Abstract
The COVID-19 pandemic has had a significant impact on the structure and operation of healthcare services worldwide. We highlight a case of a 64-year-old man who presented to the emergency department with acute dyspnoea on a background of a 2-week history of fever, dry cough and shortness of breath. On initial assessment the patient was hypoxic (arterial oxygen saturation (SaO2) of 86% on room air), requiring 10 L/min of oxygen to maintain 98% SaO2. Examination demonstrated left-sided tracheal deviation and absent breath sounds in the right lung field on auscultation. A chest radiograph revealed a large right-sided tension pneumothorax which was treated with needle thoracocentesis and a definitive chest drain. A CT pulmonary angiogram demonstrated segmental left lower lobe acute pulmonary emboli, significant generalised COVID-19 parenchymal features, surgical emphysema and an iatrogenic pneumatocoele. This case emphasises the importance of considering coexisting alternative diagnoses in patients who present with suspected COVID-19.
Keywords: pneumothorax, respiratory medicine, pulmonary embolism, tropical medicine (infectious disease), radiology
Background
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in December 2019 in Wuhan, China and has now had a significant effect on populations worldwide in the form of the COVID-19 pandemic. As of 4 June 2020, there have been 6 416 828 confirmed cases of COVID-19 reported worldwide with 382 858 deaths, with an overall case fatality rate of approximately 6%1; this has therefore led to a substantial burden on health and intensive care services. The presenting features reported are general and non-specific, including dry cough, fever, malaise, difficulty in breathing, and in severe cases profound hypoxaemia and cardiorespiratory arrest. In the early part of 2020, it has been a common presenting cause of significant hypoxia to the emergency department. Despite its high prevalence, clinicians must ensure not to allow themselves to be distracted by this relatively new phenomenon, and to continue to practise good history taking and examination skills to identify coexisting, alternative potential life-threatening diagnoses and to provide optimal, timely treatment to patients.
We exhibit a case of a 64-year-old man with suspected COVID-19 pneumonia who presented acutely to the emergency department with tension pneumothorax and acute pulmonary emboli.
Case presentation
A 64-year-old man presented to the emergency department with intermittent fevers, dry cough and progressive shortness of breath over a 2-week period. On the day of attendance, he acutely deteriorated with increased difficulty in breathing and pleuritic chest pain.
His medical history included hypertension, type II diabetes mellitus and hypercholesterolaemia. He had no known drug allergies and was taking simvastatin 20 mg once daily, ramipril 2.5 mg once daily and metformin 1 g two times per day as his regular medications. He is a socially independent man and a lifelong non-smoker with no pre-existing respiratory conditions and no significant family history.
On examination, he was significantly breathless at rest with a respiratory rate of 30 breaths/min and an arterial oxygen saturation of 86% on air. Oxygen saturations were stabilised at 98% on 10 L/min of oxygen (60% fractional inspired oxygen (FiO2)). Other vital signs measured included a heart rate of 115 beats/min, blood pressure of 125/95 mm Hg and temperature of 37.0°C. His trachea was deviated to the left and he had asymmetrical chest expansion (reduced on the right). Chest auscultation revealed absent breath sounds throughout the right hemithorax and widespread crepitations on the left. Heart sounds were unremarkable, abdomen was soft and non-tender with no organomegaly, and calves were non-tender bilaterally with no evidence of peripheral oedema.
