Abstract
Antipsychotic (AP) medications are the mainstay for the treatment of schizophrenia spectrum disorders (SSD), but their efficacy is unpredictable and widely variable. Substantial efforts have been made to identify prognostic biomarkers that can be used to guide optimal prescription strategies for individual patients. Striatal regions involved in salience and reward processing are disrupted as a result of both SSD and cannabis use, and research demonstrates that striatal circuitry may be integral to response to AP drugs. In the present study, we used functional magnetic resonance imaging (fMRI) to investigate the relationship between a history of cannabis use disorder (CUD) and a striatal connectivity index (SCI), a previously developed neural biomarker for AP treatment response in SSD. Patients were part of a 12-week randomized, double-blind controlled treatment study of AP drugs. A sample of 48 first-episode SSD patients with no more than 2 weeks of lifetime exposure to AP medications, underwent a resting-state fMRI scan pretreatment. Treatment response was defined a priori as a binary (response/nonresponse) variable, and a SCI was calculated in each patient. We examined whether there was an interaction between lifetime CUD history and the SCI in relation to treatment response. We found that CUD history moderated the relationship between SCI and treatment response, such that it had little predictive value in SSD patients with a CUD history. In sum, our findings highlight that biomarker development can be critically impacted by patient behaviors that influence neurobiology, such as a history of CUD.
Keywords: schizophrenia, cannabis, functional magnetic resonance imaging, biomarker, antipsychotics
Introduction
Antipsychotic drug (AP) treatment is the mainstay of treatment for patients with schizophrenia spectrum disorders (SSD), but clinical response to these medications is variable and unpredictable.1,2 Identifying effective treatments often depend on trial and error, which can delay clinical improvement for patients. Thus, the identification of biomarkers that can predict antipsychotic treatment response is critically needed. Biomarkers based on data derived from brain imaging have recently shown considerable promise in estimating individual clinical outcome.3,4 Magnetic resonance imaging (MRI) studies have found neuroanatomical differences such as structural morphology,5 volume,6,7 ventricle size,8 and cortical thickness,9,10 as well as differences in functional connectivity11–13 to be associated with likelihood of AP response.
However, such efforts have not yet considered the heterogeneous nature of this patient population and how individual characteristics or lifestyle patterns may impact the utility of such biomarkers. For example, cannabis use among patients with SSD is disproportionate relative to healthy individuals.14,15 The effects of cannabis on the neural circuitry implicated in clinical improvement has been largely ignored in SSD patients despite evidence that it influences these brain regions in healthy individuals. Without this knowledge, the prognostic accuracy of biomarkers may not be generalizable to the substantial number of patients who use cannabis prior to AP treatment. This study aims to examine the extent to which a history of cannabis use disorder (CUD) impacts a previously established neural biomarker of treatment response in first-episode SSD patients.
Compared to the general population, patients with SSD have a 10-fold increased risk of CUD,14 an illness characterized as a pattern of repeated cannabis use that results in harmful consequences leading to significant distress or functional impairment.16 A meta-analysis of the epidemiology of CUD in SSD patients (N = 5572 from 35 studies) reports that 44.4% of first-episode SSD populations will meet DSM-V criteria for CUD in their lifetime and that 28.6% meet criteria for current CUD.17 Moreover, patients with CUD evidence significantly worse outcomes than patients without a comorbid diagnosis, including symptom exacerbation, increased likelihood of psychotic relapse,18,19 medication non-adherence and poorer global functioning.20–23
The neural mechanisms relating cannabis use to poorer prognosis in SSD patients are poorly understood. However, abnormalities in the striatum, a neural structure involved in reward and salience processing that contains a substantial quantity of dopamine receptors,24,25 have long been acknowledged to play a prominent role in both psychotic symptomology and addiction.26–28 Theories of SSD etiology propose that an aberrant striatal dopaminergic system contributes to psychosis and have been supported by neuroimaging studies that demonstrate SSD to be associated with hyperactive dopamine activity in striatal regions coupled with hypoactive dopamine activity in the prefrontal cortex.29–32 Similarly, models of drug addiction propose a dysfunctional striatal dopamine system in which there is reduced functional connectivity between the pleasure-seeking striatum with the prefrontal cortex’s behavioral and cognitive control centers. Attenuated connectivity between these regions is hypothesized to make individuals susceptible to the overuse of pleasure-inducing substances.33
Notably, efforts to develop imaging-based biomarkers of antipsychotic treatment response have targeted the striatum. For example, using data derived from resting-state functional magnetic resonance imaging (rs-fMRI), our group reported that a baseline striatal connectivity index (SCI), a measure of the strength of striatum’s connectivity with other neural regions, could be used as a biomarker to distinguish responders from nonresponders to antipsychotic treatment response in 2 cohorts of SSD patients. Specifically, Sarpal et al34 found that antipsychotic treatment response was associated with lower baseline functional SCI. This is consistent with other studies that have found the striatum to be involved with treatment outcomes. For example, a longitudinal positron emission tomography study (PET) of 29 patients with SSD demonstrated that greater resting cerebral blood flow in the ventral striatum was associated with a subsequent reduction in psychotic symptoms.35 Additionally, treatment responders have been found to exhibit elevated striatal dopamine synthesis and release, whereas treatment-resistant patients do not show such dopamine alterations in the striatum. These results suggest that APs may be effective by way of stabilizing striatal dopaminergic aberrancies36 and support targeting the striatum in the development of biomarkers for treatment response.
