Abstract
Spontaneous brain activity has been widely used to map brain connectivity. The interactions between task-evoked brain responses and the spontaneous cortical oscillations, especially within the low frequency range of ~0.1Hz, are not fully understood. Trial-to-trial variabilities in brain’s response to sensory stimuli and the ability for brain to detect under noisy conditions suggest an appreciable impact of the brain state. Using a multimodality imaging platform, we simultaneously imaged neuronal Ca2+ and cerebral hemodynamics at baseline and in response to single-pulse forepaw stimuli in rat’s somatosensory cortex. The high sensitivity of this system enables detection of responses to very weak and strong stimuli and real time determination of low frequency oscillations without averaging. Results show that the ongoing neuronal oscillations inversely modulate Ca2+ transients evoked by sensory stimuli. High intensity stimuli reset the spontaneous neuronal oscillations to an unpreferable excitability following the stimulus. Cerebral hemodynamic responses also inversely interact with the spontaneous hemodynamic oscillations, correlating with the neuronal Ca2+ transient changes. The results reveal competing interactions between spontaneous oscillations and stimulation-evoked brain activities in somatosensory cortex and the resultant hemodynamics.
Introduction
The brain is a complex and dynamic system that spontaneously activates and responds to external stimulation (Buzsaki, 2006). Spontaneous brain activity has implications in playing critical roles in influencing perception and learning as well as formation and maintenance of cortical connections and memory formation (Fell and Axmacher, 2011). The interactions between spontaneous brain activity and task- or stimulation-evoked brain responses are not fully understood.
Various studies have been conducted to address this question. Earlier electrophysiological recordings showed that neuronal responses to sensory stimulation are modulated by the spontaneous neuronal oscillatory activities (Rice and Hagstrom, 1989). For instance, neuronal responses evoked by visual stimuli were dependent on the phase of spontaneous neuronal oscillations across multiple oscillatory bands, e.g., alpha, delta, theta and gamma bands (Lakatos et al., 2007). There were preferable and unpreferable phases of ongoing neuronal activities for sensory-stimulation-evoked neuronal responses (Fries et al., 2001; Kruglikov and Schiff, 2003). Studies also reported that the excitability of a neuronal network could be tuned by changing the phase of the ongoing neuronal oscillations. For instance, somatosensory inputs could reset the phase of the ongoing neuronal oscillations in auditory cortex to a preferable excitable phase (Lakatos et al., 2007) and the neuronal network tended to balance its excitability to recurrent sensory stimuli (Kohn and Movshon, 2003). These studies focused mostly on frequencies above 4Hz.
The finding that there is large power in low frequency oscillations (LFOs) (He and Raichle, 2009) combined with the ability of fMRI to make connectivity maps using low frequency hemodynamic changes (Smitha et al., 2017) has led to a large interest in the interaction between evoked activity and ongoing low-frequency activity. fMRI studies revealed that trial-to-trial variabilities in sensory-stimulation-evoked blood-oxygen-level dependent (BOLD) responses were affected by the resting-state LFOs. A linear-superposition model has been used to account for the variabilities. This model assumed that the stimulation-evoked brain responses were linearly superimposed on any ongoing activities, so the baseline effects could be canceled by averaging across multiple stimulation trials (Fox et al., 2006). However, recent studies suggest that the BOLD responses can interact with the ongoing brain activities in a more complicated fashion (He, 2013; Huang et al., 2017; Murphy et al., 2009). For example, simultaneous EEG and fMRI showed an inverse relationship between peristimulus EEG alpha oscillations (8–12 Hz) and the BOLD responses to auditory and visual stimulations (Becker et al., 2011). It has also become clear that the state of the brain as characterized by ongoing activity can influence performance especially when performing hard tasks or tasks in noisy environments (Birn, 2007). This is consistent with the idea that the brain states can be more or less sensitive for information processing (Fries et al., 2002; Schroeder and Lakatos, 2009).
Studies on interaction between stimulation-evoked activity and ongoing activity have been usually handicapped by limited specificity and sensitivity. Electrophysiology records broadband neuronal activity ranging from fast-spiking activity to synchronized lower-frequency oscillatory activity of neuronal populations; however, it is technically challenging to detect infra-slow LFP oscillatory activity (e.g., ≤0.1Hz) from brain (Palva and Palva, 2012). fMRI BOLD can detect single stimulation-evoked responses, but the ongoing signals are difficult to characterize without extensive temporal averaging. Furthermore, fMRI signals are low-pass filtered compared to neuronal activity due to the hemodynamic response; whereas electrophysiology techniques are very sensitive to low-frequency artifacts making it more difficult to characterize the frequency range most represented in fMRI data. These problems make it difficult to compare these techniques to study stimulation-evoked rapid neuronal responses and spontaneous low frequency oscillations.
Recent advances in ultrasensitive genetically encoded Ca2+ indicators (e.g., GCaMP6f) permit high-spatiotemporal-resolution optical recording of stimulation-evoked neuronal activation with sufficient sensitivity to detect single stimulations. Furthermore, spontaneous oscillatory activity can be detected with Ca2+ indicators and have begun to be widely used to study mesoscopic connectivity in the rodent brain (Du et al., 2014; Gu et al., 2018a; Ma et al., 2016b; Mitra et al., 2018; Vanni and Murphy, 2014; Wright et al., 2017; Xiao et al., 2017). Using genetic-encoded Ca2+ indicators, studies also pointed out that the complex BOLD signal might result from both neuronal and astrocytic activations (Schulz et al., 2012) and unveiled the correlation between cortical-wide BOLD fluctuations with local Ca2+ slow oscillations (Schwalm et al., 2017). Cerebral hemodynamic responses to both a single stimulus and low-frequency ongoing activity can be monitored to enable comparison with fMRI-related signals (Gu et al., 2018b). Here, we applied a custom multimodality imaging platform that combined GCaMP6f Ca2+ fluorescence imaging, optical intrinsic signal imaging (OISI) and laser speckle contrast imaging (LSCI) to simultaneously measure spontaneous resting neuronal and hemodynamic oscillations and their interactions with stimulation-evoked responses. We analyzed the amplitude and phase dependences between stimulus-evoked neuronal and hemodynamic responses versus the phases of spontaneous brain oscillations via cross-frequency-coupling. In turn, we studied the effects of sensory stimulation on spontaneous Ca2+ oscillations. Results showed that neuronal Ca2+ responses evoked by sensory stimuli were inversely modulated by the preceding neuronal Ca2+ oscillations and this inverse dependency was more prominent with a weak stimulation. Strong stimuli reset the phase of resting neuronal Ca2+ oscillations to an unpreferable excitability. The hemodynamic response (e.g., ΔHbT) evoked by single stimulation correlated with the corresponding neuronal Ca2+ response and interacted inversely with the ongoing hemodynamic slow oscillations. Taken together, simultaneous hemodynamic and Ca2+ imaging demonstrates competing interactions between stimulation-evoked activities and ongoing spontaneous slow oscillations.
Methods
Neuron-specific expression of GCaMP6f in somatosensory cortex of rats in vivo
Sprague Dawley rats (male SD rats, n=12) were used in the study. The genetically-encoded Ca2+ indicator GCaMP6f (AAV1.Syn.GCaMP6f.WPRE.SV40, Penn Vector Core, 0.4μl) was virally delivered into the somatosensory cortex (A/P: −0.25, M/L: +3.0, Depth: 1.2) of rats in Dr Koretsky’s laboratory at NIH. These animals were shipped to Stony Brook University after 2–3 weeks for imaging studies. All experiment procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of NIH and Stony Brook University, which were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal preparation for in vivo imaging
The rats (300–350g/each) were imaged after 4 weeks of viral expression. Animals were intubated and mechanical ventilated (CWE, SAR-830/P). Anesthesia was induced with 3% isoflurane and then maintained with 2–3% isoflurane in a ~70% oxygen / 30% air mixture. The left femoral artery was cannulated for continues arterial blood pressure monitoring (Small Animal instrument Inc. SA monitoring System, Model 1025) and periodical blood gas sampling (Radiometer America, ABL80 FLEX), whereas the left femoral vein was catheterized for drug administration (e.g., α-chloralose anesthetic). The rat was then positioned in a stereotaxic frame (Kopf 900, Tujunga, CA, USA) and a cranial window (~4×5 mm2) was created on the right somatosensory cortex (A/P: −0.25mm, M/L+3.0mm). The dura was carefully removed, and the exposed cortical surface was covered with 1.25% agarose gel. A glass coverslip was cemented to the skull to reduce motion of the exposed brain. After the surgery, the animal was transferred to the imaging platform and two electrodes were inserted under the skin of the left forepaw in the space between digits 2 to 3 and between digits 4 to 5. The anesthesia was switched from isoflurane to α-chloralose for functional brain imaging using an initial bolus of 50 mg/kg, followed by a continuous infusion of 25 mg/kg/hr through the femoral vein. During the imaging session, the animal was mechanically ventilated at 1.0Hz to avoid potential effects of physiological regulation (e.g., respiration and heartbeat) on the low frequency oscillations at <1Hz. During imaging, the physiology of the animals was monitored (Small Animal Instrument, Model 1025), including mean arterial blood pressure (MABP), respiration rate and body temperature. The end-tidal CO2 was also monitored continuously using a Poet IQ2 (Criticare Technologies).
Sensory Stimulation
Electrical stimuli were delivered through a pair of electrodes implanted under the skin of the forepaws and connected to an electrical stimulator (A-M System 2100, Sequim, WA, USA). Stimulation paradigms were programmed to generate single-forepaw-stimulus of varied stimulation intensities (i.e., 2mA, 2.5mA, 3mA and 3.5mA) at varied repetitive periods (i.e., 20s or 0.05Hz, 30s or 0.03Hz, and 50s or 0.02Hz). All stimuli were synchronized with image acquisition using a custom LabVIEW program. Rats were allowed to rest for 5min between 2 adjacent imaging cycles to minimize baseline drift.
Simultaneous imaging of neuronal Ca2+ and hemodynamics
A custom multimodality optical imaging platform (Fig.1a) was developed for simultaneous imaging of neuronal Ca2+ fluorescence (λEx=488nm, λEm=515nm), the blood volume/total hemoglobin (HbT, λHbT=568nm, an isopiestic wavelength of hemoglobin) (Du et al., 2005), deoxygenated hemoglobin (HbR, λHbT=630nm), and cerebral blood flow (CBF, λCBF=830nm) from rat cortex over a large field of view (~4×5mm2) and at a spatial resolution of 6.5μm (1X/0.22NA Plan Apo objective). A multi-channel light engine (Spectra Light Engine, Lumencor), synchronized with a 14-bit sCMOS camera (Zyla4.2, Andor, pixel size=6.5 μm), was coupled into a fiber bundle to sequentially deliver multispectral light to illuminate rat brain through the cranial window. For imaging of forepaw stimulation, 100fps was used for single channel Ca2+ imaging and 50fps was used for simultaneous Ca2+/HbT imaging. For imaging of resting activity, 4 channels (Ca2+/HbT/HbR/CBF) were acquired at 12.5Hz as previously reported (Chen et al., 2016). The illuminations and image acquisitions were controlled by a workstation via a high-speed digital time base. Image stacks were streamed into a high-speed hard disk array for post processing.