Investigations
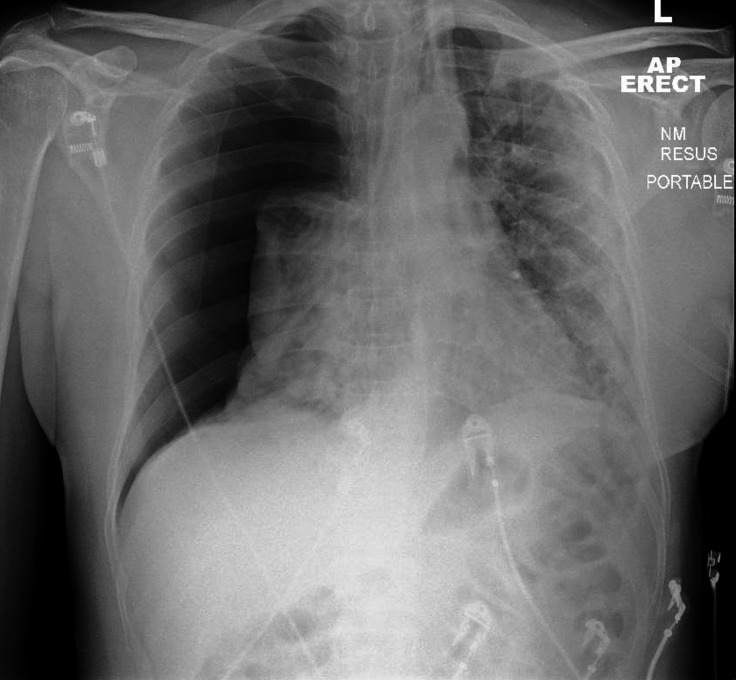
A portable chest radiograph in the emergency department demonstrated a large right-sided tension pneumothorax with mediastinal shift and diffuse airspace shadowing throughout the left lung, which was more pronounced peripherally (figure 1). A 12-lead ECG showed sinus tachycardia at a rate of 120 beats/min.
Figure 1.
Initial anteroposterior (AP) chest radiograph depicting a large right-sided pneumothorax. Note the considerable leftward mediastinal shift. Diffuse alveolar opacity is present throughout the left lung, which is more pronounced in the periphery. There is also alveolar opacity in the collapsed right lung.
Arterial blood gas performed on FiO2 of 60% showed pH 7.44, partial pressure of oxygen (pO2) 8.06 kPa, partial pressure of carbon dioxide (PCO2) 4.46 kPa, bicarbonate (HCO3) 23.6 mEq/L, base excess −0.8 mmol/L, glucose 7.4 mmol/L and lactate 1.8 mmol/L, in keeping with type 1 respiratory failure.
Routine blood test results on admission were as follows: haemoglobin: 129 g/L; white cell count: 11.2×109/L; platelets: 538×109/L; neutrophils: 9.91×109/L; lymphocytes: 0.72×109/L; D-dimer: 2560 ng/mL; ferritin: 421 µg/L; C reactive protein: 35 mg/L; alanine aminotransferase: 140 IU/L; and alkaline phosphatase: 131 IU/L. These were in keeping with a systemic inflammatory response with likely superimposed bacterial infection and possible embolic aetiology.
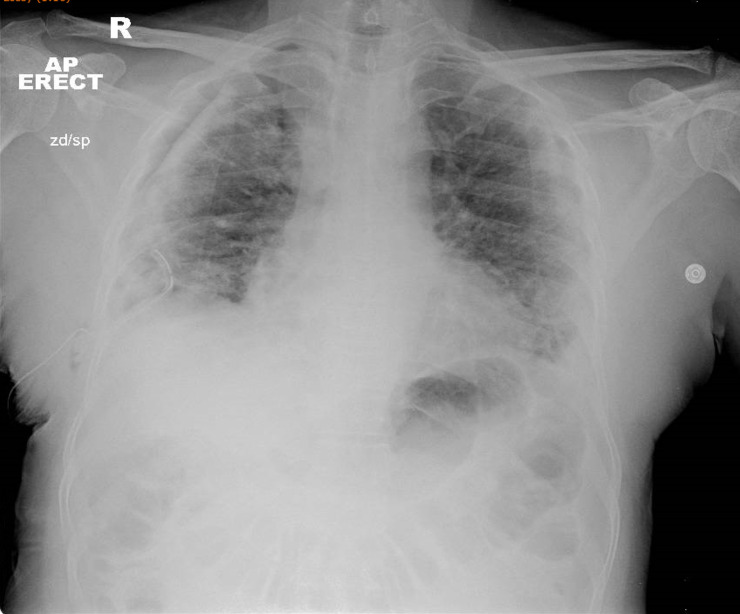
Following initial management for the tension pneumothorax, a repeat chest radiograph demonstrated lung re-expansion with a small residual pneumothorax, but with no mediastinal shift; peripheral ground glass airspace opacities were accentuated, in keeping with COVID-19 infection (figure 2).
