Abstract
Schizophrenia (SCZ) is a neurodevelopmental disorder characterized by positive symptoms (hallucinations and delusions), negative symptoms (anhedonia, social withdrawal) and marked cognitive deficits (memory, executive function, and attention). Current mainstays of treatment, including medications and psychotherapy, do not adequately address cognitive symptoms, which are essential for everyday functioning. However, recent advances in computational neurobiology have rekindled interest in neurofeedback (NF), a form of self-regulation or neuromodulation, in potentially alleviating cognitive symptoms in patients with SCZ. Therefore, we conducted a systematic review of the literature for NF studies in SCZ to identify lessons learned and to identify steps to move the field forward. Our findings reveal that NF studies to date consist mostly of case studies and small sample, single-group studies. Despite few randomized clinical trials, the results suggest that NF is feasible and that it leads to measurable changes in brain function. These findings indicate early proof-of-concept data that needs to be followed up by larger, randomized clinical trials, testing the efficacy of NF compared to well thought out placebos. We hope that such an undertaking by the field will lead to innovative solutions that address refractory symptoms and improve everyday functioning in patients with SCZ.
Keywords: neurofeedback, schizophrenia, treatment refractory, EEG, rt-fMRI, self-regulation
Introduction
Schizophrenia (SCZ) is a mental illness that affects approximately 1% of the population.1–3 The disorder is characterized by positive symptoms, such as hallucinations and delusions, negative symptoms such as social withdrawal and anhedonia, and cognitive deficits. Recent research has focused much attention on the neurological changes responsible for the symptoms of SCZ, including decreases in both gray and white matter.3–6 For example, the presence of auditory hallucinations has been associated with anatomical changes in the superior temporal gyrus (STG) as well as structural and functional abnormalities in other areas involved in auditory perception, such as the primary and secondary auditory cortex.7–9 Hypofrontality, or decreased function of the prefrontal cortex, and dysfunctional brain networks have been associated with both negative symptoms10 and cognitive deficits.11 Cognitive deficits have been found in memory, slow processing speed, and executive function,12,13 that negatively affect social functioning.6 While cognitive deficits can greatly impair everyday function and affect the majority of patients with SCZ,14 there is currently no successful treatment.13,15,16 Likewise, up to 30% of patients suffering from auditory verbal hallucinations (AVHs) do not respond to medication,17,18 prompting the need for additional treatments for refractory symptoms.
Neurofeedback (NF) has emerged as a possible novel treatment option. In brief, by allowing patients to directly perceive specific neural events (eg, by using visual or auditory representations of a patient’s own brain activity as targets), NF, through operant conditioning, allows patients to practice modulating their own neural activity.19 Collura described NF as “…an art, and [that] there can be very different ways to apply general principles, in the form of a clinical intervention.20”
There are several different methods of recording brain activity during NF, each with its own advantages and weaknesses. Electroencephalography NF (EEG NF) is a noninvasive technique. Electrodes are placed on a subject’s scalp to detect electrical activity generated by the brain, which is recorded and displayed on a computer screen in the form of a visual metaphor, such as a flying airplane. Positive feedback (the plane flying successfully) is given when the subject maintains brain activity within prespecified parameters. The parameters are made incrementally more difficult on each successive training session and brain activity is modified over time. Feedback is sometimes provided during quantitative EEG (qEEG), which measures brain electrical activity and compares it to recordings from healthy individual normative data. It then provides a signal to up- or downregulate the activity in the direction of the population mean. Some advantages of EEG NF include a well-established safety profile, ie, portable and uses relatively inexpensive equipment.21 However, EEG’s spatial resolution is less than ideal and can be affected by muscle artifacts.21
Real-time functional magnetic resonance imaging NF (rt-fMRI NF) reflects brain activity by measuring blood oxygen-level dependent (BOLD) responses in the brain, where BOLD increases in areas of brain activity.22 Similar to EEG NF, visual metaphors are used to display areas of activity to subjects, which they are asked to either up- or downregulate. Although rt-fMRI NF provides higher spatial resolution and fidelity than EEG NF,23 its utility is limited by high cost, patient discomfort at being in an enclosed scanner and low temporal resolution.24 In addition, the BOLD signal can also be influenced by nonneuronal artifacts, such as breathing or heart rate.22
While EEG and rt-fMRI are the most common NF techniques, other techniques have been used in a few studies. For instance, functional near-infrared spectroscopy (fNIRS) is similar to functional magnetic resonance imaging (fMRI) and measures the metabolic activity of neurons by assessing differences in oxygenated and deoxygenated hemoglobin.25 fNIRS is limited to the outer cortex and as such has lower spatial resolution than rt-fMRI.25 In comparison to EEG, fNIRS has higher spatial resolution and is less prone to motion artifacts. However, its temporal resolution is lower.25 Hemoencephalography (HEG) is another technique where infrared light is used to measure local blood flow through the skull.26 The equipment used is a headband with a light source and a light receiver, making this technique both cost-effective and easy to use26 (at least in the single study that was available for inclusion in our review).
Regardless of the technique utilized, the principle of NF is the same; A subject’s brain activity is represented on a computer screen and feedback like music or a visual metaphor is provided depending on whether the subject achieves the desired change in brainwave activity.27 NF is advantageous in that during training the subject becomes aware of his/her brain activity and is therefore able to knowingly alter it.27–29 As a treatment protocol, NF has been used successfully to address a variety of neuropsychiatric disorders, including attention-deficit hyperactivity disorder (ADHD),19,30 depression,19 post-traumatic stress disorder,19,31 Alzheimer’s disease,19 and anorexia.19 Furthermore, NF treatment has been associated with structural changes in brain composition in healthy subjects, including an increase in gray matter volume of the target area.32
These findings support the use of NF as a promising adjunct method for treatment-resistant and difficult to treat symptoms of SCZ.16,33 NF, however, can be administered in a large variety of protocols varying in length and targets. We conducted a systematic review of the literature on the topic of NF in SCZ to identify successful protocols, dose-response effects, and other training parameters that can then be used to design the next generation of NF studies.
