Abstract
The most common type of stroke, i.e. ischemic stroke, is a great challenge for contemporary medicine as it poses both diagnostic and therapeutic difficulties. Atherosclerosis, which is rapidly beginning to affect more and more social groups, is the main cause of cerebrovascular accidents. Atherosclerosis is currently defined as a generalized, dynamic and heterogeneous inflammatory and immune process affecting arterial walls. Atherosclerotic plaque is the emanation of this disease. As the paradigm of the diagnosis of atherosclerosis has changed, it has become crucial to properly identify plaque instability within the carotid arteries by evaluating parameters and phenomena that signify a developing cascade of complications, eventually leading to stroke. Irrespective of the ultrasound technique employed, proper morphological evaluation of atherosclerotic plaque, involving observation of its echogenicity, i.e. subjective analysis of its structure, with the classification to Gray-Weale–Nicolaides types as well as assessment of the integrity of its surface, makes it possible to roughly evaluate plaque morphology and thereby its stability. This enables treatment planning and therapy monitoring. This evaluation should be a prelude to further diagnostic work-up, which involves non-invasive examinations that enable unambiguous assessment of plaque stability. These examinations include contrast-enhanced ultrasound to assess progression or recession of inflammation, which presents as plaque neovascularization, or shear wave elastography to objectively define tissue stiffness, and thereby its mineralization.
Keywords: ultrasound, unstable atherosclerotic plaque, ischemic stroke
Introduction
Ischemic stroke, which is the most common type of stroke, is a great challenge for contemporary medicine. It poses both diagnostic and therapeutic difficulties.
Advances in the treatment of ischemic strokes have offered patients a greater chance for recovery and return to normal life. That is why early diagnosis and identification of pathological conditions that might lead to this outcome are of particular importance.
Each year, 15 million cases of ischemic stroke are noted worldwide (214 cases per 100,000 inhabitants)(1). In Poland, there are about 70,000 cases annually, 30,000 of which are fatal. A snowball increase is noted in the number of cerebrovascular accidents (CVAs), ranging from transient ischemic accidents (TIA) to ischemic strokes. This increase is estimated at 1.9% per year and is clearly linked with ageing society.
Atherosclerosis: a new paradigm in diagnosis
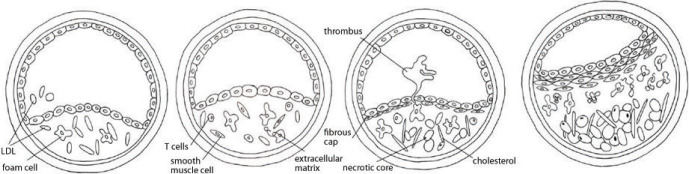
The fundamental and one of the main causes of CVAs is atherosclerosis, a disease which is beginning to affect more and more social groups. Atherosclerosis is currently defined as a generalized, dynamic and heterogeneous inflammatory and immune process that involves arterial walls. It may lead to blood flow obstruction in the affected vessel, which entails the occurrence of certain defined clinical signs and symptoms. A structure called atherosclerotic plaque is central to the disorders induced by atherosclerosis(2) (Fig. 1).
Fig. 1.
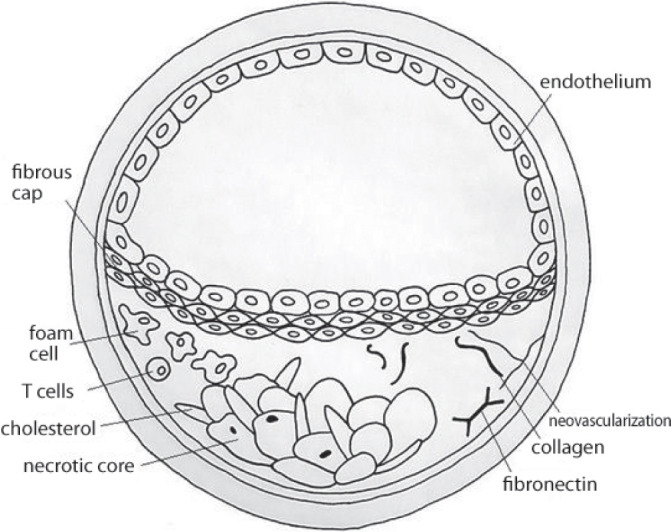
Atherosclerotic plaque: a schematic
In accordance with the Mannheim consensus(3–6) published in 2007 and later revised, atherosclerotic plaque is defined as a focal structure that builds up into the arterial lumen to at least 0.5 mm or 50% of the surrounding intima-media thickness. The ability to evaluate plaque formation dynamics and its complications (including rupture and displacement) is a very important and not yet well-explored problem. Most patients with atherosclerotic plaque present no signs of vascular disorders, which means that most plaques are silent and asymptomatic.
Disorders do appear only with hemodynamically significant stenosis or vascular occlusion and blood flow obstruction or blockage. These changes occur during the natural development of atherosclerosis that leads to the formation or development of changes within the plaque itself, such as its enlargement or mineral remodeling,(7) as well as changes within its lipid core and on its surface (so-called fibrous cap)(8).
In light of contemporary studies, the paradigm of the relationship of atherosclerosis and CVAs has changed as there are also plaques of vast damage potential that do not cause vascular stenoses or occlusion by their sole presence, but since they are capable of initiating a cascade leading to the formation and activation of plaque-related clots, they may cause ischemic strokes that in consequence result in sudden death(9). Owing to this potential, these plaques are called unstable or “vulnerable”(10,11).
The main problem in atherosclerotic plaque imaging is to identify the moment of potential transformation of a stable plaque into vulnerable plaque. The identification of a precise time of plaque destabilization defines the moment of proper treatment initiation, whether it be conservative treatment (anti-aggregation therapy, statins, cilostazol and other phosphodiesterase 3 inhibitors, peroxisome proliferator-activated receptors (PPAR) and their agonists, metalloproteinase inhibitors) or a surgical procedure (endarterectomy, endovascular procedures and sonothrombolysis or ultrasound-accelerated thrombolysis enhanced with contrast agents).
Vulnerable plaque as a diagnostic target
In patients at risk of cerebral ischemia, a site that is both crucial for the build-up of pathologic changes and convenient for clinical assessment is the carotid artery, a vessel representative for the evaluation of atherosclerosis progression.
The detection of features indicating active inflammation within atherosclerotic plaque, based on neovascularization within the lipid core structures or monitoring the levels of inflammatory products, is a criterion of certain atherosclerotic plaque instability(12,13).