Cannabis has also been demonstrated to impact the striatum in both animal and human studies. fMRI research demonstrates that in nonpsychotic samples, both acute inductions of cannabis and chronic use are associated with decreased resting-state functional connectivity in neural pathways known to be impaired in SSD patients, specifically between the dorsal striatum and prefrontal cortical areas (eg, dorsolateral prefrontal cortex, or DLPFC).37 Acute inductions of cannabis cause an increase in dopamine release and striatal neuronal firing38–40 while chronic cannabis use is linked to blunted striatal dopamine release.41,42 Although few studies have examined cannabis use in SSD populations, cross-sectional structural MRI studies demonstrate that cannabis-using SSD patients exhibit reduced gray matter in striatal regions.43–46 The sole longitudinal structural MRI study to track SSD patients over a 5-year period showed that cannabis users evidenced greater gray matter volume reductions and ventricle enlargement than nonusers.47
Taken together, evidence suggests that cannabis may influence neural processes related to treatment response in SSD and underscores the importance of research that accounts for cannabis use in the development of neural biomarkers to ensure their prognostic accuracy. To our knowledge, no functional magnetic resonance imaging (fMRI) studies have examined the impact of CUD on the striatal connectivity underlying antipsychotic treatment response. Thus, in this study, we used rs-fMRI to investigate the relationship between a putative striatal biomarker for AP treatment response and a history of CUD in patients experiencing their first episode of SSD. We hypothesized that CUD history would attenuate the relationship between baseline striatal connectivity and treatment outcome, given the impact of cannabis on the striatum.
Methods
Participants
The sample was comprised of 48 first-episode SSD (schizophrenia, schizophreniform disorder, schizoaffective disorder, or psychotic disorder not otherwise specified) patients who were part of a 52-week double-blind randomized controlled treatment study of second-generation AP drugs (risperidone or aripiprazole)48; the present analysis only includes data from the first 12 weeks of treatment, the acute treatment phase. Forty-one patients of the present sample were previously used in the original research that developed our putative biomarker of interest34; the additional 7 patients included here were part of the same study but had data that was unavailable at the time the prior manuscript34 was published.
Patients had no more than 2 weeks of cumulative lifetime exposure to AP medications and met the following inclusion criteria based on our previous first-episode SSD research: (1) DSM-IV criteria for schizophrenia, schizophreniform disorder, schizoaffective disorder or psychotic disorder NOS; (2) Current positive symptoms rated ≥4 (moderate) on at least one of the following Brief Psychiatric Rating Scale- Anchored Version (BPRS-A)49 items: hallucinatory behavior, unusual thought content, grandiosity or conceptual disorganization; (3) Within age 16–40; (4) Competent to provide informed consent; and (5) Negative pregnancy test. Patients were excluded if any of the following criteria were present: (1) Neurological or endocrine disorder; (2) Any medical condition requiring medication with known psychotropic effects; (3) Suicidal or homicidal behavior risk; (4) Cognitive or language impairments, or other factors compromising informed consent; (5) Contraindications to risperidone or aripiprazole monotherapy; (6) MRI contraindications (eg, pacemaker, claustrophobia); and (7) Presence of substance-induced psychosis as defined by DSM-IV.
Diagnosis
Patients completed the Structured Clinical Interview for DSM-IV (SCID-IV)50 to confirm they were diagnostically eligible for the study and to evaluate lifetime history of CUD, as well as other substance use disorders. In order to rule out a diagnosis of substance-induced psychosis, patients were required to have a 1-month period in which psychotic symptoms were present without the influence of substance intoxication or withdrawal. The Brief Psychiatric Rating Scale-A49 and the Clinical Global Impressions Scale (CGI)51 were used to index clinical improvement of psychotic symptoms and were administered weekly during the initial 4 weeks of treatment, and biweekly for the remaining 8 weeks of the acute treatment phase. A resting-state fMRI scan was conducted at baseline of AP treatment.