Figure 1.
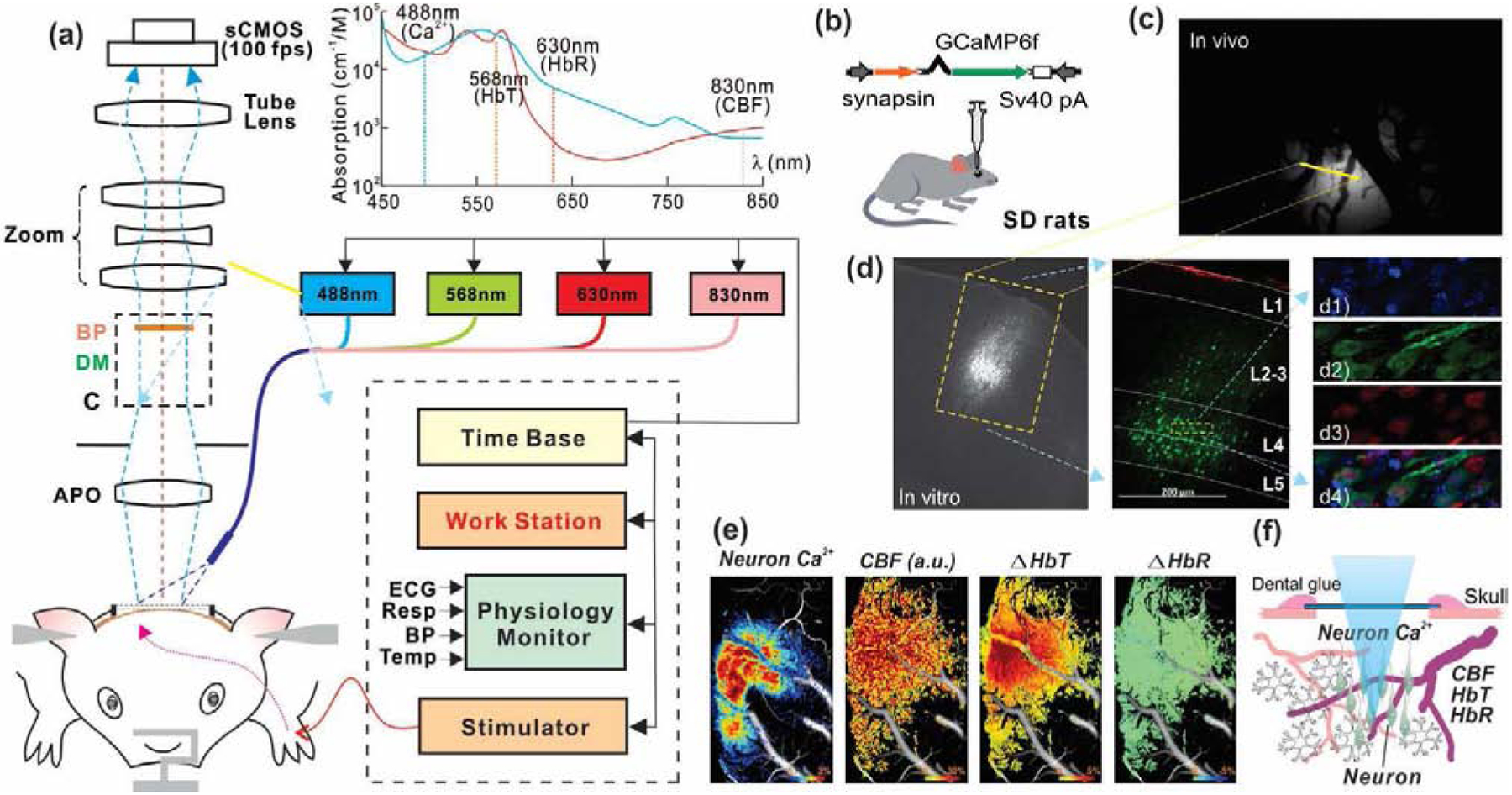
A schematic diagram to illustrate the experimental approach. (a) Multimodality imaging platform that combines GCaMP6f Ca2+ fluorescence / spectral imaging and laser speckle contrast imaging for simultaneous detection of neuronal activity, cerebral metabolic and hemodynamic changes. Sensory stimulation (electrical forepaw stimuli) was synchronized with the imaging platform via a shared time base. (b) Viral injection to express GCaMP6f in neurons within somatosensory cortex. (c) In vivo image of neuronal Ca2+ signal from rat cortex. (d) Brain slice to show the GCaMP6f injection spot in the cortex at layer IV-V and confocal fluorescence images of brain slice with DAPI (d1), GCaMP6f (d2), NeuN (d3) and merged image (d4). (e) Simultaneous imaging of neuronal Ca2+, CBF, HbT and HbR responses to forepaw electrical stimulation (Image size: 3×5mm2). (f) Illustration of simultaneous imaging of synchronized Ca2+ from neuronal population and the local hemodynamics.
ΔHbT, ΔHbR and ΔCBF were quantified as percentage changes relative to their baselines, which were derived in our previous studies (Chen et al., 2016; Yuan et al., 2011). The mean Ca▪ 2+ fluorescence was calculated based on the functionally activated brain regions where the large blood vessels were avoided (e.g., dashed yellow circle in Fig.2c). The relative change in Ca2+ fluorescence signal (ΔF/F) was obtained after correcting the local HbT fluctuation induced light absorption change via ratiometric analysis (Ma et al., 2016a; Yuan et al., 2011), as described in Supplementary Fig.S1.
Figure 2.
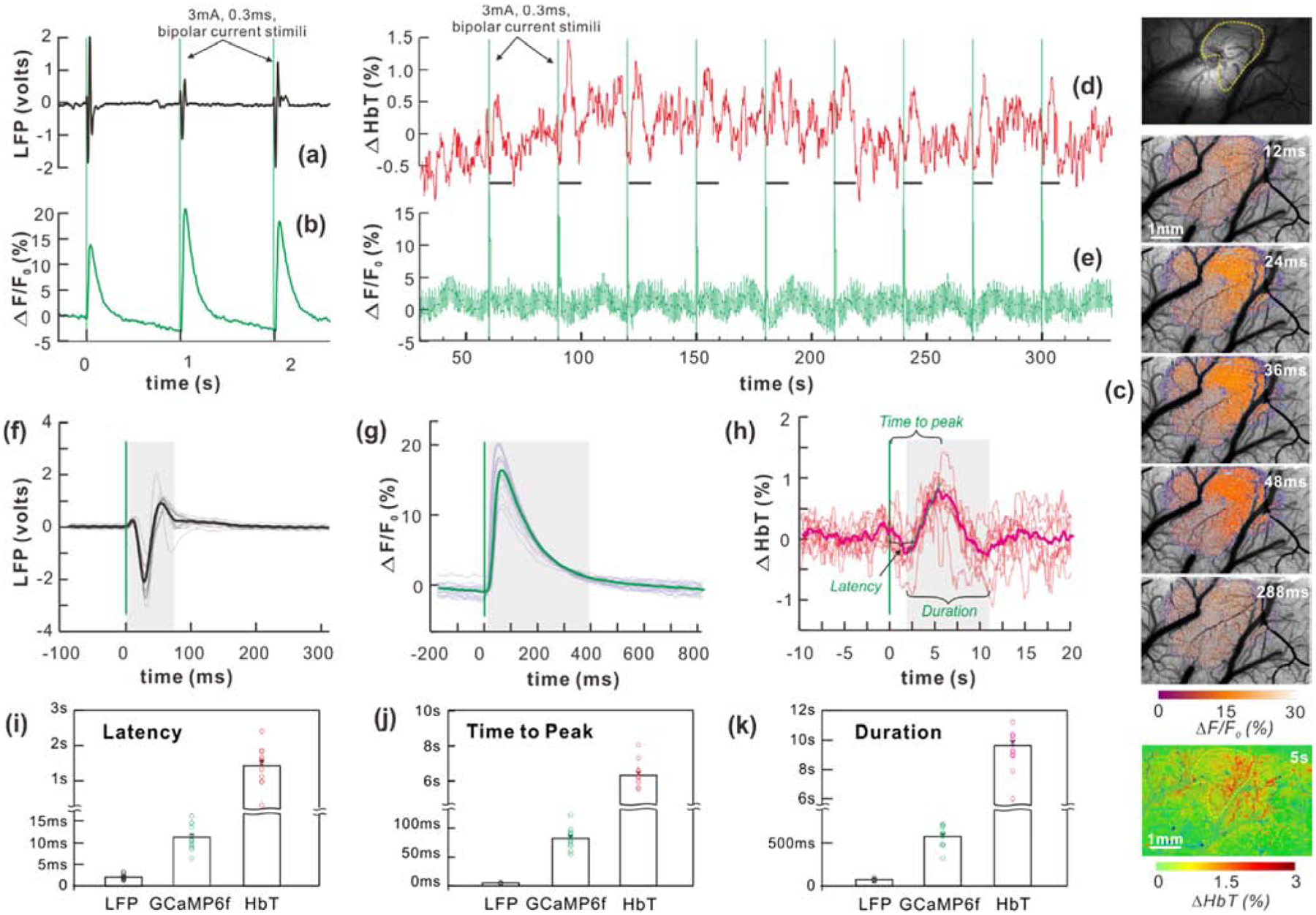
(a, b) Comparison of local field potential (LFP) impulses with Ca2+ transients evoked by single-forepaw-stimulation. (c) Forepaw response region (dashed yellow circle) in which Ca2+ transients show neuronal activations at 12-, 24-, 36-, 48-, and 288-ms post the stimulation onset. The Ca2+ response region was masked to quantify the ΔHbT change. (d-e) Simultaneously recorded ΔHbT and Ca2+ oscillations and responses evoked by forepaw stimuli per every 30s (dashed green lines). (f-h) Superimposed temporal LFP activations, Ca2+ transients, and ΔHbT responses, respectively. (i-k) Statistic results of response latency, time to peak and duration for LFP, Ca2+ and ΔHbT, respectively (n=12 stimuli-trials were tested). All data are presented as mean ± s.e.m.
