Objectives:
To describe the use of hemostatic transfusions in children following cardiac surgery with cardiopulmonary bypass and the association of hemostatic transfusions postoperatively with clinical outcomes.
Design:
A retrospective cohort study.
Setting:
PICU of a tertiary care center from 2011 to 2017.
Patients:
Children 0–18 years old undergoing cardiac surgery with cardiopulmonary bypass.
Interventions:
None.
Measurements and Main Results:
Four-hundred twenty children underwent cardiac surgery with cardiopulmonary bypass. The median (interquartile range) age was 0.8 years (0.3–5 yr) and 243 (58%) were male. The majority of cases were classified as Risk Adjustment for Congenital Heart Surgery 2 (223, 54%) or Risk Adjustment for Congenital Heart Surgery 3 (124, 30%). Twenty-four percent of children (102/420) received at least one hemostatic transfusion with the most common first product being platelet transfusions (47/102), followed by plasma (44/102), and cryoprecipitate (11/102). The children who received hemostatic transfusions were younger (p = 0.006), had lower body weights (p = 0.004), less likely to be their initial operation with cardiopulmonary bypass (p = 0.003), underwent more complex surgeries (p = 0.001) with longer bypass runs (p < 0.001), and had more use of hypothermic circulatory arrest (p = 0.014). The receipt of hemostatic blood products postoperatively was independently associated with more days of mechanical ventilation (p < 0.001) and longer PICU lengths of stay (p = 0.001) but not with time receiving vasoactive mediations (p = 0.113) or nosocomial infections (p = 0.299).
Conclusions:
Nearly one-quarter of children undergoing cardiac repair with cardiopulmonary bypass receive hemostatic transfusions postoperatively. These blood products are independently associated with worse clinical outcomes. Larger studies should be performed to determine the hemostatic efficacy of these products, as well as to clarify associated morbidities, in order to inform proper blood management.
Keywords: cardiopulmonary bypass, critical illness, pediatrics, plasma, platelet transfusions
Children who require cardiac surgery with cardiopulmonary bypass (CPB) have a significant risk of bleeding due to impaired hemostasis (1). Their diminished ability to clot is multifactorial and may result from circuit-induced platelet dysfunction, hemodilution, induced hypothermia and required anticoagulation (2–5). In response to bleeding or concern for the risk of bleeding, many cardiac anesthesiologists and pediatric intensivists often prescribe hemostatic blood products, such as platelet concentrates, plasma, or cryoprecipitate, either intraoperatively or postoperatively (6).
Although these products may offer therapeutic benefits, hemostatic transfusions have been associated with increased morbidity and mortality in critically ill children including increased organ dysfunction, length of stay, and the development of nosocomial infections (7, 8). Few studies have examined the epidemiology of hemostatic transfusions, specifically in pediatric patients undergoing CPB, and most of the work to date has focused on intraoperative blood use (9–11). Furthermore, although RBC transfusions have been associated with increased mortality and perioperative morbidity in adults undergoing CPB (12), few studies have examined the associations between the receipt of hemostatic transfusions postoperatively and clinical outcomes in children undergoing CPB. Although some blood product replacement may be required for hemostasis, a greater understanding of the morbidities associated with the receipt of hemostatic transfusions may help guide clinicians’ transfusion decisions or development of postoperative transfusion guidelines.
In this study, we sought to describe the use of hemostatic transfusions in children following cardiac surgery with CPB and the association of hemostatic transfusions postoperatively with clinical outcomes.
MATERIALS AND METHODS
Population and Setting
We performed a retrospective cohort study of all children 18 years old who underwent cardiac surgery with the use of CPB at a single, academic tertiary center from 2011 to 2017. The study was approved by the Institutional Review Board at Weill Cornell Medicine. All children were transferred to a 20-bedded mixed (medical and cardiac) PICU postoperatively. In general, the perfusion team primed the CPB circuit intraoperatively with RBCs if the patient was less than 10 kg and added plasma when children were less than 3 kg. Otherwise, for larger children, the circuit was primed with crystalloid as in adults. Prior to initiation of CPB, therapeutic anticoagulation with heparin was dosed centrally (400 U/kg) and reversed after separation from CPB with protamine (6 mg/kg) with additional protamine given if initial dose did not adequately bring the activated clotting time to the baseline value measured prior to initial heparin administration. A formal transfusion algorithm was not used, and blood products were given based on the clinical scenario, specifically hemodynamic monitoring and arterial blood gas hemoglobin and hematocrit values. Since viscoelastic data have not been extensively studied in the pediatric population, it was not used to guide transfusion in the intraoperative period. All children had a mediastinal drain that communicated with one or both pleural spaces (at the discretion of the cardiothoracic surgeon) but did not communicate with the peritoneum. All output from the surgical drain was recorded in the electronic medical record from the time of admission to the PICU. Children who required extracorporeal life support or died within the first 24 hours following surgery were excluded.
