Abstract
Leptomeningeal metastasis is an uncommon and typically late complication of cancer with poor prognosis and limited treatment options. Diagnosis is often challenging with nonspecific presenting symptoms ranging from headache and confusion to focal neurologic deficits such as cranial nerve palsies. Standard diagnostic evaluation involves a neurologic examination, MRI of the brain and spine with gadolinium, and cytologic evaluation of the cerebral spinal fluid (CSF). Therapy entails a multimodal approach focused on palliation with surgery, radiation, and/or chemotherapy, which may be administered systemically or directly into the CSF. Limited trial data exists to guide treatment, with current regimens based primarily on expert opinion. Although newer targeted and immunotherapeutic agents are under investigation and show promise, an improved understanding of the biology of leptomeningeal metastasis and treatment resistance, as well as additional randomized controlled studies, are needed to guide optimal treatment of this devastating disease.
Keywords: leptomeningeal metastasis, carcinomatous meningitis, leptomeningeal carcinomatosis, leptomeningeal disease, brain metastasis
Precis:
Leptomeningeal metastasis is an uncommon complication of cancer with poor prognosis and nonspecific symptomatic presentation that often develops late in the course of disease progression. Treatment options remain limited, and improved strategies should be guided by better understanding of the biology of leptomeningeal metastasis and treatment resistance, as well as additional randomized controlled studies.
Introduction
Incidence of leptomeningeal metastasis (LM), also known as carcinomatous meningitis or leptomeningeal carcinomatosis, typically varies by primary tumor type, occuring in approximately 5–8% of patients with solid tumors and 5–15% of patients with hematologic malignancies.1 While it can also be seen in hematologic malignancies and primary brain tumors such as gliomas, medulloblastomas, and ependymomas, this review will focus on involvement of the subarachnoid space and leptomeninges (arachnoid and pia mater) by solid tumors. Dural involvement can also occur; however, as the dura is not protected by the blood brain barrier (BBB), treatment is not subject to the same limitations as leptomeningeal involvement and falls outside the scope of this review. Nonetheless, it is important to note that leptomeningeal involvement is often seen concurrently with parenchymal or dural disease. LM usually confers a poor prognosis with an average survival of 2 to 4 months despite treatment, although response to treatment can vary with some patients surviving significantly longer.1 While treatment options remain limited, advances in the molecular and genetic understanding of systemic malignancies has yielded new opportunities for clinically effective therapies and better tools to predict therapeutic response.
Pathogenesis and Epidemiology
Unfortunately, understanding of disease pathogenesis has not improved markedly since LM was initially described in the late 19th century.2 Recent studies have started to shed light on the pathogenesis, however, with one study showing that cancer cells within the CSF upregulate production of complement component 3.3 This in turn leads to disruption of the BBB and entry of plasma growth factors into CSF, promoting cancer cell growth. Cancerous involvement of the leptomeninges is thought to occur by several mechanisms, including direct extension from brain parenchyma, dura, or bone; hematologic spread, particularly through venous plexi; or perineural extension. LM involvement most commonly occurs in the basal cisterns of the brain, posterior fossa, and cauda equina.4,5 Invasion of the leptomeninges can lead to local inflammation and impaired CSF resorption, which can then obstruct CSF flow and cause hydrocephalus and/or increased cranial pressure.
Although nearly every systemic tumor has been reported to metastasize to the leptomeninges, common solid tumors include lung, breast, and melanoma. Incidence varies by tumor type and ranges from 5–8% of metastatic breast cancers,6 9–25% of lung cancers (higher in small cell lung cancer),7 and 6–18% of melanomas.8 Overall, the incidence of LM may be increasing in the setting of improved systemic control and treatments that poorly penetrate the BBB, leading to longer survival and a reservoir of tumor cells in the central nervous system (CNS).9–13 Progressive systemic disease is also seen in 60–70% of patients at time of diagnosis.14,15 In a large case series of 187 patients, including 150 patients with solid malignancies (primarily breast and lung cancer), 58% had concurrent or prior parenchymal brain involvement.16 The median time from systemic cancer diagnosis to diagnosis of LM ranges from 1.2 to 2.0 years in solid tumors and averages 11 months in hematologic malignancies.14,16,17
Clinical Presentation & Differential Diagnosis
Signs and symptoms of LM depend on the location of involvement. Given the frequent multifocality, clinical presentation may be nonspecific and index of suspicion must be high. Common clinical findings are often attributable to cranial and spinal nerve dysfunction, increased intracranial pressure (ICP), or meningeal irritation (Table 1). Cranial nerves VI, VII and VIII are commonly affected, leading to diplopia, facial weakness and changes in hearing, respectively. Spinal signs include dermatomal sensory loss, radicular pain, bowel and bladder dysfunction, and limb weakness. Other general symptoms include headache, nausea, vomiting, and changes in mental status. Involvement or compression of small vessels in the subarachnoid space may also lead to ischemic infarct.
Table 1.
Signs and symptoms of leptomeningeal metastasis
| Brain | ||
| Headache | ||
| Confusion | ||
| Nausea/vomiting | ||
| Cranial nerve palsies | Vision changes (particularly double vision) | |
| Tinnitus, decreased hearing | ||
| Facial numbness, weakness | ||
| Dysarthria | ||
| Dysphagia | ||
| Seizure | ||
| Ataxia | ||
| Cognitive impairment | ||
| Spine | ||
| Bowel/bladder dysfunction | ||
| Pain (neck, back, or radicular) | ||
| Paresthesias | ||
| Focal weakness | ||
| Nucal rigidity | ||
| Hyporeflexia | ||
| Clinical Syndromes | ||
| Multiple cranial neuropathies | ||
| Syndrome of inappropriate diuretic hormone secretion (SIADH) | ||
| Rapidly progressive dementia | ||
Given the broad presenting features and frequently complex treatment histories, consideration should also be given to alternative diagnoses including chronic infectious meningitis, autoimmune disorders (e.g. sarcoidosis), meningeal reaction to brain abscess, side effects of chemotherapy or radiation, paraneoplastic syndromes, and toxic-metabolic encephalopathy (Table 2). In immunocompromised cancer patients, causes of infectious meningitis or encephalitis include bacterial (e.g. tuberculosis, listeriosis), fungal (e.g. Cryptococcus, candidiasis), or viral (e.g. cytomegalovirus, varicella zoster virus, Epstein-Barr virus, herpes simplex virus, and JC virus).18
Table 2.
Differential diagnoses
| Infectious meningitis | |
|---|---|
| Chemical meningitis/arachnoiditis (secondary to intrathecal chemotherapy) | |
| Multiple brain metastases | |
| Paraneoplastic syndrome | Limbic encephalitis |
| Encephalomyelitis | |
| Paraneoplastic cerebellar degeneration | |
| Intracranial hypotension (post lumbar puncture) | |
| Toxic metabolic encephalopathy | |
| Metabolic or chemotherapy-induced neuropathy | |
| Steroid myopathy | |
| Cord compression | |
Diagnostic Evaluation
The diagnosis of LM remains challenging with no test sufficiently sensitive to rule out involvement. Magnetic resonance imaging (MRI) of the brain and spine is recommended if there is clinical suspicion, and may show leptomeningeal enhancement, which is often irregular and nodular (Figure 1).19 Subependymal deposits and hydrocephalus may also be seen. Imaging should be interpreted with caution if a recent lumbar puncture has been performed as resulting low ICP or inflammation may lead to transient enhancement. Sensitivity of MRI with gadolinium is approximately 70% with specificity of 77–100% (higher for solid tumors than hematologic malignancies).20–22 In the presence of typical clinical features, an abnormal MRI is sufficient to make the diagnosis.22 11-indium or 99-technetium ventriculography may be performed to evaluate CSF flow in select circumstances when this may help guide treatment, described below.
Figure 1:
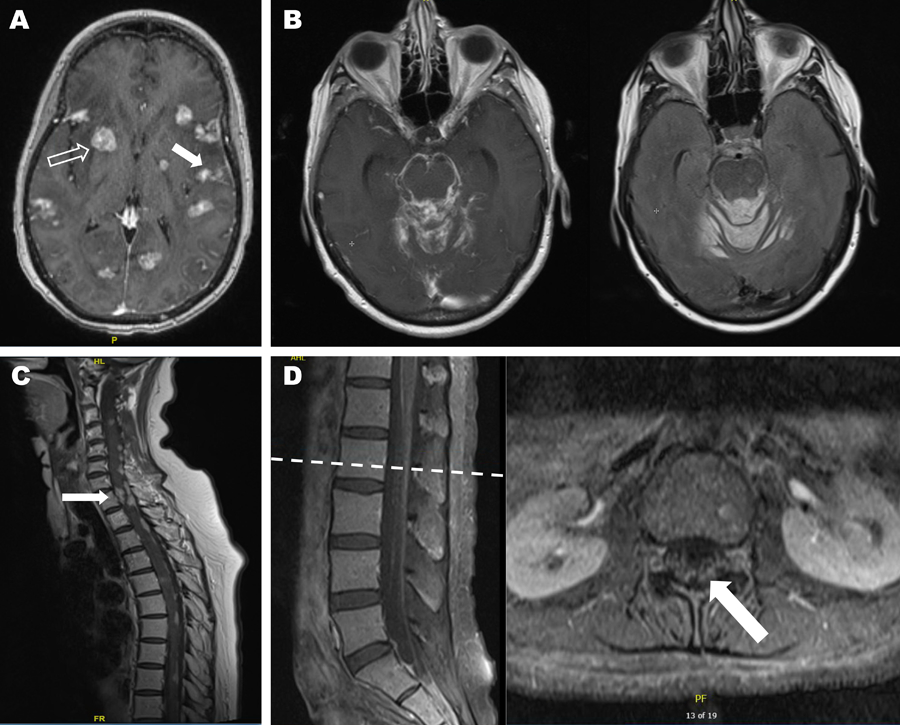
Magnetic resonance imaging of leptomeningeal metastasis.