Analysis of resting-state Ca2+ and hemodynamic low frequency osccilations
Based on long-duration imaging (e.g., 300~360s), original temporal traces for neuronal Ca2+ and hemodynamic oscillations (Fig. S2a) were selected from the same brain region defined by the Ca2+ responsive area while avoiding major vascular regions. The spontaneous Ca2+ and hemodynamic traces were then band-pass filtered (zero-phase 3rd Butterworth filtering 0.03Hz~0.5Hz) to remove high-frequency noise and irrelevant physiological frequencies (e.g., respiration at ~1Hz, heartbeat rate at ~5Hz) as shown in Fig.S2b. For spectral analysis, signals were converted from time domain to frequency domain using fast-Fourier-transform (FFT) to show the static spectral properties or using short-time-Fourier-transform (STFT) to show their evolution over time (Fig.S2c). Hilbert-transform (HT) was used to extract instant phase profiles of the spontaneous Ca2+ and hemodynamic oscillations (Fig.S2d). See Supplemental Information for more details.
Immunohistochemistry and ex vivo imaging
After in vivo imaging, animals were perfused transcardially with 0.1M PBS (pH7.4) followed by fixation with 4% paraformaldehyde in 0.1M PBS (pH7.4). After 24 hours, the cryoprotected rat brains were sliced to 40–50μm thick slides. For immunostaining, GCaMP6f signal was enhanced by a primary chicken anti-GFP antibody followed by an Alexa Fluoro 488 anti-chicken conjugated secondary antibody. Neurons were identified using Anti-Fox3 mouse primary antibodies. Alexa Fluoro 594 anti-mouse conjugated secondary antibody was used to visualize the location of NeuN. Brain slices were then imaged using confocal microscope (A1, Zeiss).
Statistics and cross-correlation
Sample size for this study was chosen and validated to provide enough power for statistical analysis. All data are presented as mean ± s.e.m. Comparisons between two different groups (e.g., Ca2+ vs. HbT) or two different time periods (pre- vs. post-stimulation) were analyzed using paired t-test. Comparisons across multiple stimuli trials at different stimuli intensities (e.g., Isti=2mA, 2.5mA, 3mA, 3.5mA) were analyzed using repeated measurement one-way analysis of variance (RM One-way ANOVA). A p-value <0.05 was considered statistically significant for both cases. For cross-correlation between two temporal traces (e.g., Ca2+ and HbO2), the Pearson correlation coefficient was calculated with a p-value provided. A p-value <0.05 was considered linearly correlated.
Results
1. Multimodality optical platform for simultaneous imaging of spontaneous and stimulation- evoked neuronal Ca2+ activity and hemodynamics
To image the interaction between stimulation-evoked brain responses, including neuronal Ca2+ and hemodynamic responses with their ongoing brain activity, a custom multimodality imaging platform (Fig.1a) was applied to simultaneously acquire Ca2+ fluorescence and hemodynamic images at high temporal resolution. Genetically encoded Ca2+ indicator was delivered via viral injection (AAV1.Syn.GCaMP6f.WPRE.SV40) into the rat’s somatosensory cortex to express GCaMP6f in neuron populations mostly in layers IV-V of the forepaw region (Fig.1b). After the 4 weeks of GCaMP6f injection, in vivo imaging was performed with subsequent ex vivo imaging to confirm the neuronal specificity of GCaM6f expression within the cortex (Fig.1c–d, Supplemental Fig.S3). In ex-vivo imaging, both GFP antibody (to enhance GCaMP6f signaling) and NeuN antibody (to label neurons) were applied to determine the efficiency of GCaMP6f expression in neurons (Fig.1d). It should be noted that unlike previous studies that utilized non-cell-specific voltage-sensitive dyes (Arieli et al., 1996) and Ca2+ indicators (e.g., Rhod2) (Du et al., 2014) to monitor regional activities, GCaMP6f Ca2+ fluorescence enables imaging of both spontaneous and stimulation-evoked activities solely from neuronal populations, and avoids the ambiguity of mixed Ca2+ signaling from other cell types such as astrocytes (Gu et al., 2018a; Winship et al., 2007). Multi-channel images acquired by the multimodality imaging platform for simultaneous detection of neuronal Ca2+ and hemodynamic changes (Fig.1f) enabled us to investigate the interactions between spontaneous and stimulation-evoked neuronal and hemodynamic activities independently (Fig.1e).
During imaging, the average values of MABP and pCO2 were 101.75±2.48 mmHg and 41±0.82 mmHg, respectively, thus indicating that the physiology of the animals was under normocapnic conditions (Supplemental Tab.S1 and Fig.S4).
2. Synchronized Ca2+ transient and hemodynamic responses to single forepaw stimulus
A recent wide-field fluorescence microscopy study showed that resting-state neuronal Ca2+ fluctuations reflect multi-unit activities of neuronal populations (Ma et al., 2016b; Mitra et al., 2018; Xiao et al., 2017). Here, we compare the local-field potential (LFP) and Ca2+ transient responses evoked by single forepaw stimuli (3mA/0.3ms). The LFP signal was recorded with a single-point electrode (A/P: −1mm, L/R: +4mm, at ~0.1mm below the dura; ϕ0.3mm EL450 electrode, Biopac) to represent the synchronized neuronal response to forepaw stimuli; the neuronal Ca2+ signal was imaged to represent the synchronized Ca2+ from local neuronal populations. Both LFP and Ca2+ transients were synchronized with forepaw stimuli (Figs.2a–b), indicating the ultrahigh sensitivity of our optical imaging that enables detection of Ca2+ transients by using GCaMP6f fluorescence to map neuronal activities, even for weak response to a single-pulse sensory stimulus.
Fig.2c shows spatiotemporal responses of Ca2+ fluorescence to the stimulation. Together with the spatiotemporal changes in Ca2+ fluorescence and hemodynamics shown in Fig.S5, these results indicate that Ca2+ transient was confined in the forepaw response region while the overall hemodynamic increase (ΔHbT) was detectable in a relatively larger surrounding area, likely due to draining of blood from the active region. For consistency, the cortical area that showed Ca2+ transient was selected to quantify both Ca2+ and HbT changes. Figs.2d–e show that both Ca2+ and HbT transient responses are well synchronized to the onsets of single sensory stimuli pulses (3mA/0.3ms, 0.03Hz), reflecting the neurovascular coupling process.
By averaging the LFP traces, the neuronal Ca2+ and hemodynamic responses according to the stimuli onsets, Figs.2f–h illustrate their temporal properties (response latency τ, response duration Δt and time to peak t). Specifically, the response latency of Ca2+ (τCa2+=11.41±0.72ms) was significantly longer than that of LFP (τLFP=2.15±0.15ms) (p<0.001, n=12), and τHbT=1.46±0.13s of HbT was significantly longer than both τCa2+ and τLFP (p<0.001, n=12) (Fig.2i). The response latency of HbT was likely prolonged from the surface compared to deeper cortex (Yu et al., 2014). Similarly, regarding the time to reach peak response, tLFP=5.64±0.11ms for LFP is shorter than tCa2+=83.29±4.49ms for Ca2+ (p<0.001, n=12), and dramatically shorter than tHbT=6.31±0.186s (p<0.001, n=12) for HbT (Fig.2j). The duration of transient response ΔtLFP=74.83±3.67ms of LFP is shorter than ΔtCa2+=571.8±34.21ms of Ca2+ (p<0.001, n=12), both of which are drastically shorter than ΔtHbT=9.63±0.325s of ΔHbT (p<0.001, n=12) (Fig.2k). It is noteworthy that the temporal property of Ca2+ transients obtained with the multimodality imaging platform (Fig.2g) is similar to that imaged with single-neuron-resolution two-photon microscopy (Chen et al., 2013), suggesting that the activity from the entire neuronal ensemble detected here is well synchronized to within the time scale of the Ca2+ transient from a single forepaw stimulus.
3. Resting-state spontaneous hemodynamic fluctuations correlate with neuronal Ca2+ oscillations
The representative signals of LFOs in the absence of stimulation (resting state) in neuronal Ca2+ (green), HbT (red), HbR (blue) and CBF (black) were simultaneously imaged from somatosensory cortex (Figs.3a–b). The Ca2+ and hemodynamic LFOs were characterized either by their normalized power spectral density (PSD) via fast Fourier transform (FFT, Fig.3e–h) or by their PSD changes via short-time Fourier transform (STFT, insets of Fig.3e–h), in which a bandpass filter of 0.03Hz~0.5Hz was used to remove the physiological fluctuations such as those from respiration (~1Hz) and heart beat (~5Hz). Neuronal Ca2+ oscillation (Fig.3e) was centered at fCa2+=0.046±0.003Hz (n=12); HbT and HbR fluctuated within a similar frequency band (Figs.3f,g) at fHbT=0.048±0.012Hz (p=0.855, n=12) and fHbR=0.055±0.0175Hz (p=0.183, n=12), which is consistent with previous work imaged by Rhod2 (Du et al., 2014). In contrast, CBF oscillated at two different frequency bands (Fig.3h), i.e., one at lower frequency (cellular band) of fCBF-L=0.061±0.011Hz, which is coincident with fCa2+, fHbT, and fHbR (p=0.171) and another one at higher frequency (vascular band) of fCBF-H=0.152±0.008Hz (p<0.001, n=12). The higher CBF frequency band which was also detected in previous studies (Du et al., 2014); but the origin is not clear. The temporal correlations between Ca2+ and hemodynamic LFOs over a 30s period (Figs.3c,d) show that the Ca2+ increase preceded a HbT increase with a delay of 1.96±0.25s (r=0.575±0.023, p<0.001) and a subsequent HbR decrease of 2.01±0.26s (r=−0.713±0.023, p<0.001; n=12).
Figure 3.