Patient demographics, surgical details, postoperative bleeding, transfusions, and clinical outcomes were extracted from the electronic medical record. Surgical complexity was captured by Risk Adjustment for Congenital Heart Surgery (RACHS) scoring (13). Hemostatic transfusions were defined as platelet concentrates, plasma concentrates, and cryoprecipitate. No formal transfusion protocols were in place in the PICU; transfusions were prescribed at the discretion of the pediatric intensivist based on the bleeding observed in the mediastinal drain and laboratory values. No viscoelastic testing was used to guide transfusion therapy postoperatively. Clinical outcomes of interest included length of mechanical ventilation, length of treatment with vasoactive medications, PICU length of stay, thrombotic complications, development of nosocomial infections, and in-hospital mortality. Thrombotic complications included stroke and clinically symptomatic deep venous thrombosis (DVT) and/or pulmonary embolism. Nosocomial infections were defined as a positive culture (blood, urine, respiratory, wound) secondary to central line-associated bloodstream infections, catheter-associated urinary tract infections, surgical site infections, or ventilator-associated pneumonia or a positive respiratory viral panel in the setting of new respiratory symptoms. Study data were managed using Research Electronic Data Capture tools hosted at Weill Cornell Medicine.
Statistical Analysis
Demographic and clinical characteristics were described as n (%) or median and interquartile range (IQR), as appropriate. Children who received hemostatic transfusions were compared to children who did not receive hemostatic transfusions by chi-square test/Fisher exact tests or Wilcoxon rank-sum tests. To examine receipt of hemostatic blood products with outcomes, multivariable linear and logistic regressions were constructed; models for all outcomes adjusted for patient age, RACHS score, CPB time, and blood loss within the first 4 hours postoperatively. All p values were two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in R Version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Use of Hemostatic Transfusions
Four-hundred twenty children were included. The median (IQR) age of the children was 0.8 years (0.3–5 yr) and 243 (58%) were male. The majority of the cases were classified as RACHS 2 (223, 54%) or RACHS 3 (124, 30%). Intraoperative records were located in 95% of the cases (399/420). Nearly all patients received either aminocaproic acid (87%, 348/399) or tranexamic acid (6%, 24/399). One patient received factor VIIa and no children received prothrombin complex concentrate or von Willebrand factor. The median (IQR) dose of protamine received intraoperatively was 7.0 mg/kg (5.9–8.4 mg/kg). One-hundred two children (24%) received hemostatic transfusions postoperatively. Of the different blood products given first, children most commonly received platelet concentrates (46%), followed by plasma (43%) and cryoprecipitate (11%). Of the 102 children who received hemostatic transfusions, 45 (44%) received a second transfusion (17 received platelets, 14 received plasma, and 14 received cryoprecipitate). Nineteen children received a third transfusion (eight received platelets, six received plasma, and five received cryoprecipitate). In total, the median (IQR) doses of hemostatic products were as follows: platelets 10 mL/kg (9–14 mL/kg); plasma 10 mL/kg (10–15 mL/kg); and cryoprecipitate 5 mL/kg (3–5 mL/kg). In addition to hemostatic transfusions, one child received factor VIIa and no children received tranexamic acid postoperatively.
Table 1 describes the demographics and surgical characteristics of the two groups. The children who received hemostatic transfusions were younger (p = 0.006), had lower body weights (p = 0.004), less likely to be their initial repair (p = 0.003), underwent more complex repairs (p = 0.001) with longer bypass runs (p < 0.001), and had more use of hypothermic circulatory arrest (p = 0.014). The children who received hemostatic transfusions had significantly more bleeding at all recorded time points (p < 0.001) as depicted in Figure 1. Specifically, in the first hour after admission to the PICU during which time the majority of the blood products were transfused, the children who received hemostatic transfusions had a median (IQR) blood loss of 3.4 mL/kg/hr (1.8–5.3 mL/kg/hr) as compared to a median (IQR) blood loss of 1.4 mL/kg/hr (0.8–2.6 mL/kg/hr) (p < 0.001) in those who did not receive hemostatic transfusions.