If safe to perform, lumbar puncture is recommended (Figure 2) and often reveals mild pleocytosis with elevated protein and hypoglycorrhachia. In cases of profound hypoglycorrhachia, infectious etiologies (described above) should be considered, particularly bacterial and fungal meningitis. An elevated opening pressure may be seen in 50–70% of cases depending on extent of leptomeningeal involvement.23 False negative cytology results can be minimized in several ways.17 First, sufficient CSF volume of at least 10 mL should be obtained for cytologic analysis. Second, the CSF specimen should be processed as soon as possible to reduce the risk of cell death. Glantz et al. found a false-negative error rate of 36% in samples refrigerated for 48 hours versus samples collected from the same patients that showed positive cytology upon immediate processing. Third, obtaining the CSF from a site of known leptomeningeal disease may increase the likelihood of detecting abnormal cells, although this may be more relevant in untreated patients screened for LM than in patients who have received intrathecal or systemic treatment. Finally, the procedure should be repeated at least once if initial sample is negative and LM is suspected. CSF cytology is positive in over 90% of patients with suspected LM after three high volume lumbar punctures, and specificity is over 95%.15,24 False positives may be seen in infectious or other inflammatory conditions with reactive lymphocytes. Flow cytometry and additional molecular studies may be valuable in select clinical scenarios. Flow cytometry has increased sensitivity compared to cytomorphologic analysis in the setting of hematologic malignancies.25
Figure 2:
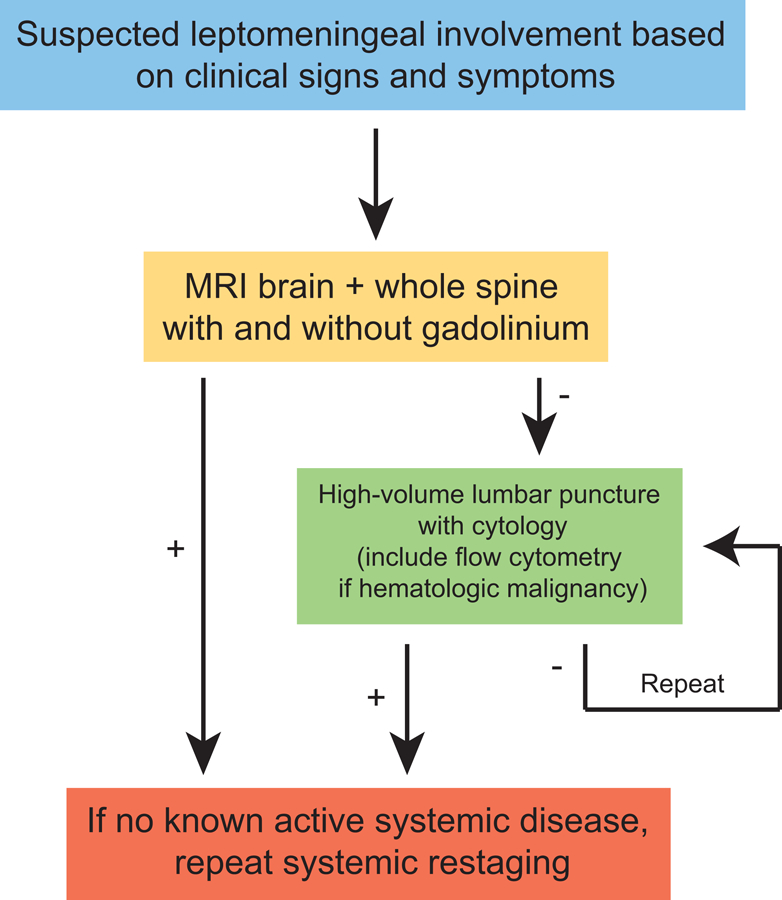
Diagnostic Algorithm.
The use of CSF tumor markers has been limited by their low sensitivity and specificity as well as significant assay variability. However, they may support the diagnosis in the face of an otherwise equivocal diagnostic evaluation. Particularly, CSF levels greater than 1% of serum levels of specific tumor markers such as carcinoembryonic antigen (CEA) from adenocarcinomas, α–fetoprotein from hepatocellular and testicular carcinoma, and β–human chorionic gonadotropin from choriocarcinoma and testicular carcinomas are relatively specific for CSF involvement.26,27 These markers may also have value in following response to treatment. More recently, cell-free DNA present in the CSF has been used to detect tumor-specific somatic alterations through next generation sequencing.28–30 Detection of tumor-specific mutations may increase sensitivity and specific of diagnostic CSF evaluation, aid in the assessment of treatment response, and shed light on mechanisms of CNS resistance to systemic therapy.
Lastly, if there is no known active systemic disease, systemic restaging should be performed as this may guide further treatment.
Assessing Response to Therapy
Of the six randomized controlled trials (Table 3) conducted in leptomeningeal metastases, the majority have incorporated neurologic examination and CSF cytology to determine response to treatment. However, assessment of neurologic response was often based on subjective neurologic evaluations, MRI criteria were not used or not stated, and cytologic evaluation was not uniform.31 Site of CSF sampling is also important in assessing response, as negative cytology at one site such as via an Ommaya reservoir does not necessarily define cytologic response when initial diagnosis was made based on cytologic evaluation at another site, such as via lumbar puncture. Primary endpoints varied across trials, including overall survival, neurologic response rate, time to neurologic progression, and progression free survival. Secondary endpoints have included neurologic progression, neurologic response rate, safety and toxicity profile, cause of death, Karnofsky performance status (KPS) evolution over time, quality of life, LM-specific survival, and survival. Secondary endpoints such as patient-reported quality of life and neurologic progression may be important considerations in settings where disease is often advanced and overall survival is unlikely to be prolonged, but symptom palliation remains a central goal of therapy.
Table 3.
Randomized controlled trials in leptomeningeal metastasis
| Trial | N | Tumor Type | Treatment Arms | Endpoint | Significance |
|---|---|---|---|---|---|
| Hitchins et al. 1987105 | 44 | 29% SCLC, 25% breast, 9% primary brain, 7% NSCLC, 7% lymphoma | IT MTX | RR 61% OS 12 wks |
RR: P > 0.10 OS: P = 0.084 |
| IT MTX + Ara-C | RR 45% OS 7 wks |
||||
| Grossman et al. 1993106 | 52 assessable | 48% breast, 23% lung, 19% lymphoma | IT MTX | OS 15.9 wks SD 32% |
RR: unknown OS: P = 0.36 |
| IT thiotepa | OS 14.1 wks SD 12.5% |
||||
| Glantz et al. 1999107 | 28 | 100% lymphoma | IT DepoCyt | RR 71% TTP 78.5 OS 99.5 d |
RR: P = 0.006 TTP, OS: P > 0.05 |
| IT Ara-C | RR 15% TTP 42 d OS 63 d |
||||
| Glantz et al. 1999108 | 61 | 36% breast, 10% NSCLC, 23% primary brain, 8% melanoma, 7% SCLC | IT DepoCyt | RR 26% TTP 58 d OS 105 d |
RR: 0.76 TTP: P = 0.007 OS: P = 0.15 |
| IT MTX | RR 20% TTP 30 d OS 78 d |
||||
| Boogerd et al. 200436 | 35 | 100% breast cancer | Systemic therapy + RT + IVT MTX | Neurologic improvement/stabilization 59% TTP 23 wks OS 18.3 wks |
Neurologic response: unknown TTP: unknown OS: P = 0.32 |
| Systemic therapy + RT | Neurologic improvement/stabilization 67% TTP 24 wks OS 30.3 wks |
||||
| Shapiro et al. 2006109 | 128 | 80% solid tumors, 20% lymphoma | Combined IT DepoCyt (solid tumor and lymphoma) | PFS 35 d | PFS: P=0.7321 HR = 0.98 |
| Combined IT MTX (solid tumor) + IT Ara-C (lymphoma) | PFS 43 d | ||||
| IT DepoCyt (lymphoma) | PFS 34 d Cytologic response 33.3% |
PFS: HR = 0.12 Cytologic response: P=0.3640 |
|||
| IT Ara-C (lymphoma) | PFS 50 d Cytologic response 16.7% |
Abbreviations: Ara-C, cytarabine; DepoCyt, liposomal cytarabine; d, days; IT, intrathecal chemotherapy; IVT, intraventricular; MTX, methotrexate; NSCLC, non-small cell lung cancer; OS, median overall survival; PFS, progression free survival; RR, response rate; RT radiotherapy; SCLC, small cell lung cancer; TTP, time to progression; wks, weeks.
