Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the strain of coronavirus that causes coronavirus disease (COVID-19), is spread through human-to-human contact.1 The World Health Organization declared COVID-19 a pandemic on March 11, 2020, and as of August 1, 2020, there were almost 5 million cases, with more than 52 million people who have been tested for SARS-CoV-2 (positivity rate of 10%) in the United States.2, 3, 4, 5 On February 4, 2020, the U.S. Health and Human Services (HHS) Secretary determined that COVID-19 posed significant public health threats, and multiple Emergency Use Authorizations (EUA) were subsequently issued by the Food and Drug Administration (FDA).6 An EUA allows FDA to facilitate the availability of countermeasures more rapidly during a public health emergency, including for SARS-CoV-2 testing. Each in vitro diagnostic test requires an EUA for distribution unless developed by state laboratories.7
Testing is 1 of the cornerstones of controlling the spread of infection.8 There are currently 3 types of in vitro tests available for the detection of current or previous SARS-CoV-2 infection: (1) molecular-based (reverse transcription polymerase chain reaction), (2) antigen, and (3) serologic (antibody) tests.9 , 10 Molecular-based tests identify the presence of viral RNA, and antigen tests detect the presence of the nucleocapsid protein antigen and are used to determine active infection. Serologic tests have been developed to identify the immune response to SARS-CoV-2 through the identification of antibodies.9 Although the tests are detecting the presence of SARS-CoV-2, the tests are commonly called COVID-19 tests. The inadequate response of the United States to develop and distribute such tests left states initially with few options except for expedited vaccine and drug development and physical distancing.11 With increasing demands to reopen the country and noncompliance with Centers for Disease Control and Prevention (CDC) recommendations to prevent infection, such as washing your hands, avoiding close contact, and covering your mouth and nose with a mask when around others, there is an even greater need now for expanded testing.12 In addition, employees may be required to have a least 1 or even 2 negative tests for SARS-CoV-2 before returning to work, increasing testing demand.
Evidence exists for the quality, safety, and effectiveness of pharmacist-administered Clinical Laboratory Improvement Amendments (CLIA)–waived point-of-care (POC) tests for infectious diseases, including Streptococcal pharyngitis, influenza, Helibactor pylori, HIV, and hepatitis C.13, 14, 15, 16 In addition, pharmacists are already using POC devices to test for Streptococcal pharyngitis and influenza that are similar to the devices in use for SARS-CoV-2 testing.14 In an analysis, Gubbins et al.17 determined that POC testing in community-based pharmacies could benefit patients. Pharmacists are fundamental in the solution to expand SARS-CoV-2 testing in the United States because of their accessibility to patients, relationships with other health care providers, skills and ability for patient assessment, POC testing, and referral.18, 19, 20 Furthermore, pharmacies ranked fourth in most number of CLIA-waived locations in 2015, with more than 10,000 sites, and can play a critical role in expanding SARS-CoV-2 testing with the use of CLIA-waived POC tests.21 On April 8, 2020, HHS Secretary, Alex Azar, issued a guidance giving pharmacists the authority to order and administer tests for SARS-CoV-2 under the Public Readiness and Emergency Preparedness (PREP) Act.22 On May 19, 2020, an advisory opinion provided clarification that the federal guidance preempts any state and local legal requirements that prevent pharmacists from ordering and administering FDA-approved SARS-CoV-2 tests.23
Even with HHS authority for testing, challenges exist for the widespread implementation of pharmacist SARS-CoV-2 diagnostic testing. Some pharmacists may have difficulty securing proper personal protective equipment (PPE).24 , 25 An anterior nares specimen collected by the patient has been recommended by CDC to decrease the exposure and required PPE.24 Pharmacists in pharmacies with limited staff may be concerned about exposure and impact on staffing issues. Pharmacies along with other private sector facilities and organizations should strive to maximize PPE through normal supply chains, exercising CDC-recommended conservation guidelines.25 A distribution allocation may be determined by specific state and territorial health departments for pharmacists who are implementing community-based testing. The biggest challenge continues to be payment for pharmacist testing. Pharmacists should work with their local Medicare administrative contractors, state Medicaid, and commercial payers to secure payment before testing. This article is intended to provide a framework for pharmacist SARS-CoV-2 diagnostic testing, including a review of the currently available CLIA-waived tests, best practices for testing, and a framework with models and potential payment opportunities.
Review of SARS-CoV-2 diagnostic tests
The types of diagnostic tests for SARS-CoV-2 vary by item of detection, specimen sample, and clinical use (Table 1 ).10 Molecular-based diagnostic tests range in complexity from high complexity requiring skilled operators to collect the sample and manually perform the test to simple, automated tests available for use in CLIA-waived testing facilities.10 CLIA-waived tests are deemed simple tests that require little manipulation, with low risk for error or incorrect results.26 For both molecular and antigen tests that require upper respiratory tract samples, CDC has established which specimens are acceptable for SARS-CoV-2 testing (Table 2 ).27 Molecular-based diagnostic tests have been the gold standard for accurately identifying positive COVID-19 cases, but the process can be lengthy if the samples must be sent to a laboratory for analysis, with a turnaround time often exceeding 48 hours.27 Antigen tests produce results faster but also produce more false negatives owing to lower sensitivity than that of molecular diagnostic tests, making it harder to manage patients appropriately.10 , 28, 29, 30 A negative antigen test may require confirmatory testing with a molecular-based diagnostic test before making treatment or prevention decisions. Novel CLIA-waived molecular POC tests can produce results in less than 20 minutes, but they must be performed in CLIA-waived laboratories.31, 32, 33, 34 SARS-CoV-2 testing technology is rapidly evolving with new technology such as the Cue COVID-19 test (Cue Health Inc) and the Cue Monitoring System that allows health care providers or patients to test and connect to an application on a smart phone to read the results of the test.34 , 35 Some of the devices that test for SARS-CoV-2 can also run tests for influenza A and B and group A Streptococcus, maximizing the tests that pharmacists may be able to offer in their pharmacies. Table 3 provides a comparison of available CLIA-waived diagnostic tests for SARS-CoV-2 at the time of writing; however, it should be noted that the technology is changing rapidly.29, 30, 31, 32, 33, 34, 35
Table 1.
Comparison of different types of in vitro SARS-CoV-2 diagnostic tests10
| Diagnostic test type | Qualitative or quantitative | Detects | Determines active or prior infection | Specimen sample collection type |
|---|---|---|---|---|
| Molecular | Qualitative | SARS-CoV-2 RNA | Active | Upper respiratory tract sample |
| Antigen | Qualitative | SARS-CoV-2 protein | Active | Upper respiratory tract sample |
| Serologic | Qualitative | Human antibodies to SARS-CoV-2 | Active and priora | Blood, serum, and plasma |
Abbreviation used: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Serologic tests are not recommended by the Centers for Disease Control and Prevention to be used to diagnose an active infection.
Table 2.
Upper respiratory tract specimen samples acceptable for SARS-CoV-2 diagnostic testing per the Centers for Disease Control and Prevention27
| Upper respiratory tract specimen samples acceptable for SARS-CoV-2 diagnostic testing | |
|---|---|
| Collector | Type of specimen collection |
| Health care provider | Nasopharyngeal swab Oropharyngeal swab Nasal midturbinate swab Anterior nares swab Nasopharyngeal wash or aspirate Nasal wash or aspirate |
| Patient (supervised) | Nasal midturbinate swab Anterior nares swab |
| Patient (unsupervised) | Anterior nares swab |
Abbreviation used: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 3.