For nonspecialists or clinicians new to the field, NF may appear chaotic, disorganized, and atheoretical. There seems to be little agreement as to what protocol or electrode site to use, or what theoretical underpinning motivates one approach vs another. Undertaking a systematic review of this field, even with the focus narrowed to NF approaches to SCZ, may appear unfruitful for the same reasons. However, understanding its historical trajectory and effectiveness in the context of clinical and therapeutic approaches can help make more sense of the chaos.34 It may even be possible to draw useful conclusions with an understanding of the foundations for the methodology35,23 (ISNR website, In Defense of Neurofeedback).
Methods
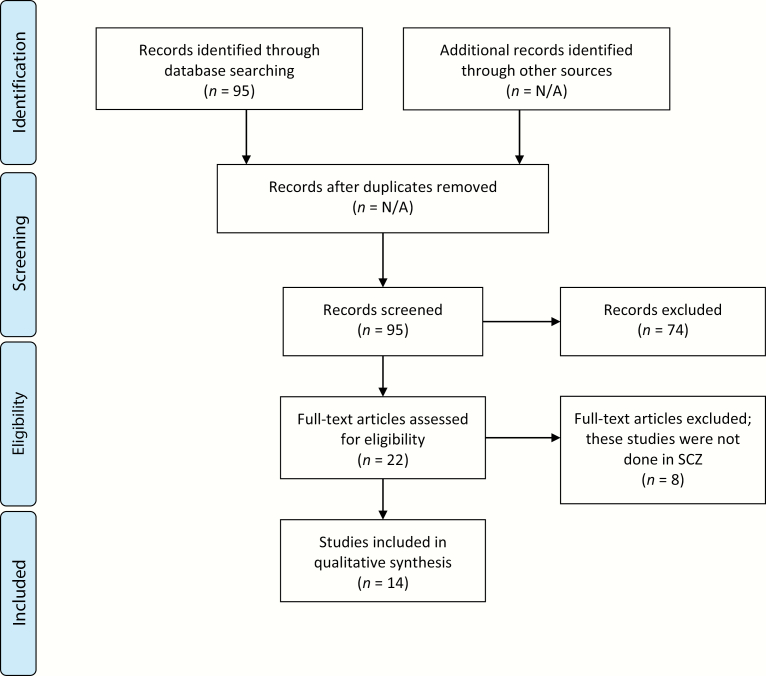
We used the search terms “(neurofeedback OR biofeedback)” AND schizophrenia in PubMed. This produced 95 articles published from 1964 to 2019. Of these 95 articles, 67 were excluded for either being review articles, nonneuronal biofeedback, or if the intervention consisted of a combination of treatments (one case study with cognitive remediation, NF, and family therapy). Another 8 studies were excluded where NF was performed on healthy subjects, or subjects with ADHD, thereby not investigating the treatment of SCZ. Lastly, 6 articles were excluded because they were written in a language other than English (Russian, Spanish, Japanese, and German) (figure 1: PRISMA flowchart). Although only 14 studies met inclusion criteria, many of the studies assessed changes in brain function as a primary outcome, an objective measure that significantly increased the strength of these studies. The patients, intervention, comparator, outcomes, study design (PICOS) criteria for inclusion and exclusion of studies are presented in table 1.
Fig. 1.
PRISMA 2009 flow diagram. From Moher et al.36
Table 1.
Inclusion and Exclusion Criteria
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | SCZ | Healthy individuals only |
| Intervention | Neurofeedback | Multiple interventionsa, biofeedback |
| Comparator | Patients with SCZ, healthy subjects, no control | N/A |
| Outcomes | Neurophysiological changes, improvement in symptoms | Not adequately described outcomes |
| Study design | Randomized clinical trials, single blinded, proof-of-concept studies, case studies | Review articles |
Note: SCZ, schizophrenia.
aOne case study included simultaneous neurofeedback, cognitive retraining, and family intervention.
Results
EEG NF in Treatment of SCZ
Of the 14 articles included in this systematic review, 7 studies used EEG NF to treat symptoms of SCZ (table 2). Three of these studies included a control group. One control group consisted of healthy subjects (HC), another used SCZ patients continuing treatment as usual, and the last was SCZ patients that received sham NF. Of the 7 EEG NF studies, 2 were case studies. Only one of the studies (14%), conducted by Rieger et al, met the standards of a randomized controlled trial. EEG NF treatment dose ranged from 3.75 hours to 58.5 training hours.
Table 2.
