Abstract
Background:
Orthopaedic trauma etiologies are a common cause for amputation. Targeted muscle reinnervation (TMR) is a technique aimed at reducing or preventing pain and improving function. The purpose of this study was to examine postoperative phantom limb pain and residual limb pain following TMR in orthopaedic trauma amputees. In addition, postoperative rates of opioid and neuromodulator medication use were evaluated.
Methods:
Twenty-five patients (60% male) prospectively enrolled in a single-institution study and underwent TMR at the time of major limb amputation (48% nonmilitary trauma, 32% infection secondary to previous nonmilitary trauma, and 20% other, also secondary to trauma). Phantom limb pain and residual limb pain scores, pain temporality, prosthetic use, and unemployment status were assessed at the time of follow-up. The use of opioid and neuromodulator medications both preoperatively and postoperatively was also examined.
Results:
At a mean follow-up of 14.1 months, phantom limb pain and residual limb pain scores were low, with 92% of the patients reporting no pain or brief intermittent pain only. Pain scores were higher overall for male patients compared with female patients (p < 0.05) except for 1 subscore, and higher in patients who underwent amputation for infection (odds ratio, 9.75; p = 0.01). Sixteen percent of the patients reported opioid medication use at the time of the latest documented follow-up. Fifty percent of the patients who were taking opioids preoperatively discontinued use postoperatively, while 100% of the patients who were not taking opioids preoperatively discontinued postoperative use. None of the patients who were taking neuromodulator medication preoperatively discontinued use postoperatively (0 of 5). The median time to neuromodulator medication discontinuation was 14.6 months, with female patients taking longer than male patients (23 compared with 7 months; p = 0.02). At the time of the latest follow-up, the rate of reported prosthetic use was 85% for lower-extremity and 40% for upper-extremity amputees, with a rate of unemployment due to disability of 36%.
Conclusions:
The use of TMR in orthopaedic trauma amputees was associated with low overall pain scores at 2-year follow-up, decreased overall opioid and neuromodulator medication use, and an overall high rate of daily prosthetic use.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Major limb amputation is a common operation in the United States, with approximately 185,000 new amputations performed each year1. Many of the nearly 2 million amputees in the United States experience various limitations in activities of daily living, and adjusting to, and coping with, these changes and/or potential mobility challenges contribute to further physical and psychological morbidity. Previous literature suggests that at least 25% of those who have undergone major limb amputation will develop chronic localized pain due to symptomatic neuromas within the residual limb2-6. Prosthetic use can further aggravate neuroma-related pain and preclude the desire to continue wearing a prosthesis, thus reducing an amputee’s functional ability and quality of life. In addition to neuroma-related pain, amputees also often experience phantom limb pain as well as residual limb pain. While the exact mechanisms of phantom and residual limb pain are poorly understood, it is surmised that spontaneous and abnormal peripheral nerve signals, along with central changes including cortical reorganization and gray-matter changes, play a role7-13.
Given the disabling nature of any type of pain, efforts have been made to reduce the rate at which it occurs. One recent strategy for amputees is targeted muscle reinnervation (TMR), which is a surgical technique developed in the early 2000s. Initially used for patients with shoulder disarticulation or who have had a transhumeral amputation, TMR transfers the transected peripheral nerves that no longer have motor or sensory end-organs into recipient motor nerves of residual muscle in the amputated extremity2-4,6,14. While initially developed in an effort to “bioamplify” myoelectric signals for advanced bioprosthetic limbs, early clinical results demonstrated an improvement in neuroma-related pain and other postoperative pain symptoms15-21.
The most recent data on TMR have shown not only improvement in the occurrence of neuroma-related pain but also a decrease in phantom limb pain and residual limb pain as well20-24. A recent randomized controlled trial demonstrated decreased phantom limb pain and residual limb pain with TMR compared with traditional amputation methods of neurectomy25. This has led many surgeons to strongly consider preemptive surgical intervention for amputated nerves with TMR at the time of limb loss in order to reduce these complications20.