Figure 2.
Right-sided chest drain in situ. The pneumothorax has reduced in size with 11 mm of depth remaining. No mediastinal shift is noted. Peripheral ground glass airspace opacities within both lungs are consistent with COVID-19 infection. AP, anteroposterior.
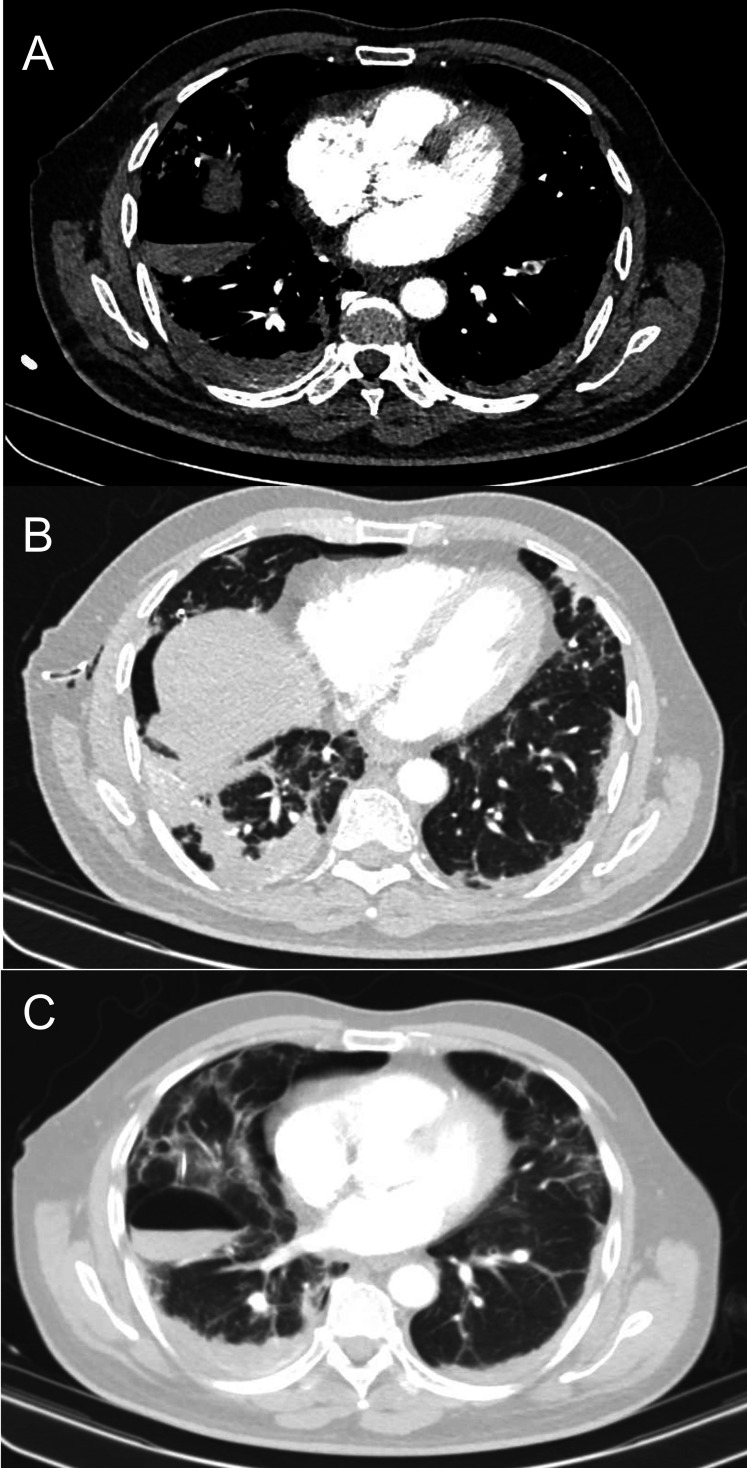
A CT pulmonary angiogram (CTPA) was performed on the second day of inpatient admission which highlighted multiple abnormalities contributing to this patient’s hypoxia. There was evidence of acute segmental pulmonary emboli in the left lower lobe alongside generalised COVID-19 alveolar opacities in the lower lobes. Residual right-sided pneumothorax was visible alongside surgical emphysema as well as an iatrogenic secondary pneumatocoele, likely related to the depth of the chest drain tip (figure 3). The patient had a positive reverse transcriptase (RT)-PCR nasopharyngeal swab for COVID-19.