Neurofeedback Treatment Studies in Patients With Schizophrenia, 1964–2019
| Authors | Year | N | NF Modality | Protocol | Treatment Dose (h) | Control | Outcomes |
|---|---|---|---|---|---|---|---|
| Zweerings J et al | 2019 | n = 56 | rt-fMRI | 8 NF sessions, ↑↓ left anterior IFG and left posterior STG activity | 0.56 ha | HC | Successful ↑↓ regulation, improved functional connectivity, decreased perceived illness severity, and decreased symptom severity |
| Balconi M et al | 2018 | n = 18 | EEG | 10 NF sessions, ↑ power in .5–5.5 Hz (delta/low theta) to treat hemispheric imbalance | 3.75 h | SCZ, TAU | Experimental group experienced ↑ hemispheric balance in delta power after NF |
| Storchak H et al | 2018 | n = 1 | fNIRS | 47 NF sessions, ↓ activity in STG during AVH, and ↑ activity in STG before AVH | — | — | Able to regulate activity in STG before AVH occurred, but not during AVH. Significant decrease in AVHs |
| Pazooki K et al | 2018 | n = 2 | EEG | 20 NF sessions, ↑ SMR and ↓ 4–8 Hz theta at C4/C3 (contralateral side to handedness). Followed by 2 wk NF to ↑ 13–18 Hz at F3 | 10 h | — | Both participants were able to regulate their alpha, beta, theta, and SMR activity, accompanied by significant improvements of negative symptoms |
| Rieger K et al | 2018 | n = 10 | EEG | 16 NF sessions, ↑ amplitude of N100 ERP component | 5.9 h | Sham NF | No significant improvement in AVH in regards to NF or sham NF |
| Orlov ND et al | 2018 | n = 12 | rt-fMRI | 4 NF sessions, ↓ regulate voice-sensitive regions in left STG | 1.93 ha | — | Patients learned to ↓ STG activation and increased functional connectivity |
| Gomes JS et al | 2018 | n = 20 | HEG | 10 NF sessions, ↑ activity at F7, Fp1, Fp2, and F8 | 1 h | HC | Both groups were able to regulate brain activity, improved cognitive function in multiple domains in both SCZ and HC |
| Nan W et al | 2017 | n = 1 | EEG | 12.5 h of NF, to ↑ alpha and ↓ beta2 at P4 | 12.5 h | — | Alpha/beta2 ratio increased over sessions. Significant improvement in + and − symptoms after 22 mo |
| Dyck MS et al | 2016 | n = 3 | rt-fMRI | 9 NF sessions, ↑ ACC activation | 1.28 ha | — | Significant ↑ activation of ACC and improvements in AVH |
| Cordes JS et al | 2015 | n = 22 | rt-fMRI | 3 NF sessions, ↑ ACC activation | 1.28 ha | HC | Experimental group ↑ activity in dorsal ACC, healthy subjects ↑ activity in rostral ACC |
| No description of effects on cognition | |||||||
| Ruiz S et al | 2013 | n = 9 | rt-fMRI | 12 NF sessions, ↑ activation of bilateral insular cortex | 1.30 ha | — | Improved control of anterior insula cortex: better control of negative symptoms |
| Surmeli T et al | 2012 | n = 51 | EEG | Average of 58.5 h of NF, targeting deviations in individual qEEG | Ave. 58.5 h | — | Significant improvements in qEEG deviations and symptoms |
| Schneider F et al | 1992 | n = 24 | EEG | 20 NF sessions, regulate activity of SCP (recorded from Cz) | 4.89 h | HC | Experimental group required more NF training to achieve similar control of SCP compared to healthy controls |
| Schneider SJ et al | 1982 | n = 9 | EEG | 5 NF sessions, ↑ 8–13 Hz and ↓ power densities over 15 Hz at O2 | 2.75 h | — | Successful within-session regulation of brain activity, no change between sessions |
Note: ACC, anterior cingulate cortex; AVH, auditory verbal hallucinations; EEG, electroencephalogram; ERP, event-related potential; fNIRS, functional near-infrared spectroscopy; HEG, hemoencephalography; IFG, inferior frontal gyrus; NF, neurofeedback; rt-fMRI, real-time functional magnetic resonance imaging; qEEG, quantitative EEG; SCP, slow cortical potentials; SCZ, schizophrenia; SMR, sensorimotor rhythm; STG, superior temporal gyrus; TAU, treatment as usual; ↑, increase; ↓, decrease.
art-fMRI alternates rest blocks with NF. Estimated NF dose includes both training and rest time.
Balconi et al aimed to improve emotion regulation in patients with SCZ by means of EEG NF.37 Patients were divided into 2 groups: the treatment group consisted of 9 subjects who received NF (n = 9), vs the control group (n = 9) where SCZ patients received treatment as usual. Only subjects on stable doses of medications for a minimum of 4 weeks were included. The NF group received 10 sessions of 25 min of feedback (total of 3 h and 45 min). For the experimental group, increased power in the delta range (0.5–5.5 Hz) at electrode sites F3 and F4 was rewarded. The rationale for this was that reductions in frontal delta activity in SCZ have been previously reported,38 and therefore, the authors aimed to (1) increase frontal delta activity and (2) reduce frontal delta asymmetry. Pre-post testing included (1) emotion recognition of 40 negative, 40 positive, and 20 neutral International Affective Picture System (IAPS) images. Subjects were asked to rate each picture for valence and arousal while EEG and fNIRS were recorded, and (2) Self-Assessment Manikin (SAM) rating of emotional experience. At post-treatment, EEG and fNIRS collected during IAPS revealed significant reduction in frontal delta asymmetry in the NF group. Subjects in the treatment group also rated negative IAPS images as more positive at post-treatment, a change that was not observed in the control group.
Rieger et al investigated the use of EEG NF to treat AVHs in patients with SCZ.39 Subjects were randomized to receive feedback to either increase N100 (treatment group, n = 4) or increase P200, presumably, an unrelated event related potential (ERP) in the control group (n = 6). The N100 ERP reflects an essential component in the neural processing of auditory stimuli and has been shown to be reduced in SCZ. Therefore, it was chosen as a target in subjects experiencing AVH. Subjects performed 16 sessions of EEG NF for a total of 5.9 h over 2 weeks. No significant change was detected between the groups on any ERP component. The authors did note that subjects with a learning pattern, one that reflected within-session improvements, showed overall improvements in AVH. However, this change was noted in both groups. Such results must be interpreted with caution given the small sample size. But, they point to the potential impact of learning style and motivation on NF’s efficacy.
Surmeli et al used quantitative electroencephalography NF (qEEG NF) on 51 patients with SCZ to normalize brain activity in deviating regions.40 Prior to recording baseline qEEG, patients discontinued medications, which were washed out for 7 half-lives. On average, patients performed 58.5 one-hour qEEG NF sessions over the course of 24–91 days. qEEG NF training resulted in significant improvement on the Positive and Negative Syndrome Scale (PANSS). The authors did not comment on the relationship between treatment dose and response. For 19 participants, brain activity was no longer classified as abnormal after qEEG NF treatment, and 27 participants remained medication-free at follow-up. Brain changes continued to be present in the subset of subjects that were followed for as long as 22 months following treatment.