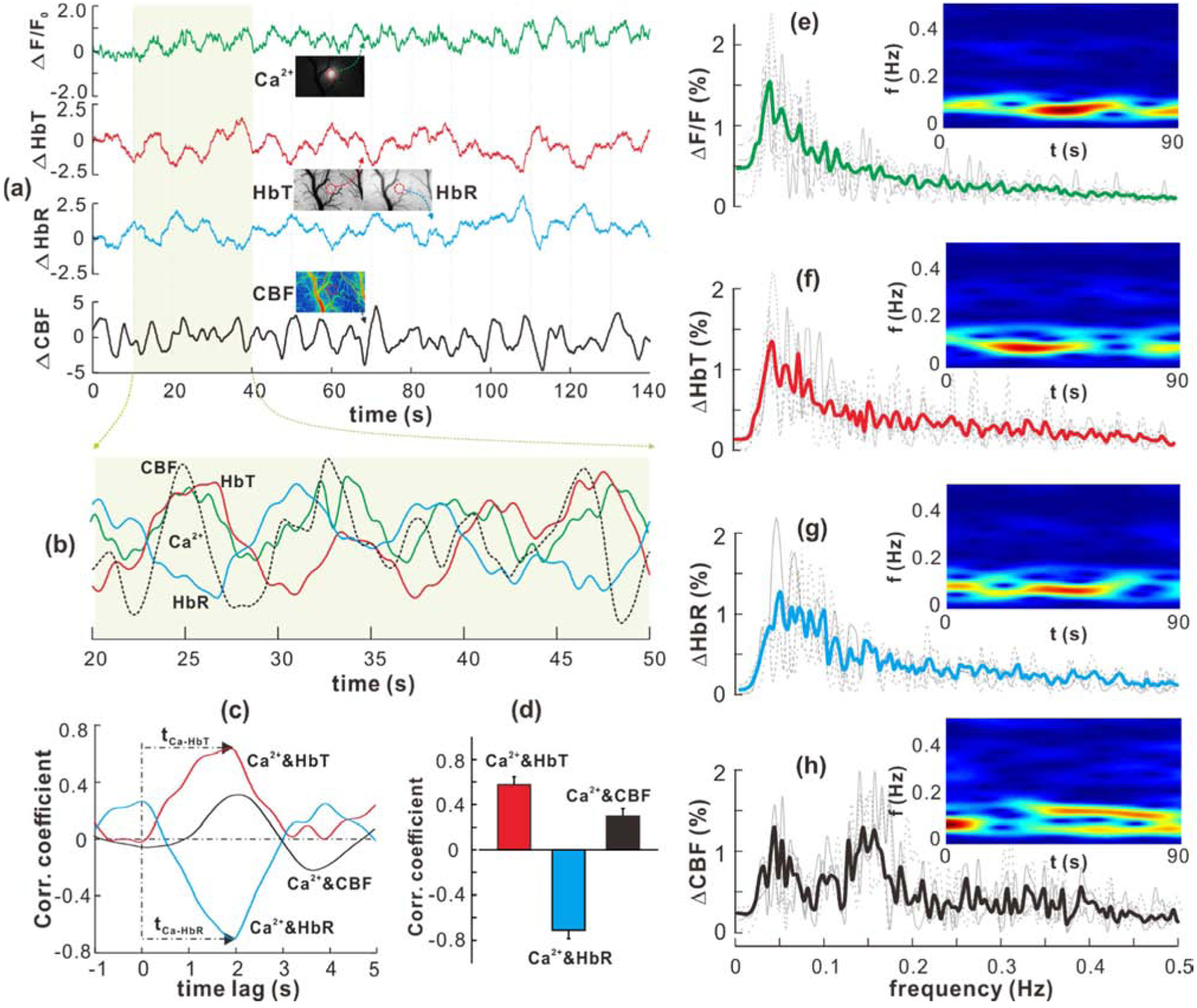
Resting spontaneous hemodynamic fluctuations correlate with neuronal Ca2+ oscillations. (a)Resting spontaneous oscillations of Ca2+ (green), HbT (red), HbR (blue) and CBF (black). (b) Zoom-in view of the superimposed neuronal Ca2+ and hemodynamic oscillations in 30s. (c, d) Temporal cross-correlations between Ca2+-HbT, -HbR, and -CBF oscillations and their correlation coefficients (n=12). (e-h) Normalized spectral power densities of LFOs (0.03~0.5Hz) in Ca2+, HbT, HbR, and CBF. The spectrograms (insets) computed by short-time-Fourier-transform (STFT) present evolution of Ca2+ and hemodynamic LFOs. All data are presented as mean ± s.e.m.
4. Spontaneous Ca2+ LFOs conversely modulate single-forepaw-stimulation-evoked Ca2+ response
Fig.2 and Fig.3 demonstrate the utility of the multimodality imaging platform to image neuronal Ca2+ transient responses to sensory stimulus and spontaneous neuronal Ca2+ LFOs. Previous electrophysiological studies demonstrated modulation of neuronal responses to auditory stimuli by phase (ϕ) of the ongoing oscillatory activities based on cross-frequency-coupling analysis (Kruglikov and Schiff, 2003). Figs.4a–c show the data from spontaneous neuronal Ca2+ LFOs in somatosensory cortex over 6min, during which different-frequency forepaw stimuli (i.e., 0.02-, 0.03- and 0.05-Hz) were applied under both strong (e.g., ≥3mA) and weak (e.g., ≤2.5mA) stimulation intensities so as to ensure stimuli occurred throughout the phase of ongoing activity. ‘Strong stimulation’ (with 3mA) always elicited a maximal response; ‘weak stimulation’ (with 2mA) was set so as to evoke a detectable response at about 50% occurrence rate. The slow and varying stimulation frequency ensured that stimuli were delivered at different phases (ϕ) of the ongoing LFOs. For each stimulus, ϕ was extracted by Hilbert transform and the evoked Ca2+ transient was quantified by the relative fluorescence change (ΔF/F). For each animal (n=12), repeated forepaw stimuli trials (m=8) were tested at three stimulation frequencies, yielding a total of 288 stimulation trials for both strong and weak stimulation paradigms. The trial-to-trial dependence of Ca2+ transient amplitude on the phase of ongoing Ca2+ LFOs showed higher Ca2+ transients towards the trough of Ca2+ LFOs, suggesting a higher Ca2+ response to a stimulation if occurred in a lower LFO state (Fig.4d). Both weak and strong stimulations showed a similar ΔF/F-ϕ relation. Statistical results in Fig.4e show that the Ca2+ transient amplitudes evoked near the LFO peaks (ϕ≈0, 2.53±0.433%, n=34 for strong and 0.3±0.12%, n=15 for weak stimulations) were significantly lower than those evoked near the LFO troughs (ϕ≈π, 3.89±0.362%, n=97, p<0.001; 1.48±0.13%, n=12; p<0.001), suggesting that the Ca2+ response to forepaw stimulation is inversely modulated by the ongoing Ca2+ LFOs. The response to weak stimulation increased by ~4-fold ((1.48%−0.3%)/0.3%) depending on the phase of the LFO, while the response to strong stimulation increased by only ~50% ((3.89%−2.53%)/2.53%). Thus, on a percent basis the weak stimulation was much more affected than the strong stimulation. Interestingly, in terms of absolute Ca2+ signal the change due to the phase of the LFO was similar for both stimulations, e.g., ~1.4% (3.89%−2.53%) for strong stimulation and ~1.2% (1.48%−0.3%) for weak stimulation.
Figure 4.
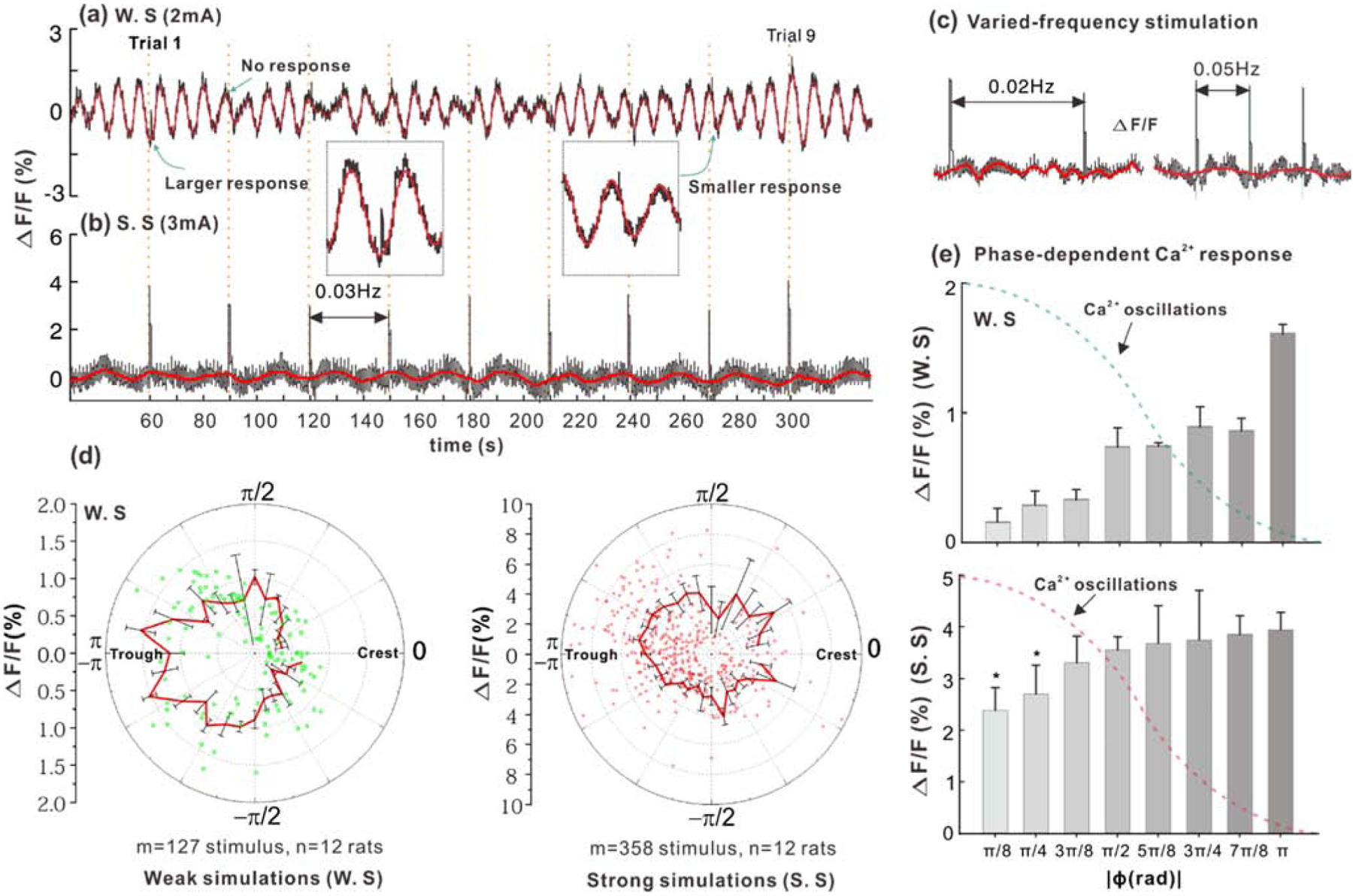
(a) Ca2+ LFOs and Ca2+ transients evoked by weak forepaw stimulations (0.3ms/0.03Hz/2mA), where ϕ is the phase of ongoing Ca2+ LFOs at which the stimuli onsets occurred, ΔF/F is the evoked Ca2+ transient amplitude. (b) ΔF/F evoked by strong forepaw stimulations (0.3ms/0.03Hz/2mA). (c) ΔF/F varied with ϕ and stimulation frequency (0.02Hz, 0.05Hz). (d) Polar graphs to show ΔF/F~ϕ (red/green dots: weak/strong stimulations in left/right panels). (e) Statistical results to show that the evoked ΔF/F is inversely modulated by the ongoing Ca2+ LFOs. Upper/lower panels: weak/strong stimulations. All data are presented as mean ± s.e.m.