Comparison of different types of Clinical Laboratory Improvement Amendments–waived SARS-CoV-2 point-of-care test29, 30, 31, 32, 33, 34, 35
| Diagnostic test | Manufacturer | Diagnostic test type | Time for results | Specimen collection type |
|---|---|---|---|---|
| ID NOW COVID-19 test | Abbott | Molecular | 13 min | Throat, nasal, or nasopharyngeal swab |
| Accula SARS-CoV-2 test | Mesa Biotech Inc | Molecular | 30 min | Throat or nasal swab |
| Xpert Xpress SARS-CoV-2 test | Cepheid | Molecular | 30 min | Oropharyngeal, nasal, or midturbinate swab or nasal wash or aspirate |
| Cue COVID-19 test | Cue Health Inc | Molecular | 25 min | Nasal swab |
| Sofia 2 SARS Antigen FIA test | Quidel | Antigen | 15 min | Nasopharyngeal or nasal swab |
| BD Veritor System for Rapid Detection of SARS-CoV-2 | Becton, Dickinson and Company | Antigen | 15 min | Nasal swab |
Abbreviations used: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease.
Best practices for pharmacist-based SARS-CoV-2 diagnostic testing
Best practices during a public health emergency begin with education. Similar to the adoption of vaccination practices, pharmacists can assume one of 3 roles (advocacy and education, facilitation, and testing) for SARS-CoV-2 diagnostic testing.36 Advocacy and education are the cornerstones of best practices for a public health emergency. Pharmacists are a source of education for patients who need to understand CDC recommendations regarding how to protect themselves and others.37 At the POC and during telephone calls or telehealth visits, pharmacists should check with patients to verify that they are following safety precautions. Pharmacists must model appropriate protection and ensure that proper controls are in place in their pharmacies.37
Furthermore, pharmacists must adopt best practices for SARS-CoV-2 diagnostic testing. This includes maintaining a safe testing site with an efficient workflow, following guidelines for whom to test, interpreting test results, reporting, and follow-up.24 , 27 , 36, 37, 38, 39, 40 Important for pharmacist testing is proper training and awareness of testing guidelines for pharmacists and staff (Table 4 ). Pharmacists should understand the pathophysiology of SARS-CoV-2 and the principles of testing, including the accuracy of the test. All pharmacy staff should understand good laboratory practices and cleaning of sites and spaces. Pharmacists and staff may need training on when to use PPE and what PPE is necessary; how to properly don, use, and doff PPE in a manner to prevent self-contamination; how to properly dispose of, or disinfect and maintain, PPE; and the limitations of PPE. State and territorial health departments may be able to assist pharmacists in providing training, especially in the area of properly fitting masks and face shields.
Table 4.
Training for pharmacists and staff
| Topic | Pharmacist | Staff |
|---|---|---|
| SARS-CoV-2 tests and principles | X | |
| Cleaning site or area | X | X |
| Patient assessment and screening | X | |
| Testing criteria | X | |
| Personal protective equipment | X | X |
| Good laboratory practices | X | X |
Collecting specimen
|
X | |
| Specimen delivery | X | |
| Testing procedures | X | |
| Notification and follow-up procedures | X | X |
| Billing and coding | X | X |
Abbreviation used: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
CDC has issued guidelines for 5 distinct populations who should receive SARS-CoV-2 diagnostic testing, including individuals with signs or symptoms consistent with COVID-19, asymptomatic individuals with recent known or suspected exposure to SARS-CoV-2 to control transmission, asymptomatic individuals without known or suspected exposure to SARS-CoV-2 for early identification in special settings, individuals being tested to determine resolution of infection, and individuals being tested for purposes of public health surveillance for SARS-CoV-2.38 Pharmacists should partner with state and territorial health departments to understand the priorities for testing in their states.
Guidance for collecting specimens is available from multiple sources.27 , 39 , 40 Many SARS-CoV-2 diagnostic tests allow for patients to self-collect a specimen. Pharmacists should be able to provide proper instruction for patients to self-collect a specimen. Pharmacists will need to understand the test technology and be able to interpret results to provide education for patients. Patients will need to understand how they will receive notification of the results and the role of contact tracers. Patients who test positive will require notification and follow-up. Patients who test negative will require notification and may require follow-up on the basis of symptoms and exposure to SARS-CoV-2. Pharmacists should coordinate with primary care providers to determine who should conduct the home management follow-up. Some newer laboratory platforms are using a text notification or providing access to a patient portal for results. However, access to results is not a substitute for post-test education and follow-up. A positive patient who is determined to be eligible for home management will receive follow-up on the basis of their risk for severity of the disease.41, 42, 43, 44 Typically, a telehealth follow-up should be conducted on days 4, 7, and 10 after the start of clinical illness.43 Pharmacists should follow all applicable state reporting laws and reach out to the state and territorial health department to discuss these requirements. Pharmacists partnering with laboratories and other entities will need to confirm reporting responsibilities.