Definition of vulnerable plaque
The density of neovascular network within atherosclerotic plaque correlates with the stage of atherosclerosis(14). These vessels are believed to be the primary cause of complications in the development of atherosclerotic plaque as they are a site of bleeding or inflammation and may in consequence lead to the loss of plaque stability. The degree of their “maturity” is proportional to a decrease in plaque stability as they are more susceptible to damage, and therefore also to blood extravasation to the plaque, formation of microthrombi and abrupt plaque enlargement(15,16).
The plaque transforms from stable plaque into plaque with thin fibrous cap atheroma/thin cap atheromatic plaque, (TFCA/TCAP) and finally into unstable or vulnerable plaque(11,17) (Fig. 2).
Fig. 2.
Atherosclerotic plaque evolution into vulnerable plaque (Virmani34)
In the case of fibrous cap rupture, when the lipid core is damaged and there is potential bleeding into the plaque, the material is released into the vascular lumen, which might lead to vascular occlusion and a CVA(16,18).
It is then currently crucial to properly identify plaque instability within the carotid arteries by evaluating parameters and phenomena that signify an initiating cascade of complications eventually leading to stroke.
The evaluation of atherosclerotic plaque should then include:
fibrous cap thickness;
size of the lipid necrotic core;
identification of plaque neovascularization;
identification of the direction of plaque remodeling;
detection of fibrous cap damage or risk of its occurrence.
Ultrasonography is the most available, the cheapest and noninvasive method of examining arteries to identify and assess the morphology and condition of atherosclerotic plaque. Within the past 20 years, two-dimensional ultrasound (2D, B-Mode) and Doppler imaging have become the mainstay of assessment of pre-cranial carotid artery segments. These modalities enable the evaluation of both vascular morphology and blood flow dynamics, including: flow direction, velocity, volume, laminar flow or pressure gradient.
Ultrasonographic methods of atherosclerotic plaque evaluation
Atherosclerotic plaques can be examined using several ultrasound-based techniques, including:
real-time ultrasound (B-Mode, 2D Mode);
Doppler ultrasound: color flow mode, power Doppler (in various configurations), including directional power Doppler, superb microvascular imaging;
non-Doppler flow evaluation methods (B-Flow);
spatial (volumetric) ultrasound;
contrast-enhanced ultrasound (CEUS);
shear wave elastography (SWE).
The three last techniques (i.e. volumetric ultrasound, CEUS and elastography) will be discussed in the next parts of this publication series.
Types of atherosclerotic plaque
The echogenicity of atherosclerotic plaque mainly depends on lipids in its core and necrotic elements of the lipid core as well as on core microvascularization(15,19–21) and mineralized or fibrous components.
In order to introduce uniform terminology regarding atherosclerotic plaque types, an ultrasound-based classification, called Gray-Weale–Nicolaides (GWN) classification(22,23), has been introduced. It is based on the subjective image of plaque—its echogenicity—on B-Mode (2D) images. This classification distinguishes between five types (classes) of plaque, as described below.
Type (class) I: uniformly echolucent plaque
Plaque is almost entirely “translucent”. It is homogeneous, hypoechoic and composed mainly of lipid elements and necrotic structures (Fig. 3).
Fig. 3.
Uniformly echolucent plaque on the posterior wall of the LCCA (left common carotid artery). A. B-Mode. B. CFM mode. Author’s own material
When imaging this type of plaque, attention must be paid to the presence and echogenicity of the fibrous cap. The hypoechoic structure of the plaque, similar to the echogenicity of fluid, is the primary difficulty. The level of echogenicity depends on the relationship between lipid–necrotic elements and fibrous structures of the plaque matrix.
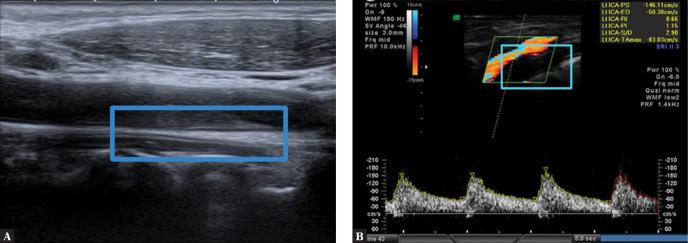
The plaque border is visualized using Doppler imaging (Fig. 4 and Fig. 5) or non-Doppler methods (B-Flow), and the visualization depends on the image of blood flow in the vessel. The detection of a thin fibrous cap defines the plaque as TCAP (thin cap atheromatic plaque) and places it in the category of vulnerable plaques. It must be noted, however, that the fibrous cap with the thickness that defines it as TCAP is not visible on classical ultrasound, and its evaluation is highly subjective.
Fig. 4.
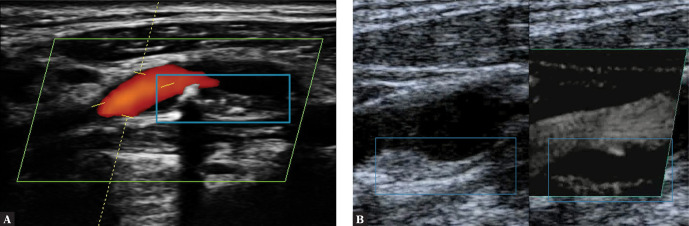
Uniformly echolucent plaque on the posterior wall of the ICA (internal carotid artery). A. Plaque is visible thanks to flow visualization in a directional power Doppler examination. B. Plaque visualized in a volumetric examination. Author’s own material
Fig. 5.
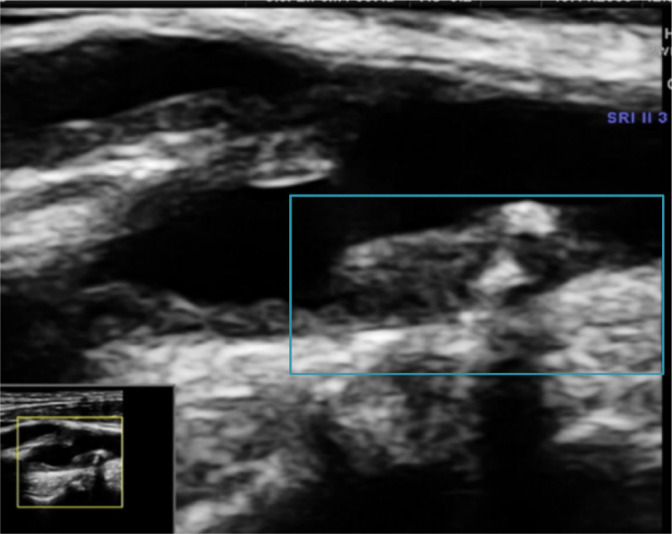
Type (class) II plaque on the posterior wall of the CCA bifurcation. Author’s own material
To sum up, type I plaques in the GWN classification are indistinguishable from fluid inside the vessel as seen on a B-Mode image due to their low echogenicity. They may be therefore overlooked in a diagnostic test. That is why most plaques are classified as type II plaques.