To eliminate the variability across animals. we also reprocessed the data by normalizing the Ca2+ responses for each animal to their own strongest Ca2+ responses and plotted the amplitude-phase dependences in Supplemental Fig. S6, for weak stimulation (i. e., W. S, 2mA) and strong stimulation (i.e., S. S, 3mA) respectively. As shown in Figs. S6a, c, the polar diagrams after normalization keep the same trends in amplitude-phase coupling as shown in Fig. 4 above. As summarized in Supplemental Figs.S6b, d, strong Ca2+ responses occurred at the trough of the spontaneous Ca2+ oscillations, which is similar to the non-normalized patterns as shown in Fig. 4e.
5. Single forepaw stimulation resets the phase of spontaneous Ca2+ LFOs
While Fig.4 shows that neuronal LFOs modulate sensory-stimulation-evoked neuronal responses, studies have also reported that sensory stimulation in turn might affect the ongoing spontaneous neuronal oscillations (Makeig et al., 2004; Shah et al., 2004), including phase reset at several oscillatory bands from low delta (~1.3Hz) to γ band (Lakatos et al., 2007). Neuronal Ca2+ imaging allows us to directly visualize the phase reset of the LFOs below 0.1Hz.
Fig.5a shows neuronal Ca2+ changes (black traces) that include spontaneous LFOs and stimulation-evoked transient spikes and their instantaneous phase changes (blue traces) under different stimulation intensities (2-, 2.5-, 3-, and 3.5-mA) to evaluate their effects on resetting LFO phases. Similarly, 0.02-, 0.03- and 0.05-Hz stimulations were applied to randomly sample stimulation onset at different Ca2+ LFO phases. Weak stimulations (e.g., 2-, 2.5-mA) evoked small Ca2+ transients about 50% of the time and when detected these transients did not cause significant interruption to the ongoing Ca2+ LFO phases. Stronger stimulations (e.g., 3-, 3.5-mA) evoked strong Ca2+ transients and reset the phases of ongoing Ca2+ LFOs as indicated by transient phase disruptions (Fig.5b). Results based on 288 stimulation trials (Fig.5c) show a uniform phase distribution before stimulation (e.g., at 0.5s prior to stimulation onset) as assessed by using Kuiper V tests (Pycke, 2010). For post stimulation analysis, LFO phase changes were measured at 1s after the stimulation onset to take into account for the 571.8±34.21ms of GCaMP6f Ca2+ transient duration evoked by single forepaw stimulation. Fig.5d shows that the LFO phases after weak stimulations were not significantly different from the uniform distribution; whereas stronger stimulations resulted in significant deviations from uniform distributions (p < 0.001) towards a more concentrated ϕ≈0 phase region. The probability of post-stimulation phase within [−π/6, π/6] was 30.37% for 3mA and 50.22% for 3.5mA, which were higher than 27.69% at 2.5mA, 22.61% at 2mA. Such phase reset effects may suggest adaptation of spontaneous neuronal activity to sensory stimulation by tuning its LFO phases to unpreferable excitable states for subsequent stimulations.
Figure 5.
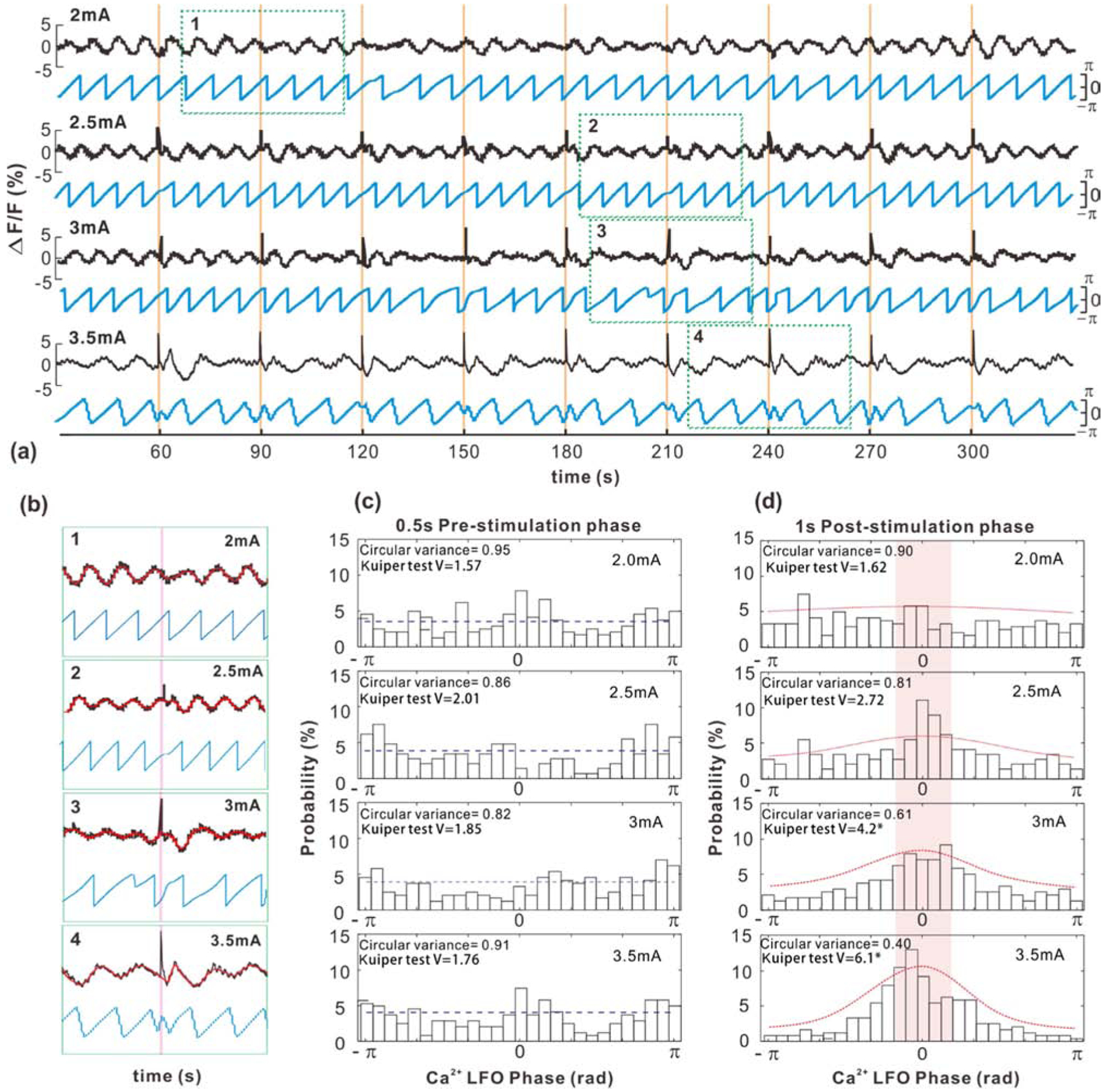
(a) Representative neuronal Ca2+ LFOs and transient spikes evoked by forepaw stimulations (black traces) at various stimulation intensities (2-, 2.5-, 3-, 3.5-mA; 0.03Hz) and the instantaneous LFO phases (blue traces) calculated by Hilbert transform from an experimental animal. (b) Zoom-in views in dashed green boxes of (a) where red and blue traces are ongoing Ca2+ LFOs and their instantaneous phases. (c) Uniform phase distributions at 0.5s before stimulation tested by modified Kuiper V statistic (smaller circular variance implies higher concentration, * p<0.001). (d) Nonuniform phase distributions at 1s after stimulation, showing increasingly significant phase distortion under strong stimulations (3mA, 3.5mA; * p<0.001). All data are presented as mean ± s.e.m.
6. Hemodynamic LFOs conversely modulate single-forepaw-stimulation-evoked ΔHbT response.
The simultaneous imaging capability of the multimodality imaging platform enabled us to analyze the complex interactions between stimulation-evoked neuronal and hemodynamic responses and their ongoing spontaneous LFOs. Fig.6a shows spontaneous neuronal Ca2+ LFOs including stimulation-evoked Ca2+ transient spikes (grey traces) simultaneously with the ΔHbT LFOs and stimulation-evoked responses (red curve) in the somatosensory cortex of a representative animal. Single forepaw stimulations (3mA/0.3ms) were applied every 30s (dashed orange lines), including the stimulation onsets at trough and crest of the ΔHbT oscillations (Fig.6b). The corresponding Ca2+ and ΔHbT phases were computed using Hilbert transform (Fig. S2). Unlike Ca2+ transients that are clearly differentiable from background LFOs, the ΔHbT responses due to their longer duration and lower amplitude were more difficult to extract from the background HbT LFOs. In order to reliably quantify stimulation-evoked ΔHbT responses, we first computed the absolute ΔHbT as the difference between the peak HbT value and the averaged HbT level over the resting LFO (dashed green line, Fig.6c); then the normalized ΔHbT response was quantified as the first rising slope following the stimulus onset (Fig.6c). The trial-to-trail dependence of the normalized ΔHbT transient amplitude on the phase of ongoing LFOs shows a tendency of higher ΔHbT transients towards the trough of ΔHbT LFOs, suggesting a stronger ΔHbT response with a lower LFO state (Fig.6d). Indeed, statistical analysis shows significantly stronger ΔHbT responses evoked at the troughs than at the crests (Fig.6e). Meanwhile, regression analysis showed a linear correlation between the normalized ΔHbT responses and the corresponding Ca2+ transients (r=0.567, p<0.001, n=128 trials), as shown in Fig.6f. Taking together, both neuronal Ca2+ transient and ΔHbT responses evoked by single forepaw stimulations were inversely modulated by the ongoing Ca2+ and hemodynamic LFOs.
Figure 6.