Standardized assessment was only recently proposed by the Response Assessment in Neuro-Oncology group in 2016 after recognition of the limitations in assessing outcomes.32 The proposed criteria include a standard neurologic examination, MRI of the brain and spine, and CSF evaluation. Therapeutic response can only be determined in the setting of negative cytologic evaluation (as well as flow cytometry in hematologic malignancies), definite improvement in CNS imaging, decreased or absent steroid dose (in hematologic malignancies only), and improved symptoms. Importantly, definitive worsening of CNS imaging is sufficient to determine progressive or refractory disease. Response based on CSF cytology is considered when cytology converts from positive to negative at all sites previously shown to be positive and is subsequently confirmed after one month. Of note, there was lack of consensus regarding response determination in a patient with persistently positive cytology in the setting of stable or improved clinical and radiographic status. Although suggested, the criteria do not include patient-reported outcomes such as the MD Anderson Cancer Center Symptom Inventory Brain Tumor Module (MDASI-BT), MD Anderson Cancer Center Symptom Inventory Spine Tumor Module (MDASI-SP), or Functional Assessment of Cancer Therapy-Brain. These experts acknowledge that the proposed criteria to standardize LM response assessment require validation and refinement, however they serve as a new standard that can be incorporated into future clinical trials to better enable comparison across trials and more rigorous assessment of therapeutic response.
Prognosis
Despite advances in care, prognosis remains poor with an overall survival of approximately 4–6 months from time of diagnosis if treated.33 Untreated, death occurs from progressive neurologic deterioration in 4–6 weeks.15 KPS greater than 70, chemosensitivity of primary cancer, impaired CSF flow, CSF protein less than 50 mg/dL, and active treatment have been identified as favorable prognostic factors.34,35 One study of patients with solid and hematologic malignancies and cytologically confirmed LM found that those with KPS of 70 or greater had a median survival of 15.5 weeks compared to 6 weeks in patients with KPS less than 70.35 The U.S. National Comprehensive Cancer Network (NCCN) identifies poor prognostic factors as KPS less than 60, severe neurologic deficits, extensive systemic disease with few treatment options, bulky CNS disease, or encephalopathy.36 Primary tumor type also plays an important role. In one patient series, those with hematologic malignancies had slightly improved survival of 4.7 months compared to 2.3 months for those with solid tumors.16 Within solid tumors, breast cancer LM has a superior prognosis compared to other tumor types with a median survival of 5–7 months.16,37–40
Treatment
Treatment of LM has traditionally been directed toward palliation, although new therapies show promising response rates. Systemic chemotherapies have been limited in their ability to cross the blood brain barrier but are often combined with radiation and other palliative surgical interventions with a goal of preventing neurologic deterioration, maintaining quality of life, and prolonging survival. Intrathecal chemotherapy is frequently considered, however clinical trial data is limited. Due to the paucity of prospective, randomized trials, optimum therapy is poorly defined and treatment is mostly guided by expert opinion.
Radiation
Radiation is typically geared toward symptom management and thus often targets bulky, symptomatic sites of disease, particularly in the spine. Frequently, whole brain radiotherapy (WBRT) at doses between 30 to 40 Gy in 2 to 3 Gy fractions is administered, although an abbreviated course of 20 Gy in 4 Gy fractions is sometimes considered in patients with a poor prognosis or who are less likely to tolerate treatment.26,41 Radiation may also restore CSF flow and relieve hydrocephalus by reducing tumor bulk, and in doing so, facilitate the use of intrathecal chemotherapy.42 In addition to the long-term side effects of radiotherapy alone, there may also be increased risk of late leukoencephalopathy when combined with other chemotherapeutic agents, such as intravenous or intrathecal methotrexate.43–48 Radiation is unlikely to prolong survival based on retrospective studies in breast and lung cancer patients, but it can result in rapid symptom improvement.49,50 Eradication of tumor cells from the leptomeninges would require craniospinal irradiation, which carries significant potential CNS and systemic toxicities, including myelosuppression, which may compromise future cytotoxic chemotherapy options. Additionally, it is often considered impractical in the setting of poor overall prognosis. While not standard practice, craniospinal irradiation may be used in the setting of LM from hematologic malignancies as these are frequently highly radiosensitive.45,51,52
Intrathecal chemotherapy
While intrathecal (IT) delivery of chemotherapy bypasses the BBB and minimizes systemic side effects, it carries some limitations. Agents can be administered by lumbar puncture or through surgical placement of a reservoir that directly feeds into the ventricular system through a catheter (such as an Ommaya reservoir). Commonly used agents include methotrexate (a folate antagonist), thiotepa (an alkylating agent), cytarabine (a pyrimidine analogue), and sustained release liposomal cytarabine (DepoCyt®). Several retrospective studies have demonstrated survival benefit to intrathecal therapy.40,42 Of the six randomized clinical trials conducted in LM, all focused on intrathecal therapy (Table 3). It is important to note that most trials and series excluded patients who were deemed too sick for treatment, which may constitute a significant proportion of patients at presentation. The study by Boogerd et al.46 was the only trial to compare IT chemotherapy to standard therapy without IT treatment. In 35 breast cancer patients with 17 randomized to receive IT chemotherapy, there was no difference in survival or neurologic response, and the trial was closed prematurely due to low accrual. Another retrospective study of 104 patients with LM from any solid tumor who received systemic therapy and radiation with or without IT therapy also found no difference in median survival.47 Quality of life measures were not assessed in either study, and both studies showed increased rates of treatment-related neurotoxicity in patients who received IT chemotherapy. A study of liposomal cytarabine in breast cancer LM is currently underway (NCT01645839).
Aseptic or chemical meningitis is one of the more common complications, seen in up to 43% of patients, and is characterized by sterile CSF pleocytosis as well as clinical signs and symptoms of meningitis.53,54 While Chamberlain et al. found that the frequency of this complication was independent of the type of IT chemotherapy (between methotrexate, cytarabine, and thiotepa) administered via Ommaya reservoirs, the frequent occurrence of chemical arachnoiditis with intrathecal liposomal cytarabine has led to it being standardly co-administered with dexamethasone.53 Corticosteroids and intravenous hydration can be used to treat and mitigate the symptoms of this complication. However, infectious meningitis should be ruled out when aseptic meningitis is being considered and is present in 8 to 24% of patients receiving intraventricular therapy.55 The most common organism is Staphylococcus epidermidis and treatment requires intravenous and intraventricular antibiotics; removal of the reservoir may be indicated as well.56,57 Other complications include leukoencephalopathy (particularly when combined with radiation), myelopathy, seizure, and inadvertent subdural or epidural delivery if administered via lumbar puncture. Despite the method of administration, myelosuppression can also be seen in up to 18% of patients.53
The site and pattern of involvement is an important consideration when considering IT chemotherapy. Penetration is limited in areas of bulky leptomeningeal disease with penetration of approximately 2–3 mm.54 If there is evidence of complete or partial obstruction of CSF flow, excessive build-up of the chemotherapy may lead to neurotoxicity and treatment failure. Radionucleotide flow studies may be helpful to evaluate CSF flow prior to therapy. However, these studies are more invasive than conventional imaging and are often technically challenging, requiring cisternograms immediately following tracer injection as well as 4 to 6, 24, 48, and sometimes even 72 hours post injection.58 In the setting of ventriculoperitoneal shunts (VPS), there are also concerns about accumulation of chemotherapy leading to neurotoxocity should there be shunt malfunction or intraperitoneal toxicity from draining of the IT drug. However, a small retrospective study showed that IT chemotherapy could safely be administered through a reservoir-on/off valve-VPS.59
Systemic chemotherapy
While systemic chemotherapy is limited by the ability of agents to penetrate the blood-brain barrier (BBB), there is breakdown of the BBB in the setting of LM, and a number of chemotherapies have been shown to achieve therapeutic levels in the CSF when given systemically in patients with this disease. Systemic chemotherapy is additionally not dependent on CSF flow, is able to penetrate bulky nodular disease, concurrently addresses any systemically active disease, and avoids the potential procedural complications associated with intrathecal therapy. The type of malignancy should guide choice of systemic chemotherapy. Options include high dose methotrexate (3 to 8 g/m2),60,61 high dose cytarabine (3 g/m2),62,63 capecitebine (particularly for breast cancer),64–67 thiotepa,68 and temozolomide.69 Response has also been reported with high dose etoposide in 5 patients with small cell lung cancer.70 Systemic chemotherapy, particularly when combined with radiation, can lead to acute or delayed leukoencephalopathy, subacute encephalopathy, or acute cerebellar syndrome associated with high dose cytarabine.
Numerous retrospective studies have demonstrated improved survival in patients treated with systemic chemotherapy.37–39,71,72 Some argue, based on the randomized trial by Boogerd et al.36 and other retrospective studies, that intrathecal chemotherapy adds little value to systemic chemotherapy.46,47,60,73 Conversely, however, a prospective series of patients with LM from NSCLC found no added survival benefit from systemic chemotherapy when combined with radiotherapy and intraventricular chemotherapy.42 The role of systemic versus intrathecal chemotherapy may vary based on primary tumor type, as the studies showing little added value of intrathecal therapy primarily consisted of patients with lymphoma or breast cancer.