Type (class) II: predominately echolucent plaque
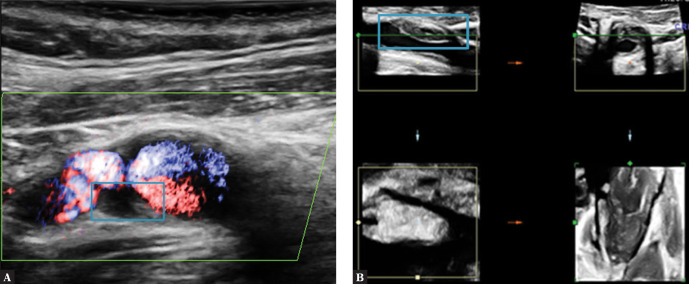
The plaque composition is as in type I with additional presence of single calcifications which perfectly aid in plaque imaging but prevent the precise visualization of the fibrous cap border due to reverberation at the calcification edges on a B-Mode image. The calcified part of the plaque should not exceed 25% of the plaque volume (in a volumetric examination) or 20–25% of the plaque size (in 2D assessment) (Fig. 5, Fig. 6, Fig. 7).
Fig. 6.
Atherosclerotic plaque on the ICA anterior wall. A. Type II plaque, TCAP with elements of JBA (juxtaluminal black area) – blue arrow, and calcification – red arrow. B. Plaque visualized in a volumetric examination. Author’s own material
Fig. 7.
Mixed type II plaque. A. On the posterior wall of the CCA bifurcation, CFM image. B. On the ICA posterior wall – SMI (superb microvascular imaging). Author’s own material
Type (class) III: predominantly echogenic plaque
Plaque composition is as in type II with numerous calcifications constituting up to 50% of the plaque structure (Fig. 8).
Fig. 8.
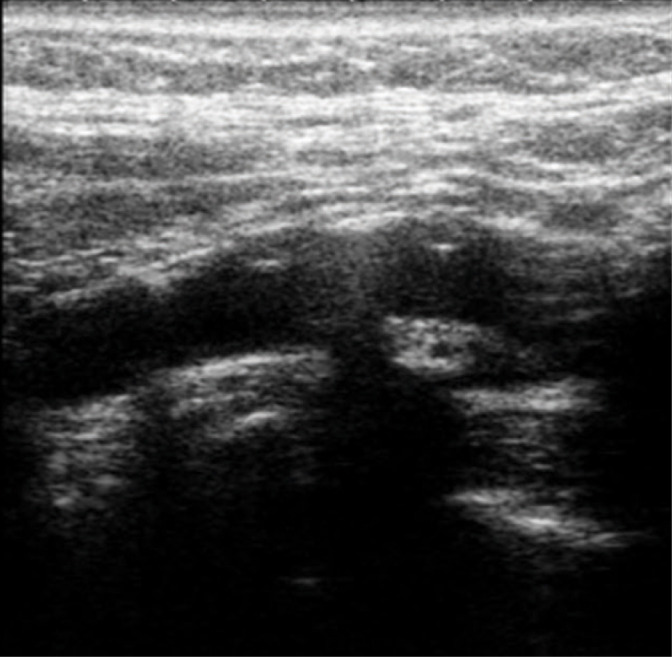
Heterogeneous type III plaque. Authors’ own material
Numerous reverberations at the calcification edges make the visualization of the borders and course of the fibrous cap extremely difficult on a B-Mode image. Plaque evaluation and its potential classification as TCAP is dubious due to relatively numerous reverberations associated with the presence of mineralized components.
In this group of plaques, flow imaging with Doppler or non-Doppler methods facilitates classification of plaque surface defects and enables visualization of potential irregularities and ulcerations.
Type (class) IV: uniformly echogenic plaque
Plaque composition is as in type II with numerous calcifications constituting over 50% of the plaque structure (Fig. 9).
Fig. 9.
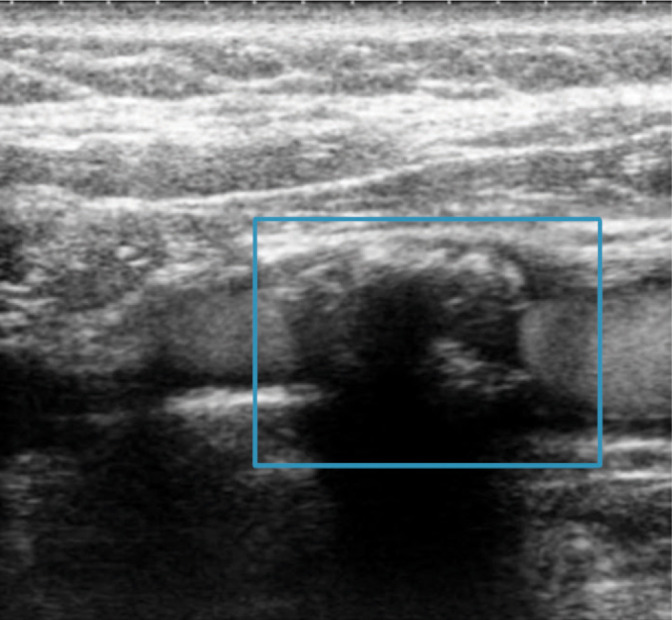
Type IV plaque; ICA flow with visible contrast enhancement. Authors’ own material
Difficulties are similar to those in type III plaques with a significant increase in the number of reverberations.
The classification of plaques to type IV is difficult based on subjective assessment because of potential irregular distribution of echogenic areas that prevent imaging.
Type (class) V: heavy calcification
In this case, the evaluation of echogenicity, which in practice means the number of calcifications, is impossible on B-Mode and Doppler imaging.
Any attempts to assess plaque surface in this type of plaque are feasible only with specialized techniques: STIC B-Flow (spatiotemporal image correlation using the B-Flow technique) (Fig. 10 B), CEUS (Fig. 10 C) and possibly also intravascular ultrasound (IVUS) as well as volumetric ultrasound. Numerous reverberations prohibit plaque structure assessment and evaluation of the borders of its individual elements. Fibrous cap imaging in type V plaques is almost infeasible (Fig. 10).
Fig. 10.