(a) Hemodynamic (ΔHbT, red traces) and neuronal Ca2+ transient (black traces) responses to single forepaw stimulations (3mA/0.3ms). (b) Zoom-in views of dashed boxes in (a) for stimulation onset at trough and crest of the ΔHbT oscillations, respectively. (c) Zoom-in views of Ca2+ and ΔHbT responses in (a), in which the normalized ΔHbT response is defined as 1st rising slope following the stimulus onset while the absolute ΔHbT response is defined as the peak ΔHbT to the averaged baseline level (green dash line). (d) Polar graph to show the dependence of evoked ΔHbT amplitude on the LFO phase onset based on the stimuli-evoked ΔHbT responses (m=162). (e) Statistical results to show inverse modulation of the evoked ΔHbT amplitudes by the ongoing ΔHbT LFOs. (f) Regression analysis to show a linear correlation between ΔHbT and Ca2+ transient responses (R=0.567, p<0.001). All data are presented as mean ± s.e.m.
Discussion
Stimulation-evoked brain responses have been observed to exhibit trial-to-trial variability, which is believed to be associated with the underlying spontaneous brain activity and shapes our behavioral response to the external world (Kisley and Gerstein, 1999). Conventional electrophysiological methods are highly suitable for studying the ultrafast transmembrane electrical signals; however, such signals containing broad frequency components are very sensitive to motion-induced low frequency fluctuations, thus rendering it difficult to study low-frequency oscillations widely observed in fMRI. The brain mapping techniques based on hemodynamic signals suffer from the compound effects that exist in the neurovascular coupling process (Chen et al., 2015). Thus, there is always a demand for simultaneous tracking of the neuronal response along with the hemodynamic responses to each single stimulus trial to uncover the complex neuronal and hemodynamic changes (Debener et al., 2006; O’Herron et al., 2016). Despite reports on modulation of stimulation-evoked brain responses by the ongoing brain oscillatory activities, it is challenging to provide side-by-side comparisons to correlate the results among different methods that interrogate brain activities at vastly different spatiotemporal scales, especially at low frequencies in the ongoing activity. Recent advances in ultrasensitive genetically encoded Ca2+ indicators have improved the optical detection of neuronal transient spikes with sufficient signal-to-noise ratio in a cell-specific fashion. The kinetic property of GCaMP6f is over 300ms (Chen et al., 2013), which makes it challenging for the Ca2+ imaging to capture individual Ca2+ transients while stimulation rate is ≥3Hz due to the insufficient time gap between repetitive stimuli (≤330ms) to restore Ca2+ back to the baseline for each stimulation (Fig. S5). Some alternative strategies have been used to overcome this issue, for example by averaging across multiple stimulation trials (van Alst et al., 2019) or using endoscopic photometry to target small neuro ensembles(Wang et al., 2018). In this study, we combined the multimodality imaging platform and GCaMP6f to simultaneously study stimulation-evoked neuronal and hemodynamic responses concurrently with the resting low frequency oscillatory activities in the cortex and analyzed the interactions between the stimulation-evoked activation with spontaneous brain oscillations. Specifically there were three major advantages: (1) Neuron specific Ca2+ imaging with genetically-encoded Ca2+ indicator (GCaMP6f) was used, thus excluding the contributions of Ca2+ signaling from other cell types; (2) Simultaneous imaging of neuronal Ca2+ and cerebral hemodynamic changes enabled correlation between the neuronal and hemodynamic activities; (3) Excellent sensitivity to detect even weak single-stimulation-evoked neuronal Ca2+ transients as well as adequate sensitivity to measure ongoing activity without averaging enabled extracting the phase of ongoing activity at the time of stimulus induced Ca2+ transients and ΔHbT responses.
Important findings of this study include: (1) Spontaneous neuronal Ca2+ and hemodynamic oscillations are correlated as previously shown; (2) The ongoing low-frequency neuronal oscillations inversely modulate neuronal Ca2+ response evoked by single forepaw stimulation; (3) The ongoing hemodynamic oscillations inversely modulate single-stimulation-evoked hemodynamic response and linearly correlate with the evoked neuronal Ca2+ response; (4) Strong single-forepaw-stimulation in turn resets the phase of the ongoing neuronal oscillations to a non-preferable excitable state. These findings provide new insights in the complex interactions between spontaneous neuronal oscillations and the task or stimulation-evoked neuronal responses. The results using Ca2+ evoked responses confirm that the variability in fMRI results to single-forepaw-stimulation-evoked hemodynamic responses can be due to when the stimulus is presented during ongoing neural oscillations (Duann et al., 2002). They also confirm that the phase of the ongoing activity can impact the level of stimulus-evoked responses, making it possible that the state of the brain can impact detectability and performance in noisy situations.
1. Ca2+ oscillations reflect activities of neuronal population that drive spontaneous hemodynamic oscillations
Spontaneous cerebral hemodynamic fluctuations and neuronal oscillations have been observed in BOLD signals (Fransson, 2005), HbO2/HbR/Ca2+ fluctuations (Du et al., 2014; Rayshubskiy et al., 2014) and local field potentials (Buzsaki and Draguhn, 2004; Obrig et al., 2000; Rayshubskiy et al., 2014). It is now widely accepted that cerebral hemodynamic oscillations of ~0.04–0.1Hz reflect underlying neuronal activity (Auer, 2008; Du et al., 2014; Fox and Raichle, 2007; Ma et al., 2016b; Tong and Frederick, 2010; Wright et al., 2017; Xie et al., 2016). However, the coupling between the spontaneous neuronal and hemodynamic oscillations below ~0.1Hz is not well understood due to the difficulty in direct comparison at different spatiotemporal scales by electrophysiology and hemodynamic imaging techniques. The genetically-encoded Ca2+ indicator GCaMP6f is specifically expressed in the neuron populations in this work, and thus allowed monitoring spontaneous Ca2+ oscillations solely from neurons without the confounding Ca2+ signals from other cell types. The results show that neuronal Ca2+ oscillation (0.046±0.003Hz, n=12) is temporally correlated with the cerebral hemodynamic oscillations in HbT (0.048±0.012Hz, r=0.57±0.023, p<0.05) and in HbR (0.048±0.012Hz, r=−0.71±0.023, p<0.05). The HbT fluctuations temporally followed the neuronal Ca2+ oscillations with an average time-lag of 1.96±0.25s and their temporal correlation was r=0.575±0.023 (p<0.001).
Total hemoglobin (HbT) showed the least time-delay to the intracellular Ca2+ oscillation while HbR showed the strongest correlation (negative) with Ca2+ after time shifting (Fig. 3d). Similar results were also observed in both optical imaging (Vazquez et al., 2014) and fMRI (He et al., 2018), which showed stronger correlation between Ca2+ fluctuation and BOLD signal than the correlation between Ca2+ fluctuation and total hemoglobin (HbT) with similar time-delays. This could be caused by the delays in the metabolic and vascular responses to the Ca2+ increase or the transient time of hemoglobin through the vasculature, which is on the order of a few seconds (Hutchinson et al., 2006). Indeed, in rodent models peak BOLD response occurs a few seconds after the onset of stimulation(Kennerley et al., 2012; Silva and Koretsky, 2002). Meanwhile, we should also be aware of that the correlation coefficients could be altered due to slightly tuned temporal profiles of hemodynamic components, which are extracted from brain regions consisting of different small arterioles and veins(Ma et al., 2016a). To avoid the hemodynamic comtaination in Ca2+ fluoresence signal due to varied tissue absoprtion, the relative change in Ca2+ fluorescence signal (ΔF/F) was corrected by ratiometric analysis (Ma et al., 2016a; Yuan et al., 2011), as described in Supplementary Fig. S1, showing that hemodynamic fluctuation induced light absorption change (<5%) does not substantially change the Ca2+ signal (<1% before and after correction).
Previous studies combining fMRI and calcium measurement have advanced our understanding of BOLD response with the neuronal activity (Schmidt et al., 2016; Schulz et al., 2012; Wang et al., 2018). However, with limited spatiotemporal resolution of fMRI, it is difficult to capture the hemodynamic responses evoked by a single-pule stimulus (most studies implemented a burst stimulation for several seconds) as well to detect ongoing activity without significant temporal averaging. Therefore, the correlation between single-pulse evoked neuronal response with real time assessment of ongoing low frequency activity has been difficult. Recently, Schwalm et al (Schwalm et al., 2017) applied the calcium imaging probe into the deep cortex and analyzed the correlation between cortical-wide BOLD fluctuations with local Ca2+ slow oscillations. Here, we studied the single-pulse sensory evoked neurovascular response versus the slow oscillatory activity, providing new insights into the regulatory role of such slow oscillation in brain response to sensory stimuli.
Comparatively, the lower temporal correlation between the CBF oscillations and the neuronal oscillations might suggest that other factors such as astrocytes may be involved in regulating the CBF oscillations (He et al., 2018; Schmidt et al., 2016). Additionally, despite GCaMP6f (AAV1.Syn.GCaMP6f.WPRE.SV40) used in the present study is neuron specific but it expresses into all neuron types including excitatory and inhibitory neurons, both of these neuron subtypes might contribute to the Ca2+ fluorescence changes. However, the previous studies reported that the density of excitatory neurons was higher than that of inhibitory neurons in rat’s primary somatosensory cortex (Narayanan et al., 2017), it might imply that the Ca2+ oscillatory activity detected in the somatosensory cortex is primarily attributed to the excitatory neuron populations. It will be interesting to separate excitatory and inhibitory neurons in future studies.
2. Ongoing Ca2+ and hemodynamic oscillations inversely modulate Ca2+ transient and hemodynamic responses to single stimulation
The trial-to-trial variability of brain response to functional stimulation has been well documented in fMRI and electrophysiological studies (Arieli et al., 1996; Duann et al., 2002; Sutton et al., 1965). This variability is not quantitatively understood but is believed to be associated with the difference in pre-stimulus states of the spontaneous neuronal dynamics or variation in neurovascular coupling.