Orthopaedic etiologies are commonly a source of amputation, whether traumatic, infectious, or oncologic in nature. The effects and outcomes of TMR in the orthopaedic amputee are not fully known. Given the frequency and wide clinical relevancy of orthopaedic amputations performed for trauma or infection-related reasons, the main aim of the current study was to determine whether TMR performed at the time of major limb amputation improved postoperative phantom limb pain and residual limb pain. Given the current state of opioid use, the secondary aim of this study was to report rates of opioid and neuromodulator medication use for our TMR amputee cohort. We hypothesized that amputees receiving TMR would have improved outcomes when compared with findings in the reported literature for traditional amputation techniques.
Materials and Methods
Patient Selection
Patients were eligible for inclusion if scheduled for major limb amputation with concurrent TMR for any nonmilitary orthopaedic trauma-related cause, including infection secondary to trauma. While routinely performed under the same anesthesia, the time from amputation to TMR was required to be ≤2 weeks. Those requiring amputation for oncologic or congenital indications were excluded. Indications for amputation included various nonmilitary traumatic injuries, failed limb salvage secondary to previous traumatic injury, or acute or acute-on-chronic infection secondary to previous traumatic injury, all at the discretion of the senior attending surgeon involved in the case. All amputations performed were considered primary in nature, meaning none were performed for a residual symptomatic neuroma, and all patients were nonneuropathic. The amputation portion of the procedure was performed by various fellowship-trained orthopaedic surgeons, while the TMR portion of the case was performed exclusively by the senior attending surgeon and co-author (I.L.V.). All patients provided written informed consent using institutional review board-approved consent forms and procedures. All surgeries occurred at a single academic teaching institution between March 2015 and December 2018. Patients were excluded if they were <18 years of age, had cognitive impairment, or were imprisoned. Data were collected as part of routine postoperative follow-up care.
Outcome Measures
Basic demographic data were collected for every patient. Also recorded were the indication for surgery, time from amputation to the TMR procedure, level of amputation, total number of nerves utilized in the transfer, and whether a perioperative nerve block was used. Preoperative use of opioid pain medication and neuromodulator medication (e.g., gabapentin) were recorded by survey. Outcome data were collected by the senior author and his clinical physician assistant and research team at 3-month, 6-month, 1-year, and 2-year postoperative follow-up visits. An in-office survey was used to collect data including opioid pain medication use, neuromodulator medication use, phantom limb pain (PLP) scores, residual limb pain (RLP) scores, pain temporality (changes in pain over time), prosthetic use, and postoperative employment status. PLP and RLP scores were further categorized by interference, intensity, and behavior using Patient-Reported Outcomes Measurement Information System (PROMIS) instruments26-28. Opioid consumption was further verified using a statewide monitoring access system.
Surgical Technique
TMR can be performed on any major extremity at any level with a standardized amputation technique29-31. The technique for a below-the-knee amputation with TMR is briefly outlined below, but the same principles can be extrapolated to more proximal lower-extremity amputations as well as to upper-extremity amputations, with the corresponding nerves at that particular anatomic level being utilized instead to create the appropriate neurorrhaphy.
Amputation with TMR at the below-the-knee (transtibial) level begins by utilizing the traditional posterior gastrocnemius and soleus myocutaneous flap32,33. Care is taken to identify and not cut or burn through the nerves to be identified. Each nerve is identified, dissected distally for additional length, marked with a 6-0 nonabsorbable polypropylene suture, and then divided to preserve length for transfer. The muscular compartments are divided in a manner preserving sites of motor innervation for future neurorrhaphy. The tibia is then cleared and divided with an anterior bevel proximally, followed by the fibula at a level slightly more proximally. The major vessels of the leg are ligated and divided when encountered.
With the proximal nerves identified and tagged as outlined above, attention is turned to locating motor nerve recipients for nerve transfer. A nerve stimulator can help identify motor nerve branches entering the target muscle with minimal additional dissection. The identified motor nerve is transected with straight microscissors near its entry into the muscle in preparation for pending nerve transfer. An end-to-end neurorrhaphy from the peripheral nerve stump to the desired motor nerve branch is then performed. These neurorrhaphies are performed with interrupted 8-0 nylon epineural stitches interspaced so that the fascicles remain within the nerve and not flaring out from the edges of the repair. Of note, there is often a size mismatch between the proximal major peripheral nerve and the target motor nerve at the neurorrhaphy site. With our technique, we dissect a portion of the surrounding muscle and then cerclage this muscle cuff around the nerve coaptation using 4-0 or 5-0 absorbable sutures. This muscle cuff completely encircles the nerve coaptation, ensuring that nerve regeneration can thus optimally enter the target motor nerve-muscle unit construct. In following these principles, the most commonly performed nerve transfers are outlined in Table I. After performing the nerve transfers (i.e., TMR procedures), closure of the below-the-knee amputation wound can be completed in the standard fashion.