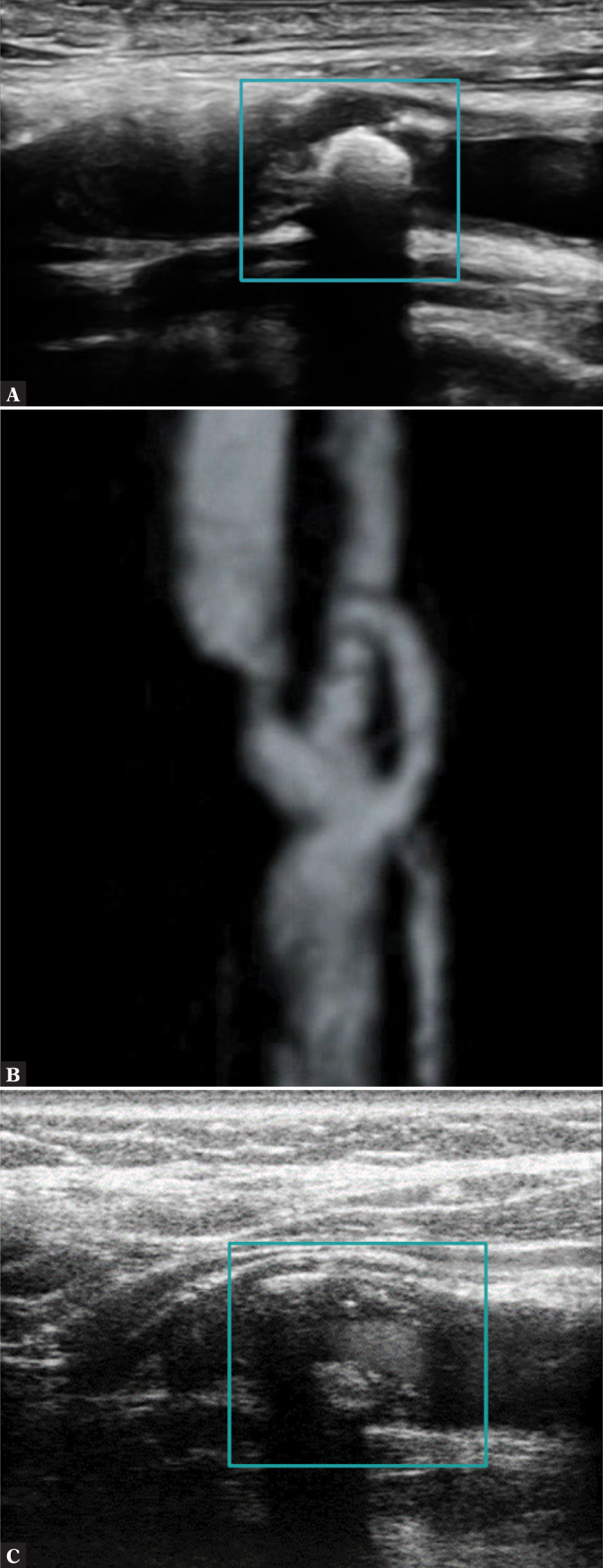
Completely calcified plaque. A. Acoustic shadow covers the field of view; B. B-Flow STIC imaging. C. CEUS. Author’s own material
The visualization of the arterial wall of pre-cranial arteries is an integral part of an ultrasound examination. This includes wall structure assessment with the measurement of the intima-media (IM) thickness, i.e. the total thickness of the tunica intima and tunica media. This measure is abbreviated as IMT. The IM complex is described(24) as a linear structure visible on both arterial walls (CCA, ICA, ECA) that can be imaged simultaneously in the longitudinal view. On ultrasound, it presents as a linear echogenic region that begins at the border of the vividly hypoechoic vascular lumen and reaches the hypoechoic linear area between the adventitia and tunica media. In physiological conditions, the intima-media thickness ranges from 0.5 to 0.9 mm(10,11,25) (Fig. 11).
Fig. 11.
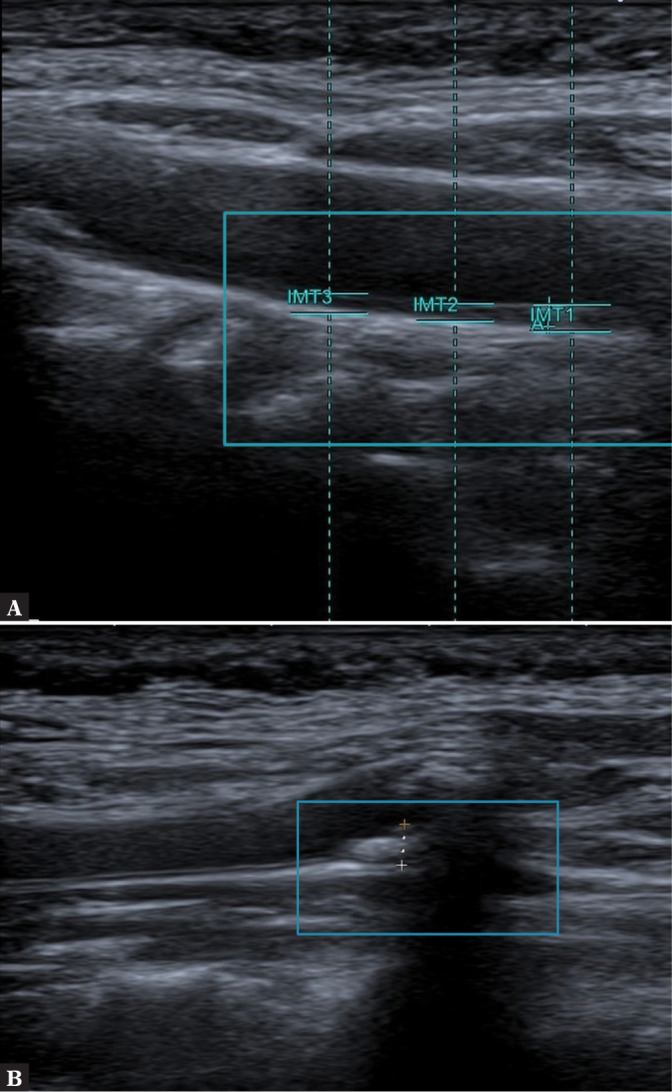
Measurements. A. IMT; B. VWT. Author’s own material
IMT is assessed by taking measurements in strictly specified points in individual arteries and then by calculating the average value (in proximal, medial and distal segments of the CCA and ICA and in the CCA bifurcation as well as, if needed, in the ECA and, when high-end ultrasound equipment is available, also in the vertebral artery). Alternatively, IMT can be calculated in the examined segment of the artery using an IMT-dedicated tool integrated with the ultrasound calculation software (Fig. 12 A).
Fig. 12.