Pharmacist models for SARS-CoV-2 diagnostic testing
Even with clarification about pharmacists’ authority to order and administer tests for SARS-CoV-2 under the PREP Act, how testing might work in community-based pharmacies and how it could be scaled more broadly is evolving.22 , 23
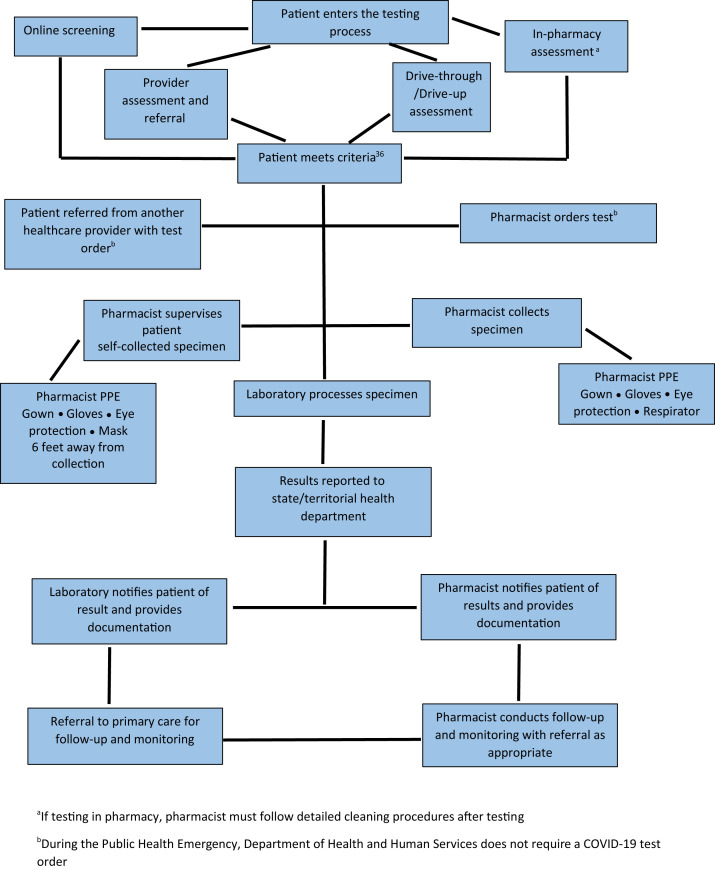
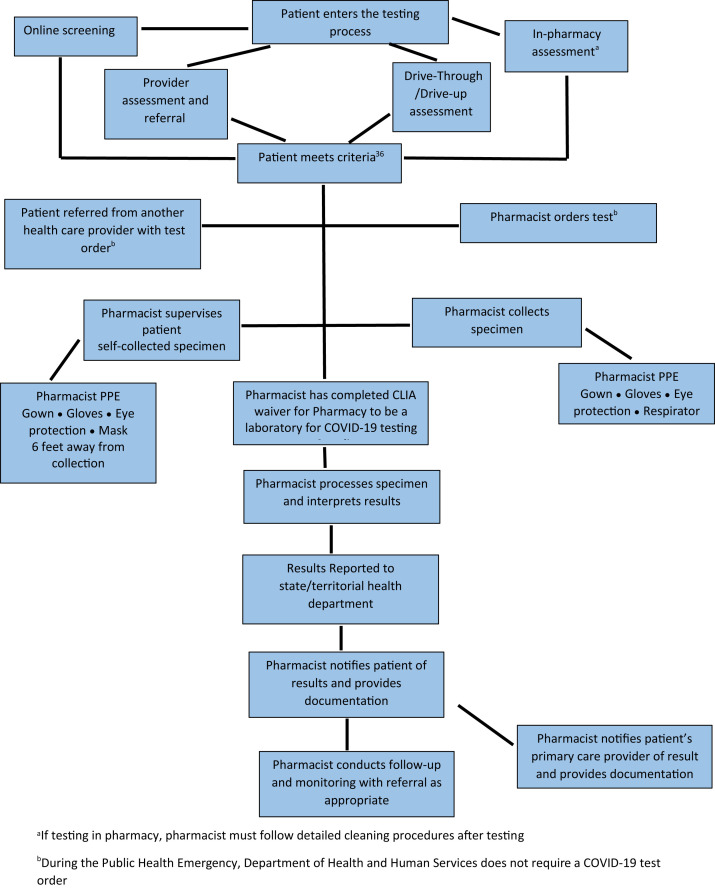
Testing in community-based pharmacies is based on 2 pathways.45 In the first, the pharmacist facilitates testing through collecting, or supervising the patient collecting, the specimen and partnering with a laboratory for processing the specimen and conducting the test. In the second pathway, the pharmacist or patient collects the specimen and the pharmacist conducts the POC test, with the pharmacy being registered as a CLIA-waived laboratory. Since May 8, 2020, 425 CLIA-waivers have been processed for pharmacy-based laboratories.46 However, under these 2 pathways there are multiple workflows for conducting SARS-CoV-2 diagnostic testing that include differences in who assesses the patient and orders the test, and where and how the specimen is collected for testing. Figures 1 and 2 depict the differences in workflows for pharmacist-based testing under these 2 pathways (laboratory testing and pharmacist POC testing). Table 5 outlines the strengths and weakness of the different workflows. In the first pathway, the pharmacist may perform the screening and assessment of patients, collect the specimen, and send it to the laboratory for processing. Two types of payment structures may be negotiated in this model: the laboratory or another entity pays the pharmacist for SARS-CoV-2 testing–related services or the pharmacist contracts with the laboratory or other entity to collect, or supervise collection of, the specimen. Depending on the contract, the pharmacist or the laboratory or other entity may conduct the patient result notification. In the pathway where the pharmacist processes the test as a CLIA-waived laboratory, payment opportunities exist for payment to the pharmacy-based laboratory to conduct the test and for COVID-19 testing–related services.
Figure 1.
Laboratory processes the severe acute respiratory syndrome coronavirus 2 diagnostic test. Abbreviation used: PPE, personal protective equipment. aIf testing in pharmacy, pharmacist must follow detailed cleaning procedures after testing. bDuring the public health emergency, Department of Health and Human Services does not require a coronavirus disease test order.
Figure 2.
Pharmacist processes the severe acute respiratory syndrome coronavirus 2 diagnostic test. Abbreviations used: CLIA, Clinical Laboratory Improvement Amendments; COVID-19, coronavirus disease; PPE, personal protective equipment. aIf testing in pharmacy, pharmacist must follow detailed cleaning procedures after testing. bDuring the public health emergency, Department of Health and Human Services does not require a COVID-19 test order.
Table 5.
Strengths and weaknesses of workflow frameworks
| Workflow | Strengths | Weaknesses |
|---|---|---|
| Laboratory processes the test | Lower cost to pharmacy for supplies and staff | Testing turnaround time |
| Pharmacist processes the test | Rapid testing turnaround time Device can be used for other testing |
Initial cost for testing devices Staffing and resource requirements |
| Patient collects specimen | Decreased potential for exposure Less stringent PPE requirements |
Potential for patient collection error Decreased relationship building with patient |
| Pharmacist collects specimen | Decreased potential for collection error | Potential exposure to staff Stringent PPE requirement |
| In-pharmacy testing | Relationship building with patient | Potential exposure to staff Cost of extra PPE Cost of cleaning Time for cleaning Lower volume of testing owing to cleaning |
| Drive-through or drive-up testing | PPE requirements not as stringent (lower cost) Less potential for exposure for staff Higher volume of testing |
May require additional staffing |
Abbreviation used: PPE, personal protective equipment.
Within the 2 pathways, different models for partnering organizations and payment for testing and pharmacist-provided testing–related services exist. The following models represent existing partnerships and emerging partnerships for pharmacist testing for SARS-CoV-2 at the time of manuscript writing:
-
•
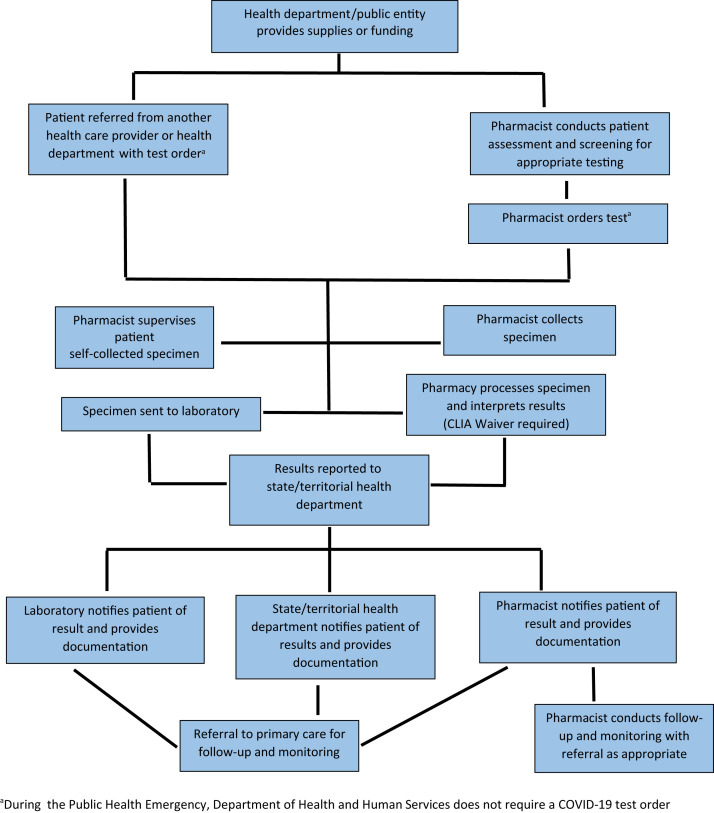
Pharmacy and pharmacist partners with the state and territorial health department or other public entity under a public-private partnership (Figure 3 ).