Treatment Response
Treatment response was based on prior work in first-episode patients49 and defined as a binary (response/nonresponse) variable defined a priori as achieving 2 consecutive ratings during the 12-week treatment period that fulfilled the following criteria: (1) a rating of 3 (mild) or less on all of the following items of the BPRS: unusual thought content, hallucinatory behavior, grandiosity, and conceptual disorganization; and (2) CGI improvement rating “very improved” or “much improved.”
Resting-State fMRI Image Acquisition and Preprocessing
A GE 3-T scanner was used to collect 5-minute resting-state functional scans on each patient (150 whole-brain volumes). Participants were asked to close their eyes and instructed not to think of anything in particular. They were spoken to between scan sequences to ensure they were awake. Methods for fMRI image acquisition, preprocessing and motion correction have been previously described in Sarpal et al34 and are included in Supplementary Materials.
Functional Connectivity Analyses
The methodology to develop the SCI is adapted from the original Sarpal et al. manuscript34; the same process was used to calculate the SCI for the present analyses. The rationale for our functional connectivity method is that this is the methodology we have used for previous functional connectivity analyses.34,52 As our prior data was based upon this, and we were aiming to demonstrate the influence of cannabis on the utility of this metric, we elected to use this methodology in the current manuscript. The original SCI was developed using a sample of 41 first-episode psychosis patients. The aim was to establish a biomarker of treatment response based on intrinsic functional connectivity between striatal subregions and the rest of the brain.
For the original SCI development, a seed-based approach was applied to the striatum utilizing the methods of Di Martino et al.53 Using their coordinates, 3.5-mm spherical regions of interest (ROI) were created, bilaterally, in the dorsal caudate, ventral caudate, nucleus accumbens, dorsal rostral putamen, dorsal caudal putamen, and ventral rostral putamen. Once these ROIs were defined, the mean time course of resting-state blood-oxygen-level-dependent activity was extracted from each seed region. Whole-brain voxel-wise correlation maps for each ROI were created with the extracted waveform as a reference. The Fisher z-transformation was applied to the subsequent correlation maps.
The striatal connectivity z maps were used to develop the SCI using the following steps: (1) for each voxel located within gray matter (181 144 voxels total), the corresponding connectivity strength for each patient was entered into a univariate Cox regression analysis along with clinical outcome (response or nonresponse), and time to outcome or study dropout (in weeks); (2) entering the z scores for each voxel from this analysis in Montreal Neurological Institute standard brain space to generate whole-brain maps; and (3) performing one-sample t-tests at the group level on these maps for each ROI. Though it is typical in case-control studies of psychiatric illness to use Bonferroni-style corrections on functional neuroimaging data, the goal was to capture the greatest amount of treatment-related variance for the potential biomarker; thus, a threshold of P < .005 was used for the analysis of the data set. A total of 91 functional connections across the 12 input ROIs predicted treatment response, which are included in the Supplementary Material of Sarpal et al.34
The SCI was then calculated using data from the 91 predictive striatal functional connections. In order to minimize overfitting and reduce circularity, the data from the first-episode psychosis patients was normalized using data from age- and-sex matched healthy participants. To do so, raw correlational values of the 91 predictive striatal functional connections were extracted from connectivity maps of every participant in both a healthy control group and the first-episode psychosis cohort. The mean and standard deviation were calculated on correlational values at each of the 91 functional connections from the healthy control sample and used to z-transform the patient data. Then a principal-components analysis was conducted on the extracted functional correlational values from the healthy volunteer sample and the first principal component was calculated. Loadings onto the first principal component across all 91 functional connections were applied to connectivity values in the first-episode cohort. For every patient, the sum of the products of every correlational value and loading onto the first principal component was calculated. This value was called the SCI, representing the expression of the first principal component of striatal connections of interest from the healthy volunteer cohort. The association between SCI and outcome was also replicated in an independent generalizability cohort of patients with chronic psychosis who were undergoing AP drug treatment. In the present analyses, we used these loadings to generate SCI values for our expanded sample.
Analyses
To investigate our hypothesis that CUD history would attenuate the relationship between the previously established prognostic value of SCI and treatment response, we reexamined these data while accounting for a lifetime CUD history. A logistic regression was conducted to examine whether baseline SCI, CUD history (dichotomous), and a SCI and CUD interaction were associated with treatment response, after adjusting for age and sex. Our conclusion will be based on whether there is a statistically significant interaction between SCI and CUD that is associated with treatment response.
