other manifestations of disease, consistent with lay dictionary definitions of “mild” as gentle, slight, and not marked or extreme.
Regardless of its definition, the prevalence of mild asthma may be underestimated [4], due to under- or mis-diagnosis by healthcare professionals and under-reporting by patients who, for various reasons, do not present to primary care [12–16]. Equally, asthma may be over-diagnosed [16, 17], with attribution of symptoms to asthma by patients and/or doctors without the diagnosis being confirmed by spirometry or other objective measurements [16]. Many recent clinical studies have focused on severe asthma, as these patients account for the majority of the morbidity and healthcare resource utilisation associated with asthma [8, 18], and because new options are available for treatment of such patients.
Limited data are available on the burden and optimal management of mild asthma. One analysis found that people with mild asthma reported significantly more absenteeism, physician visits, emergency department (ED) visits, and hospitalisations than otherwise healthy matched controls [5]. An analysis from Canada estimated that mild asthma was responsible for 67% of total asthma patient-years, but for 14% of the total direct costs of asthma [18]. It has also been observed that healthcare resource use seems disproportional to clinical features in patients with mild asthma [4]. While asthma exacerbation rates are lower in patients with mild than those with severe asthma, defined by treatment step [19], studies in which pre-exacerbation symptom frequency was recorded have found that 30–52% of exacerbations requiring emergency care occurred in patients reporting symptoms less than weekly or only on exertion in the previous 3 months [6]. In addition, sudden-onset asthma deaths are well-recognised, with distinctive pathological features [20].
We therefore conducted a systematic review to gain a better understanding of how mild asthma has been defined in the literature and to understand the burden of mild asthma as related to exacerbations, the single most burdensome outcome for patients, clinicians and healthcare systems alike.
Methods
Search strategy and selection criteria
A structured search of published literature was conducted electronically using Medline and Medline In-Process (PubMed), and Embase in OVID (OVID Technologies, Inc.), to capture publications between January 1990 and February 2019. The search was limited to English-language publications, and excluded animal studies, preclinical studies, in-vitro studies, case reports, editorials, letters, and review articles, as well as other systematic reviews and meta-analyses. Records had to contain the relevant indication plus at least one therapy plus at least one outcome/keyword. Search terms for the indication were “mild” or “persistent” or “intermittent” or “seasonal” or “episodic” or “mild–moderate” plus “asthma”, OR “Global Initiative for Asthma” or “GINA” plus “step? 1” or “step? 2”. Therapies included monotherapy with inhaled corticosteroid (ICS), leukotriene receptor antagonist (LTRA), short-acting β2-agonist (SABA), methylxanthine, co-therapy (combined or separate) with ICS+SABA, ICS+long-acting β2-agonist (LABA) and/or LTRA+SABA (see table S1 for full search terms). The search was restricted to publications in which the search terms appeared in the title and/or abstract, to increase the relevance of the articles identified. Any duplicates were removed during the electronic search process. Relevant records were identified using the search terms above with a prior pilot stage to test criteria and refine, if necessary.
After exclusion of congress abstracts, full-text versions of any records identified as definitely or possibly relevant via the title and abstract (level 1 filtering) were obtained so that the inclusion/exclusion criteria could be re-applied to the full article (level 2 filtering). Records were limited to those reporting only randomised controlled trials (RCTs) and non-RCTs (observational or retrospective studies, including database analyses) in patients stated to have mild asthma or, if “mild” was not specified by the study authors, with clinical characteristics consistent with those used in other studies reported here. Studies in patients with mild-to-moderate asthma were included only if data for patients stated to have mild asthma were reported separately. Studies were included if they contained data on exacerbations or exacerbation-related data. Filtering was undertaken by professional medical writers with several years of experience of asthma and the respiratory therapeutic area.
Data extraction
Data from each patient population were then extracted from each identified record using pre-agreed parameters. Data extraction was undertaken by two investigators who subsequently reviewed each other's data. A third investigator had overall responsibility for the project. Any conflicts over record or data inclusion were resolved via discussion between the three members of the team.
Data extracted included: the definition of mild asthma used in each identified study; study inclusion and exclusion criteria; any data on pre-study history of exacerbations, systemic corticosteroid use or hospitalisations/ED visits documented from retrospective studies and at baseline in prospective studies; and, for RCTs of ≥24 weeks’ duration, any outcome data in the placebo arm related to exacerbations, systemic corticosteroid use, hospitalisation/ED visits or study-defined “severe asthma-related events (SAREs)”. Data were also collected on the study definition of an exacerbation, including severity of exacerbation if provided. Other data extracted from each record included study design, study duration, number of patients and age of the study population, and the nature of the intervention and comparator arms if relevant. Data were extracted into a spreadsheet and checked to ensure no duplication of data.
Data analysis
While the original aim of this study was to conduct a meta-analysis, due to the heterogeneity of study designs, mild asthma and exacerbation definitions, and format in which exacerbation data were reported (e.g. annualised exacerbation rate versus incidence of exacerbations), this was not possible.
Outcomes
The primary outcomes of interest were:
- the definition of mild asthma,
- the study definition of an exacerbation (including severity of exacerbation, if stated) used in collecting patient baseline clinical characteristics/history or as a study outcome,
- retrospective exacerbation data collected from patient self-report; exacerbation data from administrative databases and medical records; prospective exacerbation data, and
- exacerbation rates in the placebo arm of prospective studies, as a prospective study outcome.
Secondary outcomes included exacerbation rates collected retrospectively in administrative databases and medical records.
For each included study, the extracted inclusion and exclusion criteria were assessed in relation to the study definition of mild asthma and as related to patient history of exacerbations.
This review has been registered on the PROSPERO database with the number CRD42018093352.
Results
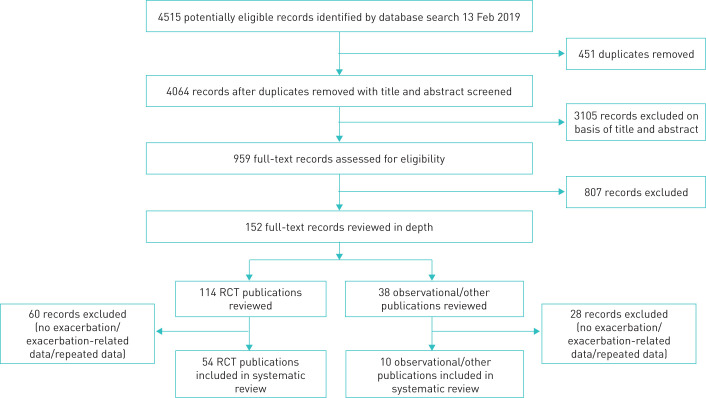
Of the 4064 records identified, 152 were reviewed in-depth, 114 RCTs and 38 observational and other non-RCT studies, from here on referred to as “observational/other” publications (figure 1). A total of 64 articles (54 RCT and 10 observational/other publications) were included in the systematic review, with 32 919 RCT subjects (including 5326 patients randomised to placebo, and 1435 paediatric/adolescent patients) and 16 824 observational/other subjects (including 98 paediatric/adolescent patients).
FIGURE 1.
Study selection process. RCT, randomised controlled trial.
Definitions of “mild asthma”
Among the articles included, there was heterogeneity in the way that mild asthma was defined. In order to make meaningful comparisons amongst the studies, we classified these definitions broadly into six categories according to the criteria the original authors had used to define mild asthma at study entry (table S2). In brief, these were: 1) treatment level; 2) symptom frequency criteria; 3) symptoms <daily and a minimum permitted forced expiratory volume in 1 s (FEV1) of 80% predicted; 4) symptoms <daily and a minimum permitted FEV1 ranging between 60–80% predicted; 5) other criteria; and 6) definitions for children <5 years (table 1). The majority of RCTs used a composite definition based on symptom frequency and FEV1 (categories 3 and 4), whereas for observational/other studies, except those limited to preschool children, similar numbers of studies used treatment level (category 1) and categories 3 or 4 to define mild asthma.
TABLE 1.
Definitions of mild asthma: classification used in this review
| Main criteria for mild asthma | RCTs (n=54) | Observational/other studies (n=10) | ||
| All n (%) | Studies permitting past history of exacerbations n (%) | All n (%) | Studies permitting past history of exacerbations n (%) | |
| 1) Treatment level | 3 (5.6) | 0 (0) | 5 | 2 (20.0) |
| 2) Symptom frequency criteria | 3 (5.6) | 3 (5.6) | 0 | – |
| 3) FEV1 ≥80% pred and symptoms<daily | 19 (35.2) | 15 (27.8) | 2 | 1 (10.0) |
| 4) FEV1 >60–80% pred and symptoms<daily | 15 (27.8) | 13 (24.1) | 1 | 1 (10.0) |
| 5) Miscellaneous definitions# | 9 (16.7) | 8 (14.8) | 0 | – |
| 6) Included patients aged <5 years | 5 (9.3) | 4 (7.4) | 2 | 2 (20.0) |
RCT: randomised controlled trial; FEV1: forced expiratory volume in 1 s. # Miscellaneous definitions not captured by the other five categories (e.g. “doctor-diagnosed mild asthma”, or “mild asthma based on airway hyper-responsiveness” alone), or, studies that did not state that patients had “mild asthma” but included patients with disease characteristics similar to those in the other five categories.