-
•
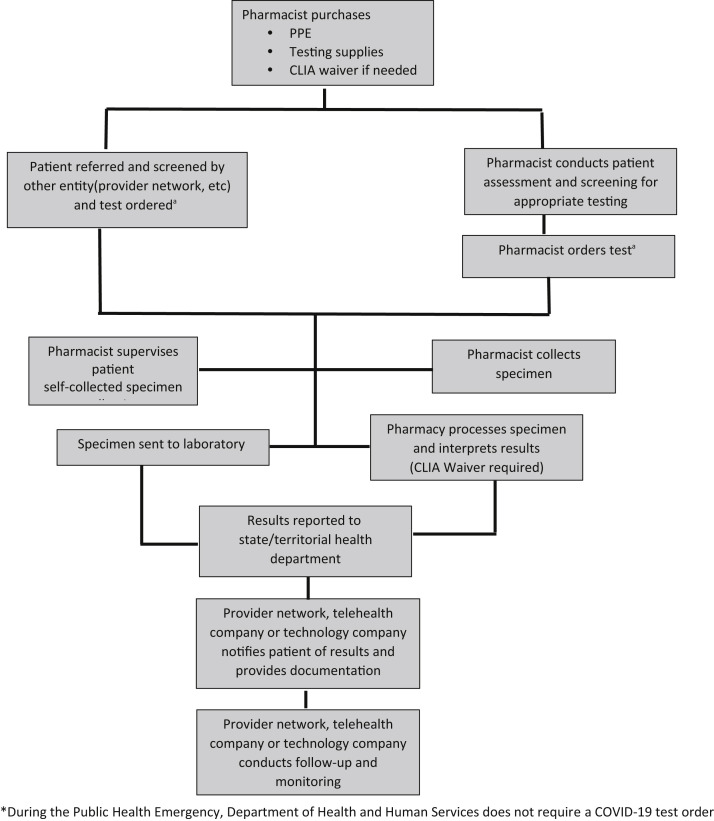
Pharmacy and pharmacist partners with a health care entity (provider network or technology company) (Figure 4 ).
-
•
Pharmacist/pharmacy partners with a telehealth company (Figure 5 ).
-
•
Pharmacy and pharmacist partners with a health care clinic or primary care practice (Figure 6 ).
-
•
Pharmacy and pharmacist Medicare COVID-19 testing and COVID-19 testing–related services.
-
•
Pharmacy and pharmacist provides the location, but the pharmacy is not involved in testing.
Figure 3.
Pharmacy and pharmacist partners with state health department or other public entity. Abbreviation used: CLIA, Clinical Laboratory Improvement Amendments. aDuring the public health emergency, Department of Health and Human Services does not require a coronavirus disease test order.
Figure 4.
Pharmacy and pharmacist partners with provider network, telehealth company, or technology company. Abbreviations used: CLIA, Clinical Laboratory Improvement Amendments; PPE, personal protective equipment. aDuring the public health emergency, Department of Health and Human Services does not require a coronavirus disease test order.
Figure 5.
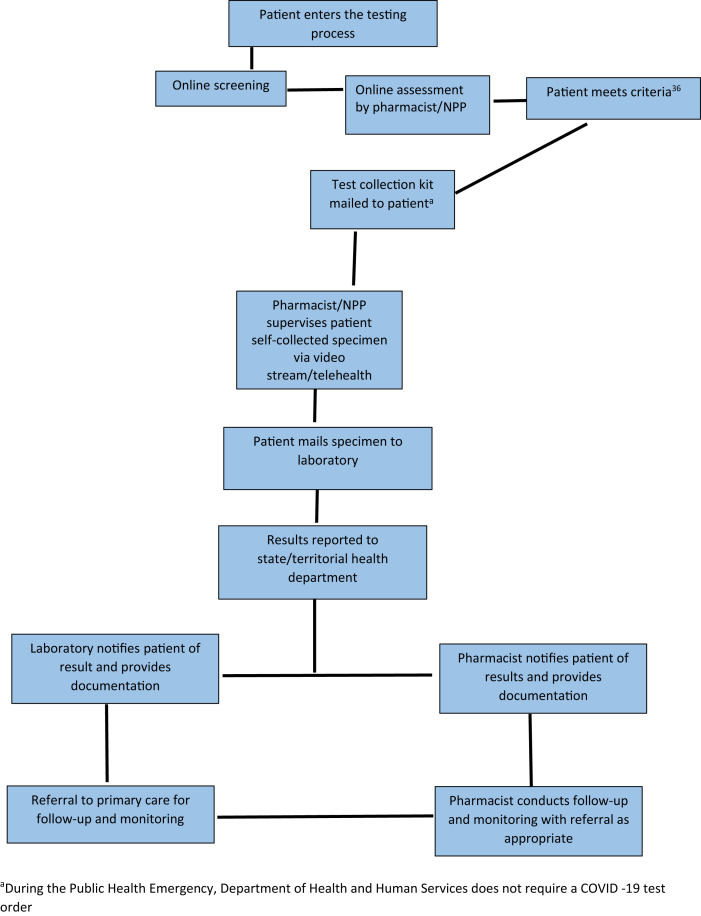
Pharmacy and pharmacist partners with laboratory for at-home test collection kit. Abbreviation used: NPP, nonphysician provider. aDuring the public health emergency, Department of Health and Human Services does not require a coronavirus disease test order.
Figure 6.
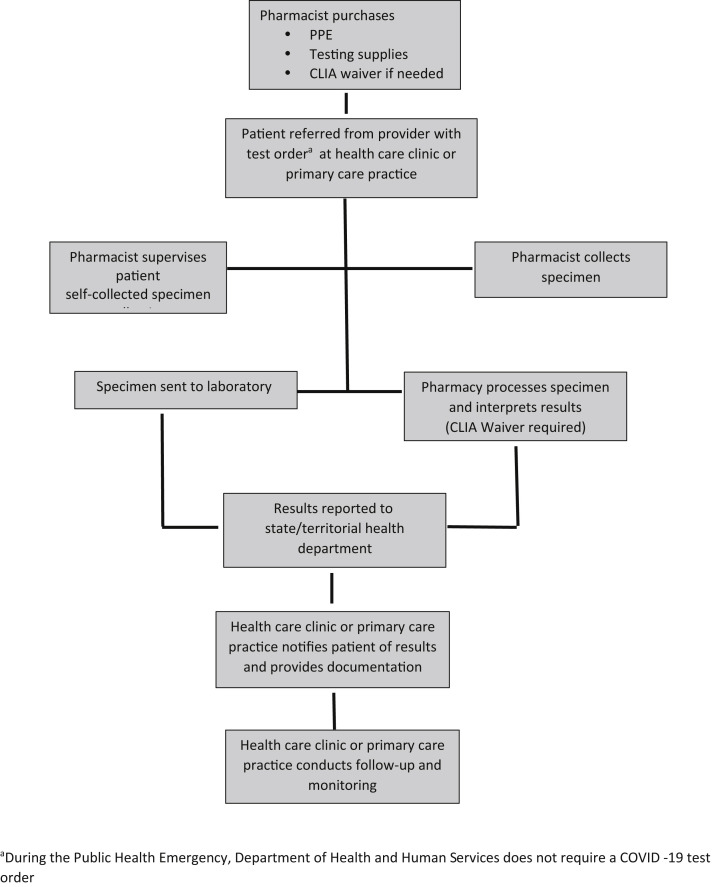
Pharmacy and pharmacist partners with health care clinic or primary care practice. Abbreviations used: CLIA, Clinical Laboratory Improvement Amendments; PPE, personal protective equipment. aDuring the Public Health Emergency, Department of Health and Human Services does not require a coronavirus disease test order.