Schneider et al41 used EEG NF to target slow cortical potentials (SCPs), which are thought to reflect regulation of the brain’s attentional resources and cortical excitability.42,43 Attentional deficits are well described in SCZ,42 and hence, the authors hypothesized that improved self-regulation of SCPs would lead to symptom reduction. Male patients with SCZ (n = 12) and HC (n = 12) were enrolled in the study. Patients were maintained on antipsychotic medications throughout the study. Scalp electrical recordings from Cz electrode site were used to monitor SCPs. Subjects were required to either increase or decrease SCP during 20 EEG NF sessions, each consisting of 110 NF trials (approximately 4.89 h of NF). Trials required subjects to either increase or decrease SCPs randomly by the appearance of the letter A or B to indicate what was required. On some trials, success was shown as a rocket moving on the screen (NF trials). Additional trials showed a letter to indicate whether increase or decrease was required, but subsequent feedback was not provided in the form of a moving rocket. These trials (transfer trials) were meant to assess whether subjects had learned strategies that they could evoke and utilize without the presence of NF. The results indicated that compared to HC, subjects in the SCZ group learned to control SCPs at a much slower rate. Furthermore, those with greater symptom severity showed reduced learning.
Schneider and Pope performed a qEEG NF to investigate if treatment with NF could achieve EEG changes comparable to those induced by antipsychotic medication in patients with SCZ.44 Previous studies had shown diminished power in higher alpha (11–14 Hz) but greater power in theta (3–6 Hz) and beta (24–33 Hz) in SCZ compared to healthy controls.45,46 Neuroleptics tend to normalize these responses, showing increases in alpha while decreasing theta and beta power. This study used auditory and visual stroboscopic flashes as the NF signal. It compared the EEG power spectrum characteristics of SCZ patients with patients showing neuroleptic-induced clinical improvement. It also included 9 patients with chronic SCZ who continued with treatment as usual. Patients performed five 33-min EEG NF sessions to increase alpha (12 Hz activity) at O2 electrode site. EEG was recorded and analyzed during the last 7 min of each session, as well as during a baseline 7-min period before each session began. There was a significant increase in power densities at lower frequencies (8, 9, 10, and 12 Hz), and a significant decrease in power densities at higher frequencies (16 and 27–35 Hz) for within-session analysis. However, no change in EEG was detected in the between-session analysis. These results indicate that EEG deviations characteristic of SCZ can be changed to look like the EEG associated with neuroleptic-induced clinical improvement. However, this effect was detected during NF training and not between consecutive sessions suggesting short-term improvement, compared to the longer-term effects of neuroleptics.
In a case study of 2 patients, Pazooki and colleagues explored the utility of EEG NF to treat negative symptoms associated with SCZ.10 Since negative symptoms can be partially attributed to decreased attention, the NF protocol targeted increasing sensorimotor rhythm (SMR, 12–15 Hz),47 and inhibiting theta (4–8 Hz)48 both of which are associated with attention in SCZ. Both subjects continued to receive treatment as usual and no medication changes were made. The subjects received a total of 10 h of NF administered in 20, 30-min sessions. In the first phase, both patients received 2 weeks of NF to increase SMR and decrease theta in the contralateral hemisphere to their handedness at C3/C4. During this phase, the patients received instructions for how to succeed at NF before each session. A second phase used the same protocol for 2 weeks but without instructions and added augmentation of beta-I (13–18 Hz),49 which is thought to reduce impulsivity. Both participants showed the ability to regulate alpha, beta, theta, and SMR activity after NF training, regardless of whether they received instructions before the session. Neuropsychological testing (Go/No-go, GAF, and PANSS) further revealed a significant improvement in negative symptoms in both subjects.
In a case study, Nan and colleagues performed intensive EEG NF on a 51-year-old woman with chronic SCZ with AVH.50 The treatment targeted the right parietal cortex (P4 electrode site) and aimed to increase the alpha/beta251–53 ratio, which has been implicated in AVH in SCZ. During 4 consecutive days, the patient completed 13.5 h of EEG NF along with medications. Post-treatment testing showed improved working memory. Additionally, both negative and positive symptom severity were significantly improved at 22 months post completion of NF.
rt-fMRI NF in Treatment of SCZ
Five of the studies included in this systematic review utilized rt-fMRI NF (table 2). Two of these studies included a control group, both consisting of healthy controls receiving the same NF, whereas one of the 5 studies was a case report. One study, conducted by Zweerings et al54 qualified as a randomized controlled trial. The average number of rt-fMRI NF sessions was 7.2 (ranging from 3 to 12), and the average length of each session was 15.2 min (ranging from 4 to 54 min). All of the rt-fMRI NF trials found a significant improvement in treatment target as a result of NF training in much shorter time frames compared to EEG NF studies.
Zweerings et al (2019) used rt-fMRI NF to target left-hemispheric language nodes in 21 patients with SCZ with AVH and 35 healthy controls.54 Subjects in the SCZ group continued with medications without any changes. The study design was a double blind, randomized, crossover intervention. All subjects received feedback to up- or downregulate in 2 regions of interest (ROIs) for approximately 0.5 h. Two nodes in the left-hemispheric language network, the inferior frontal gyrus (IFG) and posterior STG, were chosen as seed areas. The rationale being that improved self-regulation in these areas would lead to reductions in AVH. Results revealed increased coupling between language nodes and the default mode network post NF, with greater functional connectivity in the SCZ group compared to HC. Additionally, post-treatment behavioral improvement was associated with increased functional coupling in SCZ patients between the left IFG and left inferior parietal lobe (IPL).