The main characteristics for which data were extracted are summarised in table 2, with active treatment group regimens summarised in table S3. Many studies, in order to ensure clinical stability at enrolment, excluded patients with a recent exacerbation (e.g. in the previous 1–3 months). However, if patients with an exacerbation more than 3 months prior to study enrolment were excluded, we considered this study to have excluded patients with an exacerbation history. The majority of RCTs permitted patients to have such a history of exacerbations (43 of 54). One RCT in children, examining the effect of intermittent LTRA, required the children to have a history of 3–6 exacerbations within the previous 12 months, with no symptoms between exacerbations, for study inclusion [21]. One observational study included patients experiencing exacerbations (timeframe unspecified) and who had recurrent, but not daily, symptoms [22].
TABLE 2.
Mild asthma studies grouped by study type (RCT or observational/other) and by definition of mild asthma
| Study (year) [ref.] | Age range or average age years | Treatment duration | Placebo | Exacerbation definition | Baseline exacerbation data | Patients with history of exacerbation not excluded# | |
| Yes n, treatment allowed | No | ||||||
| RCTs | |||||||
| Category 1: RCTs that primarily defined mild asthma by treatment level | |||||||
| Bateman et al. (2018) [26] | 12–83 | 52 weeks | ✓ | ✓ | ✓ | ||
| Camargos et al. (2018) [31] | 10.6 (2.8)¶, 9.9 (2.7)¶ | 6 weeks | ✓ | ✓ | |||
| O’Byrne et al. (2018) [38] | 12–85 | 52 weeks | ✓ | ✓ | |||
| Category 2: RCTs that primarily defined mild asthma by symptom frequency | |||||||
| Martinez et al. (2011) [36] | 5–18 | 44 weeks | 74 P bid + P/ALB rescue |
✓ | ✓ | ✓ | |
| Pauwels et al. (2003) [41] | 5–66 | 3 years | 3568 P + usual asthma medication |
✓ | ✓ | ✓ | |
| Wongtim et al. (1995) [80] | 33.2 (7.46)¶, 32.8 (8.6)¶ | 8 weeks | 10 | ✓ | |||
| Category 3: RCTs that primarily defined mild asthma with a composite definition of FEV1 ≥80% pred and symptoms <daily | |||||||
| Basyigit et al. (2004) [81] | 38 (8.2)¶, 42.4 (9.6)¶, 45.5 (10.9)¶ | 8 weeks | ✓ | ✓ | |||
| Bousquet et al. (2005) [29] | 15–80 | 48 weeks (12 DB, 36 OL) | ✓ | ✓ | ✓ | ||
| Chrousos et al. (2005) [82] | 18–65 | 14 days | ✓ | ✓ | |||
| Chuang et al. (2007) [83] | 6–14 | 8 weeks | ✓ | ✓ | |||
| Chuchalin et al. (2005) [33] | 6–87 | 12 months | ✓ | ✓ | ✓ | ||
| Chuchalin et al. (2008) [32] | 12–79 | 52 weeks | 315 | ✓ | ✓ | ||
| Currie et al. (2003) [84] | 36 (4)+ | 2×3 weeks | ✓ | ✓ | |||
| Garcia Garcia et al. (2005) [35] | 6–14 | 12 months | ✓ | ✓ | ✓ | ||
| Karaman et al. (2004) [85] | 8–14 | 12 weeks | ✓ | ✓ | |||
| Maiti et al. (2011) [86] | 18–70 | 4 weeks | ✓ | ✓ | |||
| Ng et al. (2007) [37] | 6–14 | 2×8 weeks | ✓ | ✓ | |||
| Reddel et al. (2008) [42] | 18–80 | 11 months | 21 | ✓ | |||
| Renzi et al. (2010) [43] | ≥12 | 24 weeks | ✓ | ✓ | ✓ | ||
| Riccioni et al. (2002) [87] | 26.9 (12.3)¶, 26.7 (8.6)¶, 28.2 (10.1)¶ | 16 weeks | ✓ | ||||
| Shimoda et al. (2005) [88] | 36.2 (12.8)¶, 35.6 (14.4)¶ | 6 months | ✓ | ✓ | |||
| Tamaoki et al. (2008) [89] | ≥21 | 8 weeks | ✓ | ✓ | |||
| Vatrella et al. (2002) [90] | 18–48 | 16 weeks | ✓ | ✓ | |||
| Zeiger et al. (2005) [91] | 15–85 | 48 weeks (12 DB, 36 OL)§ | ✓ | ✓ | |||
| Zietkowski et al. (2006) [92] | 45.2 (10.9)ƒ, 42 (14)ƒ, 51 (7.6)ƒ | 12 weeks | ✓ | ✓ | |||
| Category 4: RCTs that primarily defined mild asthma with a composite definition of FEV1 approximately >60–80% and symptoms <daily | |||||||
| Berger et al. (2009) [48] | ≥12 | 16 weeks | 177 | ✓ | |||
| Boulet et al. (2000) [93] | ≥12 | 12 weeks | ✓ | ✓ | |||
| Drazen et al. (1996) [34] | 12–55 | 20 weeks (16 weeks active treatment + 4 weeks withdrawal (OL ALB as needed)) | 129 P + ALB as needed## |
✓ | ✓ | ||
| Herjavecz et al. (1999) [55] | 17–67 | 22 weeks (6 DB; 16 OL) | ✓ | ✓ | ✓ | ||
| O'Byrne et al. (2001) [39] | ≥12 | 1 year | Group A: 239 | ✓ | ✓ | ||
| O'Sullivan et al. (2003) [94] | 19–50 | 2×8 weeks | ✓ | ✓ | |||
| Papi et al. (2007) [40] | 18–65 | 6 months | 118 P bid + ALB 100 μg as needed |
✓ | ✓ | ||
| Peters et al. (2007) [56] | ≥6 | 16 weeks | ✓ | ✓ | ✓ | ||
| Stone et al. (2001) [95] | ≥16 | 4 weeks | ✓ | ✓ | |||
| Tattersfield et al. (2001) [45] | 20–60 | 2 years | ✓ | ✓ | |||
| Tomlinson et al. (2005) [46] | 20–60 | 12 weeks | ✓ | ✓ | ✓ | ||
| van Grunsven et al. (1996) [47] | ≥30 | 2 years | ✓ | ✓ | ✓ | ||
| Verberne et al. (1996) [58] | 7–16 | 4 months | ✓ | ✓ | ✓ | ||
| Vermetten et al. (1999) [96] | 18–66 | 12 weeks | ✓ | ✓ | |||
| Woodcock et al. (2002) [59] | 18–65 | 6 weeks | ✓ | ✓ | |||
| Category 5: RCTs that defined mild asthma by other/miscellaneous criteria | |||||||
| Arets et al. (2002) [23] | 5–10 | 12 weeks | 33 | ✓ | ✓ | ||
| Boushey et al. (2005) [28] | 18–65 | 1 year | ✓ | ✓ | ✓ | ||
| Villaran et al. (1999) [97] | 14–45 | 8 weeks | ✓ | ✓ | |||
| Category 5: RCTs that did not describe their patients as having “mild asthma” but included patients with disease characteristics similar to the categories described above | |||||||
| Bailey et al. (2008) [24] | 12–65 | 52 weeks | ✓ | ✓ | ✓ | ||
| Barnes et al. (2007) [98] | ≥12 | 12 weeks | ✓ | ✓ | |||
| Bateman et al. (2012) [25] | ≥12 | 8 weeks | 94 | ✓ | ✓ | ✓ | |
| Busse et al. (2001) [30] | >15 | 24 weeks | ✓ | ✓ | ✓ | ||
| Busse et al. (2001) [53] | 12–75 | 12 weeks | 114 | ||||
| van der Molen et al. (1998) [99] | 18–50 | 12 weeks | ✓ | ✓ | |||
| Category 6: RCTs that included patients <5 years old | |||||||
| Bisgaard et al. (2005) [27] | 2–5 | 48 weeks | 271 | ✓ | ✓ | ✓ | |
| Shah et al. (2014) [100] | 2–18 | 12 weeks | ✓ | ✓ | |||
| Szefler et al. (2007) [44] | 2–8 | 52 weeks | ✓ | ✓ | ✓ | ||
| Category 6: RCTs that included patients <5 years old that did not describe their patients as having “mild asthma” but included patients with disease characteristics similar to mild asthma | |||||||
| Robertson et al. (2007) [21] | 2–14 | 12 months | 113 | ✓ | ✓ | ||
| Skoner et al. (2005) [57] | 2–5 | 3 weeks | 50 | ✓ | |||
| Observational/other | |||||||
| Category 1: Observational/other studies that primarily defined mild asthma by treatment level | |||||||
| Ding and Small (2017) [49] | ≥12 | – | NA | ✓ | ✓ | ||
| Friedman et al. (2010) [50] | 12–25 | – | NA | ✓ | |||
| Friedman et al. (2010) [51] | 12–65 | – | NA | ✓ | |||
| McIvor et al. (2009) [22] | ≥6 | Survey + 6-week treatment | NA | ✓ | ✓ | ||
| Navaratnam et al. (2009) [52] | 12–65 | – | NA | ✓ | |||
| Category 3: Observational/other studies that primarily defined mild asthma with a composite definition of FEV1 ≥80% and symptoms<daily | |||||||
| Giraud et al. (2006) [54] | ≥18 | 4–8 weeks | NA | ✓ | ✓ | ||
| Lai et al. (2003) [61] | Total (not just mild asthma) Children: 7.4 (3.8)¶Adults: 40.5 (18.5)¶ | – | NA | ✓ | |||
| Category 4: Observational/other studies that primarily defined mild asthma with a composite definition of FEV1 approximately >60–80% and symptoms<daily | |||||||
| Soyer et al. (2009) [60] | 6–18 | – | NA | ✓ | |||
| Category 6: Observational/other studies that included patients <5 years old | |||||||
| König et al. 1996 [62] | ≤17 | – | NA | ✓ | ✓ | ||
| Robertson et al. (1992) [63] | ≤20 | – | NA | ✓ | |||
RCT: randomised controlled trial; bid: twice daily; P: placebo; ALB: albuterol; DB: double-blind; OL: open-label; FEV1: forced expiratory volume in 1 s; NA: not applicable. # History of exacerbations considered to be exacerbation, hospitalisation or emergency department visit or oral corticosteroid use that occurred prior to enrolment/screening (RCTs or prospective observational studies) or in the pre-index period (retrospective studies); ¶: mean (sd) age in years; +: mean (se) age. §: 10% of participants (determined at randomisation) switched therapies to preserve the masking in the preceding period; ƒ: median (range) age; ##: no dose given.