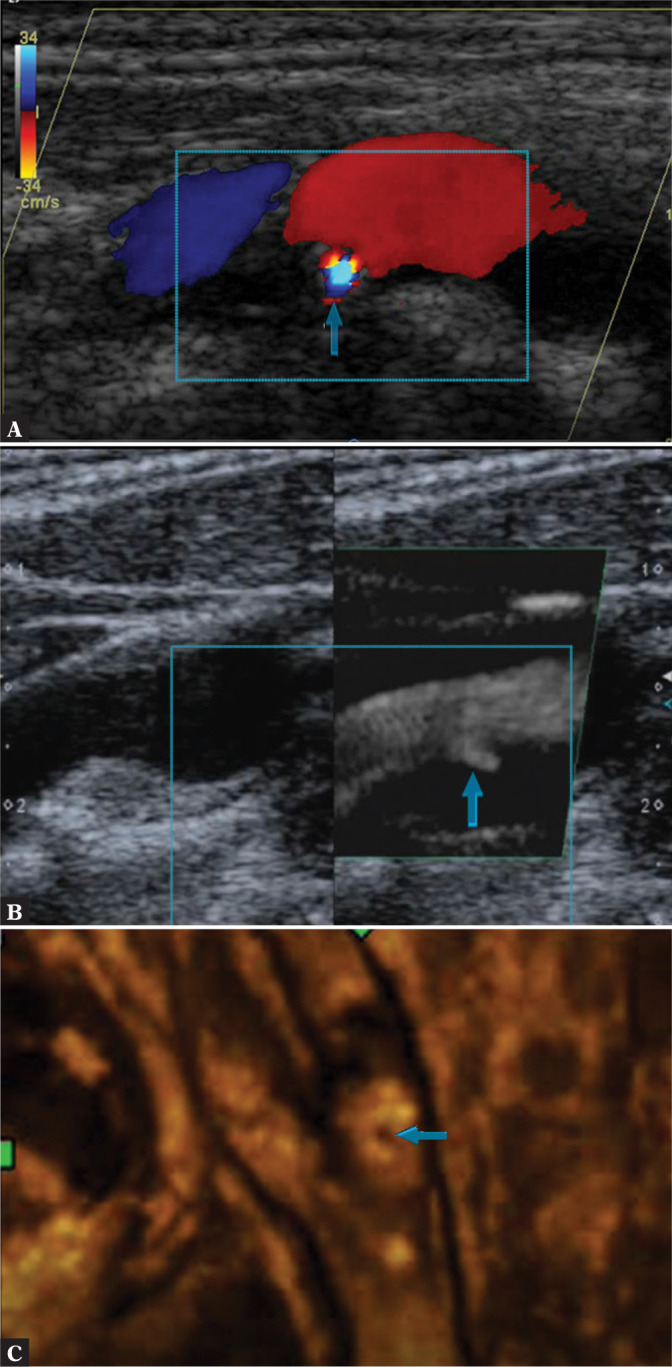
Plaque ulceration. A. CFM image. B. SMI. C. Volumetric examination – surface rendering. Authors’ own material
In normal conditions, when there is no atherosclerotic plaque, the arterial wall is clearly visible on a 2D (B-Mode) examination.
In multicenter ECST (European Carotid Surgery Trial) and NASCET (North American Symptomatic Carotid Endarerectomy Trial) trials(7), whose goal was to establish the eligibility of patients to endarterectomy (the criterion of plaque stability was not taken into account in these trials), the fundamental parameters defining the atherosclerotic plaque were, next to IMT: the direction of plaque modeling and the total plaque size expressed as VWT (vessel wall thickness). This parameter is used to monitor treatment effects and assess the size of atherosclerotic plaque. It is also used when IMT is over 2 mm(26) (Fig. 11 B).
The term VWT(7,27) was first used in studies on atherosclerotic plaques at the time when a vessel was considered a rigid “pipe,” where flow was evaluated as only a physical image of fluid rheological changes with no consideration devoted to dynamic changes in the walls of the described “pipe” and without taking into account any oscillation disorders or laminar flow. The wall thickness was the only parameter measured, and only when the value was significantly increased was the change described as atherosclerotic plaque.
In the 1970s, a relationship was found between the presence of defects on the surface of atherosclerotic plaque (irregularities, fissures, ulcerations) and CVAs (Clark, Koch, Constantinides)(28,29). It was concluded that the presence of changes (irregularity or ulceration) on the plaque surface indicates potential plaque instability and its greater susceptibility to damage (vulnerable plaque)(30). It was also stated that the presence of ulceration on the plaque surface is linked with the development of necrotic changes in the plaque lipid core that cause deformity and damage of the fibrous cap, which in turn leads to the transformation of stable plaque into vulnerable plaque(31).
In most of the available literature, authors mention difficulties linked with adequate assessment of defects on the surface of vascular endothelium (continuity defects). They prohibits the visualization of laminar blood flow directly adjacent to plaque structures. Laminar flow disorders cause thrombocyte adhesion at the site of the endothelial defect, thus initiating a cascade of pathologies leading to CVA.
In accordance with contemporary statements(32–35), plaque surface irregularity should be distinguished from its ulceration. Ulceration is defined as a defect on the plaque surface measuring at least 2×2 mm and reaching the nearest visible IM complex. Smaller defects should be treated as irregularities, which are changes of lower clinical relevance.
Ulceration, i.e. plaque erosion, is described as an “acute,” abruptly developing thrombus, found directly adjacent to the intima media in the vessel wall with no endothelium(16,18,36) (Fig. 12).
On ultrasound, this form of lesion presents as a hypoechoic intrusion on the plaque surface; its echogenicity depends on the size of the “fresh” thrombotic component. With thrombocytes being predominant, this component appears clearly hypoechoic. The image becomes slightly less hypoechoic when there are more collagen fibers making up the clot matrix; it may even appear iso-/normoechogenic in relation to the remaining plaque structures. The image can be echogenic when fibrous elements prevail. This structure is defined by some authors(19,37,38) as JBA (juxtaluminal black area) or JHA (juxtaluminal hypoechoic area) (Fig. 7)(39,40).
As for ulceration, its atypical position in relation to atherosclerotic plaque, usually at the margin of the plaque, and the presence of marginal calcification at the edges of ulceration, are characteristic features(41–43) (Fig. 13).
Fig. 13.