Targeted therapies
Melanoma
In subsets of solid tumors, targeted therapies have demonstrated promising results. Approximately 50% of melanomas harbor an activating mutation in BRAF, most commonly BRAFV600E, which constitutively activates the MAP-kinase pathway. In LM from melanoma, there are reports of response to BRAF inhibitors such as vemurafenib74 and dabrafenib.75 Most mechanisms of resistance to BRAF inhibition are mediated through MEK with three randomized Phase III studies in metastatic melanoma now showing superiority of combination BRAF and MEK inhibition compared to BRAF inhibition alone.76–78 This strategy has not been evaluated in patients with LM involvement to date although all three trials included patients with stable brain metastases.
Breast Cancer
Human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately 30% of primary breast cancers and is associated with increased risk of CNS involvement.79 Multiple reports describe response to intrathecal trastuzumab, a humanized monoclonal antibody against HER2, in LM from HER2-positive breast cancer.80–85 Preliminary results from a phase I trial of IT trastuzumab in patients with HER2 positive breast cancer and LM showed that it was well tolerated, and several Phase II trials are ongoing (NCT01325207, NCT01373710).86 Combination approaches are also being studied, with a Phase I trial of lapatinib, a small molecule dual tyrosine kinase inhibitor that targets HER2 and EGFR, in combination with capecitabine, an antimetabolite chemotherapeutic, currently underway in HER2 positive patients with LM (NCT02650752). The Phase II LANDSCAPE trial of lapatinib and capecitabine in HER2-positive patients with brain metastases (not specifically LM) showed a promising CNS response rate of 65.9%, all partial responses.87
Non-small cell lung cancer
In non-small cell lung cancer, first generation tyrosine receptor kinases (TKIs) such as erlotinib and gefitinib do not readily cross the BBB and may be actively removed by drug efflux proteins.88,89 However, CSF concentration may reach therapeutic levels at high doses.88 Although there have been no randomized trials, responses have been described to erlotinib90–98 and gefitinib,99,100 particularly at high doses. Several retrospective studies have shown prolonged survival in epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC) patients with LM treated with first generation EGFR TKIs.101,102 Second and third generation EGFR TKIs are thought to have better BBB penetration. A report of patients with pretreated EGFR-mutant NSCLC and brain metastases or LM who received afatinib on compassionate use basis showed a 35% response rate and CSF concentrations of up to 1 nMol.103 Additional case reports support the efficacy of afatinib in patients with leptomeningeal disease who have progressed on first-generation TKIs.104,105 Preliminary data for the third-generation TKI omesartinib (AZD9291) in heavily pretreated patients with EGFR-mutant NSCLC and LM showed promising response rates (7 of 12 patients with radiographic improvement, 8 of 9 patients with EGFR mDNA copy decrease).106 Importantly, EGFR mutation status in the primary tumor and metastasis may be discordant and analysis should be performed on CSF if possible.88,107,108 There is an ongoing Phase II clinical trial of tesevatinib, a BBB-penetrant oral TKI, in patients with EGFR-activating mutations and brain or leptomeningeal metastases (NCT02616393).
Anaplastic lymphoma kinase (ALK) rearrangements are another important therapeutic target in NSCLC and is associated with an increased risk of CNS involvement.109,110 Presence of the rearrangement confers sensitivity to ALK tyrosine kinase inhibitors. Data suggest that second generation inhibitors have improved BBB penetration compared to the first generation inhibitor crizotinib. Several case reports document response in leptomeningeal disease with alectinib and ceritinib in patients with crizotinib-resistant disease.110–112 The efficacy of ceritinib in treating LM in ALK-rearranged NSCLC patients is being further evaluated in an ongoing Phase II clinical trial (NCT02336451).
Supportive Care
Symptomatic management should always be pursued in addition to any disease-directed therapies. As symptoms may be caused by inflammation as well as direct tumoral involvement, steroids may play a role in symptom management, although the role of steroids is often greater in the setting of LM due to hematologic malignancies. Nausea, vomiting, and headache should also be treated with appropriate medications, and if present, seizures should be controlled with antiepileptic drugs. Fatigue related to treatment, particularly radiation, may be treated with psychostimulants. If there are clinical signs of increased ICP such as nausea, headache, or encephalopathy, a high volume lumbar puncture may be pursued. If pressure is elevated, a palliative VPS should be considered.113 Pain due to cranial and spinal nerve involvement can be managed with palliative focal radiation, opioids or opioid-sparing agents, but is unfortunately often refractory in the setting of poor response to treatment of the underlying disease.
Novel Approaches
Given the remarkable response to checkpoint blockers in many systemic malignancies, multiple clinical trials (Table 4) are underway to evaluate the efficacy in the setting of LM, including pembrolizumab for LM (NCT02886585) and combination ipilimumab and nivolumab for melanoma LM (NCT02939300). Immune-based approaches are often associated with inflammation, which even if transient, may contribute to significant neurotoxicity in the CNS. For example, despite responses seen with intrathecal interleukin 2 or interferon alpha, both had significant toxicity (particularly signs of meningitis, edema, and increased ICP), limiting widespread use.114,115 There is so far only one case report of the anti-CTLA-4 antibody ipilimumab combined with WBRT demonstrating efficacy in a patient with melanoma LM.116
Table 4.
Active Therapeutic Clinical Trials for Patients with Leptomeningeal Metastases
| NCT | Phase | Primary Histology | Site, Sponsor | Drug | Primary Outcome(s) | Secondary Outcome(s) | Study Arms | Key Inclusion Criteria | Key Exclusion Criteria | Recruiting? |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT02939300 | II | Melanoma | Massachusetts General Hospital, Bristol-Myers Squibb | Nivolumab and ipilimumab | OS | IC/EC RR LM RR IC/EC PFS Toxicity |
Single arm: combination of nivolumab with ipilimumab followed by nivolumab monotherapy | • Adults only • ECOG ≤2 or KPS ≥60 • Life expectancy ≥3 wks • LM confirmed by cytology |
• Active, known, or suspected autoimmune disease • Condition requiring systemic corticosteroid treatment • Prior systemic treatment with anti-CTLA4 antibody • Known history of active TB |
Yes |
| NCT01645839 | III | Breast cancer | Multiple sites in France, Centre Oscar Lambret | Liposomal cytarabine | Neurological PFS | Neurological, physical, cognitive, cytological, radiological improvement PFS (radiological, clinical, cytological) OS Toxicity |
A: Standard systemic treatment without liposomal cytarabine B: Standard systemic treatment with liposomal cytarabine |
• Female adults only • ECOG ≤2 • Life expectancy ≥2 mo • New diagnosis of LM by cytology OR clinical signs and symptoms • Measurable CNS disease < 0.5 cm or > 0.5 cm if focused radiation therapy |
• Symptomatic BM or BM requiring WBRT • Previous CSI or IT therapy • Previous systemic treatment with ARA-C or high-dose systemic methotrexate • Contraindication to LP and ventricular catheterization • VPS |
Yes |
| NCT01325207 | I/II | HER2+ breast cancer | Multiple US sites; Northwestern University | IT trastuzumab | Safety MTD |
Response (radiological, cytological, clinical) CSF PK |
Single arm, dose escalation: twice weekly for 2 wks, then weekly for 4 wks, then every 2 wks | • Adults only • HER2+ by IHC or FISH breast cancer • LM determined by MRI or cytology • Life expectancy ≥8 wks • KPS ≥50 • Willing to have Ommaya reservoir placed • May continue on IV trastuzumab, lapatinib or hormonal agents if controlling ECD and developed LM while on therapy |
• Previously-treated BM • BM requiring active treatment • Systemic agents (chemotherapy) that have CNS penetration, unless LM developed while on these agents and ECD controlled |
Ongoing, not recruiting |
| NCT01373710 | I/II | HER2+ breast cancer | Multiple sites in France, Institut Curie | IT/IVent trastuzumab | MTD | CNS TTP QoL OS PFS PK Radiological, CSF response |
Single arm, dose escalation: 1 injection/wk during 8 wks by lumbar puncture or Ommaya Reservoir, 4 dose levels expected from 30 mg to 150 mg | • Adults only • Life expectancy ≥2 mo • HER2+ by IHC and/or FISH • LM diagnosis by cytology and/or clinical signs and symptoms of LM with abnormal MRI |
• Symptomatic untreated BM • Symptomatic BM, unless surgery and/or RT were performed ≥3 wks before treatment initiation and lesion(s) accessible to IT or IVent treatment • Obstructive hydrocephalus • On lapatinib, unless wash out >2 wks before first dose of IT study drug • VPS or atrial shunt, unless can be turned off during treatment |
Yes |
| NCT02650752 | I | HER2+ breast cancer | 3 US sites, Memorial Sloan Kettering Cancer Center | High-dose lapatinib + capecitabine | MTD | Not specified | Single arm: weekly treatment cycle consisting of lapatinib 3 d on/11 d off + capecitabine 7 d on/7 d off. Both drugs administered orally with dose escalation. | • Female adults only • HER2+ by IHC or FISH • Life expectancy ≥12 wks • ECOG ≤2 • Non-escalating corticosteroid dose (≤16 mg dexamethasone daily) for ≥ 5 d • Radiological evidence of new and/or progressive BM/LM or CSF cytological evidence of LM |