Definitions of exacerbations for baseline characteristics and outcome measures
Of the 54 RCTs included in this review, 26 [23–48] reported a definition of an exacerbation or SARE and of the 10 observational/other studies, four [49–52] did so (table S4). There was a wide variety of definitions of exacerbations.
Of the studies included in table 2 defining an exacerbation, exacerbation severity was defined in all but 10 RCTs [21, 24, 25, 27, 28, 34, 36, 37, 48, 53], but only in one observational/other study [49]. Most definitions of mild exacerbations related to changes in peak expiratory flow, symptoms and/or reliever use (table S4). Two studies included a definition of moderate exacerbations which included worsening of asthma requiring initiation of additional asthma treatment [32, 38]. In one of these, SYGMA 1 (SYmbicort Given as needed in Mild Asthma [38]), the definition was consistent with the ATS/ERS Task Force definition (table S5) [11]. One cross-sectional survey study included a wide definition of moderate-to-severe exacerbations that included physician-assessed worsening, ED visits, hospitalisations, use of systemic corticosteroids and antibiotics [49].
The majority of definitions for severe exacerbations related to use of systemic corticosteroids, hospitalisation/ED visits or unscheduled physician visits. Of publications for which severe exacerbations are reported, three publications completely aligned [26, 38, 42] and four broadly aligned [32, 39–41] with the ATS/ERS Task Force criteria [11] defining a severe exacerbation (table S6).
An additional category of severe exacerbations, SAREs, was defined in the START study (inhaled Steroid Treatment As Regular Therapy in early asthma study) [41] as asthma-related ED visits, hospitalisations and deaths, i.e. excluding exacerbations identified only by oral corticosteroid use; these were therefore likely to be more severe than the majority of severe exacerbations defined in other studies by the ATS/ERS criteria.
In general, RCTs for which baseline data (pre-study history of exacerbations) are included in the review (table 3) did not provide a separate definition for exacerbations occurring prior to study entry. It is assumed that the same definition applied to both pre-study and within-study exacerbations.
TABLE 3.
Retrospectively collected data on asthma exacerbations, hospitalisation and emergency department admissions/visits
| Study (year) [ref.] | Age range years | Study groups n | Exacerbation parameter(s) | Data for exacerbations by specified parameter, for each study group |
| Exacerbations reported in ≤6 months prior to study entry | ||||
| Bateman et al. (2012) [25] | ≥12 | 97, 100, 110, 95, 102, 94 | Pts with ≥1 exacerbation in last 6 months | 18%, 19%, 16%, 25%, 17%, 21% |
| McIvor et al. (2009) [22]# | ≥6 | 534 | Patients with any exacerbation in 6 weeks prior to study entry | 51.7% |
| Skoner et al. (2005) [57] | 2–5 | 58, 51, 52, 50 | No. of exacerbations in last 30 days | Mean: 1.8, 1.3, 1.5, 1.2 Median: 0,1,0, 0 |
| Pts with ≥1 exacerbation in last 30 days | 48.3%, 52.9%, 48.1%, 36.7% | |||
| Woodcock et al. (2002) [59] | 18–65 | 86, 86 | Mean no. daytime asthma attacks in 7-day run-in | 0.25, 0.18 |
| Mean no. night-time asthma attacks in 7-day run-in | 0.10, 0.10 | |||
| Exacerbations reported in 12 months/1 year prior to study entry | ||||
| Bateman et al. (2018) [26] | 12–83 | 2089, 2087 | Pts with 1 severe exacerbation in last 12 months | 17.5%, 17.3% |
| Pts with ≥1 severe exacerbations in last 12 months | 22%, 21.9% | |||
| Pts with ≥2 severe exacerbations in last 12 months | 4.5%, 4.7% | |||
| Ding and Small (2017) [49] | ≥12 | 524, 591 | Pts with 1 exacerbation in last 12 months | 9.0%, 13.1% |
| Pts with ≥3 exacerbations in last 12 months | 3.4%, 1.9% | |||
| Mean (sd) no. of moderate-to-severe exacerbations in last 12 months | 0.2 (0.6)¶ | |||
| Mean (sd) no. of exacerbations treated in ED or hospital in last 12 months | 0.1 (0.4), 0.1 (0.3) | |||
| Mean (sd) no. of exacerbations treated with OCS, antibiotics, ED or hospital admission in last 12 months+ | 0.1 (0.5), 0.2 (0.6) | |||
| Pts with 1 exacerbation treated with OCS, antibiotics, ED or hospital admission in last 12 months+ | 5.8%, 10.8% | |||
| Pts with ≥3 exacerbations treated with OCS, antibiotics, ED or hospital admission in last 12 months+ | 1.0%, 1.5% | |||
| Giraud et al. (2006) [54] | ≥18 | 94 | Pts hospitalised for asthma in previous 12 months | 4.3% |
| Herjavecz et al. (1999) [55] | 17–67 | 90, 91 | Time since last exacerbation | 12.5 months, 13.0 months |
| Lai et al. (2003) [61] | Mean 7.4–40.5 | 1709, 633 | Pts with hospital admissions in last year | 7.3%, 15.4% |
| Pts with any hospital ED/unscheduled emergency visit in the last year | 33.1%, 41.3% | |||
| Martinez et al. (2011) [36] | 5–18 | 71, 72, 71,74 | Mean no. hospital visits for asthma in last 1 year | 0.3, 0.3, 0.2, 0.2 |
| O’Byrne et al. (2018) [38] | ≥12 | 1277, 1277,1282 | Pts with ≥1 severe exacerbation in last 12 months | 20.0%, 20.1%, 18.8% |
| Peters et al. (2007) [56] | ≥6 | 166, 165, 169 | Pts with ≥1 urgent visit for asthma in last 1 year | 30.7%, 35.8%, 35.5% |
| Robertson et al. (2007) [21]§ | 2–14 | 97, 105 | Median no. of ED attendances for asthma in last 1 year | 1, 1 |
| Median no. of hospital admissions for asthma in last 1 year | 1, 1 | |||
| Soyer et al. (2009) [60] | 6–18 | 522 | Mean (sd) no. of unscheduled visits per patient | 1.2 (0.2) |
| Mean (sd) no. of ED visits per patient | 0.6 (0.05) | |||
| Verbene et al. (1996) [58] | 7–16 | 30 | Pts hospitalised for asthma in last 1 year | 0% |
All studies were randomised controlled trials except Giraud et al. [54], McIvor et al. [22], Ding and Small [49], Lai et al. [61] and Soyer et al. [60]. pts: patients; ED: emergency department; OCS: oral corticosteroids; no.: number. # Study included patients with a history of exacerbations (time frame not specified); ¶ n=1076; + time frame not specified in Results section of [49] although Methods section suggests 12-month timeframe; §: Study included only patients with a history of 3–6 exacerbations (hospitalisation or ED visit or general practitioner visits) in the 12 months prior to enrolment/screening.
Retrospective exacerbation data collected from patient self-report
Fourteen prospective studies in patients with mild asthma incorporated data on exacerbation or exacerbation-related healthcare utilisation during the pre-study period as reported in the baseline characteristics [21, 22, 25–27, 36, 38, 41, 54–59]; these included exacerbations (n=7 studies) hospitalisation or ED admissions/visits (n=8) (table 3), and systemic corticosteroid use (n=5) (table S7). In addition, exacerbation data were collected in a prospective survey [49] and hospitalisation/ED visit data were collected from a patient questionnaire [60] and population survey [61] (table 3).
There was a wide range across studies in the proportion of patients who in the preceding year were hospitalised for asthma (i.e. presumably for a severe exacerbation) or had at least one severe exacerbation: 0–22% [26, 38, 54, 58, 61]. Two of the largest and most recent of these studies found that 18.8–22.0% of patients reported having had at least one severe exacerbation in the year prior to enrolment [26, 38].
In the START study of over 7000 patients with mild recent-onset asthma, close to 5% of patients had received systemic corticosteroids in the 6 weeks prior to study entry (table S7) [41], whereas a pre-post study recruiting patients with uncontrolled asthma, despite low-dose ICS, reported that 52% of patients had an exacerbation (affecting activities and sleep) in the 6 weeks prior to study entry [22]. However, it is difficult to interpret exacerbation incidence over such a short period of time. An RCT in patients with poorly controlled asthma, not taking ICS, found 16–25% of patients across all groups had at least one exacerbation in the 6 months prior to study entry [25].
In a paediatric study that required children to have a history of multiple exacerbations in the previous year, without any interval symptoms, the rate of hospital admissions/ED visit for asthma during that year was low (table 3) [21]. The mean±sd number of ED visits per year was 0.6±0.5 in another study in paediatric patients, of whom ≈50% were untreated and ≈45% received ICS with or without bronchodilators [60].