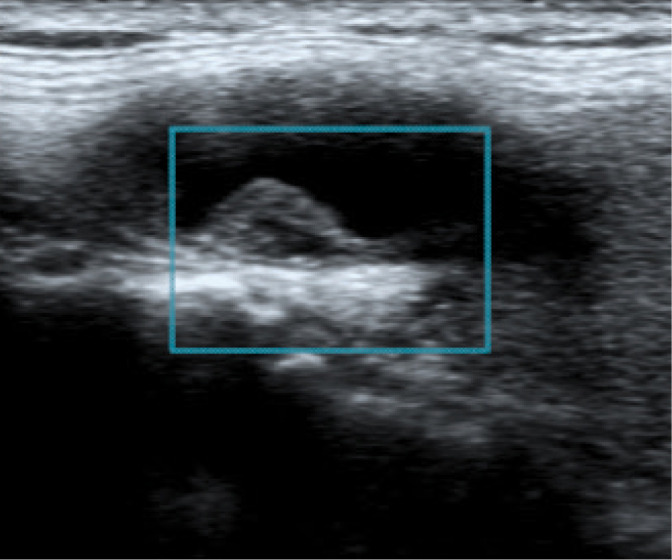
Atherosclerotic plaque with smooth surface GWN IIIa. Authors’ own material
Plaque ulceration is a much more frequent cause of thrombi than plaque rupture (74% of cases: ulceration and its complications; 40% of cases: rupture and its complications)(11). Attention must be paid to the fact that plaque ulceration and its complications are considered a cause of approximately 20% of sudden deaths from cerebrovascular accidents and cardiovascular events.
The ultrasound appearance of plaque surface (irregularity or ulceration) helps identify its instability. This way, it supplements the description of plaque echogenicity in the Gray-Weale–Nicolaides classification, and is described with letters as:
Fig. 14.
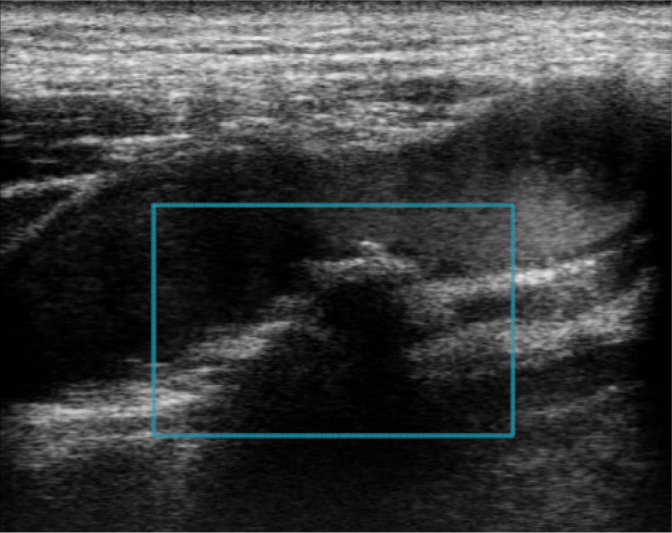
Atherosclerotic plaque with irregular surface GWN IIIb. Authors’ own material
Fig. 15.
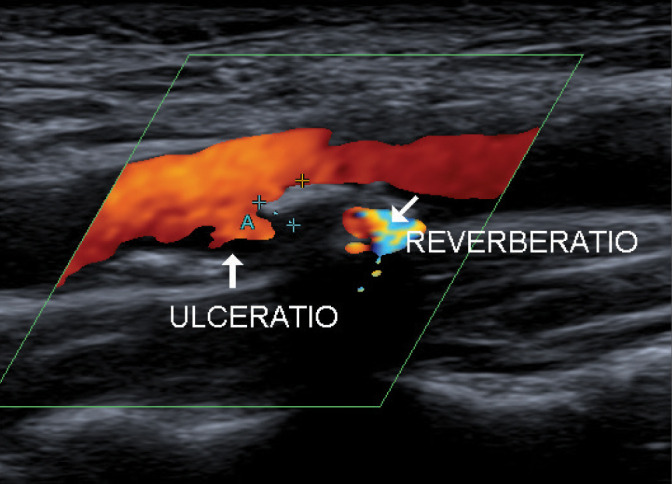
Atherosclerotic plaque with ulceration GWN IIIc. Authors’ own material
For instance, the plaque from Fig. 16 with calcification of over 50% of the plaque volume and with the irregular surface should be described as type IIIb plaque in the Gray-Weale–Nikolaides classification.
Fig. 16.
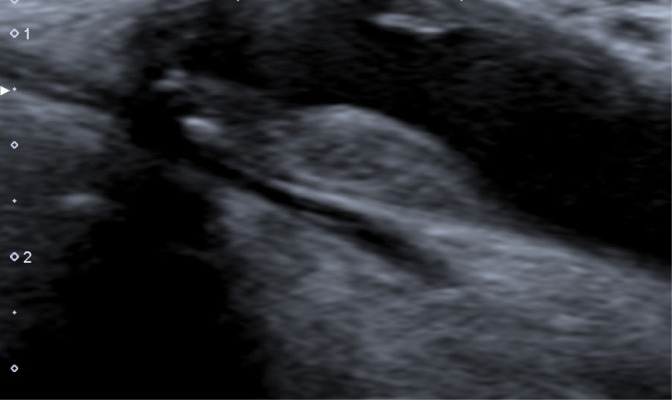
Type IIIb plaque in the Gray-Weale–Nikolaides classification. Authors’ own material
Conclusion
Irrespective of the ultrasound technique employed, proper morphological evaluation of atherosclerotic plaque by means of subjective observation of echogenicity with classification to Gray-Weale–Nicolaides types as well as assessment of the integrity of plaque surface, makes it possible to roughly evaluate plaque morphology and thereby its stability.
The contemporary techniques make this classification more objective. Computer software enables assessment and measurement of the plaque with the calculation of mean percentage values regarding the size of echogenic zones that correspond with plaque containing a greater number of fibrous or calcified structures.
Moreover, other ultrasound-based plaque classifications are appearing, taking into account both individual images of the plaque surface and plaque echogenicity. However, they are all derived from the original GWN classification(44).
The subjective assessment (visual scoring) should therefore be supplemented with the grey-scale median (GSM) evaluation, representing median tonal distribution of pixels in a scale from 0 (black) to 255 (white). On ultrasound, fluid (blood) corresponds with the lowest values, ranging from 0 to 5 in the GSM, while the highest values, from 180 to 200 in the GSM, represent solid tissues (adventitia)(18,24,45). Assigning the values to the observed parts of the plaque should be performed using as broad region of interest (ROI) as possible, i.e. the largest possible region encompassing the examined structure as allowed by the transducer.
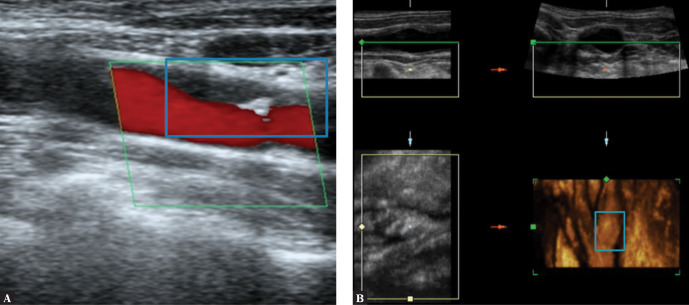
Attention should be paid to the need for further, multidirectional diagnosis of atherosclerotic plaque in high-risk patients, including volumetric (spatial) imaging examinations(8,11,20), contrast-enhanced ultrasound(14,46–49) (Fig. 17) or strain and shear wave elastography(50,51) (Fig. 18).