TABLE I.
Common Below-the-Knee Amputation Nerve Transfers
| Donor Nerve | Target Motor Nerve Branches* |
| Posterior tibial nerve | Medial or lateral gastrocnemius |
| Medial or lateral soleus | |
| Tibialis posterior | |
| Common peroneal nerve | Tibialis anterior |
| Peroneus longus | |
| Peroneus brevis | |
| Medial soleus | |
| Superficial peroneal nerve | Peroneus brevis |
| Peroneus longus | |
| Saphenous nerve | Medial gastrocnemius |
| Medial soleus | |
| Sural nerve | Lateral gastrocnemius |
| Lateral soleus | |
| Tibialis posterior |
The most common target nerves are shown in bold.
Statistical Analysis
All analyses were performed using a standard software package (JMP Pro 14.2; SAS Institute). Descriptive statistics were generated for the entire sample. Normally distributed continuous data were reported as the mean and standard deviation, whereas non-normally distributed data were reported as the median with the interquartile range (IQR; 25th to 75th percentile). PLP and RLP scores were non-normally distributed, and comparisons between groups were performed via a Wilcoxon rank-sum test. Times to discontinuation of opioid and neuromodulator medication use were summarized by Kaplan-Meier plots with consideration for length of follow-up, and comparisons between groups were performed via a Wilcoxon rank-sum test.
Results
Descriptive Data
A total of 34 consecutive TMR cases for nononcologic and noncongenital indications met all inclusion criteria. Of these patients, 3 had died from causes unrelated to the TMR procedure. Of the remaining 31 patients, 25 (81%) were successfully evaluated at a mean (and standard deviation) of 14.1 ± 7.6 months of follow-up. Sixty percent (15) of the patients were male. Forty-eight percent (12) of the patients underwent amputation for nonmilitary orthopaedic trauma, 32% (8) for infection, 8% (2) for vasopressor-induced necrosis, 4% (1) for burns, 4% (1) for arteriovenous malformation, and 4% (1) for complex regional pain syndrome (Table II). The most common level of amputation was below-the-knee (transtibial; 48%, 12 patients), followed by above-the-knee (transfemoral; 32%, 8 patients), above-the-elbow (transhumeral; 12%, 3 patients), and below-the-elbow (transradial; 8%, 2 patients). One TMR case was performed 14 days after the initial amputation. The number of nerves used in the procedure ranged from 2 to 6, with a mean of 3.8 ± 1.1 nerves used. A perioperative regional nerve block was utilized for 60% (15) of the patients, and 32% (8) of the patients reported preoperative opioid use and 20% (5) reported preoperative neuromodulator medication use for at least 1 month prior to the date of amputation.
TABLE II.
Summary Data (N = 25 Patients)*
| Age† (yr) | 47.5 ± 13.1 |
| Sex | |
| Male | 60% (15) |
| Female | 40% (10) |
| Caucasian | 92% (23) |
| African-American | 8% (2) |
| History of diabetes mellitus | 16% (4) |
| Educational level | |
| Some high school | 4% (1) |
| High school graduate/equivalent | 32% (8) |
| Some college, associate degree, or trade school | 44% (11) |
| Bachelor’s degree or higher | 20% (5) |
| Preop. opioid use | 32% (8) |
| Preop. neuromodulator use | 20% (5) |
| Indication for surgery | |
| Nonmilitary trauma | 48% (12) |
| Infection | 32% (8) |
| Other‡ | 20% (5) |
| Amputation level | |
| Below-the-knee (transtibial) | 48% (12) |
| Above-the-knee (transfemoral) | 32% (8) |
| Below-the-elbow (transradial) | 8% (2) |
| Above-the-elbow (transhumeral) | 12% (3) |
| Time from amputation to TMR§ (days) | 0 (0-1) [14] |
| No. of nerves used in procedure† | 3.8 ± 1.1 (2-6) |
| Periop. regional nerve block used | 60% (15) |
The values are given as the percentage, with the number of patients in parentheses, except as otherwise noted.