Exacerbation data from administrative databases and medical records
Five studies collected data on exacerbations from claims databases or medical records (table S8) [50–52, 62, 63], with all but one covering a time period of 1 year. Several outcomes were captured, including mean number of exacerbations (n=3) [50–52], and hospitalisations or ED visits (n=2) [62, 63]. In the three database claims studies in patients with mild asthma aged 12–65 years [50–52], the mean number of exacerbations ranged from 0.12–0.19, depending on which of three maintenance ICS they received. In a study of asthma-related deaths in 51 children aged 2–14 years, 17 (33%) patients had mild asthma (based on features such as symptoms less than monthly, exercise limitation only on active sports, or less than 1 week's school absence in the previous year) [63].
Prospective exacerbation data
A total of nine RCT publications reported data on exacerbation/exacerbation-related outcomes in a placebo arm (table 4), including exacerbations (n=8) [27, 32, 36, 38–42], SAREs (n=1) [41], systemic corticosteroid use (n=3) [21, 27, 41], and hospitalisation, ED admissions/visits and unscheduled visits/healthcare resource use (n=4) [21, 27, 32, 38]. Data on exacerbations were reported using a variety of parameters, including patients with exacerbations (percentage or number; n=6) [27, 38–42], exacerbation rate (n=5) [27, 32, 38–40], probability of a first exacerbation by the end of the trial (n=1) [36], time to first exacerbation (n=1) [27], withdrawal/discontinuation due to exacerbation (n=1) [32] and treatment failure (n=1) [36].
TABLE 4.
Prospective data on exacerbations and exacerbation-related outcomes in mild asthma from placebo arms of RCTs of ≥24 weeks' duration
| Study (year) [ref.] | Age range years | Placebo n | Other asthma medication in placebo arm | Treatment duration | Exacerbation parameter | Placebo arm |
| Studies not excluding patients with exacerbation history+ | ||||||
| Bisgaard et al. (2005) [27] | 2–5 | 271 | Rescue OCS or ICS or β2-agonist | 48 wks | Pts with exacerbation§ | 56% |
| Exacerbation rate/year (n=257)ƒ | 2.34 | |||||
| Median time to first exacerbation | 147 days | |||||
| Pts with ≥1 unscheduled visit to physician for asthmaƒ | 42.4% | |||||
| Pts hospitalised for asthmaƒ | 5.8% | |||||
| Rate of OCS courses/yearƒ | 0.64 | |||||
| Chuchalin et al. (2008) [32] | 12–79 | 315 | Rescue ALB | 52 wks | Mean exacerbation rate per pt per year (mild, moderate, severe) | 2.88 |
| Moderate (OCS) or severe exacerbation (hospitalisation) rate/year | 0.33 | |||||
| No. unscheduled asthma-related healthcare contacts | 7 | |||||
| Pt withdrawal/discontinued due to exacerbation, n | 8 | |||||
| Martinez et al. (2011) [36] | 5–18 | 74 | Rescue ALB | 44 wks | Probability (95% CI) of first exacerbation by end of trial requiring prednisone course | 49 (37–61)% |
| Proportion with treatment failure (all defined by requirement for a second course of prednisone) | 23% | |||||
| O'Byrne et al. (2001) [39] | ≥12 | 239 | Yes - only after first exacerbation (n=104)¶¶ | 1 yr | Pts with severe exacerbation | 33.3% |
| No. of pts with severe exacerbation, pts treated with OCSs | 70.9% | |||||
| Pts receiving systemic corticosteroids | 23.6% | |||||
| Severe exacerbation rate per pt per year | 0.77 | |||||
| O’Byrne et al. (2018) [38] | ≥12 | 1277 | TERB 0.5 mg as needed | 1 yr | Pts with ≥1 moderate or severe exacerbation | 21.5% |
| Pts with ≥1 severe exacerbation | 11.9% | |||||
| Annualised severe exacerbation rate | 0.20 | |||||
| Papi et al. (2007) [40] | 18–65 | 118 | ALB as-needed | 6 months | Pts with severe exacerbation | 3.4% |
| Pts with ≥1 exacerbation | 17.80% | |||||
| Mean no. of exacerbations/pt/year | 1.63 | |||||
| Pauwels et al. (2003) [41] | 5–66 | 3568 | Usual asthma treatment (SABA 64.6% of placebo pts) plus ICS or systemic corticosteroid if needed | 3 yrs | Pts with life-threatening exacerbation over 3 years, n | 24 (0.67%) |
| Pts with ≥1 SARE over 3 years, n | 198 | |||||
| Pts with ≥2 SAREs over 3 years, n | 49 | |||||
| Mean no. of courses of systemic corticosteroids per year | 0.21 | |||||
| Pts using systemic corticosteroids | 3 months: 4.1% | |||||
| 12 months: 3.1% | ||||||
| 24 months: 3.3% | ||||||
| 36 months: 2.0% | ||||||
| Pts with ≥1 systemic corticosteroid course | 23% | |||||
| Studies excluding patients with exacerbation history+ | ||||||
| Reddel et al. (2008) [42] | 18–80 | 21 | ALB as-needed | 11 months | Pts with ≥1 mild exacerbation, n | 13 |
| Pts with severe exacerbation, n | 3 | |||||
| Studies requiring patients to have a history of frequent exacerbations# | ||||||
| Robertson et al. (2007) [21] | 2–14 | 113 | Inhaled β2-agonist or OCS for acute asthma episode | 12 months | Proportion of children with ≥1 episode treated with short course of randomised therapy, n (%) | 105 (92.9%) |
| Total number of treated episodes of asthma | 336 | |||||
| Proportion of treated asthma episodes utilising ≥1 health resource, n (%) | 134 (39.9%) | |||||
| Proportion of treated asthma episodes requiring ED visit, n (%) | 46 (13.7%) | |||||
| Proportion of treated asthma episodes requiring hospitalisation, n (%) | 13 (3.9%) | |||||
| Proportion of treated asthma episodes with OCS use, n/N pts with diary data (%) | 78 of 321 (24.3%) | |||||
RCT: randomised controlled trial; ED: emergency department; wks: weeks; pt: patient; OCS: oral corticosteroid; ICS: inhaled corticosteroid; CI: confidence interval; SABA: short-acting β2-agonist; SARE: severe asthma-related event; ALB: albuterol; TERB: terbutaline; GP: general practitioner; LABA: long-acting β2-agonist. # Study included only patients with a history of 3–6 exacerbations (hospitalisation or ED visit or GP visits) within 12 months prior to enrolment/screening); ¶ Unscheduled visits to GP, specialist paediatrician, ED or admission to hospital; +: Exacerbation history defined as an exacerbation, hospitalisation or ED visit or OCS use occurring ≥3 months prior to enrolment/screening; §: data appear to relate to 48-week double-blind period only (total study duration 1 year including screening and single-blind, placebo run-in period); ƒ: source publication refers to “yearly” data, but double-blind treatment period only 48 weeks (total study duration 1 year including screening and single-blind, placebo run-in period); ##: defined as the requirement for a second dose of prednisone within any 6-month period; ¶¶: most common extra medication in placebo group was systemic corticosteroids (n=56), ICS (n=15) and LABAs (n=11).
Study-reported annual exacerbation rates of any severity in placebo recipients ranged from 0.36–2.88 (table 4) [27, 32, 40]. Few studies reported the proportions of patients with one or more exacerbations of any severity, and only one did so for a time period of 1 year [38]. In an RCT of adult patients previously stable on medium-dose ICSs, 21 of 118 (17.8%) of those randomised to placebo plus as-needed salbutamol had ≥1 exacerbation over a 6-month period [40]. In the 48-week double-blind period of a 1-year study in preschool children aged 2–5 years with intermittent asthma symptoms triggered by viral infection, 56% had ≥1 exacerbation and 5.8% were hospitalised for asthma [27]. In a small 11-month RCT that excluded patients with an exacerbation in the previous year, 13 of 21 placebo recipients were reported as having ≥1 “mild” asthma exacerbations [42]. In an RCT of adolescents and adults with asthma well-controlled on low dose ICS or LTRA or uncontrolled on short-acting bronchodilators alone, 274 of 1277 (21.5%) of those randomised to placebo plus as-needed terbutaline had ≥1 moderate or severe exacerbations, and 152 (11.9%) had ≥1 severe exacerbations, over a 12 month period [38].
Regarding use of healthcare resources, in an RCT in paediatric patients, 113 placebo recipients had 228 unscheduled acute healthcare utilisations during 12 months [21].
Exacerbation rates in placebo recipients
Six of the seven publications in which the definition of severe exacerbations aligned/broadly aligned with the ATS/ERS Task Force definition (table S6) [11] had relevant outcomes data from a placebo arm [32, 38–42]. The annual rate of severe exacerbations was reported from the placebo arms of three trials in mild asthma (table 4): 0.77 in ICS-free patients aged ≥12 years (OPTIMA A) [39], 0.33 in patients aged 12–79 years [32], and 0.20 in 1277 patients aged ≥12 years (SYGMA 1) [38]. In the START trial in patients aged 5–66 years with newly diagnosed mild asthma, exacerbation rate was not reported per se, but the annualised mean number of courses of systemic corticosteroids was 0.21 [41], which may be considered a proxy for the exacerbation rate in this study, according to the ATS/ERS Task Force definition of exacerbations based on systemic corticosteroid use [11]. In three of these studies, additional medications were allowed: other asthma medications in both the OPTIMA A study (after the first severe exacerbation) [39] and the START study [41], and open-label ICSs for persistent poorly controlled asthma or prolonged exacerbation in SYGMA 1; [38] in the remaining study [32], placebo recipients were only allowed SABA medication.