Independent-samples t-tests were conducted using SPSS (version 24; IBM Corp.) to compare group differences between patients with and without a lifetime diagnosis of CUD in regards to baseline age, symptom severity (as measured by the BPRS), duration of psychosis, and SCI. Chi-square analyses were performed to evaluate group differences in sex, frequency of treatment response, and medication condition. An independent-samples t-test was conducted to evaluate differences in the SCI between patients with a lifetime history of CUD who had comorbid substance use disorders vs those who did not.
Results
Baseline Characteristics
Based on diagnostic interview, 20 patients met criteria for a lifetime history of CUD (CUD+) and 28 patients had no lifetime history of CUD (CUD−). There were no significant differences between CUD+ and CUD− patients on baseline age, symptom severity, duration of psychosis, SCI, frequency of treatment response, or medication condition. There was a significantly larger amount of males in the CUD+ group relative to the CUD− group. A comparison of patients is displayed in table 1.
Table 1.
A Comparison of Patients With (CUD+) and Without (CUD−) a Lifetime Diagnosis of CUD
| CUD− (N = 28) | CUD+ (N = 20) | Statistics | ||
|---|---|---|---|---|
| Age (SD) | 20.25 (5.49) | 21.35 (4.73) | t = −.72 | P = .47 |
| % Male (N) | 53% (15) | 95% (19) | X2 = 9.70 | P = .002 |
| Duration of untreated psychosis (SD) | 79.93 (121.05) | 135.28 (278.02) | t = −.93 | P = .36 |
| Mean BPRS total score at baseline (SD) | 43.75 (8.58) | 42.85 (7.17) | t = .38 | P = .70 |
| % of Responders (N) | 64.3% (18) | 55% (11) | X2 = .42 | P = .52 |
| % on risperidone (vs aripiprazole) | 50% | 45% | X2 = .12, | P = .73 |
| Mean SCI at baseline (SD) | −.05 (4.32) | .31 (3.02) | t = −.33 | P = .75 |
Note: BPRS, Brief Psychiatric Rating Scale; SCI, Striatal Connectivity Index.
Of the 48 participants, 8 met criteria for at least one other substance use disorder other than CUD. Specifically, 6 participants met criteria for history of an Alcohol Use Disorder, 3 for history of a Stimulant Use Disorder (ie, cocaine, amphetamines), and 1 participant for a Sedative, Hypnotic or Anxiolytic Use Disorder. 2 participants met criteria for more than one substance use disorder. There were no significant differences (t = .77, P = .45) in mean SCI between CUD+ patients who also met criteria for another substance use disorder (Mean (M) = −.33, SD = 3.47), vs those who only met criteria for CUD (M = .74, SD = 2.77).
Interaction Analysis
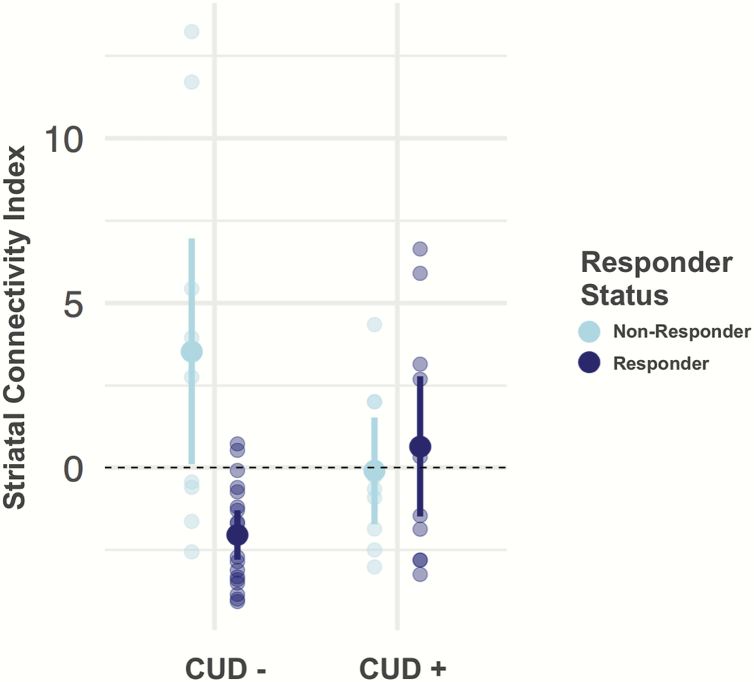
We found evidence for an interaction of SCI and CUD (χ 2 = 16.16, R2 = .39, P = .005, figure 1) to suggest a moderating effect of CUD history on the SCI’s ability to distinguish responders from nonresponders to antipsychotic treatment. Specifically, for CUD− patients, the baseline SCI was more negative in responders compared to nonresponders, a relationship consistent with original findings.34 For CUD+ patients, SCI no longer separated responders from nonresponders.
Fig. 1.