Figure 3.
CT pulmonary angiogram. (A) Segmental pulmonary emboli in the left lower lobe. (B) Generalised peripheral alveolar opacities in keeping with COVID-19 with dense bilateral lower lobe consolidation, more pronounced in the right lung. Chest drain is noted in the right axilla with residual right pneumothorax and subcutaneous surgical emphysema. (C) Iatrogenic secondary loculated pneumatocoele due to the chest drain traversing the lung parenchyma.
Treatment
On recognition of the tension pneumothorax, the patient was initially treated with needle decompression with a large-bore, 14-gauge cannula inserted in the right second intercostal space in the mid-clavicular line. This was followed with a 12-French Seldinger chest drain inserted into the ‘triangle of safety’ on the right side to achieve definitive management. The patient received supplemental oxygen to maintain target saturations of >94% and commenced on broad spectrum intravenous antibiotics (co-amoxiclav and clarithromycin) for treatment of superadded bacterial pneumonia. Treatment dose low molecular weight heparin (tinzaparin sodium) was initiated for the pulmonary emboli visualised on CTPA.
Outcome and follow-up
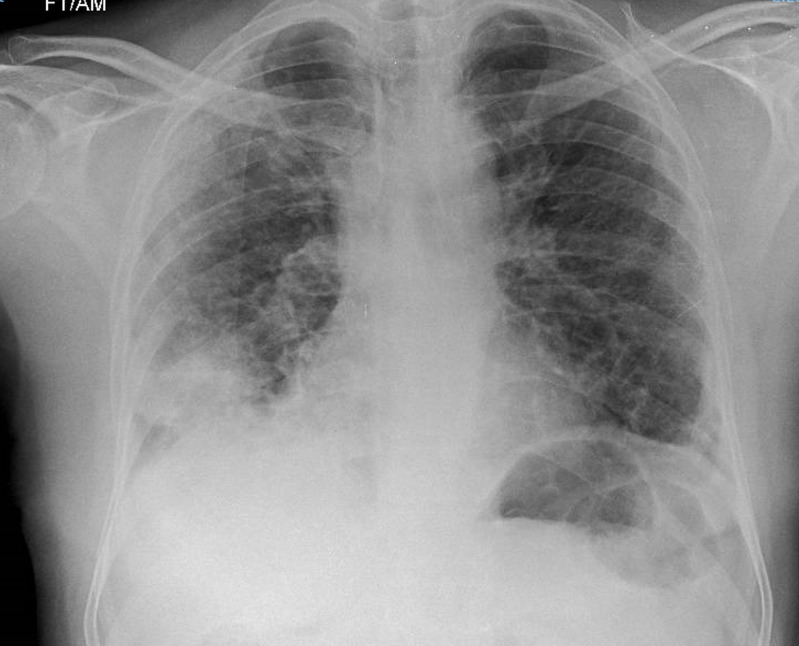
The chest drain was removed on day 3 of admission following the CTPA findings of iatrogenic pneumatocoele, and a repeat chest radiograph showed significant reinflation of the right lung with no residual pneumothorax. Once oxygen saturations normalised without supplementary oxygen (96% on air), the patient was discharged after a 4-day admission with a 3-month course of apixaban 5 mg two times per day and follow-up in the anticoagulation clinic. He was reviewed in the outpatient ambulatory care clinic 5 days postdischarge; at this point, he remained normoxic and a repeat chest radiograph showed complete resolution of pneumothorax. However, right basal consolidation persisted, for which he received a further 1-week course of antibiotics (figure 4). He is now due for routine follow-up with the respiratory team in 4–6 months’ time. He was advised to remain isolated until his symptoms resolved after discharge.