• Prior capecitabine therapy allowed if ≥6 mo since last dose • Everolimus therapy • Craniotomy, other major surgery, open biopsy, or significant traumatic injury ≤4 wks of enrollment • HIV infection or chronic hepatitis B or C • Concurrent chemotherapy, hormonal therapy, radiation therapy, surgery, immunotherapy, tumor embolization, or biologic therapy, except for trastuzumab or hormonal therapy |
Yes |
| NCT02422641 | II | Breast cancer | Wake Forest University and Sidney Kimmel Comprehensive Cancer Center, Wake Forest University Health Sciences | High-dose methotrexate | 3 mo OS | 1 yr OS PFS Tolerability Cost Cytologic sterilization |
Single arm: high dose methotrexate (8 gm/m2 IV every 2 wks) | • Adults only • ECOG 0–1 • Triple negative, HER2+, or HR+ hormone refractory breast cancer • Cytologic or radiographic confirmation of LM with/without BM |
• Chemotherapy or SRS within 2 wks, WBRT within 6 mo • Heart failure (>NYHA Class 3) • Prior treatment with any methotrexate-containing systemic regimen within 1 yr (excluding IT methotrexate) • Concurrent or planned systemic chemotherapy, radiotherapy, or new hormonal/anti-HER2 directed therapy |
Yes |
| NCT02616393 | II | EGFR-mutant NSCLC | Multiple US sites, Kadmon Corp. LLC | Tesevatinib | Clinical activity using RECIST 1.1 (Cohorts A, C), symptom resolution (Cohort B) | QoL Median PFS CNS TTP Median OS PK |
Dosing the same among all arms: 300 mg orally once daily A: NSCLC who have progressed with BM B: NSCLC who have progressed with LM C: NSCLC with BM at initial presentation |
• Adults only • EGFR mutation that has clinical response to erlotinib, afatinib, or gefitinib • BM occurrence or progression while receiving erlotinib, afatinib, or gefitinib • Measurable BM (≥10 mm) • ECOG ≤2 • No clinically significant progression outside of the CNS on most recent EGFR inhibitor therapy |
• First day of dosing with tesevatinib • <2 wks from the last treatment of cytotoxic chemotherapy, biological therapy, or immunotherapy, • <6 wks for nitrosoureas and mitomycin C • <2 wks since surgical procedure • <4 wks since last CNS-direct RT • <3 d since discontinuing erlotinib, afatinib, or TKI • Any concurrent BM (Cohorts A and C), LM (Cohort B) therapy other than study treatment |
Yes |
| NCT02336451 | II | NSCLC with ALK rearrangement | Multiple US and international sites, Novartis | Ceritinib | ORR | TTIR and TTR IC/EC DOR IC/EC ORR IC/EC DCR PFS Safety PK |
Dosing the same among all arms: 750 mg orally once daily • ALK+ NSCLC with BM, without LM, with previous exposure to crizotinib • ALK+ NSCLC with BM, without LM, without previous exposure to crizotinib • ALK+ NSCLC with LM, with or without previous exposure to crizotinib |
• ≥1 measurable EC lesion • ECOG ≤2 • Life expectancy ≥6 wks |
• Prior ALK inhibitor other than crizotinib • BM requiring WBRT • Previously-treated BM, unless progressive or new since WBRT • Unstable or increasing dosage of corticosteroids • Planning to receive local treatment to BM (e.g. surgery, SRS, WBRT, IT chemotherapy) |
Yes |
| NCT00445965 | II | GD2-positive LMD (primarily neuroblastoma, primary CNS tumors) | Memorial Sloan Kettering Cancer Center, Same | IVent 131-I-labeled monoclonal antibody 3F8 | 6mo OS RR (alive at 6 mo) |
Toxicity | Single arm: 10 mCi injected IT weekly for up to 4 courses as tolerated | • Children and adults • Histologically-confirmed diagnosis of a malignancy known to express GD2 |
• Rapidly progressing or deteriorating neurologic examination • Obstructive or symptomatic communicating hydrocephalus • CSI or systemic chemotherapy <3 wks prior to start of protocol • >45 Gy CSI or >72 Gy focal brain radiation |
Ongoing, not recruiting |
| NCT00089245 | I | Malignancy known to be 8H9 reactive, confirmed by IHC or bone marrow IF | Memorial Sloan Kettering Cancer Center, Same | 131-I-labeled 8H9 | MTD over 2 yrs | Not specified | Single arm, dose escalation with patients entering in cohorts of: 3 patients at each dose level from 10–60 mCi 6 patients at each dose level from 70–100 mCi |
• Children and adults• LMD refractory to conventional therapies or recurrent brain tumors with predilection for LM dissemination (medulloblastoma, PNET, rhabdoid tumors) | • Rapidly progressing or deteriorating neurologic examination • Obstructive or symptomatic communicating hydrocephalus • CSI or systemic chemotherapy <3 wks prior to start of protocol |
Yes |
| NCT02308020 | II | HR+ breast cancer, NSCLC, or melanoma | Multiple US sites, Eli Lilly and Co. | Abemaciclib | IC ORR | BOIR IC DOR DCR IC DCR ICBR OS OR PFS PK at 3 mo |
Same treatment for all arms, UOS: 200mg study drug Q 12 hr, days 1–21 of each 21 d cycle • A: HER2+ breast cancer • B: HER2- breast cancer • C: Surgical resection indicated for intracranial lesions, drug days 5–14 prior to surgical resection and resumed dosing after wound healing • D: NSCLC, 150 mg drug if receiving concurrent gemcitabine or pemetrexed • E: Melanoma • F: HR+ Breast cancer, NSCLC, or melanoma |
• Adults only • KPS ≥70 • Life expectancy ≥12 wks • Completed local therapy (surgical resection, WBRT, or SRS) ≥14 d prior to initiating study drug |
• Require immediate local therapy (including WBRT, SRS, surgical resection) • Concurrent EIAED use • Evidence of symptomatic intracranial hemorrhage • ≥2 seizures within 4 wks prior to study initiation |
Yes |
| NCT02886585 | II | Multiple histologies | Massachusetts General Hospital, Merck Sharp & Dohme Corp. | Pembrolizumab | ORR OS EC ORR |
Toxicity OS rate IC/EC RR EC PFS |
Same treatment for all arms, UOS: study drug Q 3 wks • A: previously untreated BM • B: progressive BM after prior local CNS-directed therapy (e.g. WBRT, SRS, or surgery) • C: LM with positive CSF cytology • D: 1–4 BM from histologically-confirmed melanoma with clinical indication for SRS, cycles 1 and 2 of study drug administered 3 wks apart with SRS between |
• Adults only • Progressive systemic disease from any histologically or cytologically confirmed solid tumor • Measurable CNS disease (≥10 mm), except for Arm C • ECOG ≤2 or KPS ≥60 • Life expectancy ≥6 wks • Stable dose of dexamethasone ≤2 mg for ≥7 d prior to treatment initiation |
• Arm A: excludes HER2+ breast cancer, SCLC,; NSCLC with targetable genomic tumor aberrations (e.g. EGFR, ALK) • Known history of active TB • Immunodeficient HIV-positive participants on combination antiretroviral therapy • Prior treatment with an anti-PD-1, anti-PD-L1, or anti-PD-L2 agent |
Yes |
All information obtained from clinicaltrials.gov
Abbreviations:
ALK – anaplastic lymphoma kinase, BM – brain metastasis(es), BOIR – best overall intracranial response, CNS – central nervous system, CR – complete response, CSI – craniospinal irradiation, CTLA4 – Cytotoxic T-Lymphocyte Associated Protein 4, DCR – disease control rate, DOR – duration of response, EC – extracranial, ECD – extracranial disease, ECOG – Eastern Cooperative Oncology Group Performance Status, EGFR – epidermal growth factor receptor, EIAED – enzyme-inducing antiepileptic drugs, FISH – fluorescence in situ hybridization, HER2 – human epidermal growth factor receptor 2, HIV – human immunodeficiency virus, HR – hormone receptor, IC – intracranial, ICBR – intracranial clinical benefit rate, IF – immunofluorescence, IHC – immunohistochemistry, IT – intrathecal, IV – intravenous, IVent – intraventricular, KPS – Karnofsky Performance Status, LMD – leptomeningeal metastasis/disease, MTD – maximum tolerated dose, NSCLC – non-small cell lung cancer, NYHA – New York Heart Association, ORR – objective response rate, OS – overall survival, PD – progressive disease, PD/PD-L – Programmed Death/Programmed Death-Ligand, PFS – progression-free survival, PK – pharmacokinetics, QoL – quality of life, RR – response rate, RT – radiotherapy, SCLC – small cell lung cancer, SRS – stereotactic radiosurgery, TB – Bacillus Tuberculosis, TT(I)R – time to (intracranial) tumor response, TKI – tyrosine kinase inhibitor, TTP – time to progression, UOS – unless otherwise specified, VPS – ventriculoperitoneal shunt, WBRT – whole-brain radiation therapy
Intrathecally delivered monoclonal antibodies against tumor-specific antigens have also been studied as a means to selectively deliver radiation (also known as radioimmunotherapy) and/or therapeutic agents. Though the approach was first studied the 1980s, it has regained interest with the renewed focus on targeted and immune-based therapies. Retrospective data and prior Phase I trials suggest therapeutic safety and efficacy in LM across several tumor types, with particular activity seen in LM from primitive neuroectodermal tumors.117,118 More recently, a Phase I study of intraventricular iodine-131-labeled monoclonal antibody 3F8 targeting GD2-positive leptomeningeal disease (primarily neuroblastoma and primary CNS tumors) showed that the antibody reached therapeutic doses in the CSF and 3 of 13 assessed patients achieved objective and/or cytologic responses.119 A Phase II trial of this agent is ongoing (NCT00445965). This approach, as with other intrathecal therapies, is limited by toxicities such as myelosuppression, aseptic meningitis, and increased intracranial pressure. Similar to other targeted therapies, this approach is also limited by the availability of tumor-specific antibodies. There is an ongoing Phase I clinical trial of 131-I-labeled 8H9, an antibody that targets the glycoprotein 4Ig-B7H3 present on a broad spectrum of solid tumors, in patients with refractory brain or leptomeningeal disease (NCT00089245).