This graph shows the interaction of the striatal connectivity index (SCI) and cannabis use disorder in distinguishing responders from nonresponders to treatment. In patients without a history of cannabis use disorder (CUD) (CUD− = 28), baseline SCI distinguished between responders and nonresponders, results that are consistent with our prior work.34 In patients with a history of CUD (CUD+ = 20), the SCI no longer separated responders from nonresponders. The displayed values represent the mean SCI for each group. Error bars represent 95% confidence intervals and individual values are overlaid. Treatment response was defined based on stringent clinical criterion described in the Methods section.
When the sample was divided between CUD+ and CUD−, the SCI was more accurate at predicting treatment response for CUD− patients. For CUD−, 75% sensitivity and 80% specificity were observed; total accuracy was 78.57%. In contrast, for CUD+, 40% sensitivity and 53.33% sensitivity was achieved; total accuracy was 50%. The difference in sensitivity and specificity between groups was not tested for statistical significance, given the modest sample size.
Discussion
This study found that a lifetime history of CUD moderated the relationship between SCI and treatment response such that it had little predictive value in SSD patients with a CUD history. This is consistent with research that cannabis use influences striatal circuitry, thus impeding its prognostic utility in those patients with a history of CUD. To our knowledge, this is the first study that has investigated the impact of comorbid CUD on a previously developed marker of clinical improvement in patients with SSD.
The reduction of the relationship between the SCI and treatment response as a function of cannabis use is particularly noteworthy, given recent evidence that cannabis use may impede effective clinical response. For example, an observational study of over 2000 first-episode patients found that cannabis use was associated with a greater likelihood of hospital admission, and that this relationship was mediated by the number of different APs prescribed. The authors proposed that a higher number of unique APs may be considered a proxy measure of treatment nonresponse as it indicates there was a clinical judgment of antipsychotic failure, suggesting that cannabis use may be associated with worse clinical prognosis because of its contribution to failed AP treatment.54 Another study that examined the genetic and clinical correlates of treatment-resistant psychosis in 1070 individuals with SSD found an association between cannabis use and greater likelihood of treatment resistance to AP drugs.55 Furthermore, a recent study in mice found that exposure to THC reversed the neurobehavioral effects of risperidone by reducing the brain concentrations of risperidone and its active metabolite, 9-hydroxy risperidone. This reduction was mediated by THC increasing an ABC transporter, P-glycoprotein, which removes risperidone and its metabolite from brain tissue. The findings suggest that THC increases the amount of the compound responsible for metabolizing antipsychotic medications, allowing it less time to be effective.56 Taken together, it suggests an intimate relationship between cannabis, clinical outcomes, and predictors of treatment response that warrants future investigation.
Despite this overlap, it is important to emphasize that our results support conclusions about cannabis’ interference with prognostic utility and do not provide evidence for cannabis impacting the actual likelihood of response. In this sample, there were no differences in response rate between patients with and without a history of CUD. This is inconsistent with research that cannabis users have greater symptom exacerbation.18,19 However, relative to the large-scale studies that have demonstrated a detrimental impact of CUD on treatment, our sample was greatly underpowered to detect effects of CUD on clinical response. Additionally, our study only evaluated the first 12 weeks of treatment, so it may be that differences in treatment trajectory as a function of cannabis use occur at a later point. Future research would also benefit from the inclusion of a posttreatment scan in order to consider the mechanism of response, or normalization of aberrant functional connectivity patterns previously demonstrated after AP treatment.57
Our study also lacked information regarding patient’s duration of CUD and time since remission, and research suggests that neural structure and function may shift over the course of the recovery process. While some studies demonstrate long-lasting neural disruptions following heavy cannabis use,58,59 others suggest that neural recovery can occur following sustained abstinence from cannabis. For instance, hippocampal volume was demonstrated to be reduced in an MRI study of hippocampal integrity in long-term cannabis users, but able to be restored for those who discontinued use.60 Another study that examined cerebral blood volume (CBV) over 28 days of abstinence in chronic cannabis users found that CBV aberrancies normalized with continued abstinence.61 Thus, it may be that even if the prognostic utility of striatal biomarkers is compromised, the cannabis-induced neural changes that might make one vulnerable to treatment resistance may have resolved if they had discontinued use long enough ago, thereby explaining the lack of impact on actual response.
It is also possible that cannabis impacts neural circuitry for some patients and not others based on other neurobiological factors. For example, it has been theorized that there may be subtypes of SSD based on dopaminergic functioning: a hyperdopaminergic type, characterized by elevated striatal dopamine synthesis and release capacity, and a normodopaminergic type that does not show these alterations.36 If this is the case, it could be that the hyperdopaminergic type is more or less sensitive to the dopamine-altering effects of cannabis.