Figure 4.
Follow-up chest radiograph 5 days postdischarge illustrating complete resolution of the pneumothorax and mild improvement in ground glass peripheral opacification, but with persistent right basal consolidation.
Discussion
This case outlines the importance of timely identification and treatment of other contributory reversible causes of hypoxia on a background of COVID-19 infection. To our knowledge, we have reported the first documented case of a patient with COVID-19 pneumonia presenting with both spontaneous tension pneumothorax and acute pulmonary emboli.
The evolution of spontaneous pneumothorax2 3 and pneumomediastinum4–6 with concomitant COVID-19 infection, prior to ventilation procedures, are rare entities, with very few cases reported in the literature. The rupture of subpleural bullae or pneumatocoeles is the most common cause of primary spontaneous pneumothorax, and risk factors for development include pre-existing chronic lung conditions, smoking, male gender and prolonged coughing. The precise mechanism of this phenomena in the context of COVID-19 is not fully understood and a coincidental association cannot be excluded. However, studies have reported the presence of pneumatocoeles and cystic lung parenchymal features in patients with COVID-19,7 which may increase the chance of developing secondary pneumothoraces.
Several studies have reported the increased prevalence of thromboembolic events in COVID-19 infection.8–10 The systemic inflammation associated with COVID-19 and endothelial dysfunction, along with the generated hypercoagulable state, are the likely contributory factors to the pathogenesis of acute pulmonary emboli. The viscosity of blood is further increased by the hypoxia from severe pneumonia. Biomarkers such as elevated D-dimer, lactate dehydrogenase and ferritin levels are associated with a worse prognosis.11 It is essential to therefore identify and treat thromboembolic events with timely anticoagulation along with stringent prevention strategies aiming at adequate thromboembolic prophylaxis with low molecular weight heparin.
The current widely accepted and implemented method for the diagnosis of COVID-19 is the RT-PCR, with a pooled sensitivity in meta-analyses reported to be of 89%.12 However, a high false negative rate poses several clinical and social challenges. CT chest imaging is a useful evaluation tool reserved for assessing disease severity, progression, coexisting pathologies and complications. The classic radiological findings on CT are bilateral, peripheral ground glass opacifications, crazy-paving pattern, bronchovascular thickening and consolidation.13 14 Atypical CT findings reported include pleural effusions, mediastinal lymphadenopathy, pneumothoraces and cavitations, which raise the suspicion of superadded bacterial infection and are often more apparent during the later courses of the disease.15 Typical CT findings have also been observed on asymptomatic patients with COVID-19.16 Using CT as a diagnostic or screening tool for COVID-19 alone is discouraged by many radiological societies and institutions17 due to recent meta-analyses showing a pooled high sensitivity and low specificity of 94% and 37%, respectively, as well as a high rate of false positives.12 Furthermore, routine use of CT poses risks, for example, increased ionising radiation and usage of finite personal protective resources between patients in the event of shortage.18 Nevertheless, CT imaging in the context of our case had considerable benefit in the identification of additional diagnoses, namely pulmonary emboli, as well as an assessment of the severity of infection. The rationale for using CTPA as opposed to CT thorax without contrast was due to local data from our centre showing that 34.5% of patients who were SARS-CoV-2-positive were diagnosed with pulmonary embolism as an inpatient, alongside an increasingly high number of readmissions with thromboembolism in previously discharged patients.19 The identification of an iatrogenic pneumatocoele on CT assisted in the timely removal of the chest drain.
There have been noticeable changes to the structure and operation of healthcare services in the fight against this pandemic. Many trusts have adopted protocols and escalation pathways for patients with COVID-19, which may risk patients being given incorrect and potentially dangerous treatment in the event of other coexisting pathologies. For example, protocol-driven prompt continuous positive airway pressure treatment without appropriate assessment in our patient with profound hypoxia would have been extremely detrimental to his condition. In the current climate, it is important for clinicians to maintain an individualised, thorough approach to patients presenting with symptoms of COVID-19 and to exclude additional underlying pathologies in the interest of patient-centred care.