In the first model, the pharmacist may partner with the state and territorial health department or other public entity under a public-private partnership to provide testing (Figure 3). Under this model, the state and territorial health department or other public entity may provide equipment for testing, such as PPE or testing supplies, or provide funding for testing.47 HHS has launched public-private partnerships with community pharmacies, including large community chains, and some independent pharmacies.47 The pharmacist may or may not perform the rapid test under this model; it depends on the testing model used by the state and territorial health department or HHS. For example, laboratory and health care technology companies are included in some of the HHS partnerships with pharmacies.47 If the pharmacist is involved in performing the rapid test, the pharmacy must apply for a CLIA certificate of waiver for SARS-CoV-2 diagnostic testing through the state. Under this model, payment for the pharmacist testing is received through funding from the state and territorial health department or the public entity. In addition, the pharmacy may submit claims for uninsured patients through the Health Resources and Services Administration (HRSA) COVID-19 Claims Reimbursement Program (https://www.hrsa.gov/coviduninsuredclaim).48 This model may be desirable for pharmacists because payment is received from the state and territorial department or public entity. The large community-based pharmacy chains and some independents have adopted this model for early SARS-CoV-2 diagnostic testing.47 , 49, 50, 51, 52 In some cases, the large community-based pharmacy chains may be conducting SARS-CoV-2 diagnostic testing through a company-owned health clinic that may not involve pharmacists; however, it may facilitate more payment options.49
The second model provides a platform for partnering with other health care entities, including provider networks, telehealth companies, and technology companies (Figure 4). In this model, it is highly likely that the provider network has providers performing the initial screening and assessment, which may be online or through telehealth, and referring to the pharmacy for specimen collection and conducting the test or sending the specimen to the laboratory. If the pharmacist is using the POC test in this model, then a CLIA certificate of waiver will be required. However, in most of these models, the pharmacist only participates in specimen collection. The pharmacist would receive the contracted payment from the partner. This model is being adopted by some of the large pharmacy chains for pharmacy and pharmacist testing.52 However, even in this model, HHS may be providing funding to the health care entity, which is then distributed to the pharmacy and pharmacist. The benefit of this model is that the pharmacist/pharmacy is partnering with another entity with experience in the provision of health care services.
In the third model, the pharmacy and pharmacist partners with a laboratory to offer home SARS-CoV-2 testing (Figure 5). In this model, the patient completes an online screening and assessment that is reviewed by a licensed health professional employed by the company. If the patient meets the criteria for testing, a specimen collection kit is mailed to the patient and the observation of the collection is performed through a telehealth visit again by a licensed health professional. Then the patient mails the specimen to the laboratory or pharmacy for processing. Kroger Health obtained its own FDA EUA for its COVID-19 Test Home Collection Kit and is using this model for its own employees as well as expanding it to other employers, payers, and organizations through a contractual relationship.53 , 54 This model may be desirable for pharmacists because of the decreased risk of exposure to patients who are sick.
In another model, the pharmacy and pharmacist partners with a health care clinic or primary care practice to receive referrals for testing (Figure 6). The practitioners at the health care clinic or primary care practice perform the initial screening and assessment to provide the referral to the pharmacy for specimen collection, or specimen collection and testing. The payment is received from the health care clinic or primary care office for specimen collection and testing. This model will enable pharmacists to build relationships with other health care providers, which could be beneficial for the advancement of other pharmacist patient care services.
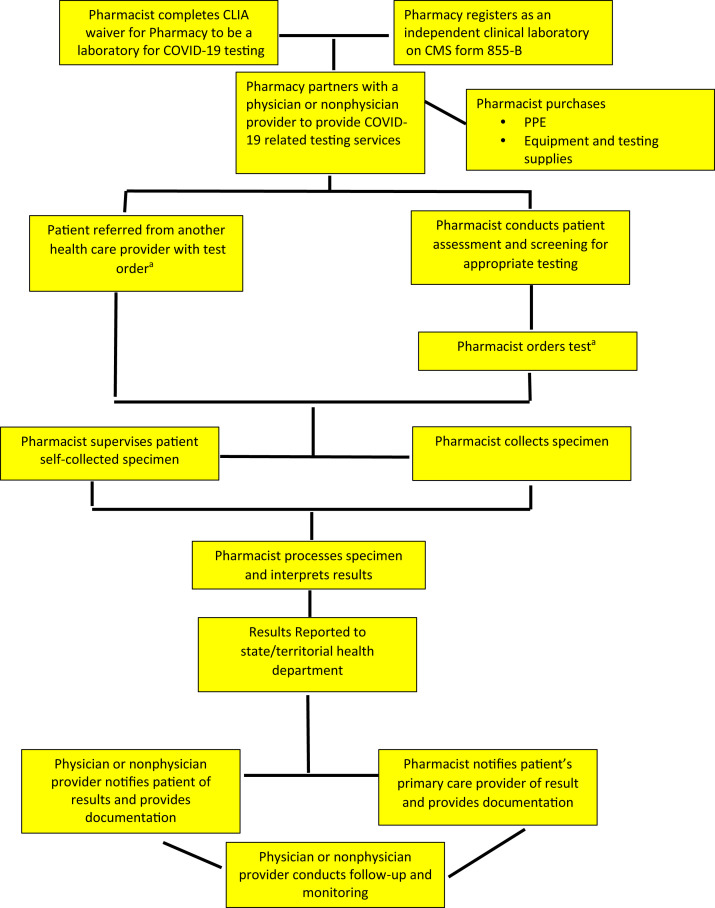
In this model, a scenario is presented for pharmacists to conduct SARS-CoV-2 tests and provide COVID-19 related testing services and be paid in the Medicare program. The pharmacist registers the pharmacy as an independent clinical laboratory, and the pharmacy bills Medicare for conducting the test (Figure 7 ). The pharmacist may also partner under an incident-to-physician-services arrangement with a physician or nonphysician provider (NPP) who can bill for the pharmacist’s COVID-related testing services. Again, this model may be beneficial to building relationships and partnerships that could be leveraged for advancing other pharmacist patient care services.
Figure 7.
Pharmacy and pharmacist Medicare coronavirus disease testing and coronavirus disease testing–related services. Abbreviations used: CMS, Centers for Medicare & Medicaid Services; CLIA, Clinical Laboratory Improvement Amendments; COVID-19, coronavirus disease; PPE, personal protective equipment. aDuring the public health emergency, Department of Health and Human Services does not require a COVID-19 test order.
Under the final model, the pharmacy and pharmacist provides the location—often a parking lot—for another entity to assess the patient, order the test, and collect the specimen at the pharmacy. The pharmacist may offer the space at no cost or receive payment for the use of the space. The pharmacist is not involved in this model of testing.
The pathways and models provide a framework for community-based pharmacy SARS-CoV-2 diagnostic testing. As the pandemic continues to evolve, the models may change, especially as the role of the pharmacist in increasing testing becomes more important.