Table 1.
Demographics and Surgical Characteristics of Children Undergoing Cardiac Surgery With Cardiopulmonary Bypass
Figure 1.

Blood loss from mediastinal drains following admission to PICU.
Clinical Outcomes
In the overall cohort, the median (IQR) length of mechanical ventilation was 1 day (0–4 d), the median (IQR) length of vasoactive therapy was 1 day (0–3 d), and the median (IQR) length of PICU stay was 5 days (4–8 d). Forty-five children (11%) developed nosocomial infections and 16 children (4%) had thrombotic complications (13 DVTs, three strokes). One patient died.
A comparison of the clinical outcomes between the two groups is presented in Table 2. Children who received hemostatic transfusions had more days of mechanical ventilation (p < 0.001), more days of vasoactive therapy (p < 0.001), longer PICU length of stay (p < 0.001), more nosocomial infections (p = 0.005), and more thrombotic complications (p = 0.002). To examine the independent association between the receipt of hemostatic transfusions and each of the clinical outcomes, multivariate regressions were performed, each adjusting for patient age, RACHS score, CPB time, and blood loss within the first 4 hours postoperatively. The receipt of hemostatic blood products postoperatively was independently associated with more days of mechanical ventilation (p < 0.001) and a longer PICU length of stay (p = 0.001) but not with the time receiving vasoactive mediations (p = 0.113) or nosocomial infections (p = 0.299) (Table 3). The prevalence of thrombotic complications was too low in the cohort to analyze its association with the receipt of hemostatic transfusions.
Table 2.
Clinical Outcomes of Children Undergoing Cardiac Surgery With Cardiopulmonary Bypass
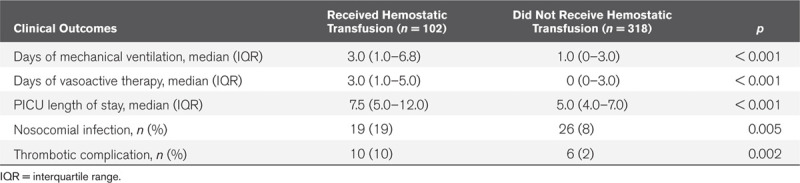
Table 3.
Independent Association of the Receipt of Hemostatic Blood Products With Clinical Outcomes
DISCUSSION
Children undergoing cardiac repair with CPB are at high risk of bleeding and receive hemostatic transfusions frequently following their surgeries. Nearly one-quarter of the children received at least one hemostatic product with platelet concentrates and plasma being the most common. Over 10% of the children received two hemostatic transfusions and nearly 5% received three. The children who received platelet concentrates, plasma, or cryoprecipitate were younger and underwent more complicated repair, with longer times on CPB and more use of hypothermic circulatory arrest. After adjusting for age, surgical complexity, CPB time, and blood loss postoperatively, the receipt of hemostatic transfusions was independently associated with a longer time of mechanical ventilation and longer PICU length of stay.
The rates of transfusion of hemostatic products reported here are lower than previously described, which range from 30% to 60% (6, 7, 14). The discrepancy may be secondary to an older population (some previous studies focused on infants alone) or less surgical complexity. The median blood loss of our patients in the first 4 hours following surgery was lower than previous reports (6, 10, 11) and would not be considered severe according to a recent consensus definition of bleeding particularly applicable to critically ill children (15). Last, the difference in rates of transfusion may be the result of practitioner cognizance of patient blood management principles.
We demonstrated that platelet concentrates are the most common blood product first transfused to children following CPB. The prescription of platelet concentrates makes physiologic sense in an effort to correct the hemodilution and platelet dysfunction induced by the CPB circuit (16). Theoretically, platelet concentrates have been shown to be the superior blood component to correct impaired thrombin generation ex vivo in this pediatric population (17). The hemostatic efficacy of plasma and cryoprecipitate is less clear (18, 19). In an observational study of 75 children undergoing CPB, the transfusion of cryoprecipitate after platelet concentrates was more efficacious when compared to plasma after platelet transfusions (20). Furthermore, other products, such as fibrinogen concentrate (21), prothrombin complex concentrate (22), and whole blood (23), may be equally, if not more effective, in hemostasis in children following CPB.