Learning points.
Spontaneous pneumothorax and acute pulmonary emboli are important coexisting respiratory pathologies to consider on a background of COVID-19 infection.
It is important to maintain an individualised approach to exclude additional underlying pathologies, which can be assisted with good history taking and examination skills.
CT chest imaging can provide assistance with the assessment of coexisting pathologies, disease severity, progression and complications.
Footnotes
Contributors: RK is the lead author in this case report and played a significant role in writing the manuscript, obtaining consent and following up the patient. FTFJ contributed to writing the manuscript and clerked the patient in the emergency department. RN is a clinical fellow in respiratory medicine who was involved in the care of the patient and contributed to writing the manuscript. SH is a consultant chest radiologist who provided expert opinion, supervised and contributed to writing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.World health organisation coronavirus disease (COVID-19) Dashboard.
- 2.Flower L, Carter J-PL, Rosales Lopez J, et al. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep 2020;13. 10.1136/bcr-2020-235861. [Epub ahead of print: 17 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ucpinar BA, Sahin C, Yanc U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: case report. J Infect Public Health 2020;13:887–9. 10.1016/j.jiph.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan V, Tauseen RA. Spontaneous pneumomediastinum in COVID-19. BMJ Case Rep 2020;13:e236519. 10.1136/bcr-2020-236519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Gao C, Xie Y, et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis 2020;20:510. 10.1016/S1473-3099(20)30156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacroix M, Graiess F, Monnier-Cholley L, et al. SARS-CoV-2 pulmonary infection revealed by subcutaneous emphysema and pneumomediastinum. Intensive Care Med 2020. 10.1007/s00134-020-06078-3. [Epub ahead of print: 19 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Zeng Y, Xie P, et al. COVID-19 with cystic features on computed tomography: a case report. Medicine 2020;99:e20175. 10.1097/MD.0000000000020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzo C, Francesca B, Francesco P, et al. Acute pulmonary embolism in COVID-19 related hypercoagulability. J Thromb Thrombolysis 2020;50:223–6. 10.1007/s11239-020-02160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hékimian G, Lebreton G, Bréchot N, et al. Severe pulmonary embolism in COVID-19 patients: a call for increased awareness. Crit Care 2020;24 10.1186/s13054-020-02931-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grillet F, Behr J, Calame P, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 2020:201544. 10.1148/radiol.2020201544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology 2020:201343. 10.1148/radiol.2020201343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323. 10.1001/jama.2020.1585. [Epub ahead of print: 07 Feb 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao W, Zhong Z, Xie X, et al. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 2020;214:1072–7. 10.2214/AJR.20.22976 [DOI] [PubMed] [Google Scholar]
- 15.Perlman S. Another decade, another coronavirus. N Engl J Med 2020;382:760–2. 10.1056/NEJMe2001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inui S, Fujikawa A, Jitsu M, et al. Chest CT Findings in Cases from the Cruise Ship “Diamond Princess” with Coronavirus Disease 2019 (COVID-19). Radiology: Cardiothoracic Imaging 2020;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mossa-Basha M, Meltzer CC, Kim DC, et al. Radiology Department Preparedness for COVID-19: Radiology Scientific Expert Review Panel. Radiology 2020;296:E106–12. 10.1148/radiol.2020200988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raptis CA, Hammer MM, Short RG, et al. Chest CT and coronavirus disease (COVID-19): a critical review of the literature to date. AJR Am J Roentgenol 2020:1–4. 10.2214/AJR.20.23202 [DOI] [PubMed] [Google Scholar]
- 19.Kumar P, Mediwake R, Rhead C. A matter of time: duration and choice of venous thromboprophylaxis in patients diagnosed with COVID-19. Br J Hosp Med 2020;81:1–2. 10.12968/hmed.2020.0210 [DOI] [PubMed] [Google Scholar]