Orlov et al performed rt-fMRI NF training in patients with SCZ to downregulate activity in the left STG, a node in the left-hemispheric language network.55 The rationale was similar to Zweerings et al, ie, to reduce AVH in treatment refractory patients with SCZ (n = 12). Subjects were maintained on stable antipsychotic regimens. They received 4 sessions of rt-fMRI NF over 2 weeks (approximately 2 h total NF) to downregulate left STG activity. During the fourth visit, a transfer run was performed without visual feedback, to assess generalization of the training. The results showed that rt-fMRI NF significantly improved the subjects’ ability to (1) downregulate left STG activity, even when feedback was not given, and (2) increase functional connectivity between IFG, STG, and inferior parietal cortex. This indicated increased connectivity in the speech motor and speech perception regions of the language network.
Cordes et al designed a NF study to target upregulation of anterior cingulate cortex (ACC) activity using rt-fMRI NF.56 The ACC was chosen as a ROI given its central role in cognitive processing, with the goal of treating cognitive deficits.57 Eleven patients with SCZ and 11 HC participated. Both groups received 3 sessions of rt-fMRI NF over the course of a week (approximately 1.28 h) to upregulate ACC activity. Both groups were successful in upregulating ACC activity post-treatment, although by using different strategies. The study was limited in that the authors did not provide any data on behavior pre/post NF.
Ruiz et al designed a study to influence activation of bilateral insula cortex (BIC) in individuals with SCZ.58 The insula was chosen given its role in emotion recognition59 and because in a previous study, HC individuals were able to upregulate insular activity using rt-fMRI.60 Nine individuals with SCZ were trained to upregulate bilateral insular hemodynamic response during a 2-week NF protocol (approximately 1.3 h of NF), while being maintained on their usual medications. A pre-post emotion recognition task was administered to assess behavioral response in addition to connectivity analyses. After NF training, subjects showed increased activation of BIC. They also showed increased identification of “disgust” faces, and decreased identification of “happy” faces on the emotion recognition task. Negative correlation between insular activation and negative symptoms was also noted indicating that more severe negative symptoms are associated with difficulties to learn self-regulation. The fact that identification of “disgust” faces was heightened, whereas identification of “happy” faces was reduced, highlights the need for careful protocols. SCZ patients are more attuned to negative emotions at baseline and so increasing this sensitivity would not be clinically indicated. Nonetheless, the study provided early proof-of-concept data that individuals with SCZ can modulate BOLD responses with associated changes in behavior.
Dyck and colleagues performed rt-fMRI to upregulate ACC activity (1.28 h, 9 sessions) in 3 patients with SCZ and AVH.17 In addition to NF, the patients also performed transfer runs without feedback on the last day of testing. Significant upregulation of the ACC was observed in all 3 subjects during NF, but only one subject was able to upregulate ACC during the transfer run. Self-reported questionnaires of subjective distress due to AVH indicated improvement. Effects on mood were mixed, suggesting that rt-fMRI NF effects on behavior may be sporadic.
fNIRS and HEG NF in Treatment of SCZ
Two of the studies included used additional methods of NF (table 2). In a case study, Storchak et al explored the use of fNIRS NF to treat severe AVH in a woman with paranoid SCZ.61 The subject performed 47 fNIRS NF sessions targeting activity in the bilateral posterior STG, a speech-activated region previously implicated in AVH in SCZ. The total length of training could not be quantified as this information was not provided in the publication. Antipsychotic medication doses were stable for the duration of the study. During NF trials without AVH and during trials in which the subject experienced AVH, subject was instructed to downregulate activity in the STG (measured by O2Hb). During NF trials when the subject felt she was about to experience AVH, she was instructed to increase activity in the STG. The subject was able to significantly increase activity in the STG before AVH began, but not during trials where she was actively experiencing AVH. After 27 NF sessions, the subject experienced a significant reduction in AVH.
Gomes et al investigated the use of near-infrared hemoencephalography NF (HEG NF) training to improve cognitive deficits in patients with SCZ (n = 8) compared to HC (n = 12).62 Subjects received HEG NF twice a week at the 4 frontal electrode sites F7, Fp1, Fp2, and F8 to improve prefrontal cortical function previously shown to be impaired in SCZ.63 After 10 HEG NF sessions (1 h of NF), the left-hemispheric sites (F7 and Fp1) showed significantly increased activation in both groups, and the right F8 site showed a near-significant change. Both groups showed post-treatment improvement in most domains of cognitive functioning (speed of processing, working memory, verbal memory, visual learning, and executive function). The authors did not explore whether there were differences in length of training required to affect change in one group vs the other.
Discussion
A recent review by Zamanpoor64 argues that “There is no central pathophysiology mechanism, diagnostic neuropathology, or biological markers (that) have been defined for schizophrenia.” The lack of answers makes it clear that the complex interactions of genetics and environmental factors implicated in the neuroetiology of SCZ need to be clarified. There is a growing need for clinicians and scientists to move beyond genomic-centric answers to an adoption of developmental, neurochemical, and biophysical perspectives in clinical practice and research. This expanded perspective must also overlap with an understanding of the environmental risk factors, such as pregnancy and birth complications, childhood trauma, migration, social isolation, and substance abuse that influence the individual’s likelihood to develop the disorder.
Thapar and Riglin65 argue, in support of this integrative perspective, that “schizophrenia typically onsets after adolescence. However, it is commonly preceded by childhood antecedents that do not resemble schizophrenia itself but do appear to index schizophrenia genetic liability.” These researchers see the necessity for considering age-at-onset, changes over time, and different developmental periods when interpreting clinical symptoms. It is precisely because NF approaches are quite flexible and customizable to the varieties of SCZ symptoms that makes them a valuable therapeutic tools and need to be studied.