Previous studies in this pediatric population have examined the relationship between the receipt of plasma and the prevalence of nosocomial infection (14, 24). Although immunomodulatory effects have been observed in critically ill children who receive blood products (25), in the most recent report of 233 infants who underwent cardiac repair with CPB (94 of whom received plasma), no association was seen between the receipt of plasma and nosocomial infections after propensity score matching (14). Although the association of RBC transfusions and clinical outcomes in adults undergoing CPB has been studied extensively (26–28), little work has been done to explore similar outcomes in either adults or children receiving hemostatic products following CPB. Likewise, the receipt of RBC transfusions has been strongly associated with the development of thrombotic complications in adult surgical patients (29), and our findings suggest that there may be a similar association in children undergoing CPB. These results provide initial epidemiologic associations that should be further explored in larger cohorts.
There are several strengths to this study. This work not only details the use of platelet concentrates, plasma, and cryoprecipitate postoperatively but importantly collects information on children who did not receive hemostatic transfusions. By doing so, we were able to relate the receipt of these products with several relevant clinical outcomes in children undergoing cardiac surgery with CPB. In addition, bleeding and hemostatic transfusions are closely related and often collinear in analyses (30). However, we were able to adjust for bleeding in our multivariate model and examine the association of hemostatic transfusions, separate from bleeding itself, on clinical outcomes.
Some limitations exist. The study is observational and, as such, can only demonstrate associations and not causations. Although the sample size is relatively large, the data are retrospective and come from a single center. Children with highly complex surgeries (RACHS 5 and 6) were not included, and therefore, the results may not be generalizable. Children undergoing CPB are highly complex patients, and there may have been some unmeasurable factors or components of their care, including intraoperative transfusions, which were not collected that may have impacted the clinical outcomes. Pathogen-reduced plasma and platelets were introduced during the end of the study and may have affected either hemostatic efficacy and/or clinical outcomes.
CONCLUSIONS
Nearly one-quarter of the children undergoing cardiac repair with CPB receive hemostatic transfusions postoperatively. These blood products are associated with worse clinical outcomes. Larger observational studies and clinical trials should be performed to determine the hemostatic efficacy of these products, as well as to clarify associated morbidities, in order to inform proper blood management in these children.
Footnotes
Dr. Dayton received funding from Zogenix. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.McEwan A. Aspects of bleeding after cardiac surgery in children. Paediatr Anaesth. 2007; 17:1126–1133 [DOI] [PubMed] [Google Scholar]
- 2.Turner-Gomes SO, Andrew M, Coles J, et al. Abnormalities in von Willebrand factor and antithrombin III after cardiopulmonary bypass operations for congenital heart disease. J Thorac Cardiovasc Surg. 1992; 103:87–97 [PubMed] [Google Scholar]
- 3.Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: A review. Intensive Care Med. 2004; 30:1873–1881 [DOI] [PubMed] [Google Scholar]
- 4.Andreasen JB, Hvas AM, Christiansen K, et al. Can RoTEM® analysis be applied for haemostatic monitoring in paediatric congenital heart surgery? Cardiol Young. 2011; 21:684–691 [DOI] [PubMed] [Google Scholar]
- 5.Bønding Andreasen J, Hvas AM, Ravn HB. Marked changes in platelet count and function following pediatric congenital heart surgery. Paediatr Anaesth. 2014; 24:386–392 [DOI] [PubMed] [Google Scholar]
- 6.Petäjä J, Lundström U, Leijala M, et al. Bleeding and use of blood products after heart operations in infants. J Thorac Cardiovasc Surg. 1995; 109:524–529 [DOI] [PubMed] [Google Scholar]
- 7.Karam O, Lacroix J, Robitaille N, et al. Association between plasma transfusions and clinical outcome in critically ill children: A prospective observational study. Vox Sang. 2013; 104:342–349 [DOI] [PubMed] [Google Scholar]