The proportion of placebo recipients in RCTs experiencing a severe exacerbation in ∼1 year varied widely (table 4), from 33.3% of placebo recipients in the OPTIMA A study (23.4% if only oral corticosteroids were considered) [39], to 11.9% in the SYGMA 1 study [38], both studies being 12-month studies in patients aged ≥12 years, and 3.4% in the 6-month BEST study in patients ≥18 years whose asthma had been well controlled on moderate-dose ICSs [40]. In a small study which excluded patients with a history of exacerbations, a severe exacerbation occurred in 3 of 21 placebo recipients (14.2%) aged 18–80 years with mild asthma and minimal symptoms at study entry [42].
Of 3568 placebo recipients in the START trial, in which patients could receive additional maintenance asthma treatment, 198 (5.5%) experienced one or more SARE during the 3-year double-blind period [41].
Discussion
This systematic review collated data on definitions of mild asthma and summarised the burden of exacerbations in this patient population. There were two key findings. The first was that there were multiple different definitions of “mild” asthma, which in RCTs were most commonly based on symptoms and lung function and, in observational/administrative studies, were often based on treatment level, independent of symptoms. The second was that, for patients considered to have mild asthma and treated with as-needed SABA alone, the mean exacerbation rate (all severities) was as high as 2.88 per patient per year [32], with up to 42% of patients requiring unscheduled physician visits for their asthma [27], although this high rate of visits was in preschool children. In adolescents and adults, the mean number of unscheduled asthma-related healthcare visits in a 52-week study was 7 [32]. The annualised severe exacerbation rate in the placebo arm of prospective studies of 24 or more weeks’ duration ranged from 0.20 [38] to 0.77 [39].The proportion of patients with a severe exacerbation also varied widely, from 3.4% [40] to 33.3% [39]. Considering even more severe events, a large placebo-controlled study found that 0.67% of patients with newly diagnosed mild asthma experienced life-threatening exacerbations over a 3-year period, despite their treating physician being able to prescribe additional asthma medications [41]. These results clearly indicate that mild asthma, although variably defined in these studies, is associated with a considerable risk of exacerbations including severe, life-threatening exacerbations, which represent a significant burden to both the patient and to healthcare systems [4].
It has previously been reported that 5–13% of investigated deaths due to asthma occurred in patients “being treated for mild asthma” (i.e. with SABA alone [64], or judged to have “trivial or mild asthma” [65, 66]). In the latter two studies, 15–20% of patients had symptoms less than weekly in the previous 3 months [65, 66]. In the current review, one study found that 17 of 51 (33%) children with asthma-related death had been assessed as having had mild asthma [63]. Few of these 17 children had features flagging them as being high risk (e.g. only three had had a hospital admission in the year preceding their death and one had ever been treated in an intensive care unit (ICU)) [63]. These data exemplify not only the risk of death across the spectrum of asthma but also the need for a more sensitive marker for identifying patients at risk of SARE who may not have necessarily had a previous hospital or ICU admission.
This systematic review highlights the very marked heterogeneity in the assessment and definition of mild asthma. Where a definition was provided, it was either the investigators’ own definition or was taken from one of the various guidelines or reports published between 1987 and 2015 (table S9). We grouped studies by their definition of mild asthma into six broad categories (tables 1 and S2); only the first category (treatment) reflects the approach taken in current consensus recommendations (e.g. GINA 2020 [1]). Indeed, only a few of the most recent studies included in this review followed mild asthma definitions according to contemporaneous GINA recommendations [26, 38, 49]. Mild asthma may be determined arbitrarily by treatment step according to patient self-report or in administrative databases (where no clinical details are available), or more reliably but probably far less often, by a systematic approach (via step-down titration) [67] based on the minimum treatment needed to maintain good asthma control. These approaches differ substantially from a common perception that mild asthma means infrequent and/or easily relieved symptoms [9].
Describing the severity of asthma in a study population by treatment step (e.g. referring to mild asthma as “Step 1 or 2 patients”), appears problematic, but in epidemiologic studies, prescribed treatment may be the only available data. In addition, a patient's asthma prescription may not have been clinically appropriate. For example, in the National Review of Asthma Deaths, 9% of deaths occurred in patients “being treated for mild asthma” [64]. For epidemiologic studies, GINA recommends naming the treatment rather than inferring severity (e.g. “being treated with low-dose ICS”) [1]. This approach would also avoid ambiguity about whether a “Step 2 patient” is someone eligible for Step 2 treatment, or taking Step 2 treatment.
Analysis of the inclusion and exclusion criteria relating to exacerbation history for each study (table 1), indicates that some authors considered that a history of exacerbations in the previous 12 months should preclude classification of asthma as mild. However, this did not apply to the majority of studies in this review (except for the common practice of excluding recent exacerbations to ensure clinical stability at baseline). This review suggests that a credible definition of mild asthma may need to focus on symptom control with or without lung function assessment. However, clinicians and patients must at the same time be made aware that patients with mild asthma may still be at risk of severe and potentially life-threatening or fatal exacerbations, albeit infrequently. This is consistent with the current GINA approach [1], highlighting potential discordance between recent symptom control and risk of future adverse outcomes, and recommending that both should be assessed.
Despite the care taken to focus only on mild asthma, the variations in, or absence of specific definitions for mild asthma in some publications, and the fact that many patients with mild asthma have normal or close to normal lung function, still allows for the possibility that some patients may not have had asthma at all, or were misdiagnosed with “mild asthma”, and hence were treated unnecessarily [12] or were under-treated [64]. Previous studies have shown that up to one-third of patients with “physician-diagnosed” asthma are found not to have asthma when assessed by a respiratory physician using a range of clinical tests [4, 12, 15, 16, 68, 69]. However, the observational studies identified in this review also confirmed that patients with infrequent symptoms had a significant risk of exacerbations, albeit less than seen in studies of patients with more severe asthma [19].
Our analysis also found considerable heterogeneity in definitions of exacerbations; however, definitions of severe exacerbations or SAREs used in prospective studies were aligned or broadly aligned with the ATS/ERS Task Force definition of a severe exacerbation (table S6) [11]. To enable meta-analyses of larger sets of exacerbation data, future studies should be conducted using standardised definitions for moderate and severe exacerbations, such as those proposed in the ATS/ERS statement [11], and utilised in the recent SYGMA studies [26, 38]. However, a similar recommendation for a uniform definition was made in a previous systematic review of the burden of mild asthma in 2005, indicating that little has changed since then [4].
This review was originally intended to include a meta-analysis, but the heterogeneity of definitions found in the available literature precluded such an analysis. However, the number of patients affected reveals the magnitude of the burden of exacerbations and the discordance with the patients’ apparent level of symptom control. The risks of mild asthma, albeit low, can be halved by the regular use of low-dose ICS [39, 41], even in patients with symptoms once a week or less [70]. However, the well-recognised poor adherence to daily maintenance ICSs by patients with mild or intermittent asthma is one reason to advocate a different approach [12, 49, 71, 72]. In addition, practical difficulties in managing patients with episodic symptoms or exacerbations occurring in response to variable environmental triggers such as allergens, respiratory viruses and pollutants, with few interval symptoms [73, 74], provide other examples of the need for a new approach.
Such situations can now be dealt with by use of an anti-inflammatory reliever (ICS/formoterol) that rapidly increases the intensity of both anti-inflammatory and bronchodilator therapy when needed and thus reduces the risks of severe exacerbations without requiring adherence to daily therapy [1, 26, 38, 75–77]. This significant change to the treatment of mild asthma has been recommended by GINA since 2019 [1], and should go some way to reducing the burden of exacerbations [77], by avoiding the discomfort and risk, the potential loss in lung function, and the risks of OCS treatment [78, 79] associated with exacerbations.
The strengths of this systematic review include the care that was taken to ensure that, as far as possible, only data from patients who were considered by the original authors to have (or who had characteristics consistent with) mild asthma were included in the analyses, and the extent of the search: ranging from 1990 to 2019. Studies described as having data on “mild-to-moderate” asthma were excluded, unless data were presented separately for the patients with “mild” asthma.
Potential limitations include the restriction to English-language publications, and that our search terms would likely have missed observational studies that classified the severity of broad asthma populations, and assessed their outcomes, without therapies or key words such as “mild” or “intermittent” having been included in the title or abstract. Some studies selected may have included people who did not have asthma at all. Exacerbation data collected retrospectively at baseline in RCTs may be subject to recall or selection bias or over-reporting by patients. For example, in one of the trials included in this review [38], 20.0% of patients in the placebo group reported having had at least one severe exacerbation in the year prior to enrolment compared to 11.9% who had a severe exacerbation during the subsequent 1-year trial. This demonstrates the difficulty of accurately estimating real world exacerbation rates in mild asthma.
Conclusions
In this systematic review we have shown that more robust, consistent and widely acceptable definitions for both mild asthma and asthma exacerbations are required. Despite being unable to complete a formal meta-analysis we have demonstrated a significant burden, in terms of exacerbations, for this patient population. This highlights not only the need for greater awareness of the risks of so-called “mild” asthma, but also access to effective interventions, such as use of an anti-inflammatory reliever, especially during the episodic periods when increasing symptoms foreshadow an impending exacerbation.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00359-2019.SUPPLEMENT (519.1KB, pdf)
Acknowledgements
The authors would like to thank David Candlish, Lisa Swanson, Matt Weitz and Tracy Harrison of inScience Communications, Springer Healthcare Ltd., UK, for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (www.ismpp.org/gpp3).