Some electrophysiological studies have shown that stimulation-evoked high-frequency neuronal responses are modulated by the neuronal low-frequency oscillations, suggesting that certain favorable pre-stimulus states would either amplify or suppress the stimulation-evoked neuronal responses in auditory cortex (Kisley and Gerstein, 1999; Kruglikov and Schiff, 2003; Lakatos et al., 2007) and visual cortex (Azouz and Gray, 1999). Although these findings might implicate the interpretation of trial-to-trial variability observed in fMRI, it is difficult to correlate the datasets obtained from these two approaches (electrophysiology and fMRI) because of their vastly different spatiotemporal scales. In this study, the discrepancy was minimized by the simultaneous Ca2+ and hemodynamic imaging of both resting spontaneous oscillations and stimulation-evoked neuronal and hemodynamic responses. The results show that single-stimulation-evoked Ca2+ transients were stronger at the troughs of the spontaneous Ca2+ oscillations; this finding is in line with previous electrophysiology studies that correct visual perceptions were linked to an optimal phase of ongoing oscillations (Busch et al., 2009) and strongest auditory responses were observed at the trough of the theta oscillations (Lakatos et al., 2005). Comparing the Ca2+ transients’ amplitude and phase dependency in strong stimulation and weak stimulation scenarios, we noticed the higher standard deviation of Ca2+ responses near the crest of fluctuations in strong stimulation case while compared with those in weak stimulation. the difference in Ca2+ transients’ variability near the crest for strong and weak stimulations likely reflects that the weak stimulation was much more affected than the strong stimulation by the states of the resting neuronal activity. This was the case in the present studies even for the LFOs studies here. The simultaneously acquired ΔHbT responses when quantified as normalized HbT increases were also negatively modulated by the spontaneous HbT oscillations, although the absolute ΔHbT increases were superposed to the ongoing HbT oscillations. Further analysis showed that the normalized HbT responses linearly correlated with the intensity of the corresponding Ca2+ transients. Due to a trade-off between the acquisition time and total channels to be imaged in our current system, we were only able to simultaneously image single-stimulus-evoked Ca2+ transients along with the ΔHbT responses at sufficient temporal resolution (50 fps). Further improvement in imaging system will be needed to capture the Ca transients currently with multiple channels of hemodynamic changes at a high speed.
3. Sensory stimulation induced phase reset of neuronal oscillations might reflect brain’s adaptation to subsequent sensory inputs.
The spontaneous slow oscillations modulated the neuronal responses to forepaw stimulation and strong forepaw stimulations in turn reset the phases to the crests of the ongoing neuronal oscillations which are a non-preferable state for the subsequent stimulations. Unlike some previous studies on the phase reset across different cortices such as somatosensory input to reset the phase of ongoing neuronal oscillations in auditory cortex within the delta, theta and gamma bands to preferable excitable states, the phase reset within the same brain region might suppress the subsequent response to sensory stimulation and play a role in desensitization of responses to subsequent sensory inputs(Kruglikov and Schiff, 2003; Lakatos et al., 2007; Lakatos et al., 2005). Thus, this phase reset can be interpreted as modulation of neuronal responses to subsequent sensory stimulation that resulted in either amplified or suppressed neuronal responses(Lakatos et al., 2007).
It is not clear how to quantitatively analyze the changes in Ca2+ signal. Changes in the number of cells that are excited can increase Ca2+ signal. In addition, changes in the frequency of firing in a cell can lead to increases in Ca2+. Assuming that the dominant effect in these studies is that Ca2+ signal primarily changes due to the number of cells that are excited enables the data to be interpreted by a simple model. Ongoing activity changes the number of cells that are spontaneously firing with fewer cells firing in the trough as opposed to the crest of the Ca2+ LFOs. A weak stimulus causes a few cells to fire and at the trough of the LFOs; there are more cells ready to respond to the stimulus than at the crest of the LFOs. These changes in cells that can fire to the weak stimulation lead to the very large percent change in cells that fire to the weak stimulation. However, since very few cells are excited by the weak stimulus, this does not affect the LFOs. The strong stimulus can also evoke more cell firing at the trough than the peak of the LFOs due to the fact that the strong stimulus causes many more cells to fire than the LFOs do, the effects of the phase of the LFO are proportionately less important. Furthermore, the strong stimulus causes so many cells to fire that it dominates the LFOs and causes a reset in the phase. The ability to monitor both ongoing activity and stimulus evoked activity with the Ca2+ indicators should enable testing of this model by monitoring Ca2+ at a single cell level and counting cells affected by LFOs and sensory stimulation.
We are aware that the variability in GCaMP6f expression ratio and the difference in stimuli-evoked Ca2+ response amplitudes are important influencing factors for the aforementioned hypothesis. To ensure the similar fluorescence expression rate across animals, we quantified the GCaMP6f neurons versus total neurons at difference cortical depths in brain slices. As shown in supplemental Fig. S3, the majority GCaMP6f expression was located in the cortical layer IV and V accounting for 63.3% of total GCaMP6f expressed neurons in the cortex. The averaged expression ratio for GCaMP6f within cortical layer IV/V is about 29.6±1.1% across experimental animals (n=12) with a small deviation range (max: 36.1%, min: 23.9%). To minimize the effect of variability in GCaMP6f expression on the amplitude-phase dependence signaling, the normalization process of Ca2+ responses to its maximal response was conducted for each animal. The normalized results are summarized in Fig. S6, showing a consistent amplitude-phase coupling pattern as shown in Fig. 4 above.
4. Limitation
A limitation of this study was that anesthetized animals were studied. Imaging awake animals would be an ideal scenario for studying brain functional connectivity (McGirr et al., 2017; Wright et al., 2017) because different anesthesia might affect neuronal activity and connectivity. For example, it has been reported that an increase of delta-band and oscillatory signal between 0.08–0.4Hz was observed in anesthetized mice (i.e., Ketamine) compared with awake mice (Wright et al., 2017). In this study, we used anesthesia to minimize the motion effect of the animal and to remove alterations of physiological effects such as blood PCO2 and arousal/stress states on the measurement. Many fMRI studies have used α-chloralose as was done here, because: 1) it preserves metabolic coupling for somatosensory stimulation (Ueki et al., 1992); 2) it provides a normal CBF baseline close to that measured in the awake state compared with other anesthetic agents such as isoflurane (Masamoto et al., 2006); and 3) it preserves cerebrovascular reactivity (Bonvento et al., 1994).
Here, a small dose of 25mg/kg/hr of α-chloralose was used in order to minimize the impacts of anesthesia. To study its effects on neuronal activities, we recorded the LFP in the cortex while the rat was under different doses of α-chloralose, i.e., 20 mg/kg/hr, 30 mg/kg/hr and 40 mg/kg/hr, respectively. Fig. S7 shows that there was no significant change in the spontaneous neuronal activity under different doses of α-chloralose. This is in agreement with the previous report (Luckl et al., 2008; Park et al., 2019), showing no significant changes in the EEG spectrum under different α-chloralose doses (30-, 70-, 100-mg/kg). Also, we compared the power spectra of Ca2+, HbO and HbR between the awake state and 30 minutes after administrating α-chloroses (114 mg/kg, i.p.)(Low et al., 2016) obtained from the same animal. Our data showed the dominant oscillations at ~0.1Hz in the spectra of Ca2+, HbO and HbR as shown in Supplemental Fig. S8b, which were consistent at the awake state and after α-chloroses, indicating no significant effects of α-chloralose on the slow frequency oscillatory activities of both neurons and hemodynamics in the cortex. In addition, the correlations of calcium signals with hemodynamic changes (i.e, Ca2+-vs- HbO and Ca2+-vs- HbR) were analyzed at awake and α-chloralose-anesthetized status as shown in Supplemental Fig. S8c. The similarity of the cross-correlation spectra between these two states indicate that the neurovascular coupling was preserved in α-chloralose condition.
Electrical stimulation induced pain might change the functional and metabolic process in animal brain(Amirmohseni et al., 2016), To make sure anesthesia was deep enough we pinched the forepaw (e.g., by forceps) to assess pain response of the animal before we proceeded to the image acquisition. In addition, the animal mean arterial blood pressure (MABP), heart rate, respiration rate and body temperature were continuously monitored (Supplemental Fig. S4). These approaches helped us to ensure no pain to influence on our signals. Indeed, the lack of change in these parameters indicates that the single pulse stimulation we used did not induce pain including at the 3 mA current level.
In conclusion, the results indicate that there are interactions between ongoing brain activity at low frequency and evoked responses in rodent somatosensory cortex. Genetically encoded Ca2+ fluorescent indicators have enough sensitivity to measure both the state of the LFOs and the response to stimulation without averaging either. Stimuli arriving at a trough of ongoing activity illicit a stronger Ca2+ response than those that arrive at a crest of ongoing activity. The effects are proportionally larger for weak stimuli. Furthermore, strong stimuli cause a reset of the ongoing low-frequency activity to be in a state that will cause smaller responses to the next stimulus. Further studies will enable the cellular origin of these effects to be determined.