Novel clinical trial designs are allowing for recruitment of patients across malignancy subtypes, often based on molecular characteristics shared across many cancers. For example, the Phase II clinical trial for the CDK inhibitor abemaciclib includes patients with leptomeningeal metastases from breast cancer, NSCLC, or melanoma, with a particular focus on hormone receptor positive patients (NCT02308020). This approach may be particularly beneficial in uncommon diseases such as LM, which has historically been excluded from clinical trials and is infrequent enough that accrual to dedicated trials in a single tumor subtype is prohibitively slow.
Conclusion
Leptomeningeal metastasis continues to remain one of the most challenging complications of cancer in terms of diagnostic complexity, poor prognosis, often devastating impact on quality of life, and mixed response to standard cytotoxic or targeted therapies. Treatment to date has been limited by effective drug delivery as well as toxicity, and as a result, it is clear that not all patients benefit from currently available therapies. Improved diagnostic tools and better biomarkers may allow for earlier diagnosis and treatment, thereby improving outcomes. Following diagnosis, optimum treatment continues to be based mostly on expert consensus due to a paucity of clinical trials. An improved understanding of the biological mechanisms underlying tumor metastasis and the molecular features of metastatic disease in comparison to the primary site will allow for more targeted treatment strategies to be tested in subsets of patients most likely to benefit. Improved patient-derived xenograft models of brain and leptomeningeal metastases will also assist in discovery of new therapeutic agents and mechanisms of resistance to therapy. Evaluation of the efficacy of new treatments will be facilitated by novel trial designs and molecular-based patient selection, which has led to increased recruitment of patients with LM into clinical trials. The newly proposed RANO criteria for assessing leptomeningeal disease will help standardize response evaluation across clinical trials, although the criteria will need to be prospectively validated and quality of life measures should be considered moving forward.
Acknowledgments
Funding: Damon Runyon Cancer Research Foundation, Foundation for the National Institutes of Health (K12CA090354), Susan G. Komen
Footnotes
Disclosures: Dr. Priscilla Brastianos received Speaker’s Honoraria from Merck and Genentech and consultant fees for Genentech and Angiochem.
References
- 1.Beauchesne P Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010;11(9):871–879. 10.1016/S1470-2045(10)70034-6. [DOI] [PubMed] [Google Scholar]
- 2.Eberth CJ. Zur Entwickelung des Epithelioms (Cholesteatoms) der Pia und der Lunge. Arch für Pathol Anat und Physiol und für Klin Med. 1869;49(1):51–63. 10.1007/BF02214196. [DOI] [Google Scholar]
- 3.Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massagué J. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell. 2017;168(6):1101–1113. 10.1016/j.cell.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle R, Thomas M, Adams JH. Diffuse involvement of the leptomeninges by tumour--a clinical and pathological study of 63 cases. Postgrad Med J. 1980;56(653):149–158. http://www.ncbi.nlm.nih.gov/pubmed/7393804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson ME, Chernik NL, Posner JB. Infiltration of the leptomeninges by systemic cancer. A clinical and pathologic study. Arch Neurol. 1974;30(2):122–137. 10.1001/archneur.1974.00490320010002. [DOI] [PubMed] [Google Scholar]
- 6.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. http://doi.wiley.com/10.1002/1097-0142%2819831215%2952%3A12%3C2349%3A%3AAID-CNCR2820521231%3E3.0.CO%3B2-B%5Cn(null). [DOI] [PubMed] [Google Scholar]
- 7.Aroney RS, Dalley DN, Chan WK, Bell DR, Levi JA. Meningeal carcinomatosis in small cell carcinoma of the lung. Am J Med. 1981;71(1):26–32. 10.1016/0002-9343(81)90254-0. [DOI] [PubMed] [Google Scholar]
- 8.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases. Incidence, diagnosis, treatment and survival. Cancer. 1978;42(2):660–668. . [DOI] [PubMed] [Google Scholar]
- 9.Frisk G, Svensson T, Bäcklund LM, Lidbrink E, Blomqvist P, Smedby KE. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. 2012;106(11):1850–1853. 10.1038/bjc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesari S, Batchelor T. Leptomeningeal Metastases. Neurol Clin. 2003;21:25–66. [DOI] [PubMed] [Google Scholar]
- 11.Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of Metastatic Brain Tum or s. Neurosurg Clin NA. 2011;22:1–6. 10.1016/j.nec.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Groves MD. New strategies in the management of leptomeningeal metastases. Arch Neurol. 2010;67(3):305–312. 10.1001/archneurol.2010.18. [DOI] [PubMed] [Google Scholar]
- 13.Tosoni A, Franceschi E, Brandes AA. Chemotherapy in breast cancer patients with brain metastases: Have new chemotherapic agents changed the clinical outcome? Crit Rev Oncol Hematol. 2008;68(3):212–221. 10.1016/j.critrevonc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53(7):626–632. http://www.ncbi.nlm.nih.gov/pubmed/8929170. [DOI] [PubMed] [Google Scholar]
- 15.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. . [DOI] [PubMed] [Google Scholar]
- 16.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waki F, Ando M, Takashima A, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009;93(2):205–212. 10.1007/s11060-008-9758-3. [DOI] [PubMed] [Google Scholar]
- 18.Pruitt AA. CNS infections in patients with cancer. Continuum (Minneap Minn). 2012;18(April):384–405. 10.1212/01.CON.0000413665.80915.c4. [DOI] [PubMed] [Google Scholar]
- 19.Collie DA, Brush JP, Lammie GA, et al. Imaging features of leptomeningeal metastases. Clin Radiol. 1999;54(11):765–771. 10.1016/S0009-9260(99)91181-9. [DOI] [PubMed] [Google Scholar]
- 20.Straathof CSM, De Bruin HG, Dippel DWJ, Vecht CJ. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol. 1999;246(9):810–814. 10.1007/s004150050459. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain MC, Glantz M, Groves MD, Wilson WH. Diagnostic Tools for Neoplastic Meningitis: Detecting Disease, Identifying Patient Risk, and Determining Benefit of Treatment. Semin Oncol. 2009;36(SUPPL. 2):S35–S45. 10.1053/j.seminoncol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38(1):51–57. 10.1002/ana.410380111. [DOI] [PubMed] [Google Scholar]
- 23.Pavlidis N The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(SUPPL. 4):285–291. 10.1093/annonc/mdh941. [DOI] [PubMed] [Google Scholar]
- 24.Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–739. . [pii] [DOI] [PubMed] [Google Scholar]
- 25.Bromberg JEC, Breems DA, Kraan J, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68(20):1674–1679. 10.1212/01.wnl.0000261909.28915.83. [DOI] [PubMed] [Google Scholar]
- 26.DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. J Neurooncol. 1998;38(2–3):245–252. 10.1023/A:1005956925637. [DOI] [PubMed] [Google Scholar]
- 27.Clifford Schold S, Wasserstrom WR, Fleisher M, Schwartz MK, Posner JB. Cerebrospinal fluid biochemical markers of central nervous system metastases. Ann Neurol. 1980;8(6):597–604. 10.1002/ana.410080609. [DOI] [PubMed] [Google Scholar]
- 28.Pentsova EI, Shah RH, Tang J, et al. Evaluating Cancer of the Central Nervous System Through Next-Generation Sequencing of Cerebrospinal Fluid. J Clin Oncol. 2016;34(20):2404–2415. 10.1200/JCO.2016.66.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Momtaz P, Pentsova E, Abdel-Wahab O, et al. Quantification of tumor-derived cell free DNA(cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAF<sup>V600</sup> mutated malignancies. Oncotarget. 2016;7(51):85430–85436. 10.18632/oncotarget.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61(3):514–522. 10.1373/clinchem.2014.235457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamberlain M, Soffietti R, Raizer J, et al. Leptomeningeal metastasis: A Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16(9):1176–1185. 10.1093/neuonc/nou089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2016;(Xx):now183 10.1093/neuonc/now183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25(2):103–119. 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]
- 34.Hyun JW, Jeong IH, Joung AR, Cho HJ, Kim SH, Kim HJ. Leptomeningeal metastasis: Clinical experience of 519 cases. Eur J Cancer. 2016;56:107–114. 10.1016/j.ejca.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain MC, Johnston SK, Glantz MJ, et al. Neoplastic Meningitis–Related Prognostic Significance of the Karnofsky Performance Status. Arch Neurol. 2009;66(1):3394–3402. 10.1001/archneurol.2008.506. [DOI] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network. Central Nervous System Cancers.; 2016.