Footnotes
This article has supplementary material available from openres.ersjournals.com.
Data availability: Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Author contributions: J.M. FitzGerald contributed to the protocol design, data review and manuscript preparation; P.J. Barnes contributed to data review and manuscript preparation; B.E. Chipps contributed to data review and manuscript preparation; C.R. Jenkins contributed to data review and manuscript preparation; P. O'Byrne contributed to the data review and manuscript preparation; I.D. Pavord contributed to data review and manuscript preparation; H.K. Reddel contributed to the data analysis and presentation and manuscript preparation.
Conflict of interest: J.M. FitzGerald is a member of the Scientific and Executive Committees of the Global Initiative for Asthma. He also reports research funds paid directly to UBC and personal fees from Astra Zeneca, GSK, Sanofi Regeneron and Novartis, personal fees from Teva, during the conduct of the study.
Conflict of interest: P.J. Barnes reports research funding from, scientific advisory boards and consultancy for, and lecture fees from AstraZeneca; research funding from, a scientific advisory board for and lecture fees from Boehringer Ingelheim; a scientific advisory board for and lecture fees from Teva; lecture fees from Novartis; and a scientific advisory board for Pieris, all during the conduct of the study.
Conflict of interest: B.E. Chipps reports personal fees for advisory boards and speaker's bureaus from AstraZeneca, Circassia, Genentech, Novartis, Regeneron, Sanofi-Genzyme and Teva, outside the submitted work.
Conflict of interest: C.R. Jenkins reports personal fees for attending advisory boards where this paper was discussed from AstraZeneca P/L, and personal fees for attending advisory boards and providing educational materials from GlaxoSmithKline, Novartis, Sanofi and Boehringer Ingelheim, during the conduct of the study.
Conflict of interest: P.M. O'Byrne reports grants and personal fees from AstraZeneca, personal fees from GSK, grants from Novartis, grants and personal fees from Medimmune, and personal fees from Chiesi, outside the submitted work.
Conflict of interest: I.D. Pavord reports speaker fees, advisory board honoraria, sponsorship to attend scientific meetings and payments for organising educational events from AstraZeneca; speaker fees, advisory board honoraria and sponsorship to attend scientific meetings from Boehringer Ingelheim; a speaker fee from Aerocrine; speaker fees and advisory board honoraria from Almirall and Novartis; speaker fees, advisory board honoraria and sponsorship to attend scientific meetings from GlaxoSmithKline; advisory board honoraria from Genentech and Regeneron; speaker fees, payments for organising educational events and sponsorship to attend scientific meetings from Teva; speaker fees from Chiesi; advisory board honoraria from Sanofi, Circassia and Knopp; and funding as a Senior Investigator from NIHR, all outside the submitted work.
Conflict of interest: H.K. Reddel reports attending data safety monitoring (DSMB) and advisory boards for, and an honorarium for providing independent medical education and a grant for a registry from AstraZeneca; attending a DSMB and advisory boards for, an unconditional research grant from, an honorarium for providing independent medical education to, consulting for, and study medication from GlaxoSmithKline; attending a DSMB for Merck; attending a DSMB and an advisory board for, and an honorarium for providing independent medical education and a grant for a registry from Novartis; honoraria for providing independent medical education from Teva and Mundipharma; an honorarium for providing independent medical education from and attending an advisory board for Boehringer Ingelheim; and attending an advisory board for Sanofi-Genzyme, all outside the submitted work. Dr Reddel is also Chair of the GINA Scientific Committee.
Support statement: This study was supported by AstraZeneca. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report, but reviewed the manuscript prior to submission. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA) 2020. Global strategy for asthma management and prevention. Date last accessed: 9 July, 2020.
- 2.Jackson DJ, Sykes A, Mallia P, et al. . Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol 2011; 128: 1165–1174. doi: 10.1016/j.jaci.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To T, Stanojevic S, Moores G, et al. . Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012; 12: 204. doi: 10.1186/1471-2458-12-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman KR. Impact of ‘mild' asthma on health outcomes: findings of a systematic search of the literature. Respir Med 2005; 99: 1350–1362. doi: 10.1016/j.rmed.2005.03.020 [DOI] [PubMed] [Google Scholar]
- 5.Ding B, DiBonaventura M, Karlsson N, et al. . A cross-sectional assessment of the prevalence and burden of mild asthma in urban China using the 2010, 2012, and 2013 China National Health and Wellness Surveys. J Asthma 2017; 54: 632–643. doi: 10.1080/02770903.2016.1255750 [DOI] [PubMed] [Google Scholar]
- 6.Dusser D, Montani D, Chanez P, et al. . Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62: 591–604. doi: 10.1111/j.1398-9995.2007.01394.x [DOI] [PubMed] [Google Scholar]
- 7.Hekking PP, Wener RR, Amelink M, et al. . The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 8.Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract 2017; 3: 1. doi: 10.1186/s40733-016-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor DR, Bateman ED, Boulet LP, et al. . A new perspective on concepts of asthma severity and control. Eur Respir J 2008; 32: 545–554. doi: 10.1183/09031936.00155307 [DOI] [PubMed] [Google Scholar]
- 10.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 2007; 120: S94–138. doi: 10.1016/j.jaci.2007.09.029 [DOI] [PubMed] [Google Scholar]
- 11.Reddel HK, Taylor DR, Bateman ED, et al. . An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009; 180: 59–99. doi: 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 12.Brozek GM, Farnik M, Lawson J, et al. . Underdiagnosis of childhood asthma: a comparison of survey estimates to clinical evaluation. Int J Occup Med Environ Health 2013; 26: 900–909. [DOI] [PubMed] [Google Scholar]
- 13.Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir Med 2006; 100: 354–362. doi: 10.1016/j.rmed.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 14.Tirimanna PR, van Schayck CP, den Otter JJ, et al. . Prevalence of asthma and COPD in general practice in 1992: has it changed since 1977? Br J Gen Pract 1996; 46: 277–281. [PMC free article] [PubMed] [Google Scholar]
- 15.van Schayck CP, van Der Heijden FM, van Den Boom G, et al. . Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA project. Thorax 2000; 55: 562–565. doi: 10.1136/thorax.55.7.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaron SD, Vandemheen KL, FitzGerald JM, et al. . Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017; 317: 269–279. doi: 10.1001/jama.2016.19627 [DOI] [PubMed] [Google Scholar]
- 17.Aaron SD, Boulet LP, Reddel HK, et al. . Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med 2018; 198: 1012–1020. doi: 10.1164/rccm.201804-0682CI [DOI] [PubMed] [Google Scholar]
- 18.Sadatsafavi M, Lynd L, Marra C, et al. . Direct health care costs associated with asthma in British Columbia. Can Respir J 2010; 17: 74–80. doi: 10.1155/2010/361071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloom CI, Nissen F, Douglas IJ, et al. . Exacerbation risk and characterisation of the UK's asthma population from infants to old age. Thorax 2018; 73: 313–320. doi: 10.1136/thoraxjnl-2017-210650 [DOI] [PubMed] [Google Scholar]
- 20.James AL, Elliot JG, Abramson MJ, et al. . Time to death, airway wall inflammation and remodelling in fatal asthma. Eur Respir J 2005; 26: 429–434. doi: 10.1183/09031936.05.00146404 [DOI] [PubMed] [Google Scholar]
- 21.Robertson CF, Price D, Henry R, et al. . Short-course montelukast for intermittent asthma in children: a randomized controlled trial. Am J Respir Crit Care Med 2007; 175: 323–329. doi: 10.1164/rccm.200510-1546OC [DOI] [PubMed] [Google Scholar]
- 22.McIvor RA, Kaplan A, Koch C, et al. . Montelukast as an alternative to low-dose inhaled corticosteroids in the management of mild asthma (the SIMPLE trial): an open-label effectiveness trial. Can Respir J 2009; 16: Suppl A, 11A–21A. doi: 10.1155/2009/429482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arets HGM, Kamps AWA, Brackel HJL, et al. . Children with mild asthma: do they benefit from inhaled corticosteroids? Eur Respir J 2002; 20: 1470–1475. doi: 10.1183/09031936.02.00292702 [DOI] [PubMed] [Google Scholar]
- 24.Bailey W, Castro M, Matz J, et al. . Asthma exacerbations in African Americans treated for 1 year with combination fluticasone propionate and salmeterol or fluticasone propionate alone. Curr Med Res Opin 2008; 24: 1669–1682. doi: 10.1185/03007990802119111 [DOI] [PubMed] [Google Scholar]