Payment for pharmacist SARS-CoV-2 diagnostic testing
Payment for SARS-CoV-2 diagnostic testing by pharmacists may be obtained from the following sources: patient self-pay, HRSA COVID-19 Claims Reimbursement Program, Medicare, Medicaid, employers, state and territorial health department funding, HHS funding, or commercial third party payers. As with all pharmacist patient care and testing services, a patient may pay out of pocket or use a health savings account. Note that Medicare beneficiaries are not expected to pay co-pays or other out-of-pocket expenses for SARS-CoV-2 tests and COVID-19 testing–related services. Many states and health plans have adopted similar policies. The methods of payment from these sources are continuing to evolve, with payment occurring through pharmacy billing at the point of sale, the pharmacy or pharmacist billing through the medical benefit, and from HHS funding. It is important to note that HHS funding is not sustainable. In addition, a scenario where the pharmacist is recognized and paid for services under the medical benefit is the most desirable for the profession.55
Depending on the SARS-COV-2 diagnostic testing framework and model for testing, pharmacists should consider the following when setting a usual and customary fee and negotiating payment contracts: (1) cost of PPE and cleaning supplies, (2) screening and assessment, (3) collecting or supervising patient self-collection, (4) cost of testing supplies and POC device, (5) cost of technology services or technology, (6) patient notification and documentation, and (7) patient follow-up and monitoring.
Under the HRSA COVID-19 Claims Reimbursement Program, the pharmacist must enroll in the program to provide testing under the Coronavirus Aid, Relief, and Economic Security Act, which provides testing to uninsured individuals in the United States without health care coverage. The requirements include enrolling as a provider participant with the UnitedHealth Group, checking patient eligibility, submitting patient information, submitting claims electronically, and receiving payment through direct deposit.48 More information may be obtained at https://coviduninsuredclaim.linkhealth.com/.
A disconnect exists at the federal level with authority versus recognition for payment. The Centers for Medicare & Medicaid Services (CMS) has issued guidance for payment under Medicare, which does not recognize payment directly to the pharmacist for services.56 Under this guidance, Medicare will pay for SARS-CoV-2 tests performed by pharmacists as long as the pharmacy is enrolled with Medicare as an independent clinical laboratory on CMS Form 855-B (https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/cms855b.pdf) and has a CLIA certificate of waiver. The pharmacist performs the test, and the pharmacy bills the appropriate code depending on the type of test conducted. Billing codes may be found here: https://www.cms.gov/files/document/covid-ifc-2-flu-rsv-codes.pdf. In the Medicare program, for a pharmacist to be paid for services associated with COVID-19 testing, such as assessing the patient and collecting the sample, the pharmacist must have a contract with a physician or NPP, and perform the services within the pharmacist’s scope of practice and state law. The contract must include a financial relationship to qualify as an incident-to-physician-services arrangement between the pharmacist and physician or other qualified NPP. There is also a supervision requirement in which, under the public health emergency, a physician or NPP must be available immediately, using real-time audio and visual technology, and the service is billed by the physician or NPP using Evaluation and Management Services Current Procedural Terminology (CPT) code 99211 for both new and established patients. Medicare billing codes are found in Table 6 .
Table 6.
Select Medicare billing codes for COVID-19 tests and COVID-19 related services
| Coding | Description |
|---|---|
| ICD-10 coding | |
| Z11.59 | Asymptomatic, no known exposure, results unknown or negative |
| Z03.8181 | Possible exposure to COVID-19, ruled out |
| Z20.828 | Contact with COVID-19, suspected exposure |
| CPT/HCPCS code | |
| U0001 | CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel |
| U0002a | 2019-nCoV Coronavirus, SARS-CoV-2/2019-nCoV (COVID-19), any technique, multiple types or subtypes (includes all targets), non-CDC |
| U0003 | Infectious agent DNA or RNA; SARS-CoV-2 amplified probe technique, making use of high throughput technologies as described by CMS-2020-01-R |
| 87635a | Infectious agent DNA or RNA; SARS-CoV-2, amplified probe technique |
Abbreviations used: CPT, current procedural terminology; HCPCS, Healthcare Common Procedure Coding System; nCoV, novel coronavirus; CMS, Centers for Medicare & Medicaid Services; CDC, Centers for Disease Control and Prevention; RT-PCR, reverse transcription polymerase chain reaction; COVID-19, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Note: Patient assessment and specimen collection can be billed by a physician or nonphysician provider using CPT code 99211, with funds being distributed under a financial relationship to the pharmacist who meets incident-to-physician-services billing requirements.
Codes that a pharmacy would use to directly bill Medicare as a clinical laboratory.
Some state Medicaid agencies are considering paying pharmacists for COVID-19 services.57 Medicaid agencies can submit a waiver amendment to pay pharmacists for services. In addition, some states have used a governor’s executive order or legislation to secure payment for pharmacists from Medicaid and commercial payers in the state. The governor in New York has issued such an executive order for Medicaid to pay pharmacists for SARS-CoV-2 diagnostic testing.57 Pharmacists may contract with employers to provide COVID-19 assessment, specimen collection, and testing at the workplace or pharmacy. Opportunities may exist for pharmacists to partner with state and territorial health departments or other public or private partnerships to provide and be paid for testing services.
Pharmacists are encouraged to evaluate how physicians and NPPs are being paid for services (non-Medicare patients) when negotiating with payers.58 , 59 A payer will need to recognize pharmacists as providers in its payer network to negotiate contracts or submit medical billing codes for payment. This recognition could be required per legislation or regulation or could be based on a private sector payer recognizing the value of pharmacist services and including them in their network. Payers may want to simplify coding and adopt the same codes for pharmacists that are being used in medical billing for SARS-CoV-2 diagnostic testing and related services for other health professionals. Pharmacists may have to be recognized as “providers” to negotiate contracts or submit medical billing codes for payment. Payment should be requested for patient assessment and screening (this would include collecting or supervising the collection of the specimen); payment for specimen collection subsequent to another health care provider conducting patient assessment and screening; performing the laboratory test (including the cost of testing supplies); reporting the results to the patient, health department, and primary care provider, as appropriate; and telehealth follow-up. Table 7 provides potential relevant non-Medicare medical billing CPT codes for these service scenarios.58 , 59
Table 7.
Example coding for pharmacist testing services based on medical coding for non-Medicare payers (information about coding from the medical model that payers may use to pay pharmacists for COVID-19 diagnostic testing and testing-related services)
| ICD-10 coding | |||
|---|---|---|---|
| Z11.59 | Asymptomatic, no known exposure, results unknown or negative | ||
| Z03.8181 | Possible exposure to COVID-19, ruled out | ||
| Z20.828 | Contact with COVID-19, suspected exposure | ||
| Assessment of patient or test ordering | Specimen collectiona | Test performed | Follow-up (telephonic) service code (02) |
| Pharmacist 99201 new patient 99212 established patient |
Included in 99201 and 99212 | 87635 if done in pharmacy by pharmacist | Pharmacist: 99441 (5–10 min) 99442 (11–20 min) 99443 (21–30 min) Non-pharmacist: 98966 (5–10 min) 98967 (11–20 min) 98968 (21–30 min) |
| Other health professional (pharmacist will not bill for this) | 99000a: pharmacist performs specimen collection 99001a: patient performs specimen collection |
87635 if done in pharmacy by pharmacist | Pharmacist: 99441 (5–10 min) 99442 (11–20 min) 99443 (21–30 min) Nonpharmacist: 98966 (5–10 min) 98967 (11–20 min) 98968 (21–30 min) |
Abbreviation used: COVID-19, coronavirus disease.