Fig. 17.
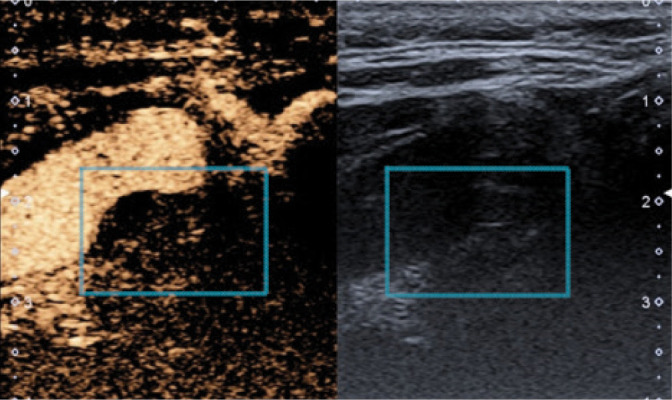
Atherosclerotic plaque, CEUS. Authors’ own material
Fig. 18.
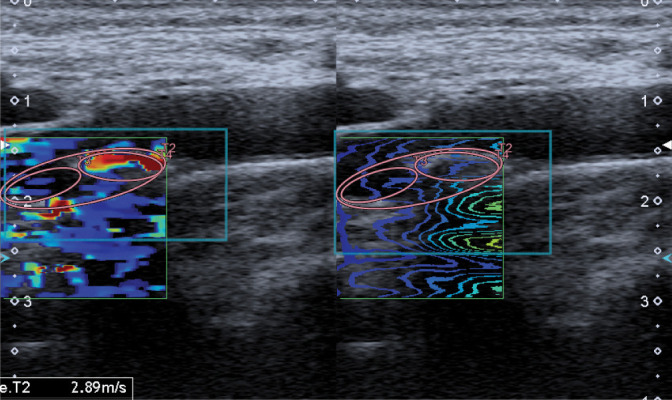
Atherosclerotic plaque, SWE. Authors’ own material
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
References
- 1.Grabowska-Fudala B, Jaracz K, Górna K: Zapadalność, śmiertelność i umieralność z powodu udarów mózgu – aktualne tendencje i prognozy na przyszłość. Przegl Emidemiol 2010; 64: 439–442. [PubMed] [Google Scholar]
- 2.The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration WHO MONICA Project Principal Investigators. J Clin Epidemiol 1988; 41: 105–114. [DOI] [PubMed] [Google Scholar]
- 3.de Bray JM: Consensus concerning the morphology and the risk of carotid plaques. Cerebrovasc Dis 1997; 7: 289–296. [Google Scholar]
- 4.Li J, Mi D, Pu Y, Zou X, Pan Y, Soo Y et al. : Comparison of carotid atherosclerotic plaque characteristics between patients with first-time and recurrent acute ischaemic stroke using B-mode ultrasound. Neurol Res 2015: 1–5. [DOI] [PubMed] [Google Scholar]
- 5.Nyman E, Vanoli D, Näslund U, Grönlund C: Inter-sonographer reproducibility of carotid ultrasound plaque detection using Mannheim consensus in subclinical atherosclerosis. Clin Physiol Funct Imaging 2020; 40: 46–51. [DOI] [PubMed] [Google Scholar]
- 6.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N et al. : Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007; 23: 75–80. [DOI] [PubMed] [Google Scholar]
- 7.North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 8.Skagen K, Skjelland M, Zamani M, Russell D: Unstable carotid artery plaque: new insights and controversies in diagnostics and treatment. Croat Med J 2016; 57: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinjikji W, Rabinstein AA, Lanzino G, Murad MH, Williamson EE, DeMarco JK et al. : Ultrasound characteristics of symptomatic carotid plaques: a systematic review and meta-analysis. Cerebrovasc Dis 2015; 40: 165–174. [DOI] [PubMed] [Google Scholar]
- 10.Casscells W, Naghavi M, Willerson JT: Vulnerable atherosclerotic plaque: a multifocal disease. Circulation 2003; 107: 2072–2075. [DOI] [PubMed] [Google Scholar]
- 11.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J et al. : From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation 2003; 108: 1772–1778. [DOI] [PubMed] [Google Scholar]
- 12.Constantinides P: Cause of thrombosis in human atherosclerotic arteries. Am J Cardiol 1990; 66: 37G–40G. [DOI] [PubMed] [Google Scholar]
- 13.Saba L, Saam T, Jäger HR, Yuan C, Hatsukami TS, Saloner D et al. : Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019; 18: 559–572. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt C, Fischer T, Rückert RI, Oberwahrenbrock T, Harms L, Kronenberg G et al. : Identification of neovascularization by contrast-enhanced ultrasound to detect unstable carotid stenosis. PLoS One 2017; 12: e0175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Stefano R, Felice F, Balbarini A: Angiogenesis as risk factor for plaque vulnerability. Curr Pharm Des 2009; 15: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 16.Kolodgie FD, Burke AP, Farb A, Gold HK, Tuan J, Narula J et al. : The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 2001; 16: 285–292. [DOI] [PubMed] [Google Scholar]
- 17.Badimon L, Vilahur G: Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med 2014; 276: 618–632. [DOI] [PubMed] [Google Scholar]
- 18.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS et al. : Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003; 349: 2316–2325. [DOI] [PubMed] [Google Scholar]
- 19.Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ et al. : Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation 2010; 121: 1941–1950. [DOI] [PubMed] [Google Scholar]
- 20.Kume S, Hama S, Yamane K, Wada S, Nishida T, Kurisu K: Vulnerable carotid arterial plaque causing repeated ischemic stroke can be detected with B-mode ultrasonography as a mobile component: Jellyfish sign. Neurosurg Rev 2010; 33: 419–430. [DOI] [PubMed] [Google Scholar]
- 21.Ota H, Tu W, Underhill HR, Oikawa M, Dong L, Zhao X et al. : Hemorrhage and large lipid-rich necrotic cores are independently associated with thin or ruptured fibrous caps: an in vivo 3T MRI study. Arterioscler Thromb Vasc Biol 2009; 29: 1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinjikji W, Huston J 3rd, Rabinstein AA, Kim GM, Lerman A, Lanzio G: Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016; 124: 27–42. [DOI] [PubMed] [Google Scholar]
- 23.Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS et al. : Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 2004; 292: 1845–1852. [DOI] [PubMed] [Google Scholar]
- 24.Simova I: Intima-media thickness: appropriate evaluation and proper measurement, described. E-J Cardiol Pract 2015; 13, 21. [Google Scholar]
- 25.Balta S, Aparci M, Ozturk C, Yildirim AO, Demir M, Celik T: Carotid intima media thickness and subclinical early atherosclerosis. Int J Cardiol 2016; 203: 1146. [DOI] [PubMed] [Google Scholar]
- 26.Porsche C, Walker L, Mendelow AD, Birchall D: Assessment of vessel wall thickness in carotid atherosclerosis using spiral CT angiography. Eur J Vasc Endovasc Surg 2002; 23: 437–440. [DOI] [PubMed] [Google Scholar]
- 27.Choi GPT, Chen Y, Lui LM, Chiu B: Conformal mapping of carotid vessel wall and plaque thickness measured from 3D ultrasound images. Med Biol Eng Comput 2017; 55: 2183–2195. [DOI] [PubMed] [Google Scholar]