Genetic differences may also contribute to differing dopaminergic responses to cannabis. The dopamine β-hydroxylase (DβH) enzyme transforms dopamine into noradrenaline, and it is theorized that relative to individuals with high activity DBH genotypes, those with low activity DBH genotypes are more sensitive to the cognitive and neural effect of cannabis on the limbic reward network because it induces a hyperdopaminergic state. A sample of 122 regular (nonpsychotic) drug users received acute inductions of cannabis and placebo prior to undergoing a resting-state fMRI to examine functional connectivity between the nucleus accumbens and subcortical regions. An interaction was observed between DBH genotype and the influence of cannabis on functional connectivity; specifically, for individuals with low-activity DBH genotypes only, cannabis reduced functional connectivity between the nucleus accumbens and limbic regions, prefrontal cortex, striatum, and thalamus.62 Thus, it may be that cannabis impacts corticostriatal connectivity only for a certain genetic subset of patients. However, limited research on the acute effects of cannabis has been conducted in SSD samples, so conclusions on how these findings may apply to SSD patients is unknown.
Our study is also limited by the categorical nature of our definition of CUD. While a diagnosis of CUD implies a clinically problematic use of cannabis, individual patterns of cannabis use among patients (eg, frequency/amount of cannabis consumption, duration of CUD, time since last use) may vary significantly among individuals who meet criteria for CUD. Our study lacked information on these variables. It is critical that future research includes this information, as well as a continuous measure of cannabis use to better capture the relationship between cannabis and striatal connectivity. It will also be important to collect information on cannabis use prior to and over the course of antipsychotic treatment to better understand how both past and ongoing cannabis use influences the neural connectivity integral to clinical response. Furthermore, while we were able to consider the potential impact of additional substance use disorders such as alcohol and stimulant use disorders, and did not find meaningful differences in the SCI based on comorbid disorders, we did not have data available on the prevalence of tobacco use in our patients. Tobacco use is highly prevalent in patients with SSD and can also impact the striatal network.63,64 Thus, our study is limited in its ability to exclude the potential effects of tobacco on SCI’s predictive utility. Additionally, there was a significant difference in sex the CUD+ and CUD− groups, in that patients with a history of CUD were predominantly male (19 out of 20 patients). While this is not surprising given prior data from epidemiological studies that demonstrate a higher use of CUD among men,65 it is possible that findings may have been influenced by this imbalance. Future studies should attempt to have a more equal distribution of male and female cannabis users to ensure neural differences are not solely a function of sex.
Despite limitations, this study is the first to use fMRI to investigate the extent to which cannabis impacts the utility of a previously established biomarker. Our conclusions are strengthened by longitudinal study design, and the use of a first-episode sample that all had less than 2 weeks lifetime exposure to AP, which reduces confounds such as long medication exposure and duration of psychosis.
In sum, our findings highlight that biomarker development can be critically impacted by patient behaviors that influence neurobiology, such as substance use. These results are an example of how biomarker utility can be diminished by patient behaviors that are independent of psychiatric diagnosis and underscore the importance of accounting for such characteristics in developing prognostic indices that generalize to heterogeneous patient populations. As the development of biomarkers to predict treatment response becomes an increasingly important priority, it is critical for investigators to consider the influence of cannabis on neural circuitry, thereby ensuring that biomarkers are indeed generalizable to the ~35% of SSD patients that use cannabis during AP treatment.66,67
Funding
Supported by National Institute of Mental Health (grants P50MH080173 and R01MH108654 to A.K.M.).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Leucht S, Cipriani A, Spineli L, et al. . Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. [DOI] [PubMed] [Google Scholar]
- 2. Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50(11):898–911. [DOI] [PubMed] [Google Scholar]
- 3. Winkelbeiner S, Leucht S, Kane JM, Homan P. Evaluation of differences in individual treatment response in Schizophrenia spectrum disorders. JAMA Psychiatry. 2019; 76(10):1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Homan P, Argyelan M, Fales CL, et al. . Striatal volume and functional connectivity correlate with weight gain in early-phase psychosis. Neuropsychopharmacology. 2019;44(11):1948–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Homan P, Argyelan M, DeRosse P, et al. . Structural similarity networks predict clinical outcome in early-phase psychosis. Neuropsychopharmacology. 2019;44(5):915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fung G, Cheung C, Chen E, et al. . MRI predicts remission at 1 year in first-episode Schizophrenia in females with larger Striato-Thalamic volumes. Neuropsychobiology. 2014;69(4):243–248. [DOI] [PubMed] [Google Scholar]