The last decade has seen renewed interest in direct brain training, or neuromodulation as the disciplines of computer science and engineering have made computational advances. Neuromodulation generally refers to the concept of direct brain-based treatment with the goal of targeting psychiatric disorders. Inherently, the field uses a research domain criteria (RDoCs) approach, in that treatment targets are biologically defined and systems based. For instance, rt-FMRI NF or EEG NF requires the identification of a brain region or electrical activity, ie, associated with a particular set of behaviors and uses it as a target of treatment. Additionally, the target is part of a neural circuit whose workings are known in healthy populations to some extent, and impaired in diseased states. Unlike other treatments, these treatments do not aim to treat syndromes or collections of symptoms, but rather, a constellation of behaviors arising from a neural network impairment. Therefore, neuromodulation treatments provide opportunities not just for treatment, but also to advance mechanistic understanding of disorders. In this context, NF strengthens an individual’s ability to gain mastery over his/her neural processing in a particular neural network. In this model, understanding factors that lead to failure may be as valuable as those that lead to success. In addition, different individuals may use different strategies to accomplish the same goals, allowing investigators to perhaps find novel treatment strategies, and/or factors that could be used to customize treatment.
As evidenced by the reviewed studies, although the principles of NF are uniform, they have been applied in SCZ in a wide variety of protocols. Additionally, despite large numbers of publications associated with the term “NF,” at present, there are few studies with empirical data in the published literature. Despite these limitations, NF treatment appears to influence neural processing, connectivity and metabolism in the brain, as shown by changes in pre/post scalp electrical activity and neuroimaging studies. Furthermore, many of the changes are noted during periods when feedback is not being provided (transfer runs), indicating that brain change has generalized and is no longer dependent on external cues (NF). Some studies with longer follow-up periods have found evidence of continued brain change even months after the training ended.
Overall, rt-fMRI NF protocols appear to reach efficacy in a shorter period of time compared to EEG NF, however, rt-fMRI’s cost, discomfort and expensive equipment may preclude its use as a clinical intervention with wide use. In addition, the pre/post measures in rt-fMRI and EEG NF studies could benefit from standardization. For instance, it may be useful to the field to include both brain oscillatory and connectivity measures pre/post in all NF studies so comparisons can be made across modalities. The single HEG study reviewed here showed promising results in just 1 h of NF. Given HEG NF’s ease of use and potential efficacy in a shorter protocol, this modality could benefit from further testing in larger samples. In general, given the flexibility and variety of NF treatment protocols (alpha, beta, alpha/beta, delta, gamma, and theta), different EEG electrode placements (frontal, temporal, central, occipital, unipolar, and bipolar), and types of NF (frequency, power, SCP, fMRI, etc.), it can easily fit into a more customized or personalized therapeutic intervention for disorders that are themselves complex and varied in their symptoms.
The promise of NF as a therapeutic tool23,35,49,66 (ISNR website, In Defense of Neurofeedback, https://www.isnr.org/in-defense-of-neurofeedback) must be balanced with certain caveats. To date, most clinical trials that have tested its efficacy are small in number and scale or case studies, do not randomize the subjects into treatment groups, do not all have proper blinded controls, and rarely compare NF to the gold standard of treatment, which is typically medication and other forms of therapy. Additionally, there is still much to uncover about what the patterns and dynamics of brain activity mean as they change or are changed through self-regulation. Clearly, future studies must address these problems.
Despite these growing concerns, a chorus of support for the safety and efficacy of NF training has been developing, including meta-analyses showing its effectiveness for epilepsy,67 ADHD,68 and other disorders. This progress gives confidence of its application in SCZ treatment. Thus, given the availability of a large amount of proof-of-concept studies in the literature, a logical next step would be to conduct studies with larger sample sizes and placebo arms, with uniform well thought out study designs. It is important to focus on suitable target regions, identification of parameters including duration of treatment, dose-response effects, etc. in successful protocols. Equally important would be translating rt-fMRI NF findings to EEG NF studies and combining these approaches when feasible. The recent article by Ros et al (Brain, in press) titled “Consensus on The Reporting and Experimental Design of Clinical and Cognitive-behavioural Neurofeedback Studies (CRED-nf checklist)” does just that. It provides an excellent framework for future studies to unify efforts in the field. This consensus is not necessarily exclusive of smaller mechanistic or parametric studies that may still be useful. Empowering individuals with SCZ with the tools to improve their brain function and functioning in everyday lives will have great benefits not just for those affected with the illness but also for society in general.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the National Institutes of Health (R61MH112793) and the University of California Chancellor’s Research Education Scholars program (UCSD-CRES).
References
- 1. Markiewicz R, Kozioł M, Olajossy M, Masiak J. Can brain-derived neurotrophic factor (BDNF) be an indicator of effective rehabilitation interventions in schizophrenia? Psychiatr Pol. 2018;52(5):819–834. [DOI] [PubMed] [Google Scholar]