- 28.Blaser T, Hofmann K, Buerger T, Effenberger O, Wallesch CW, Goertler M: Risk of stroke, transient ischemic attack, and vessel occlusion before endarterectomy in patients with symptomatic severe carotid stenosis. Stroke 2002; 33: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 29.Kim DE, Kim JY, Jeong SW, Cho YJ, Park JM, Lee JH et al. : Association between changes in lipid profiles and progression of symptomatic intracranial atherosclerotic stenosis: a prospective multicenter study. Stroke 2012; 43: 1824–1830. [DOI] [PubMed] [Google Scholar]
- 30.Carra G, Visonà A, Bonanome A, Lusiani L, Pesavento R, Bortolon M et al. : Carotid plaque morphology and cerebrovascular events. Int Angiol 2003; 22: 284–289. [PubMed] [Google Scholar]
- 31.Aly S, Bishop CC: An objective characterization of atherosclerotic lesion: an alternative method to identify unstable plaque. Stroke 2000; 31: 1921–1924. [DOI] [PubMed] [Google Scholar]
- 32.Artas H, Okcesiz I: Three-dimensional ultrasonographic evaluation of carotid artery plaque surface irregularity. Arch Med Sci 2019; 16: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nonin S, Iwata S, Sugioka K, Fujita S, Norioka N, Ito A et al. : Plaque surface irregularity and calcification length within carotid plaque predict secondary events in patients with coronary artery disease. Atherosclerosis 2017; 256: 29–34. [DOI] [PubMed] [Google Scholar]
- 34.Virmani R, Burke AP, Farb A, Kolodgie FD: Pathology of the unstable plaque. Prog Cardiovasc Dis 2002; 44: 349–356. [DOI] [PubMed] [Google Scholar]
- 35.Virmani R, Burke AP, Kolodgie FD, Farb A: Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J Interv Cardiol 2003; 16: 267–272. [DOI] [PubMed] [Google Scholar]
- 36.Zhou D, Li J, Liu D, Ji LY, Wang NQ, Deng J et al. : Irregular surface of carotid atherosclerotic plaque is associated with ischemic stroke: a magnetic resonance imaging study. J Geriatr Cardiol 2019; 16: 872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennerici MG: The unstable plaque. Cerebrovasc Dis 2004; 17 Suppl 3: 17–22. [DOI] [PubMed] [Google Scholar]
- 38.Homburg PJ, Rozie S, van Gils MJ, Jansen T, dr Weert TT, Dippel DW et al. : Atherosclerotic plaque ulceration in the symptomatic internal carotid artery isassociated with nonlacunar ischemic stroke. Stroke 2010; 41: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 39.Ohyama H, Mizushige K, Takahashi T, Hosomi N, Kohno M: Plaque rupture on the carotid artery observed by Doppler ultrasonography – a case report. Angiology 2001; 52: 867–869. [DOI] [PubMed] [Google Scholar]
- 40.Paraskevas KI, Veith FJ, Spence JD: How to identify which patients with asymptomatic carotid stenosis could benefit from endarterectomy or stenting. Stroke Vasc Neurol 2018; 3: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dweck MR, Fayad ZA: Multitarget vulnerable plaque imaging. Circ Cardiovasc Imaging 2017; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafailidis V, Chryssogonidis I, Tegos T, Kouskouras K, Charitanti-Kouridou A: Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging 2017; 8: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rafailidis V, Chryssogonidis I, Xerras C, Nikolau I, Tegos T, Kouskouras K et al. : A comparative study of color Doppler imaging and contrast-enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. Eur Radiol 2019; 29: 2137–2145. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Zhang Y, Meng L, Abbott D, Qian M, Wong KKL et al. : Evaluation of carotid plaque echogenicity based on the integral of the cumulative probability distribution using gray-scale ultrasound images. PLoS One 2017; 12: e0185261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Östling G, Persson M, Hedblad B, Gonҫalves I: Comparison of grey scale median (GSM) measurement in ultrasound images of human carotid plaques using two different softwares. Clin Physiol Funct Imaging 2013; 33: 431–435. [DOI] [PubMed] [Google Scholar]
- 46.Feinstein SB: Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol 2006; 48: 236–243. [DOI] [PubMed] [Google Scholar]
- 47.Saha SA, Gourineni V, Feinstein SB: The use of contrast-enhanced ultrasonography for imaging of carotid atherosclerotic plaques: current evidence, future directions. Neuroimaging Clin N Am 2016; 26: 81–96. [DOI] [PubMed] [Google Scholar]
- 48.van den Oord SC, Akkus Z, Bosch JG, Hoogi A, ten Kate GL, Renaud G et al. : Quantitative contrast-enhanced ultrasound of intraplaque neovascularization in patients with carotid atherosclerosis. Ultraschall Med 2015; 36: 154–161. [DOI] [PubMed] [Google Scholar]
- 49.Varetto G, Gobello L, Castagno C, Quaglino S, Ripepi M, Benintende E et al. : Use of contrast-enhanced ultrasound in carotid atherosclerotic disease: limits and perspectives. Biomed Res Int 2015; 293163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naim C, Cloutier G, Mercure E, Destrempes F, Qin Z, El-Abyad W et al. : Characterisation of carotid plaques with ultrasound elastography: feasibility and correlation with high-resolution magnetic resonance imaging. Eur Radiol 2013; 23: 2030–2041. [DOI] [PubMed] [Google Scholar]
- 51.Ramnarine KV, Garrard JW, Kanber B, Nduwayo S, Hartshorne TC, Robinson TG: Shear wave elastography imaging of carotid plaques: feasible, reproducible and of clinical potential. Cardiovasc Ultrasound 2014; 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]