Note: Based on American Medical Association Guidance58,59 and may be used by payers for pharmacists to bill for COVID-19 diagnostic testing and related services. Note that the use of billing codes is payer specific and depends on the pharmacist being recognized as a provider by the payer to be paid for the service.
It is not clear if the CPT codes 99000 and 99001 can be used for specimen collection if not included in an Evaluation and Management Services code (99201 and 99212). It may need to be billed as a 99211.59
The National Council for Prescription Drug Programs (NCPDP) has developed guidance for billing for SARS-CoV-2 specimen collection and conducting testing services through a secure pharmacy dispensing system or pharmacy point-of-sale software.60 , 61 NCPDP guidance can be used by pharmacy software systems to bill COVID-19 tests that are part of a medical benefit or a pharmacy benefit. For tests that are part of the medical benefit, the pharmacy will likely need to work with an intermediary organization to take the NCPDP claim and translate it for submission to the payer. Pharmacists and pharmacies will need a National Provider Identifier number to be able to bill for COVID-19 services. Table 8 provides coding guidance for NCPDP.
Table 8.
NCPDP coding guidance
| NCPDP field | Field name | Service | Code |
|---|---|---|---|
| 201-B1 | Service provider ID | Pharmacy | Type 2 NPI |
| 411-DB | Prescriber ID | Ordering provider | Type 1 NPI |
| 444-E9 | Provider ID | Administering provider | Type 1 NPI |
| 407-D7 436-E1 |
Product or Service ID Product or Service ID Qualifier |
Specimen collection | 99999-0992-11a 03 |
Abbreviations used: ID, identification; NCPDP, National Council for Prescription Drug Programs; NPI, National Provider Identifier.
Code to be used if the test kit does not contain a universal product code or unique device identifier code.
Although challenges exist for community-based pharmacists to implement SARS-CoV-2 diagnostic testing, there is a significant opportunity to create an impact on patient care and public health, and develop collaborations with other providers. A framework for SARS-CoV-2 diagnostic testing that includes payment for services can serve as the foundation for community-based pharmacists to provide services for SARS-CoV-2 antibody testing as the science and role of this test become well-defined.
Conclusion
Pharmacists are the most accessible health care providers and have the knowledge and skills to assume a key role in increasing SARS-CoV-2 diagnostic testing in the United States. The active engagement of pharmacists in SARS-CoV-2 testing can help to address many of the gaps in testing availability and demonstrate the value that pharmacists can provide in addressing unmet health needs.
Biographies
Jean-Venable R. Goode, PharmD, BCPS, FAPhA, FCCP, Professor and Director, Postgraduate Year 1 Community-Based Residency Program, Virginia Commonwealth University, Richmond, VA
Alexis Page, PharmD, Pharmacist-in-Charge, Kroger Health, Richmond, VA; at time of study, Fellow, Community-Based Pharmacy Leadership and Management, Virginia Commonwealth University, Richmond, VA
Anne Burns, BSPharm, Vice President, Professional Affairs, American Pharmacists Association, Washington, DC
Shaina Bernard, PharmD, BCPS, Antimicrobial Resistance Coordinator, Virginia Department of Health, Richmond, VA
Stephanie Wheawill, PharmD, Pharmacy Director, Virginia Department of Health, Richmond, VA
Sharon B.S. Gatewood, PharmD, BCACP, FAPhA, Associate Professor, Virginia Commonwealth University, Richmond, VA
References
- 1.World Health Organization Q&A on coronaviruses (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses Available at:
- 2.Johns Hopkins University and Medicine Coronavirus resource center: COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://coronavirus.jhu.edu/map.html Available at:
- 3.World Health Organization Timeline of WHO’s response to COVID-19. https://www.who.int/news-room/detail/29-06-2020-covidtimeline Available at:
- 4.Centers for Disease Control and Prevention Testing data in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/testing-in-us.html Available at:
- 5.Centers for Disease Control and Prevention Cases in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at:
- 6.Department of Health and Human Services Determination of a public health emergency. https://www.federalregister.gov/documents/2020/02/07/2020-02496/determination-of-public-health-emergency Available at:
- 7.Food and Drug Administration Emergency use authorizations. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization Available at:
- 8.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19 – 16 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---16-march-2020 Available at:
- 9.United States Department of Health and Human Services Policy for coronavirus disease-2019 tests during the public health emergency (revised) https://www.fda.gov/media/135659/download Available at:
- 10.U.S. Food and Drug Administration FDA combating COVID-19 with medical devices. https://www.fda.gov/media/136702/download Available at:
- 11.Schneider E.C. Failing the test - the tragic data gap undermining the U.S. Pandemic response. N Engl J Med. 2020;383(4):299–302. doi: 10.1056/NEJMp2014836. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention How to protect yourself and others. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html Available at:
- 13.Weber N.C., Klepser M.E., Akers J.M., Klepser D.G., Adams A.J. Use of CLIA-waived point-of-care tests for infectious diseases in community pharmacies in the United States. Expert Rev Mol Diagn. 2016;16(2):253–264. doi: 10.1586/14737159.2015.1116388. [DOI] [PubMed] [Google Scholar]
- 14.Klepser D.G., Klepser M.E., Murry J.S., Borden H., Olsen K.M. Evaluation of a community pharmacy-based influenza and group A streptococcal pharyngitis disease management program using polymerase chain reaction point-of-care testing. J Am Pharm Assoc (2003) 2019;59(6):872–879. doi: 10.1016/j.japh.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Klepser D.G., Klepser M.E., Smith J.K., Dering-Anderson A.M., Nelson M., Pohren L.E. Utilization of influenza and streptococcal pharyngitis point-of-care testing in the community pharmacy practice setting. Res Social Adm Pharm. 2018;14(4):356–359. doi: 10.1016/j.sapharm.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Darin K.M., Klepser M.E., Klepser D.E. Pharmacist-provided rapid HIV testing in two community pharmacies. J Am Pharm Assoc (2003) 2015;55(1):81–88. doi: 10.1331/JAPhA.2015.14070. [DOI] [PubMed] [Google Scholar]
- 17.Gubbins P.O., Klepser M.E., Dering-Anderson A.M. Point-of-care testing for infectious diseases: opportunities, barriers, and considerations in community pharmacy. J Am Pharm Assoc (2003) 2014;54(2):163–171. doi: 10.1331/JAPhA.2014.13167. [DOI] [PubMed] [Google Scholar]
- 18.Kelling S.E. Exploring accessibility of community pharmacy services. Innov Pharm. 2015;6(3):1–4. 210. [Google Scholar]
- 19.Berenbrok L.A., Gabriel N., Coley K.C., Hernandez I. Evaluation of frequency of encounters with primary care physicians vs visits to community pharmacies among Medicare beneficiaries. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Pharmacists Association Pharmacist as front-line responders for COVID-19 patient care: executive summary. https://www.pharmacist.com/sites/default/files/files/APHA%20Meeting%20Update/PHARMACISTS_COVID19-Final-3-20-20.pdf Available at:
- 21.Klepser M.E., Adams A.J., Srnis P., Mazzucco M., Klepser D. U.S. community pharmacies as CLIA-waived facilities: prevalence, dispersion, and impact on patient access to testing. Res Social Adm Pharm. 2016;12(4):614–621. doi: 10.1016/j.sapharm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health and Human Services Guidance for licensed pharmacists, COVID-19 testing, and immunity under the PREP act. https://www.hhs.gov/sites/default/files/authorizing-licensed-pharmacists-to-order-and-administer-covid-19-tests.pdf Available at:
- 23.Department of Health and Human Services Advisory opinion 20-02 on the Public Readiness and Emergency Preparedness Act and the Secretary’s declaration under the act May 2020. https://www.hhs.gov/sites/default/files/advisory-opinion-20-02-hhs-ogc-prep-act.pdf Available at:
- 24.Centers for Disease Control and Prevention Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html Available at:
- 25.Centers for Disease Control and Prevention Optimizing supply of PPE and other protective equipment during shortages. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html Available at:
- 26.Centers for Disease Control and Prevention Clinical laboratory improvement amendments (CLIA): test complexities. https://www.cdc.gov/clia/test-complexities.html Available at:
- 27.Centers for Disease Control and Prevention Interim guidelines for collecting, handling, and testing clinical specimens for COVID-19. https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html Available at:
- 28.Beeching N.J., Fletcher T.E., Beadsworth M.B.J. COVID-19: testing times. BMJ. 2020;369:m1403. doi: 10.1136/bmj.m1403. [DOI] [PubMed] [Google Scholar]
- 29.Becton, Dickinson and Company BD Veritor system for rapid detection of SARS-CoV-2. Product website. https://www.bd.com/en-us/offerings/capabilities/microbiology-solutions/point-of-care-testing/bd-veritor-sars-cov-2 Available at:
- 30.Sofia 2 SARS Antigen FIA. [product insert] Quidel Corporations; San Diego, CA: 2020. [Google Scholar]
- 31.ID Now. [product insert] Abbott; Scarborough, ME: 2020. [Google Scholar]
- 32.Accula Test. [product insert] Mesa Biotech, INC; San Diego, CA: 2020. [Google Scholar]
- 33.Xpert® Xpress SARS-CoV-2. [product insert] Cepheid; Sunnyvale, CA: 2020. [Google Scholar]
- 34.Cue Health COVID-19 Test. [product insert] Cue Health, Inc; San Diego, CA: 2020. [Google Scholar]
- 35.Cue Health COVID-19 Test. [user manual] Cue Health, Inc; San Diego, CA: 2020. [Google Scholar]
- 36.American Pharmacists Association Guidelines for pharmacy-based immunization advocacy and administration. https://www.pharmacist.com/sites/default/files/files/Guidelines_for_Pharmacy_Based_IMZ_Advocacy_Approved_Jan_26_2019.pdf Available at: [DOI] [PubMed]
- 37.Centers for Disease Control and Prevention Guidance for pharmacies: guidance for pharmacists and pharmacy technicians in community pharmacies during the COVID-19 response. cdc.gov/coronavirus/2019-ncov/hcp/pharmacies.html Available at:
- 38.Centers for Disease Control and Prevention Overview of SARS-CoV-2 testing. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html Available at:
- 39.Marty F.M., Chen K., Verrill K.A. How to obtain a nasopharyngeal swab specimen. N Engl J Med. 2020;382(22):e76. doi: 10.1056/NEJMvcm2010260. [DOI] [PubMed] [Google Scholar]
- 40.Office of the Assistant Secretary for Health SARS-CoV-2: nasal (anterior nasal) specimen collection for SARS-CoV-2 diagnostic testing. https://www.cdc.gov/coronavirus/2019-ncov/downloads/OASH-nasal-specimen-collection-fact-sheet.pdf Available at:
- 41.Centers for Disease Control and Prevention Interim guidance for implementing home care of people not requiring hospitalization for coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-home-care.html Available at:
- 42.Centers for Disease Control and Prevention Discontinuation of isolation for persons with COVID-19 not in healthcare settings: interim guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html Available at:
- 43.Coronavirus disease 2019 (COVID-19): outpatient management in adults. UpToDate. https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-outpatient-evaluation-and-management-in-adults Available at:
- 44.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 45.American Pharmacists Association, National Alliance of State Pharmacy Associations Pharmacy models for COVID-19 testing. https://www.pharmacist.com/sites/default/files/audience/Pharmacy%20Models%20for%20COVID19%20Testing_07292020.pdf Available at:
- 46.National Alliance of State Pharmacy Associations COVID-19: testing. https://naspa.us/resource/covid-19-testing/ Available at:
- 47.United States Department of Health and Human Services Community-based testing sites for COVID-19. https://www.hhs.gov/coronavirus/community-based-testing-sites/index.html Available at:
- 48.Health Resources and Services Administration COVID-19 claims reimbursement to health care providers and facilities for testing and treatment of the uninsured. https://coviduninsuredclaim.linkhealth.com/ Available at:
- 49.CVS pharmacy COVID-19 testing. https://www.cvs.com/minuteclinic/covid-19-testing Available at:
- 50.Walmart Inc Supporting COVID-19 drive-thru testing. https://corporate.walmart.com/covid19testing Available at:
- 51.Rite Aid Corporation Rite Aid significantly expands COVID-19 testing. https://www.riteaid.com/corporate/news/-/pressreleases/news-room/2020/rite-aid-significantly-expands-covid-19-testing Available at:
- 52.Walgreens No-cost COVID-19 testing at Walgreens. https://www.walgreens.com/findcare/covid19/testing?ban=covid_testing Available at:
- 53.U.S. Food and Drug Administration Kroger health COVID-19 test home collection kit. https://www.fda.gov/media/139681/download Available at:
- 54.Browne M. Kroger to make emergency home COVID-19 test kits available to associates. Supermarket News. https://www.supermarketnews.com/health-wellness/kroger-make-emergency-home-covid-19-test-kits-available-associates Available at:
- 55.American Pharmacists Association APhA urges congress to authorize pharmacists’ COVID testing and immunization services under Medicare in the next pandemic bill. https://www.pharmacist.com/press-release/apha-urges-congress-authorize-pharmacists-covid-testing-and-immunization-services?is_sso_called=1 Available at:
- 56.Department of Health and Human Services Centers for Medicaid and Medicare. Medicare and Medicaid Programs, Basic Health Program, and exchanges; additional policy and regulatory revisions in response to the COVID-19 public health emergency and delay of certain reporting requirements for the skilled nursing facility quality reporting program. https://www.cms.gov/files/document/covid-medicare-and-medicaid-ifc2.pdf Available at:
- 57.Balick R. APhA coronavirus watch: New York, Ohio, and Maryland make moves toward allowing pharmacists to bill Medicaid for COVID-19 testing. https://www.pharmacist.com/article/new-york-and-ohio-make-moves-toward-allowing-pharmacists-bill-medicaid-covid-19-testing?is_sso_called=1 Available at:
- 58.American Medical Association Special coding advice during COVID-19 public health emergency. https://www.ama-assn.org/system/files/2020-05/covid-19-coding-advice.pdf Available at:
- 59.American Medical Association CPT reporting for COVID-19 testing. https://www.ama-assn.org/system/files/2020-05/cpt-reporting-covid-19-testing.pdf Available at:
- 60.National Council for Prescription Drug Program NCPDP emergency preparedness information. Version 1.7. https://www.ncpdp.org/NCPDP/media/pdf/NCPDPEmergencyPreparednessInformation.pdf Available at:
- 61.National Council for Prescription Drug Programs . July 3, 2020. NCPDP News Flash: New Product/Services Identifier Added to Allow Pharmacies to Bill for Specimen Collection of COVID-19. [Google Scholar]