- 7. Bodnar M, Malla AK, Joober R, et al. . Neural markers of early remission in first-episode schizophrenia: a volumetric neuroimaging study of the parahippocampus. Psychiatry Res Neuroimaging. 2012;201(1):40–47. [DOI] [PubMed] [Google Scholar]
- 8. Lieberman J, Jody D, Geisler S, et al. . Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry. 1993;50(5):369. [DOI] [PubMed] [Google Scholar]
- 9. Szeszko PR, Narr KL, Phillips OR, et al. . Magnetic resonance imaging predictors of treatment response in first-episode schizophrenia. Schizophr Bull. 2012;38(3):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zipursky RB, Zhang-Wong J, Lambe EK, Bean G, Beiser M. MRI correlates of treatment response in first episode psychosis. Schizophr Res. 1998;30(1):81–90. [DOI] [PubMed] [Google Scholar]
- 11. Cao B, Cho RY, Chen D, et al. . Treatment response prediction and individualized identification of first-episode drug-naïve schizophrenia using brain functional connectivity [published online ahead of print June 19, 2018]. Mol Psychiatry. 2018:1–8. doi:10.1038/s41380-018-0106-5. [DOI] [PubMed] [Google Scholar]
- 12. Hadley JA, Nenert R, Kraguljac NV, et al. . Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2014;39(4):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarpal DK, Robinson DG, Lencz T, et al. . Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer AS, Whitfield-Gabrieli S, Roth RM, Brunette MF, Green AI. Impaired functional connectivity of brain reward circuitry in patients with schizophrenia and cannabis use disorder: effects of cannabis and THC. Schizophr Res. 2014;158(1-3):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wisdom JP, Manuel JI. Prevalence of substance use in people with first-episode psychosis. J Dual Diagn. 2011;7(1-2):39–49. [DOI] [PubMed] [Google Scholar]
- 16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; 2013. https://www.appi.org/Course/Book/Subscription/JournalSubscription/id-3322/Diagnostic_and_Statistical_Manual_of_Mental_Disorders_%28DSM-5®%29. Accessed February 22, 2018. [Google Scholar]
- 17. Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35(2):383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schoeler T, Monk A, Sami MB, et al. . Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. The Lancet Psychiatry. 2016;3(3):215–225. [DOI] [PubMed] [Google Scholar]
- 20. Henquet C, van Os J, Kuepper R, et al. . Psychosis reactivity to cannabis use in daily life: an experience sampling study. Br J Psychiatry. 2010;196(6):447–453. [DOI] [PubMed] [Google Scholar]
- 21. Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis. Br J Psychiatry. 2006;189:137–143. [DOI] [PubMed] [Google Scholar]
- 22. Swendsen J, Ben-Zeev D, Granholm E. Real-time electronic ambulatory monitoring of substance use and symptom expression in schizophrenia. Am J Psychiatry. 2011;168(2):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zammit S, Moore TH, Lingford-Hughes A, et al. . Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193(5):357–363. [DOI] [PubMed] [Google Scholar]
- 24. Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23(22):8092–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haber SN. Neuroanatomy of reward: a view from the ventral striatum. In: Gottfried J, ed. Neurobiology of Sensation and Reward. Boca Raton, FL: CRC Press/Taylor & Francis; 2011. http://www.ncbi.nlm.nih.gov/pubmed/22593898. Accessed July 31, 2017. [PubMed] [Google Scholar]
- 26. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wise RA. Roles for nigrostriatal–not just mesocorticolimbic–dopamine in reward and addiction. Trends Neurosci. 2009;32(10):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abi-Dargham A, Rodenhiser J, Printz D, et al. . Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brisch R, Saniotis A, Wolf R, et al. . The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. da Silva Alves F, Figee M, van Amelsvoort T, Veltman D, de Haan L. The revised dopamine hypothesis of schizophrenia: evidence from pharmacological MRI studies with atypical antipsychotic medication. Psychopharmacol Bull. 2008;41(1):121–132. [PubMed] [Google Scholar]
- 31. Walter H, Kammerer H, Frasch K, Spitzer M, Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl). 2009;206(1):121–132. [DOI] [PubMed] [Google Scholar]
- 32. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148(11):1474–1486. [DOI] [PubMed] [Google Scholar]
- 33. Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol. 2013;48(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarpal DK, Argyelan M, Robinson DG, et al. . Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 2016;173(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34(13):2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic). Br J Psychiatry. 2014;205(1):1–3. [DOI] [PubMed] [Google Scholar]
- 37. Bhattacharyya S, Falkenberg I, Martin-Santos R, et al. . Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015;40(6):1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bossong MG, van Berckel BN, Boellaard R, et al. . Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–766. [DOI] [PubMed] [Google Scholar]