- 25.Bateman ED, Bleecker ER, Lotvall J, et al. . Dose effect of once-daily fluticasone furoate in persistent asthma: a randomized trial. Respir Med 2012; 106: 642–650. doi: 10.1016/j.rmed.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 26.Bateman ED, Reddel HK, O'Byrne P, et al. . As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. N Engl J Med 2018; 378: 1877–1887. doi: 10.1056/NEJMoa1715275 [DOI] [PubMed] [Google Scholar]
- 27.Bisgaard H, Zielen S, Garcia-Garcia ML, et al. . Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am J Respir Crit Care Med 2005; 171: 315–322. doi: 10.1164/rccm.200407-894OC [DOI] [PubMed] [Google Scholar]
- 28.Boushey H, Sorkness C, King TS, et al. . Daily versus as-needed corticosteroids for mild persistent asthma. N Eng J Med 2005; 352: 1519–1528. doi: 10.1056/NEJMoa042552 [DOI] [PubMed] [Google Scholar]
- 29.Bousquet J, Menten J, Tozzi CA, et al. . Oral montelukast sodium versus inhaled fluticasone propionate in adults with mild persistent asthma. J Applied Res 2005; 5: 402–414. [Google Scholar]
- 30.Busse W, Raphael GD, Galant S, et al. . Low-dose fluticasone propionate compared with montelukast for first-line treatment of persistent asthma: a randomized clinical trial. J Allergy Clin Immunol 2001; 107: 461–468. doi: 10.1067/mai.2001.114657 [DOI] [PubMed] [Google Scholar]
- 31.Camargos P, Affonso A, Calazans G, et al. . On-demand intermittent beclomethasone is effective for mild asthma in Brazil. Clin Transl Allergy 2018; 8: 7. doi: 10.1186/s13601-018-0192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuchalin A, Jacques L, Frith L. Salmeterol/fluticasone propionate via Diskus™ once daily versus fluticasone propionate twice daily in patients with mild asthma not previously receiving maintenance corticosteroids. Clin Drug Investig 2008; 28: 169–181. doi: 10.2165/00044011-200828030-00004 [DOI] [PubMed] [Google Scholar]
- 33.Chuchalin A, Kasl M, Bengtsson T, et al. . Formoterol used as needed in patients with intermittent or mild persistent asthma. Respir Med 2005; 99: 461–470. doi: 10.1016/j.rmed.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 34.Drazen JM, Israel E, Boushey HA, et al. . Comparison of regularly scheduled with as-needed use of albuterol in mild asthma. N Engl J Med 1996; 335: 841–847. doi: 10.1056/NEJM199609193351202 [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Garcia ML, Wahn U, Gilles L, et al. . Montelukast, compared with fluticasone, for control of asthma among 6- to 14-year-old patients with mild asthma: the MOSAIC study. Pediatrics 2005; 116: 360–369. doi: 10.1542/peds.2004-1172 [DOI] [PubMed] [Google Scholar]
- 36.Martinez FD, Chinchilli VM, Morgan WJ, et al. . Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet 2011; 377: 650–657. doi: 10.1016/S0140-6736(10)62145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng DKK, Chan CH, Chow PY, et al. . Oral montelukast versus inhaled budesonide in children with mild persistent asthma: a pilot study. HK J Paediatr 2007; 12: 3–10. [Google Scholar]
- 38.O'Byrne P, FitzGerald J, Bateman E, et al. . Inhaled combined budesonide–formoterol as needed in mild asthma. N Engl J Med 2018; 378: 1865–1876. doi: 10.1056/NEJMoa1715274 [DOI] [PubMed] [Google Scholar]
- 39.O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. . Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164: 1392–1397. doi: 10.1164/ajrccm.164.8.2104102 [DOI] [PubMed] [Google Scholar]
- 40.Papi A, Canonica GW, Maestrelli P, et al. . Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med 2007; 356: 2040–2052. doi: 10.1056/NEJMoa063861 [DOI] [PubMed] [Google Scholar]
- 41.Pauwels RA, Pedersen S, Busse WW, et al. . Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 2003; 361: 1071–1076. doi: 10.1016/S0140-6736(03)12891-7 [DOI] [PubMed] [Google Scholar]
- 42.Reddel HK, Belousova EG, Marks GB, et al. . Does continuous use of inhaled corticosteroids improve outcomes in mild asthma? A double-blind randomised controlled trial. Prim Care Respir J 2008; 17: 39–45. doi: 10.3132/pcrj.2008.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renzi PM, Howard LA, Ortega HG, et al. . Low-dose fluticasone propionate with and without salmeterol in steroid-naive patients with mild, uncontrolled asthma. Respir Med 2010; 104: 510–517. doi: 10.1016/j.rmed.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 44.Szefler SJ, Baker JW, Uryniak T, et al. . Comparative study of budesonide inhalation suspension and montelukast in young children with mild persistent asthma. J Allergy Clin Immunol 2007; 120: 1043–1050. doi: 10.1016/j.jaci.2007.08.063 [DOI] [PubMed] [Google Scholar]
- 45.Tattersfield A, Town GI, Johnell O, et al. . Bone mineral density in subjects with mild asthma randomised to treatment with inhaled corticosteroids or non-corticosteroid treatment for two years. Thorax 2001; 56: 272–278. doi: 10.1136/thorax.56.4.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomlinson JEM, McMahon AD, Chaudhuri R, et al. . Efficacy of low and high dose inhaled corticosteroid in smokers versus non-smokers with mild asthma. Thorax 2005; 60: 282–287. doi: 10.1136/thx.2004.033688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Grunsven PM, Dompeling E, van Schayck CP, et al. . Treatment of mild asthma with inhaled corticosteroids: is discontinuation of therapy possible? Fam Med 1996; 28: 46–51. [PubMed] [Google Scholar]
- 48.Berger WE, Kerwin E, Bernstein DI, et al. . Efficacy and safety evaluation of ciclesonide in subjects with mild-to-moderate asthma not currently using inhaled corticosteroids. Allergy Asthma Proc 2009; 30: 304–314. doi: 10.2500/aap.2009.30.3242 [DOI] [PubMed] [Google Scholar]
- 49.Ding B, Small M. Disease burden of mild asthma: findings from a cross-sectional real-world survey. Adv Ther 2017; 34: 1109–1127. doi: 10.1007/s12325-017-0520-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman HS, Navaratnam P, McLaughlin J. Adherence and asthma control with mometasone furoate versus fluticasone propionate in adolescents and young adults with mild asthma. J Asthma 2010; 47: 994–1000. doi: 10.1080/02770903.2010.513076 [DOI] [PubMed] [Google Scholar]
- 51.Friedman HS, Urdaneta E, McLaughlin JM, et al. . Mometasone furoate versus beclomethasone dipropionate: effectiveness in patients with mild asthma. Am J Manag Care 2010; 16: e151–e156. [PubMed] [Google Scholar]
- 52.Navaratnam P, Friedman HS, Urdaneta E. Mometasone furoate vs fluticasone propionate with salmeterol: multivariate analysis of resource use and asthma-related charges. Curr Med Res Opin 2009; 25: 2895–2901. doi: 10.1185/03007990903336515 [DOI] [PubMed] [Google Scholar]
- 53.Busse W, Wolfe J, Storms W, et al. . Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50: 595–602. [PubMed] [Google Scholar]
- 54.Giraud V, Rogeaux Y, Dusser D. Asthma control following initial inhaled corticosteroid monotherapy in mild to moderate asthma: a 4- to 8-week observational study. Respiration 2006; 73: 617–622. doi: 10.1159/000089817 [DOI] [PubMed] [Google Scholar]
- 55.Herjavecz I, Blomqvist P, Serrano A. Efficacy of once- and twice-daily administration of budesonide via Turbuhaler as initial therapy in patients with mild persistent asthma. Respir Med 1999; 93: 230–235. doi: 10.1016/S0954-6111(99)90018-5 [DOI] [PubMed] [Google Scholar]
- 56.Peters SP, Anthonisen N, Castro M, et al. . Randomized comparison of strategies for reducing treatment in mild persistent asthma. N Engl J Med 2007; 356: 2027–2039. doi: 10.1056/NEJMoa070013 [DOI] [PubMed] [Google Scholar]
- 57.Skoner DP, Greos LS, Kim KT, et al. . Evaluation of the safety and efficacy of levalbuterol in 2–5-year-old patients with asthma. Pediatr Pulmonol 2005; 40: 477–486. doi: 10.1002/ppul.20288 [DOI] [PubMed] [Google Scholar]
- 58.Verberne AA, Hop WC, Creyghton FB, et al. . Airway responsiveness after a single dose of salmeterol and during four months of treatment in children with asthma. J Allergy Clin Immunol 1996; 97: 938–946. doi: 10.1016/S0091-6749(96)80068-6 [DOI] [PubMed] [Google Scholar]
- 59.Woodcock A, Williams A, Batty L, et al. . Effects on lung function, symptoms, and bronchial hyperreactivity of low-dose inhaled beclomethasone dipropionate given with HFA-134a or CFC propellant. J Aerosol Med 2002; 15: 407–414. doi: 10.1089/08942680260473489 [DOI] [PubMed] [Google Scholar]
- 60.Soyer OU, Beyhun NE, Demir E, et al. . A multicenter survey of childhood asthma in Turkey. II: utilization of asthma drugs, control levels and their determinants. Pediatr Allergy Immunol 2009; 20: 172–179. doi: 10.1111/j.1399-3038.2008.00769.x [DOI] [PubMed] [Google Scholar]
- 61.Lai CK, De Guia TS, Kim YY, et al. . Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol 2003; 111: 263–268. doi: 10.1067/mai.2003.30 [DOI] [PubMed] [Google Scholar]
- 62.Konig P, Shaffer J. The effect of drug therapy on long-term outcome of childhood asthma: a possible preview of the international guidelines. J Allergy Clin Immunol 1996; 98: 1103–1111. doi: 10.1016/S0091-6749(96)80198-9 [DOI] [PubMed] [Google Scholar]