- 2. Fromer M, Pocklington AJ, Kavanagh DH, et al. . De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen P, Modinos G, Hubl D, et al. . Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull. 2012;38(4):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell F, LoCastro E, Acosta D, et al. . Age-related changes in topological degradation of white matter networks and gene expression in chronic schizophrenia. Brain Connect. 2017;7(9):574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Donoghue S, Holleran L, Cannon DM, McDonald C. Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: a selective review of structural network analyses using diffusion MRI. J Affect Disord. 2017;209:217–228. [DOI] [PubMed] [Google Scholar]
- 6. Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry. 2015;20(1): 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mørch-Johnsen L, Nesvåg R, Jørgensen KN, et al. . Auditory cortex characteristics in schizophrenia: associations with auditory hallucinations. Schizophr Bull. 2017;43(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nenadic I, Smesny S, Schlösser RG, Sauer H, Gaser C. Auditory hallucinations and brain structure in schizophrenia: voxel-based morphometric study. Br J Psychiatry. 2010;196(5):412–413. [DOI] [PubMed] [Google Scholar]
- 9. O’Daly OG, Frangou S, Chitnis X, Shergill SS. Brain structural changes in schizophrenia patients with persistent hallucinations. Psychiatry Res. 2007;156(1):15–21. [DOI] [PubMed] [Google Scholar]
- 10. Pazooki K, Leibetseder M, Renner W, Gougleris G, Kapsali E. Neurofeedback treatment of negative symptoms in schizophrenia: two case reports. Appl Psychophysiol Biofeedback. 2019;44(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pratt JA, Winchester C, Egerton A, Cochran SM, Morris BJ. Modelling prefrontal cortex deficits in schizophrenia: implications for treatment. Br J Pharmacol. 2008;153(suppl 1):S465–S470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajeswaran J, Taksal A, Jain S. Rehabilitation in schizophrenia: a brain-behavior and psychosocial perspective. Indian J Psychol Med. 2017;39(6):797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Firth J, Cotter J, Carney R, Yung AR. The pro-cognitive mechanisms of physical exercise in people with schizophrenia. Br J Pharmacol. 2017;174(19):3161–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seidman LJ, Mirsky AF. Evolving notions of schizophrenia as a developmental neurocognitive disorder. J Int Neuropsychol Soc. 2017;23(9–10):881–892. [DOI] [PubMed] [Google Scholar]
- 15. Grent-’t-Jong T, Rivolta D, Gross J, et al. . Acute ketamine dysregulates task-related gamma-band oscillations in thalamo-cortical circuits in schizophrenia. Brain. 2018;141(8):2511–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keefe RSE, Harvey PD, Khan A, et al. . Cognitive effects of MIN-101 in patients with schizophrenia and negative symptoms: results from a randomized controlled trial. J Clin Psychiatry. 2018;79(3):17m11753. [DOI] [PubMed] [Google Scholar]
- 17. Dyck MS, Mathiak KA, Bergert S, et al. . Targeting treatment-resistant auditory verbal hallucinations in schizophrenia with fMRI-based neurofeedback—exploring different cases of schizophrenia. Front Psychiatry. 2016;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubera KM, Barth A, Hirjak D, Thomann PA, Wolf RC. Noninvasive brain stimulation for the treatment of auditory verbal hallucinations in schizophrenia: methods, effects and challenges. Front Syst Neurosci. 2015;9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markiewcz R. The use of EEG Biofeedback/Neurofeedback in psychiatric rehabilitation. Psychiatr Pol. 2017;51(6):1095–1106. [DOI] [PubMed] [Google Scholar]
- 20. Collura TF. Technical Foundations of Neurofeedback. New York: Routledge; 2014:1–266. [Google Scholar]
- 21. Ta Dinh S, Nickel MM, Tiemann L, et al. . Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. Pain. 2019;160(12):2751–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lührs M, Riemenschneider B, Eck J, et al. . The potential of MR-Encephalography for BCI/Neurofeedback applications with high temporal resolution. Neuroimage. 2019;194:228–243. [DOI] [PubMed] [Google Scholar]
- 23. Paret C, Goldway N, Zich C, et al. . Current progress in real-time functional magnetic resonance-based neurofeedback: methodological challenges and achievements. Neuroimage. 2019;202:116107. [DOI] [PubMed] [Google Scholar]
- 24. Keynan JN, Cohen A, Jackont G, et al. . Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nat Hum Behav. 2019;3(1):63–73. [DOI] [PubMed] [Google Scholar]
- 25. Gvirts HZ, Perlmutter R. What guides us to neurally and behaviorally align with anyone specific? A neurobiological model based on fNIRS hyperscanning studies. Neuroscientist. 2019;26(2):108–116; doi: 10.1177/1073858419861912 [DOI] [PubMed] [Google Scholar]
- 26. Serra-Sala M, Timoneda-Gallart C, Pérez-Álvarez F. Clinical usefulness of hemoencephalography beyond the neurofeedback. Neuropsychiatr Dis Treat. 2016;12:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hammond D. What is neurofeedback: an update. J Neurother. 2011;15:305–336. [Google Scholar]
- 28. Gray SN. An overview of the use of neurofeedback biofeedback for the treatment of symptoms of traumatic brain injury in military and civilian populations. Med Acupunct. 2017;29(4):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Razoki B. Neurofeedback versus psychostimulants in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a systematic review. Neuropsychiatr Dis Treat. 2018;14:2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci. 2009;40(3):180–189. [DOI] [PubMed] [Google Scholar]
- 31. Chiba T, Kanazawa T, Koizumi A, et al. . Current status of neurofeedback for post-traumatic stress disorder: a systematic review and the possibility of decoded neurofeedback. Front Hum Neurosci. 2019;13:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghaziri J, Tucholka A, Larue V, et al. . Neurofeedback training induces changes in white and gray matter. Clin EEG Neurosci. 2013;44(4):265–272. [DOI] [PubMed] [Google Scholar]
- 33. Tripathi A, Kar SK, Shukla R. Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clin Psychopharmacol Neurosci. 2018;16(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Orndorff-Plunkett F, Singh F, Aragon OR, Pineda JA. Assessing the effectiveness of neurofeedback training in the context of clinical and social neuroscience. Brain Sci. 2017;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marzbani H, Marateb HR, Mansourian M. Neurofeedback: a comprehensive review on system design, methodology and clinical applications. Basic Clin Neurosci. 2016;7(2):143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097; doi: 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balconi M, Frezza A, Vanutelli ME. Emotion regulation in schizophrenia: a pilot clinical intervention as assessed by EEG and optical imaging (functional near-infrared spectroscopy). Front Hum Neurosci. 2018;12:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kikuchi M, Koenig T, Wada Y, et al. . Native EEG and treatment effects in neuroleptic-naïve schizophrenic patients: time and frequency domain approaches. Schizophr Res. 2007;97(1–3):163–172. [DOI] [PubMed] [Google Scholar]