- 37.Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004;223(2):167–178. 10.1016/j.jns.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010;136(11):1729–1735. 10.1007/s00432-010-0831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant R, Naylor B, Greenberg HS, Junck L. Clinical outcome in aggressively treated meningeal carcinomatosis. Arch Neurol. 1994;51(5):457–461. http://www.ncbi.nlm.nih.gov/pubmed/8179494. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain MC, Kormanik PR. Carcinomatous meningitis secondary to breast cancer: predictors of response to combined modality therapy. J Neurooncol. 1997;35(1):55–64. http://www.ncbi.nlm.nih.gov/pubmed/9266441. [DOI] [PubMed] [Google Scholar]
- 41.Souchon R, Feyer P, Thomssen C, et al. Clinical recommendations of DEGRO and AGO on preferred standard palliative radiotherapy of bone and cerebral metastases, metastatic spinal cord compression, and leptomeningeal carcinomatosis in breast cancer. Breast Care. 2010;5(6):401–407. 10.1159/000322661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamberlain MC, Kormanik P. Carcinoma meningitis secondary to non-small cell lung cancer: combined modality therapy. Arch Neurol. 1998;55(4):506–512. 10.1001/archneur.55.4.506. [DOI] [PubMed] [Google Scholar]
- 43.Pan Z, Yang G, He H, et al. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int J cancer. 2016;139(8):1864–1872. 10.1002/ijc.30214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soussain C, Ricard D, Fike JR, Mazeron J-J, Psimaras D, Delattre J-Y. CNS complications of radiotherapy and chemotherapy. www.thelancet.com. 2009;374 http://search.proquest.com/openview/236014a72468633fb1ada5934deef75d/1?pq-origsite=gscholar&cbl=40246. Accessed June 14, 2017. [DOI] [PubMed]
- 45.Chang EL, Maor MH. Standard and novel radiotherapeutic approaches to neoplastic meningitis. Curr Oncol Rep. 2003;5(1):24–28. http://www.ncbi.nlm.nih.gov/pubmed/12493147. [DOI] [PubMed] [Google Scholar]
- 46.Boogerd W, Van Den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: A randomised study. Eur J Cancer. 2004;40(18):2726–2733. 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors: A comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer. 1998;82(9):1756–1763. . [DOI] [PubMed] [Google Scholar]
- 48.Bleyer WA, Drake JC, Chabner BA. Neurotoxicity and elevated cerebrospinal-fluid methotrexate concentration in meningeal leukemia. N Engl J Med. 1973;289(15):770–773. 10.1056/NEJM197310112891503. [DOI] [PubMed] [Google Scholar]
- 49.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal Metastasis from Non-small Cell Lung Cancer: Survival and the Impact of Whole Brain Radiotherapy. J Thorac Oncol. 2012;7(2):382–385. 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 50.Gani C, Müller AC, Eckert F, et al. Outcome after whole brain radiotherapy alone in intracranial leptomeningeal carcinomatosis from solid tumors. Strahlentherapie und Onkol. 2012;188(2):148–153. 10.1007/s00066-011-0025-8. [DOI] [PubMed] [Google Scholar]
- 51.Gunther JR, Rahman AR, Dong W, et al. Craniospinal irradiation prior to stem cell transplant for hematologic malignancies with CNS involvement: Effectiveness and toxicity after photon or proton treatment. Pract Radiat Oncol. 2017;0(0). 10.1016/j.prro.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker GV, Shihadeh F, Kantarjian H, et al. Comprehensive Craniospinal Radiation for Controlling Central Nervous System Leukemia HHS Public Access. Int J Radiat Oncol Biol Phys. 2014;90(5):1119–1125. 10.1016/j.ijrobp.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chamberlain MC, Kormanik P a, Barba D. Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J Neurosurg. 1997;87(5):694–699. 10.3171/jns.1997.87.5.0694. [DOI] [PubMed] [Google Scholar]
- 54.Chamberlain MC. Neoplastic meningitis. Oncologist. 2008;13(9):967–977. 10.1634/theoncologist.2008-0138. [DOI] [PubMed] [Google Scholar]
- 55.Chamberlain MC. Leptomeningeal metastasis. Semin Neurol. 2010;30(3):236–244. 10.1055/s-0030-1255220. [DOI] [PubMed] [Google Scholar]
- 56.Siegal T, Pfeffer MR, Steiner I. Antibiotic therapy for infected Ommaya reservoir systems. Neurosurgery. 1988;22(1 Pt 1):97–100. http://www.ncbi.nlm.nih.gov/pubmed/2449629. [DOI] [PubMed] [Google Scholar]
- 57.Szvalb AD, Raad II, Weinberg JS, Suki D, Mayer R, Viola GM. Ommaya reservoir-related infections: Clinical manifestations and treatment outcomes. J Infect. 2014;68(3):216–224. 10.1016/j.jinf.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Grossman S a., Trump DL, Chen DCP, Thompson G, Camargo EE. Cerebrospinal fluid flow abnormalities in patients with neoplastic meningitis. An evaluation using 111indium-DTPA ventriculography. Am J Med. 1982;73(5):641–647. 10.1016/0002-9343(82)90404-1. [DOI] [PubMed] [Google Scholar]
- 59.Lin N, Dunn IF, Glantz M, et al. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: a retrospective, case-controlled study. J Neurosurg. 2011;115(4):730–736. 10.3171/2011.5.JNS101768. [DOI] [PubMed] [Google Scholar]
- 60.Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: Is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16(4):1561–1567. [DOI] [PubMed] [Google Scholar]
- 61.Lassman AB, Abrey LE, Shah GD, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78(3):255–260. 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 62.Frick J, Ritch PS, Hansen RM, Anderson T. Successful treatment of meningeal leukemia using systemic high-dose cytosine arabinoside. J Clin Oncol. 1984;2(5):365–368. 10.1200/jco.1984.2.5.365. [DOI] [PubMed] [Google Scholar]
- 63.Morra E, Lazzarino M, Brusamolino E, et al. The role of systemic high-dose cytarabine in the treatment of central nervous system leukemia. Clinical results in 46 patients. Cancer. 1993;72(2):439–445. http://www.ncbi.nlm.nih.gov/pubmed/8319175. [DOI] [PubMed] [Google Scholar]
- 64.Ekenel M, Hormigo AM, Peak S, DeAngelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85(2):223–227. 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 65.Giglio P, Tremont-Lukats IW, Groves MD. Response of neoplastic meningitis from solid tumors to oral capecitabine. J Neurooncol. 2003;65(2):167–172. 10.1023/B:NEON.0000003752.89814.ca. [DOI] [PubMed] [Google Scholar]
- 66.Paydas S, Bicakci K, Yavuz S. Dramatic response with capecitabine after cranial radiation to the brain parenchymal and leptomeningeal metastases from lung cancer. Eur J Intern Med. 2009;20(1):96–99. 10.1016/j.ejim.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 67.Rogers LR, Remer SE, Tejwani S. Durable response of breast cancer leptomeningeal metastasis to capecitabine monotherapy. Neuro Oncol. 2004;6(1):63–64. 10.1215/S1152851703000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chahal J, Stopeck A, Clarke K, Livingston RB, Chalasani P. Intravenous thiotepa for treatment of breast cancer-related leptomeningeal carcinomatosis: case series. Neurol Sci. 2015;36(9):1691–1693. 10.1007/s10072-015-2259-1. [DOI] [PubMed] [Google Scholar]
- 69.Segura PP, Gil M, Balañá C, et al. Phase II trial of temozolomide for leptomeningeal metastases in patients with solid tumors. J Neurooncol. 2012;109(1):137–142. 10.1007/s11060-012-0879-3. [DOI] [PubMed] [Google Scholar]
- 70.Postmus PE, Haaxma-Reiche H, Berendsen HH, Sleijfer DT. High-dose etoposide for meningeal carcinomatosis in patients with small cell lung cancer. Eur J Cancer Clin Oncol. 1989;25(2):377–378. 10.1016/0277-5379(89)90033-3. [DOI] [PubMed] [Google Scholar]
- 71.Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis - The role of multimodality treatment. J Neurooncol. 2007;84(1):57–62. 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 72.Siegal T, Lossos A, Pfeffer MR. Leptomeningeal metastases: analysis of 31 patients with sustained off-therapy response following combined-modality therapy. Neurology. 1994;44(8):1463–1469. 10.1002/9781118321478.ch19. [DOI] [PubMed] [Google Scholar]
- 73.Siegal T Leptomeningeal metastases: Rationale for systemic chemotherapy or what is the role of intra-CSF-chemotherapy? J Neurooncol. 1998;38(2–3):151–157. 10.1023/A:1005999228846. [DOI] [PubMed] [Google Scholar]
- 74.Schäfer N, Scheffler B, Stuplich M, et al. Vemurafenib for leptomeningeal melanomatosis. J Clin Oncol. 2013;31(11):e173–4. 10.1200/JCO.2012.46.5773. [DOI] [PubMed] [Google Scholar]