Supplementary Material
Acknowledgments
We thank Kevin Clare for the help on ex vivo imaging. We thank Xin Yu and Kathyrn Sharer for helping with virus injection This research was supported in part by National Institutes of Health grants R01DA029718 and R21DA042597 (Y.P., C.D.), and by the NINDS, NIH intramural program (APK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Amirmohseni S, Segelcke D, Reichl S, Wachsmuth L, Görlich D, Faber C, Pogatzki-Zahn E, 2016. Characterization of incisional and inflammatory pain in rats using functional tools of MRI. NeuroImage 127, 110–122. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A, 1996. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273, 1868–1871. [DOI] [PubMed] [Google Scholar]
- Auer DP, 2008. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’brain. Magnetic resonance imaging 26, 1055–1064. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM, 1999. Cellular Mechanisms Contributing to Response Variability of Cortical Neurons In Vivo. The Journal of Neuroscience 19, 2209–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Reinacher M, Freyer F, Villringer A, Ritter P, 2011. How ongoing neuronal oscillations account for evoked fMRI variability. J Neurosci 31, 11016–11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, 2007. The behavioral significance of spontaneous fluctuations in brain activity. Neuron 56, 8–9. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Charbonné R, Correze J-L, Borredon J, Seylaz J, Lacombe P, 1994. Is α-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research? Brain research 665, 213–221. [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R, 2009. The Phase of Ongoing EEG Oscillations Predicts Visual Perception. The Journal of Neuroscience 29, 7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, 2006. Rhythms of the Brain. Oxford University Press. [Google Scholar]
- Buzsaki G, Draguhn A, 2004. Neuronal oscillations in cortical networks. Science 304, 1926–1929. [DOI] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu P, Volkow ND, Pan Y, Du C, 2015. Cocaine attenuates blood flow but not neuronal responses to stimulation while preserving neurovascular coupling for resting brain activity. Molecular Psychiatry 21, 1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Park K, Volkow N, Pan Y, Du C, 2016. Cocaine-Induced Abnormal Cerebral Hemodynamic Responses to Forepaw Stimulation Assessed by Integrated Multi-wavelength Spectroimaging and Laser Speckle Contrast Imaging. IEEE J Sel Top Quantum Electron 22, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Engel AK, 2006. Single-trial EEG-fMRI reveals the dynamics of cognitive function. Trends Cogn Sci 10, 558–563. [DOI] [PubMed] [Google Scholar]
- Du C, Koretsky AP, Izrailtyan I, Benveniste H, 2005. Simultaneous detection of blood volume, oxygenation, and intracellular calcium changes during cerebral ischemia and reperfusion in vivo using diffuse reflectance and fluorescence. Journal of Cerebral Blood Flow & Metabolism 25, 1078–1092. [DOI] [PubMed] [Google Scholar]
- Du C, Volkow ND, Koretsky AP, Pan Y, 2014. Low-frequency calcium oscillations accompany deoxyhemoglobin oscillations in rat somatosensory cortex. Proc Natl Acad Sci U S A 111, E4677–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann JR, Jung TP, Kuo WJ, Yeh TC, Makeig S, Hsieh JC, Sejnowski TJ, 2002. Single-trial variability in event-related BOLD signals. NeuroImage 15, 823–835. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N, 2011. The role of phase synchronization in memory processes. Nat Rev Neurosci 12, 105–118. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME, 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME, 2006. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci 9, 23–25. [DOI] [PubMed] [Google Scholar]
- Fransson P, 2005. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R, 2001. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563. [DOI] [PubMed] [Google Scholar]
- Fries P, Schroder JH, Roelfsema PR, Singer W, Engel AK, 2002. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. Journal of Neuroscience 22, 3739–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Chen W, Volkow ND, Koretsky AP, Du C, Pan Y, 2018a. Synchronized astrocytic Ca2+ responses in neurovascular coupling during somatosensory stimulation and for the resting state. Cell reports 23, 3878–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XC, Chen W, You J, Koretsky AP, Volkow ND, Pan YT, Du CW, 2018b. Long-term optical imaging of neurovascular coupling in mouse cortex using GCaMP6f and intrinsic hemodynamic signals. NeuroImage 165, 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, 2013. Spontaneous and task-evoked brain activity negatively interact. J Neurosci 33, 4672–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Raichle ME, 2009. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci 13, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang M, Chen X, Pohmann R, Polimeni JR, Scheffler K, Rosen BR, Kleinfeld D, Yu X, 2018. Ultra-Slow Single-Vessel BOLD and CBV-Based fMRI Spatiotemporal Dynamics and Their Correlation with Neuronal Intracellular Calcium Signals. Neuron 97, 925–939.e925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang J, Longtin A, Dumont G, Duncan NW, Pokorny J, Qin P, Dai R, Ferri F, Weng X, Northoff G, 2017. Is There a Nonadditive Interaction Between Spontaneous and Evoked Activity? Phase-Dependence and Its Relation to the Temporal Structure of Scale-Free Brain Activity. Cereb Cortex 27, 1037–1059. [DOI] [PubMed] [Google Scholar]
- Hutchinson EB, Stefanovic B, Koretsky AP, Silva AC, 2006. Spatial flow-volume dissociation of the cerebral microcirculatory response to mild hypercapnia. NeuroImage 32, 520–530. [DOI] [PubMed] [Google Scholar]
- Kennerley AJ, Harris S, Bruyns-Haylett M, Boorman L, Zheng Y, Jones M, Berwick J, 2012. Early and late stimulus-evoked cortical hemodynamic responses provide insight into the neurogenic nature of neurovascular coupling. Journal of Cerebral Blood Flow & Metabolism 32, 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL, 1999. Trial-to-trial variability and state-dependent modulation of auditory-evoked responses in cortex. J Neurosci 19, 10451–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Movshon JA, 2003. Neuronal adaptation to visual motion in area MT of the macaque. Neuron 39, 681–691. [DOI] [PubMed] [Google Scholar]
- Kruglikov SY, Schiff SJ, 2003. Interplay of electroencephalogram phase and auditory-evoked neural activity. J Neurosci 23, 10122–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE, 2007. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron 53, 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE, 2005. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. Journal of neurophysiology 94, 1904–1911. [DOI] [PubMed] [Google Scholar]
- Low LA, Bauer LC, Klaunberg BA, 2016. Comparing the Effects of Isoflurane and Alpha Chloralose upon Mouse Physiology. PLOS ONE 11, e0154936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckl J, Keating J, Greenberg JH, 2008. Alpha-chloralose is a suitable anesthetic for chronic focal cerebral ischemia studies in the rat: A comparative study. Brain research 1191, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Shaik MA, Kim SH, Kozberg MG, Thibodeaux DN, Zhao HT, Yu H, Hillman EMC, 2016a. Wide-field optical mapping of neural activity and brain haemodynamics: considerations and novel approaches. Sciences 371, 20150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Shaik MA, Kozberg MG, Kim SH, Portes JP, Timerman D, Hillman EMC, 2016b. Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proceedings of the National Academy of Sciences 113, E8463–E8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A, 2004. Mining event-related brain dynamics. Trends Cogn Sci 8, 204–210. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim S-G, 2006. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cerebral cortex 17, 942–950. [DOI] [PubMed] [Google Scholar]
- McGirr A, LeDue J, Chan AW, Xie Y, Murphy TH, 2017. Cortical functional hyperconnectivity in a mouse model of depression and selective network effects of ketamine. Brain 140, 2210–2225. [DOI] [PubMed] [Google Scholar]
- Mitra A, Kraft A, Wright P, Acland B, Snyder AZ, Rosenthal Z, Czerniewski L, Bauer A, Snyder L, Culver J, Lee JM, Raichle ME, 2018. Spontaneous Infra-slow Brain Activity Has Unique Spatiotemporal Dynamics and Laminar Structure. Neuron 98, 297–305 e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA, 2009. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan RT, Udvary D, Oberlaender M, 2017. Cell Type-Specific Structural Organization of the Six Layers in Rat Barrel Cortex. Front Neuroanat 11, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Herron P, Chhatbar PY, Levy M, Shen ZM, Schramm AE, Lu ZY, Kara P, 2016. Neural correlates of single-vessel haemodynamic responses in vivo. Nature 534, 378–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, Villringer A, 2000. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. NeuroImage 12, 623–639. [DOI] [PubMed] [Google Scholar]
- Palva JM, Palva S, 2012. Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. NeuroImage 62, 2201–2211. [DOI] [PubMed] [Google Scholar]
- Park K, Chen W, Volkow ND, Allen CP, Pan Y, Du C, 2019. Hemodynamic and neuronal responses to cocaine differ in awake versus anesthetized animals: Optical brain imaging study. NeuroImage 188, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycke JR, 2010. Some tests for uniformity of circular distributions powerful against multimodal alternatives. Canadian Journal of Statistics-Revue Canadienne De Statistique 38, 80–96. [Google Scholar]
- Rayshubskiy A, Wojtasiewicz TJ, Mikell CB, Bouchard MB, Timerman D, Youngerman BE, McGovern RA, Otten ML, Canoll P, McKhann GM 2nd, Hillman EM, 2014. Direct, intraoperative observation of ~0.1 Hz hemodynamic oscillations in awake human cortex: implications for fMRI. NeuroImage 87, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DM, Hagstrom EC, 1989. Some Evidence in Support of a Relationship between Human Auditory Signal-Detection Performance and the Phase of the Alpha Cycle. Perceptual and Motor Skills 69, 451–457. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Seiler S, Loitfelder M, 2016. Longitudinal change of small-vessel disease-related brain abnormalities. Journal of Cerebral Blood Flow & Metabolism 36, 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P, 2009. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Sydekum E, Krueppel R, Engelbrecht CJ, Schlegel F, Schröter A, Rudin M, Helmchen F, 2012. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nature Methods 9, 597–602. [DOI] [PubMed] [Google Scholar]
- Schwalm M, Schmid F, Wachsmuth L, Backhaus H, Kronfeld A, Jury FA, Prouvot P-H, Fois C, Albers F, van Alst T, 2017. Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves. eLife 6, e27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Bressler SL, Knuth KH, Ding M, Mehta AD, Ulbert I, Schroeder CE, 2004. Neural dynamics and the fundamental mechanisms of event-related brain potentials. Cereb Cortex 14, 476–483. [DOI] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP, 2002. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proceedings of the National Academy of Sciences 99, 15182–15187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitha KA, Akhil Raja K, Arun KM, Rajesh PG, Thomas B, Kapilamoorthy TR, Kesavadas C, 2017. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J 30, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER, 1965. Evoked-potential correlates of stimulus uncertainty. Science 150, 1187–1188. [DOI] [PubMed] [Google Scholar]
- Tong Y, Frederick BD, 2010. Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. NeuroImage 53, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki M, Mies G, Hossmann KA, 1992. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta anaesthesiologica scandinavica 36, 318–322. [DOI] [PubMed] [Google Scholar]
- van Alst TM, Wachsmuth L, Datunashvili M, Albers F, Just N, Budde T, Faber C, 2019. Anesthesia differentially modulates neuronal and vascular contributions to the BOLD signal. NeuroImage 195, 89–103. [DOI] [PubMed] [Google Scholar]
- Vanni MP, Murphy TH, 2014. Mesoscale Transcranial Spontaneous Activity Mapping in GCaMP3 Transgenic Mice Reveals Extensive Reciprocal Connections between Areas of Somatomotor Cortex. The Journal of Neuroscience 34, 15931–15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez AL, Murphy MC, Kim SG, 2014. Neuronal and physiological correlation to hemodynamic resting-state fluctuations in health and disease. Brain Connect 4, 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Sejnowski TJ, Yu X, 2018. Brain-state dependent astrocytic Ca2+ signals are coupled to both positive and negative BOLD-fMRI signals. Proceedings of the National Academy of Sciences 115, E1647–E1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH, 2007. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci 27, 6268–6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PW, Brier LM, Bauer AQ, Baxter GA, Kraft AW, Reisman MD, Bice AR, Snyder AZ, Lee J-M, Culver JP, 2017. Functional connectivity structure of cortical calcium dynamics in anesthetized and awake mice. PLOS ONE 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Vanni MP, Mitelut CC, Chan AW, LeDue JM, Xie Y, Chen ACN, Swindale NV, Murphy TH, 2017. Mapping cortical mesoscopic networks of single spiking cortical or sub-cortical neurons. eLife 6, e19976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Chan AW, McGirr A, Xue S, Xiao D, Zeng H, Murphy TH, 2016. Resolution of High-Frequency Mesoscale Intracortical Maps Using the Genetically Encoded Glutamate Sensor iGluSnFR. The Journal of Neuroscience 36, 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Qian C, Chen DY, Dodd SJ, Koretsky AP, 2014. Deciphering laminar-specific neural inputs with line-scanning fMRI. Nat Methods 11, 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Luo Z, Volkow ND, Pan Y, Du C, 2011. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. NeuroImage 54, 1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