- 39. Rieger K, Rarra MH, Diaz Hernandez L, Hubl D, Koenig T. Neurofeedback-based enhancement of single-trial auditory evoked potentials: treatment of auditory verbal hallucinations in schizophrenia. Clin EEG Neurosci. 2018;49(6):367–378. [DOI] [PubMed] [Google Scholar]
- 40. Surmeli T, Ertem A, Eralp E, Kos IH. Schizophrenia and the efficacy of qEEG-guided neurofeedback treatment: a clinical case series. Clin EEG Neurosci. 2012;43(2):133–144. [DOI] [PubMed] [Google Scholar]
- 41. Schneider F, Rockstroh B, Heimann H, et al. . Selfregulation of slow cortical potentials in psychiatric patients: schizophrenia. Biofeedback Self Regul. 1992;17(4):277–292. [DOI] [PubMed] [Google Scholar]
- 42. Elbert T, Rockstroh B, Lutzenberger W, Birbaumer N. Biofeedback of slow cortical potentials. I. Electroencephalogr Clin Neurophysiol. 1980;48(3):293–301. [DOI] [PubMed] [Google Scholar]
- 43. Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990;70(1):1–41. [DOI] [PubMed] [Google Scholar]
- 44. Schneider SJ, Pope AT. Neuroleptic-like electroencephalographic changes in schizophrenics through biofeedback. Biofeedback Self Regul. 1982;7(4):479–490. [DOI] [PubMed] [Google Scholar]
- 45. Giannitrapani D, Kayton L. Schizophrenia and EEG spectral analysis. Electroencephalogr Clin Neurophysiol. 1974;36(4):377–386. [DOI] [PubMed] [Google Scholar]
- 46. Itil TM, Hsu W, Saletu B, Mednick S. Computer EEG and auditory evoked potential investigations in children at high risk for schizophrenia. Am J Psychiatry. 1974;131(8):892–900. [DOI] [PubMed] [Google Scholar]
- 47. Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16(12):606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hyun J, Baik MJ, Kang UG. Effects of psychotropic drugs on quantitative EEG among patients with schizophrenia-spectrum disorders. Clin Psychopharmacol Neurosci. 2011;9(2):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lubar JF. Neocortical dynamics: implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Appl Psychophysiol Biofeedback. 1997;22(2):111–126. [DOI] [PubMed] [Google Scholar]
- 50. Nan W, Wan F, Chang L, Pun SH, Vai MI, Rosa A. An exploratory study of intensive neurofeedback training for schizophrenia. Behav Neurol. 2017;2017:6914216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee SH, Wynn JK, Green MF, et al. . Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophr Res. 2006;83(2–3):111–119. [DOI] [PubMed] [Google Scholar]
- 52. Merrin EL, Floyd TC. Negative symptoms and EEG alpha in schizophrenia: a replication. Schizophr Res. 1996;19(2–3):151–161. [DOI] [PubMed] [Google Scholar]
- 53. Wada Y, Takizawa Y, Kitazawa S, Jiang ZY, Yamaguchi N. Quantitative EEG analysis at rest and during photic stimulation in drug-naive patients with first-episode paranoid schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1994;244(5):247–251. [DOI] [PubMed] [Google Scholar]
- 54. Zweerings J, Hummel B, Keller M, Zvyagintsev M, Schneider F, Klasen M, Mathiak K. . Neurofeedback of core language network nodes modulates connectivity with the default-mode network: a double-blind fMRI neurofeedback study on auditory verbal hallucinations. Neuroimage. 2019;189:533–542. [DOI] [PubMed] [Google Scholar]
- 55. Orlov ND, Giampietro V, O’Daly O, et al. . Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl Psychiatry. 2018;8(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cordes JS, Mathiak KA, Dyck M, et al. . Cognitive and neural strategies during control of the anterior cingulate cortex by fMRI neurofeedback in patients with schizophrenia. Front Behav Neurosci. 2015;9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sanders GS, Gallup GG, Heinsen H, Hof PR, Schmitz C. Cognitive deficits, schizophrenia, and the anterior cingulate cortex. Trends Cogn Sci. 2002;6(5):190–192. [DOI] [PubMed] [Google Scholar]
- 58. Ruiz S, Lee S, Soekadar SR, et al. . Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum Brain Mapp. 2013;34(1):200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Makris N, Goldstein JM, Kennedy D, et al. . Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83(2–3):155–171. [DOI] [PubMed] [Google Scholar]
- 60. Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68(5):425–432. [DOI] [PubMed] [Google Scholar]
- 61. Storchak H, Hudak J, Haeussinger FB, Rosenbaum D, Fallgatter AJ, Ehlis AC. Reducing auditory verbal hallucinations by means of fNIRS neurofeedback—a case study with a paranoid schizophrenic patient. Schizophr Res. 2019;204:401–403. [DOI] [PubMed] [Google Scholar]
- 62. Gomes JS, Ducos DV, Gadelha A, et al. . Hemoencephalography self-regulation training and its impact on cognition: a study with schizophrenia and healthy participants. Schizophr Res. 2018;195:591–593. [DOI] [PubMed] [Google Scholar]
- 63. Lewis DA, Glausier JR. Alterations in prefrontal cortical circuitry and cognitive dysfunction in schizophrenia. Nebr Symp Motiv. 2016;63:31–75. [DOI] [PubMed] [Google Scholar]
- 64. Zamanpoor M. Schizophrenia in a genomic era: a review from the pathogenesis, genetic and environmental etiology to diagnosis and treatment insights. Psychiatr Genet. 2020;30(1):1–9. [DOI] [PubMed] [Google Scholar]
- 65. Thapar A, Riglin L. The importance of a developmental perspective in Psychiatry: what do recent genetic-epidemiological findings show? Mol Psychiatry. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Swingle PG. Neurofeedback treatment of pseudoseizure disorder. Biol Psychiatry. 1998;44(11):1196–1199. [DOI] [PubMed] [Google Scholar]
- 67. Bussalb A, Congedo M, Barthélemy Q, et al. . Clinical and experimental factors influencing the efficacy of neurofeedback in ADHD: a meta-analysis. Front Psychiatry. 2019;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lambez B, Harwood-Gross A, Golumbic EZ, Rassovsky Y. Non-pharmacological interventions for cognitive difficulties in ADHD: a systematic review and meta-analysis. J Psychiatr Res. 2020;120:40–55. [DOI] [PubMed] [Google Scholar]