The values are given as the mean and standard deviation; the range is given in parentheses for the number of nerves used.
Other indications included vasopressor-induced necrosis (n = 2), burns (n = 1), arteriovenous malformation (n = 1), and complex regional pain syndrome (n = 1).
The values are given as the median, with the interquartile range in parentheses and the maximum in square brackets.
Self-Reported Phantom or Residual Limb Pain
In general, PLP and RLP scores were low at the time of final follow-up, and 92% of patients reported no pain or intermittent pain events without pain between episodes (Table III). PLP behavior subscores tended to decrease with increased length of follow-up (mean 0.4-point decrease per month of follow-up, r2 = 0.21; p = 0.02); otherwise, no significant association between PLP or RLP scores and length of follow-up was identified. Pain scores were higher for male patients than for female patients for all subscores except the PLP interference subscore (Table IV). No association was identified between amputation level or site (upper versus lower extremity) and pain scores at the time of final follow-up (p > 0.25 for each comparison).
TABLE III.
Outcome Data*
| Postop. employment status (% [no.]) | |
| Employed full time | 36% (9) |
| Student | 4% (1) |
| Unemployed, seeking work | 4% (1) |
| Retired | 20% (5) |
| Unemployed, disabled | 36% (9) |
| Reported daily prosthetic use (% [no./total no.]) | |
| Overall | 76% (19/25) |
| Upper-extremity prosthetic use | 40% (2/5) |
| Lower-extremity prosthetic use | 85% (17/20) |
| Neuromodulator (NM) medication use (% [no./total no.]) | |
| NM use at latest follow-up | 56% (14/25) |
| Successful discontinuation of NM use (use preceding TMR) | 0% (5/5 continued) |
| New chronic NM use following amputation and TMR | 45% (9/20) |
| Opioid medication use (% [no./total no.]) | |
| Opioid use at latest follow-up | 16% (4/25) |
| Successful discontinuation of chronic opioid use (use preceding TMR) | 50% (4/8 continued) |
| New chronic opioid use following amputation and TMR | 0% (0/17) |
| RLP score† | |
| Interference | 8 (8-8) [32] |
| Intensity | 4 (3-6) [10] |
| Behavior | 7 (7-14.5) [33] |
| PLP score† | |
| Interference | 8 (8-8) [30] |
| Intensity | 4 (3-5.5) [10] |
| Behavior | 14 (7-16) [30] |
| Pain temporality (% [no./total no.]) | |
| No pain | 48% (12/25) |
| Pain events without pain in between | 44% (11/25) |
| Steady pain with slight changes | 4% (1/25) |
| Steady pain with intense pain attacks | 4% (1/25) |
TMR = targeted muscle reinnervation, PLP = phantom limb pain, and RLP = residual limb pain.
The values are given as the median, with the interquartile range (IQR) in parentheses and the maximum in square brackets.
TABLE IV.
Pain Scores by Patient Sex*
| Measure | Median Score (IQR) | P Value | |
| Male | Female | ||
| RLP interference | 8 (8-10) | 8 (8-8) | 0.047 |
| RLP intensity | 5 (3-7) | 3 (3-4) | 0.01 |
| RLP behavior | 14 (7-17) | 7 (7-7) | 0.01 |
| PLP interference | 8 (8-11) | 8 (8-8) | 0.27 |
| PLP intensity | 5 (4-7) | 3.5 (3-4.25) | 0.048 |
| PLP behavior | 15 (14-16) | 7 (7-15) | 0.05 |
IQR = interquartile range, RLP = residual limb pain score, and PLP = phantom limb pain score.