- 75.Simeone E, De Maio E, Sandomenico F, et al. Neoplastic leptomeningitis presenting in a melanoma patient treated with dabrafenib (a V600EBRAF inhibitor): a case report. J Med Case Rep. 2012;6(1):131 10.1186/1752-1947-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 77.Robert C, Karaszewska B, Schachter J, et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N Engl J Med. 2014;372(1):141116004513004 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 78.Ascierto PA, McArthur GA, Dr??no B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248–1260. 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 79.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira M, Braga S, Passos-Coelho JL, Fonseca R, Oliveira J. Complete response in HER2+ leptomeningeal carcinomatosis from breast cancer with intrathecal trastuzumab. Breast Cancer Res Treat. 2011;127(3):841–844. 10.1007/s10549-011-1417-2. [DOI] [PubMed] [Google Scholar]
- 81.Stemmler HJ, Schmitt M, Harbeck N, et al. Application of intrathecal trastuzumab (Herceptin) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep. 2006;15(5):1373–1377. 10.1097/CAD.0b013e32830b58b0. [DOI] [PubMed] [Google Scholar]
- 82.Zagouri F, Sergentanis TN, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: A systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13–22. 10.1007/s10549-013-2525-y. [DOI] [PubMed] [Google Scholar]
- 83.Dumitrescu C, Lossignol D. Intrathecal Trastuzumab Treatment of the Neoplastic Meningitis due to Breast Cancer: A Case Report and Review of the Literature. Case Rep Oncol Med. 2013;2013:154674 10.1155/2013/154674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu NT, Raizer J, Gabor EP, et al. Intrathecal trastuzumab: immunotherapy improves the prognosis of leptomeningeal metastases in HER-2+ breast cancer patient. J Immunother Cancer. 2015;3(1):41 10.1186/s40425-015-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Allison DL, Glantz M, Werner TL, Kirkegaard SL, Murdock K, Jensen R. Intra-CSF trastuzumab in patients with neoplastic meningitis from breast cancer or primary brain tumors. J Clin Oncol. 2009;27(15_suppl):2066 10.1200/jco.2009.27.15.19255317 [DOI] [Google Scholar]
- 86.Raizer J, Pentsova E, Omuro A, et al. AT-47 PHASE I TRIAL OF INTRATHECAL TRASTUZUMAB IN HER2 POSITIVE LEPTOMENINGEAL METASTASES. Neuro Oncol. 2014;16(suppl 5). 10.1093/neuonc/nou237. [DOI] [Google Scholar]
- 87.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 88.Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. 2013;111(1):1–10. 10.1007/s11060-012-0990-5. [DOI] [PubMed] [Google Scholar]
- 89.Elmeliegy MA, Carcaboso AM, Tagen M, Bai F, Stewart CF. Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res. 2011;17(1):89–99. 10.1158/1078-0432.CCR-10-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13(12):1364–1369. 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawamura T, Hata A, Takeshita J, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol. 2015;75(6):1261–1266. 10.1007/s00280-015-2759-y. [DOI] [PubMed] [Google Scholar]
- 92.Dhruva N, Socinski MA. Carcinomatous meningitis in non-small-cell lung cancer: Response to high-dose erlotinib. J Clin Oncol. 2009;27(22):31–32. 10.1200/JCO.2008.21.0963. [DOI] [PubMed] [Google Scholar]
- 93.Yi HG, Kim HJ, Kim YJ, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009;65(1):80–84. 10.1016/j.lungcan.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Masuda T, Hattori N, Hamada A, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol. 2011;67(6):1465–1469. 10.1007/s00280-011-1555-6. [DOI] [PubMed] [Google Scholar]
- 95.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99(2):283–286. 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuiper JL, Smit EF. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases-One with a remarkable thoracic response as well. Lung Cancer. 2013;80(1):102–105. 10.1016/j.lungcan.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 97.Xing P, Li J, Shi Y, Zhang X. Recurrent response to advanced lung adenocarcinoma with erlotinib developing leptomeningeal metastases during gefitinib therapy and two case reports. Thorac cancer. 2014;5(1):38–42. 10.1111/1759-7714.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sasaki S, Yoshioka Y, Ko R, et al. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefitinib therapy failure. Respir Investig. 2016;54(1):14–19. 10.1016/j.resinv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24(27):4517–4520. 10.1200/JCO.2006.06.6126. [DOI] [PubMed] [Google Scholar]
- 100.Jackman DM, Cioffredi LA, Jacobs L, et al. A phase I trial of high dose gefitinib for patients with leptomeningeal metastases from non-small cell lung cancer. Oncotarget. 2015;6(6):4527–4536. 10.18632/oncotarget.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park JH, Kim YJ, Lee J-O, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012;76(3):387–392. 10.1016/j.lungcan.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 102.Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer. 2012;77(1):134–139. 10.1016/j.lungcan.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10(1):156–163. 10.1097/jto.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tamiya M, Shiroyama T, Nishihara T, et al. Afatinib successfully treated leptomeningeal metastasis during erlotinib treatment in a patient with EGFR-mutant (Exon18:G719S) lung adenocarcinoma as a second-line chemotherapy. Asia Pac J Clin Oncol. 2016;18 10.1111/ajco.12643. [DOI] [PubMed] [Google Scholar]
- 105.Lin CH, Lin MT, Kuo YW, Ho CC. Afatinib combined with cetuximab for lung adenocarcinoma with leptomeningeal carcinomatosis. Lung Cancer. 2014;85(3):479–480. 10.1016/j.lungcan.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Yang JC-H, Kim D-W, Kim S-W, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): Updated results from BLOOM, a phase I study. J Clin Oncol. 2016;34(suppl abstr 9002). [Google Scholar]
- 107.Gow CH, Chang YL, Hsu YC, et al. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol. 2009;20(4):696–702. 10.1093/annonc/mdn679. [DOI] [PubMed] [Google Scholar]
- 108.Daniele L, Cassoni P, Bacillo E, et al. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non-small cell lung cancer. J Thorac Oncol. 2009;4(6):684–688. 10.1097/JTO.0b013e3181a52359. [DOI] [PubMed] [Google Scholar]
- 109.Kwak EL, Bang Y-J, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10(2):232–236. 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ou S-HI, Sommers KR, Azada MC, Garon EB. Alectinib induces a durable (>15 months) complete response in an ALK-positive non-small cell lung cancer patient who progressed on crizotinib with diffuse leptomeningeal carcinomatosis. Oncologist. 2015;20(2):224–226. 10.1634/theoncologist.2014-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arrondeau J, Ammari S, Besse B, Soria J-C. LDK378 compassionate use for treating carcinomatous meningitis in an ALK translocated non-small-cell lung cancer. J Thorac Oncol. 2014;9(8):e62–3. 10.1097/JTO.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 113.Omuro AMP, Lallana EC, Bilsky MH, DeAngelis LM. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64(9):1625–1627. 10.1212/01.WNL.0000160396.69050.DC. [DOI] [PubMed] [Google Scholar]
- 114.Herrlinger U, Weller M, Schabet M. New aspects of immunotherapy of leptomeningeal metastasis. J Neurooncol. 1998;38(2–3):233–239. 10.1023/A:1005948722912. [DOI] [PubMed] [Google Scholar]
- 115.Chamberlain MC. A phase II trial of intra-cerebrospinal fluid alpha interferon in the treatment of neoplastic meningitis. Cancer. 2002;94(10):2675–2680. 10.1002/cncr.10547. [DOI] [PubMed] [Google Scholar]
- 116.Bot I, Blank CU, Brandsma D. Clinical and radiological response of leptomeningeal melanoma after whole brain radiotherapy and ipilimumab. J Neurol. 2012;259(9):1976–1978. 10.1007/s00415-012-6488-4. [DOI] [PubMed] [Google Scholar]
- 117.Coakham HB, Kemshead JT. Treatment of neoplastic meningitis by targeted radiation using (131)I-radiolabelled monoclonal antibodies. Results of responses and long term follow-up in 40 patients. J Neurooncol. 1998;38(2–3):225–232. http://www.ncbi.nlm.nih.gov/pubmed/9696376. [DOI] [PubMed] [Google Scholar]
- 118.Bigner DD, Brown M, Coleman RE, et al. Phase I studies of treatment of malignant gliomas and neoplastic meningitis with 131I-radiolabeled monoclonal antibodies anti-tenascin 81C6 and anti-chondroitin proteoglycan sulfate Me1–14 F (ab’)2--a preliminary report. J Neurooncol. 1995;24(1):109–122. http://www.ncbi.nlm.nih.gov/pubmed/8523067. [DOI] [PubMed] [Google Scholar]
- 119.Kramer K, Humm JL, Souweidane MM, et al. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra-Ommaya 131-I-3F8. J Clin Oncol. 2007;25(34):5465–5470. 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]