- 39. Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med. 2012;2(8):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276(5321):2048–2050. [DOI] [PubMed] [Google Scholar]
- 41. Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76(Pt B):498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van de Giessen E, Weinstein JJ, Cassidy CM, et al. . Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry. 2017;22(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Batalla A, Bhattacharyya S, Yücel M, et al. . Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8(2):e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. James A, Hough M, James S, et al. . Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS). Schizophr Res. 2011;128(1–3):91–97. [DOI] [PubMed] [Google Scholar]
- 45. Malchow B, Hasan A, Fusar-Poli P, Schmitt A, Falkai P, Wobrock T. Cannabis abuse and brain morphology in schizophrenia: a review of the available evidence. Eur Arch Psychiatry Clin Neurosci. 2013;263(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martín-Santos R, Fagundo AB, Crippa JA, et al. . Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med. 2010;40(3):383–398. [DOI] [PubMed] [Google Scholar]
- 47. Rais M, Cahn W, Van Haren N, et al. . Excessive brain volume loss over time in cannabis-using first-episode Schizophrenia patients. Am J Psychiatry. 2008;165(4):490–496. [DOI] [PubMed] [Google Scholar]
- 48. Robinson DG, Gallego JA, John M, et al. . A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-month outcomes. Schizophr Bull. 2015;41(6):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robinson DG, Woerner MG, Alvir JM, et al. . Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 1999;156(4):544–549. [DOI] [PubMed] [Google Scholar]
- 50. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patients Edition. (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 51. Guy W, Bonato R. CGI: clinical global impressions. ECDEU Assess Man Psychopharmacol Revis. 1976:217–222. [Google Scholar]
- 52. Sarpal DK, Robinson DG, Fales C, et al. . Relationship between duration of untreated psychosis and intrinsic corticostriatal connectivity in patients with early phase Schizophrenia. Neuropsychopharmacology. 2017;42(11):2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Di Martino A, Scheres A, Margulies DS, et al. . Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–2747. [DOI] [PubMed] [Google Scholar]
- 54. Patel R, Wilson R, Jackson R, et al. . Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6(3):e009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Legge SE, Dennison CA, Pardiñas AF, et al. . Clinical indicators of treatment-resistant psychosis [published online ahead of print June 3, 2019]. Br J Psychiatry. 2019:1–8. doi: 10.1192/bjp.2019.120. [DOI] [PubMed] [Google Scholar]
- 56. Brzozowska NI, de Tonnerre EJ, Li KM, et al. . The differential binding of antipsychotic drugs to the ABC transporter P-Glycoprotein predicts cannabinoid-antipsychotic drug interactions. Neuropsychopharmacology. 2017;42(11):2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duan X, Hu M, Huang X, et al. . Effect of risperidone monotherapy on dynamic functional connectivity of insular subdivisions in treatment-naive, first-episode Schizophrenia [published online ahead of print September 5, 2019]. Schizophr Bull. 2019. doi: 10.1093/schbul/sbz087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yücel M, Solowij N, Respondek C, et al. . Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65(6):694–701. [DOI] [PubMed] [Google Scholar]
- 59. Zalesky A, Solowij N, Yücel M, et al. . Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135(Pt 7):2245–2255. [DOI] [PubMed] [Google Scholar]
- 60. Yücel M, Lorenzetti V, Suo C, et al. . Hippocampal harms, protection and recovery following regular cannabis use. Transl Psychiatry. 2016;6:e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sneider JT, Pope HG, Silveri MM, et al. . Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. Eur Neuropsychopharmacol. 2008;18(8):612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ramaekers JG, van Wel JH, Spronk D, et al. . Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes. Brain Imaging Behav. 2016;10(4):1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Myles N, Newall HD, Curtis J, Nielssen O, Shiers D, Large M. Tobacco use before, at, and after first-episode psychosis: a systematic meta-analysis. J Clin Psychiatry. 2012;73(4):468–475. [DOI] [PubMed] [Google Scholar]
- 64. Brody AL, Mandelkern MA, Olmstead RE, et al. . Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34(2):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kerridge BT, Pickering R, Chou P, Saha TD, Hasin DS. DSM-5 cannabis use disorder in the national epidemiologic survey on alcohol and related conditions-III: gender-specific profiles. Addict Behav. 2018;76:52–60. [DOI] [PubMed] [Google Scholar]
- 66. Burns JK. Cannabis use and duration of untreated psychosis: a systematic review and meta-analysis. Curr Pharm Des. 2012;18(32):5093–5104. [DOI] [PubMed] [Google Scholar]
- 67. Wisdom JP, Manuel JI, Drake RE. Substance use disorder among people with first-episode psychosis: a systematic review of course and treatment. Psychiatr Serv. 2011;62(9):1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]