- 8.Nellis ME, Karam O, Mauer E, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network, Pediatric Critical Care Blood Research Network (BloodNet), and the P3T Investigators. Platelet transfusion practices in critically ill children. Crit Care Med. 2018; 46:1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faraoni D, Willems A, Romlin BS, et al. Development of a specific algorithm to guide haemostatic therapy in children undergoing cardiac surgery: A single-centre retrospective study. Eur J Anaesthesiol. 2015; 32:320–329 [DOI] [PubMed] [Google Scholar]
- 10.Savan V, Willems A, Faraoni D, et al. Multivariate model for predicting postoperative blood loss in children undergoing cardiac surgery: A preliminary study. Br J Anaesth. 2014; 112:708–714 [DOI] [PubMed] [Google Scholar]
- 11.Spiezia L, Di Gregorio G, Campello E, et al. Predictors of postoperative bleeding in children undergoing cardiopulmonary bypass: A preliminary Italian study. Thromb Res. 2017; 153:85–89 [DOI] [PubMed] [Google Scholar]
- 12.Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007; 116:2544–2552 [DOI] [PubMed] [Google Scholar]
- 13.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002; 123:110–118 [DOI] [PubMed] [Google Scholar]
- 14.Chenouard A, Rozé JC, Hanf M, et al. Evaluation of the relationship between plasma transfusion and nosocomial infection after cardiac surgery in children younger than 1 year. Pediatr Crit Care Med. 2015; 16:139–145 [DOI] [PubMed] [Google Scholar]
- 15.Nellis ME, Tucci M, Lacroix J, et al. Bleeding assessment scale in critically ill children (BASIC): Physician-driven diagnostic criteria for bleeding severity. Crit Care Med. 2019; 47:1766–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AK, Leaker M, Burrows FA, et al. Coagulation and fibrinolytic profile of paediatric patients undergoing cardiopulmonary bypass. Thromb Haemost. 1997; 77:270–277 [PubMed] [Google Scholar]
- 17.Andreasen JB, Ravn HB, Hvas AM. Changes in thrombin generation in children after cardiac surgery and ex-vivo response to blood products and haemostatic agents. Blood Coagul Fibrinolysis. 2016; 27:24–30 [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Stanworth S, Hopewell S, et al. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion. 2012; 52:1673–1686; quiz 1673 [DOI] [PubMed] [Google Scholar]
- 19.Casbard AC, Williamson LM, Murphy MF, et al. The role of prophylactic fresh frozen plasma in decreasing blood loss and correcting coagulopathy in cardiac surgery. A systematic review. Anaesthesia. 2004; 59:550–558 [DOI] [PubMed] [Google Scholar]
- 20.Miller BE, Mochizuki T, Levy JH, et al. Predicting and treating coagulopathies after cardiopulmonary bypass in children. Anesth Analg. 1997; 85:1196–1202 [DOI] [PubMed] [Google Scholar]
- 21.Callum J, Farkouh ME, Scales DC, et al. Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: The FIBRES randomized clinical trial. JAMA. 2019; 322:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald J, Lenihan M, Callum J, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: A propensity score matched comparison to plasma. Br J Anaesth. 2018; 120:928–934 [DOI] [PubMed] [Google Scholar]
- 23.Manno CS, Hedberg KW, Kim HC, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991; 77:930–936 [PubMed] [Google Scholar]
- 24.Valera M, Scolfaro C, Cappello N, et al. Nosocomial infections in pediatric cardiac surgery, Italy. Infect Control Hosp Epidemiol. 2001; 22:771–775 [DOI] [PubMed] [Google Scholar]
- 25.Muszynski JA, Spinella PC, Cholette JM, et al. ; Pediatric Critical Care Blood Research Network (Blood Net). Transfusion-related immunomodulation: Review of the literature and implications for pediatric critical illness. Transfusion. 2017; 57:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaPar DJ, Crosby IK, Ailawadi G, et al. ; Investigators for the Virginia Cardiac Surgery Quality Initiative. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thorac Cardiovasc Surg. 2013; 145:796–803 [DOI] [PubMed] [Google Scholar]
- 27.Engoren MC, Habib RH, Zacharias A, et al. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002; 74:1180–1186 [DOI] [PubMed] [Google Scholar]
- 28.Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: The Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007; 835 SupplS27–S86 [DOI] [PubMed] [Google Scholar]
- 29.Goel R, Patel EU, Cushing MM, et al. Association of perioperative red blood cell transfusions with venous thromboembolism in a North American Registry. JAMA Surg. 2018; 153:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nellis ME, Dalton H, Karam O; PediECMO Investigators. Quantifiable bleeding in children supported by extracorporeal membrane oxygenation and outcome. Crit Care Med. 2019; 47:e886–e892 [DOI] [PMC free article] [PubMed] [Google Scholar]