Patients who preoperatively were using neuromodulator medication to control neuropathic pain were more likely to have had residual symptoms. Among the patients with preoperative neuromodulator medication use, 100% (5 of 5) had ≥1 RLP or PLP score above the 75th percentile of all patients in the current study; in contrast, only 25% (5 of 20) of the patients without preoperative neuromodulator medication use had ≥1 RLP or PLP score above the 75th percentile. Of the 5 patients with preoperative neuromodulator medication use, at the time of final follow-up 2 of 5 rated “no pain” on a pain temporality diagram, 1 of 5 reported pain events without pain in between, 1 of 5 reported steady pain with slight changes, and 1 of 5 reported steady pain with intense pain attacks.
In addition, there was a higher rate of residual symptoms in patients who underwent amputation for infection: 75% (6 of 8) rated high residual or phantom pain symptoms compared with 24% (4 of 17) who underwent amputation for non-infection-related reasons (odds ratio [OR], 9.75; 95% confidence interval [CI], 1.38 to 68.8; p = 0.01). However, 50% (4 of 8) of the patients with amputation for infection were using neuromodulator medication preoperatively.
Medication Use
Opioid discontinuation rates were excellent following amputation with TMR. Among all patients, 16% (4 of 25) reported opioid use at the latest follow-up. Among the 8 patients with preoperative opioid use, 50% had discontinued opioid use postoperatively, with a median time to discontinuation of 5 months (Fig. 1). Among the 17 patients without preoperative opioid use, 100% had discontinued postoperative opioid use at the time of the latest follow-up, with a significantly faster median time to discontinuation of 2 months (p = 0.02, Wilcoxon rank-sum) (Fig. 1).
Fig. 1.
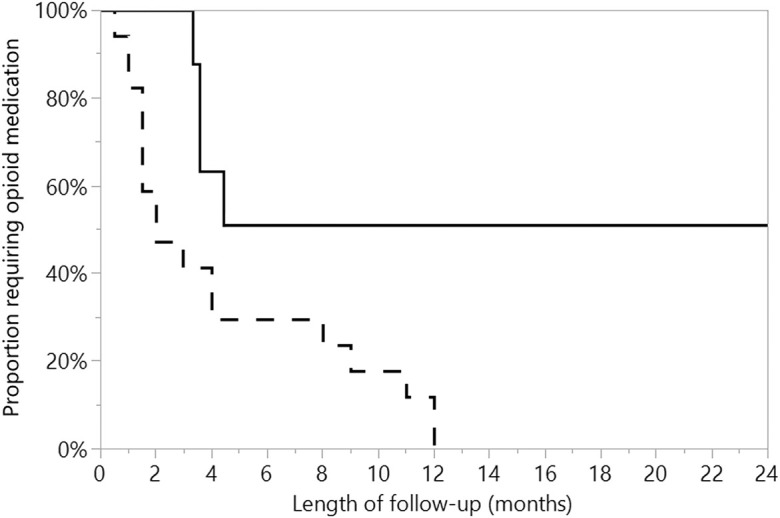
The median time to discontinuation of opioid medication was 4 months overall, 2 months for patients without a history of preoperative opioid use (dashed line), and 5 months for patients reporting preoperative opioid use (solid line) (p = 0.02, Wilcoxon rank-sum).
Neuromodulator medication discontinuation rates varied on the basis of patient sex and preoperative neuromodulator medication use. None of the 5 patients who were on neuromodulator medication for neuropathic pain preoperatively had discontinued use at the time of the latest follow-up. Among the 20 patients who were not using neuromodulator medication preoperatively, the median time to discontinuation of postoperative neuromodulator medication was 14.6 months, with male patients discontinuing neuromodulator medication sooner than female patients (median time of 7 months compared with 23 months; p = 0.02, Wilcoxon rank-sum) (Fig. 2).
Fig. 2.