- 63.Robertson CF, Rubinfeld AR, Bowes G. Pediatric asthma deaths in Victoria: the mild are at risk. Pediatr Pulmonol 1992; 13: 95–100. doi: 10.1002/ppul.1950130207 [DOI] [PubMed] [Google Scholar]
- 64.RCP Why asthma still kills: the National Review of Asthma Deaths (NRAD). Confidential Enquiry Report. London, Royal College of Physicians, 2014; Available from: https://www.rcplondon.ac.uk/file/868/download?token=3wikiuFg. [Google Scholar]
- 65.Campbell DA, McLennan G, Coates JR, et al. . A comparison of asthma deaths and near-fatal asthma attacks in South Australia. Eur Respir J 1994; 7: 490–497. doi: 10.1183/09031936.94.07030490 [DOI] [PubMed] [Google Scholar]
- 66.Robertson CF, Rubinfeld AR, Bowes G. Deaths from asthma in Victoria: a 12-month survey. Med J Aust 1990; 152: 511–517. doi: 10.5694/j.1326-5377.1990.tb125350.x [DOI] [PubMed] [Google Scholar]
- 67.NAEPP National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program: Expert Panel Report 3: guidelines for the Diagnosis and Management of Asthma, 2007. [Google Scholar]
- 68.Looijmans-van den Akker I, van Luijn K, Verheij T. Overdiagnosis of asthma in children in primary care: a retrospective analysis. Br J Gen Pract 2016; 66: e152–e157. doi: 10.3399/bjgp16X683965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Asthma: diagnosis, monitoring and chronic asthma management. National Institute for Health and Care Excellence, 2017. [PubMed]
- 70.Reddel HK, Busse WW, Pedersen S, et al. . Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet 2017; 389: 157–166. doi: 10.1016/S0140-6736(16)31399-X [DOI] [PubMed] [Google Scholar]
- 71.O'Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J 2017; 50: 1701103. doi: 10.1183/13993003.01103-2017 [DOI] [PubMed] [Google Scholar]
- 72.Barnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care 2015; 60: 455–468. doi: 10.4187/respcare.03200 [DOI] [PubMed] [Google Scholar]
- 73.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax 2006; 61: 722–728. doi: 10.1136/thx.2005.045161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thien F, Beggs PJ, Csutoros D, et al. . The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health 2018; 2: e255–e263. doi: 10.1016/S2542-5196(18)30120-7 [DOI] [PubMed] [Google Scholar]
- 75.Beasley R, Holliday M, Reddel HK, et al. . Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 2019; 380: 2020–2030. doi: 10.1056/NEJMoa1901963 [DOI] [PubMed] [Google Scholar]
- 76.Hardy J, Baggott C, Fingleton J, et al. . Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet 2019; 394: 919–928. doi: 10.1016/S0140-6736(19)31948-8 [DOI] [PubMed] [Google Scholar]
- 77.Reddel HK, FitzGerald JM, Bateman ED, et al. . GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J 2019; 53: 1901046. doi: 10.1183/13993003.01046-2019 [DOI] [PubMed] [Google Scholar]
- 78.Waljee AK, Rogers MA, Lin P, et al. . Short-term use of oral corticosteroids and related harms among adults in the United States: population-based cohort study. BMJ 2017; 357: j1415. doi: 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Price DB, Trudo F, Voorham J, et al. . Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy 2018; 11: 193–204. doi: 10.2147/JAA.S176026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wongtim S, Mogmued S, Chareonlap P, et al. . Effect of inhaled corticosteroids on bronchial hyperresponsiveness in patients with mild asthma. Asian Pac J Allergy Immunol 1995; 13: 81–85. [PubMed] [Google Scholar]
- 81.Basyigit I, Yildiz F, Kacar Ozkara S, et al. . Effects of different anti-asthmatic agents on induced sputum and eosinophil cationic protein in mild asthmatics. Respirology 2004; 9: 514–520. doi: 10.1111/j.1440-1843.2004.00631.x [DOI] [PubMed] [Google Scholar]
- 82.Chrousos GP, Ghaly L, Shedden A, et al. . Effects of mometasone furoate dry powder inhaler and beclomethasone dipropionate hydrofluoroalkane and chlorofluorocarbon on the hypothalamic-pituitary-adrenal axis in asthmatic subjects. Chest 2005; 128: 70–77. doi: 10.1378/chest.128.1.70 [DOI] [PubMed] [Google Scholar]
- 83.Chuang SS, Hung CH, Hua YM, et al. . Suppression of plasma matrix metalloproteinase-9 following montelukast treatment in childhood asthma. Pediatr Int 2007; 49: 918–922. doi: 10.1111/j.1442-200X.2007.02497.x [DOI] [PubMed] [Google Scholar]
- 84.Currie GP, Stenback S, Lipworth BJ. Effects of fluticasone vs. fluticasone/salmeterol on airway calibre and airway hyperresponsiveness in mild persistent asthma. Br J Clin Pharmacol 2003; 56: 11–17. doi: 10.1046/j.1365-2125.2003.01831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karaman O, Sunneli L, Uzuner N, et al. . Evaluation of montelukast in 8 to 14-year-old children with mild persistent asthma and compared with inhaled corticosteroids. Allergol Immunopathol (Madr) 2004; 32: 21–27. doi: 10.1157/13057766 [DOI] [PubMed] [Google Scholar]
- 86.Maiti R, Prasad CN, Jaida J, et al. . Racemic salbutamol and levosalbutamol in mild persistent asthma: A comparative study of efficacy and safety. Indian J Pharmacol 2011; 43: 638–643. doi: 10.4103/0253-7613.89817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riccioni G, Ballone E, D'Orazio N, et al. . Effectiveness of montelukast versus budesonide on quality of life and bronchial reactivity in subjects with mild-persistent asthma. Int J Immunopathol Pharmacol 2002; 15: 149–155. doi: 10.1177/039463200201500210 [DOI] [PubMed] [Google Scholar]
- 88.Shimoda T, Obase Y, Matsuse H, et al. . A study of the usefulness of anti-inflammatory treatment for mild intermittent asthma (Step 1): budesonide vs. montelukast. Allergol Int 2005; 54: 123–130. doi: 10.2332/allergolint.54.123 [DOI] [Google Scholar]
- 89.Tamaoki J, Isono K, Taira M, et al. . Role of regular treatment with inhaled corticosteroid or leukotriene receptor antagonist in mild intermittent asthma. Allergy Asthma Proc 2008; 29: 189–196. doi: 10.2500/aap.2008.29.3100 [DOI] [PubMed] [Google Scholar]
- 90.Vatrella A, Pelaia G, Parrella R, et al. . A single-blind, partial crossover clinical trial of the effects of inhaled fluticasone propionate and nedocromil sodium on airway hyperresposivenes to methacholine. Curr Ther Res 2002; 63: 316–327. doi: 10.1016/S0011-393X(02)80035-2 [DOI] [Google Scholar]
- 91.Zeiger RS, Bird SR, Kaplan MS, et al. . Short-term and long-term asthma control in patients with mild persistent asthma receiving montelukast or fluticasone: a randomized controlled trial. Am J Med 2005; 118: 649–657. doi: 10.1016/j.amjmed.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 92.Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, et al. . Effect of ciclesonide and fluticasone on exhaled nitric oxide in patients with mild allergic asthma. Respir Med 2006; 100: 1651–1656. doi: 10.1016/j.rmed.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 93.Boulet LP, Cowie RL, Negro RD, et al. . Comparison of once- with twice-daily dosing of fluticasone propionate in mild and moderate asthma. Can Respir J 2000; 7: 239–247. doi: 10.1155/2000/464639 [DOI] [PubMed] [Google Scholar]
- 94.O'Sullivan S, Akveld M, Burke CM, et al. . Effect of the addition of montelukast to inhaled fluticasone propionate on airway inflammation. Am J Respir Crit Care Med 2003; 167: 745–750. doi: 10.1164/rccm.200208-783OC [DOI] [PubMed] [Google Scholar]
- 95.Stone DWG, Mairs ML, Willits L, et al. . A comparison of fluticasone propionate, 100μg twice daily, administered via a CFC and non-CFC propellant, HFA 134a, in adult patients with asthma. Clin Drug Investig 2001; 21: 695–703. doi: 10.2165/00044011-200121100-00004 [DOI] [Google Scholar]
- 96.Vermetten FA, Boermans AJ, Luiten WD, et al. . Comparison of salmeterol with beclomethasone in adult patients with mild persistent asthma who are already on low-dose inhaled steroids. J Asthma 1999; 36: 97–106. doi: 10.3109/02770909909065153 [DOI] [PubMed] [Google Scholar]
- 97.Villaran C, O'Neill SJ, Helbling A, et al. . Montelukast versus salmeterol in patients with asthma and exercise-induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. J Allergy Clin Immunol 1999; 104: 547–553. doi: 10.1016/S0091-6749(99)70322-2 [DOI] [PubMed] [Google Scholar]
- 98.Barnes NC, Jacques L, Goldfrad C, et al. . Initiation of maintenance treatment with salmeterol/fluticasone propionate 50/100 microg bd versus fluticasone propionate 100 microg bd alone in patients with persistent asthma: integrated analysis of four randomised trials. Respir Med 2007; 101: 2358–2365. doi: 10.1016/j.rmed.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 99.van der Molen T, Meyboom-de Jong B, Mulder HH, et al. . Starting with a higher dose of inhaled corticosteroids in primary care asthma treatment. Am J Respir Crit Care Med 1998; 158: 121–125. doi: 10.1164/ajrccm.158.1.9707035 [DOI] [PubMed] [Google Scholar]
- 100.Shah MB, Gohil J, Khapekar S, et al. . Montelukast versus budesonide as a first line preventive therapy in mild persistent asthma in 2 to 18 y. Indian J Pediatr 2014; 81: 655–659. doi: 10.1007/s12098-013-1334-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00359-2019.SUPPLEMENT (519.1KB, pdf)