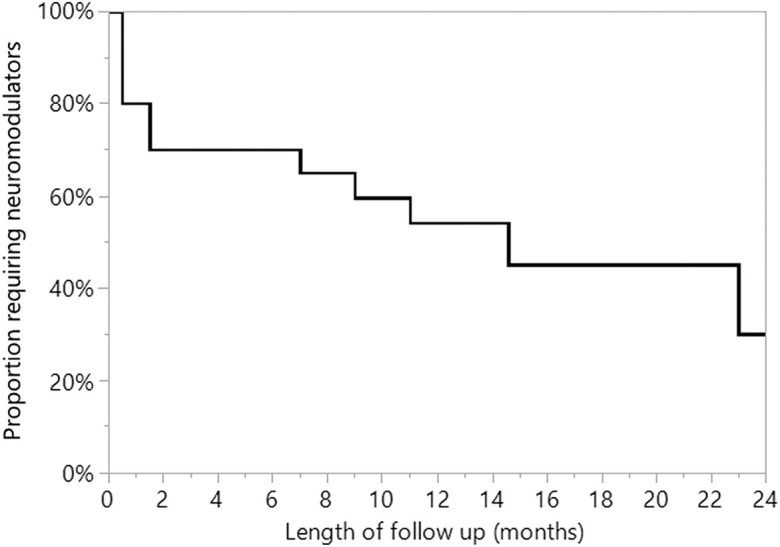
Time to discontinuation of neuromodulator medication use among patients who were not using neuromodulator medication preoperatively. The median time to neuromodulator discontinuation was 14.6 months. Of the 5 patients with preoperative use of neuromodulators (not depicted in graph), none had discontinued at a mean of 6.3 months of follow-up.
Functional Outcomes
At the latest follow-up, a high rate of daily lower-extremity prosthetic use was reported (85%, 17 of 20). The rate for daily use of an upper-extremity prosthesis was 40% (2 of 5). The rate of unemployment due to self-reported disability was 36% (9 of 25).
Discussion
Multiple studies have evaluated the prevalence of symptomatic phantom limb pain and residual limb pain in amputees to denote the magnitude of these morbidities. Reported rates of symptomatic phantom limb pain and residual neuroma pain among amputee patients are 9% to 67% and 2% to 25%, respectively2-4,6,14,15. In a military population, Carlen et al. found that 67% of soldiers with traumatic amputation had phantom limb pain in the first few months after surgery16. Early results from the current study and prior work by the senior author20,23,25,32 suggest that symptoms of phantom and residual limb pain may persist for a short period after surgery, but in the current study, 92% of the patients at the most recent follow-up reported no pain or brief intermittent pain only. This is a notable improvement from previously reported rates2-6.
Postoperative and long-term pain control among amputee patients continues to be a struggle, and multiple strategies have been attempted to aid with this, including the use of regional anesthesia, perineural anesthesia, opioid analgesics, nonopioid analgesics, and neuromodulator medications34-36. Known risk factors for increased postoperative pain in amputees include increased preoperative anxiety37, increased preoperative pain and intensity38,39, and preoperative opioid use40. Of the 32% (8 patients) in our study who were taking opioids preoperatively, 50% had discontinued their use by 5 months. This is consistent with previous literature from the senior author when examining TMR for all indications and not orthopaedic trauma-related alone21. Perhaps more striking, however, is that among the patients who did not use opioids preoperatively (17 patients), 100% had discontinued use at the time of the latest follow-up and did so, on average, 2 months sooner than their counterparts who used opioids preoperatively (Fig. 1). Overall, our findings indicate that only 16% (4 of 25) of all orthopaedic trauma TMR amputees were taking opioids at the time of latest follow-up.
The results were not the same for neuromodulator medication use, as none of the 5 patients who used neuromodulator medication preoperatively had discontinued use by the latest follow-up. For the 20 patients who used neuromodulator agents postoperatively only, the median time to discontinuation was 14.6 months, with female patients taking considerably longer than male patients to discontinue use (23 compared with 7 months). The reason for this is unclear. The indication for neuromodulator medication use can often be multifactorial, which could contribute to the difficulty in stopping their use. It should be noted that male patients had significantly higher pain scores than female patients for all but 1 measure. While the reason for this is likely multifactorial, perhaps the earlier discontinuation of neuromodulator medication by male patients was a contributing factor. Further investigation is warranted, as the efficacy of perioperative neuromodulator use remains unclear41-43.
Amputation with TMR performed specifically for infection was associated with worse residual pain, with a high rate (75%, 6 of 8) of the patients reporting residual symptoms (compared with 24%, 4 of 17, for non-infection-related indications). The etiology of this finding remains unclear, but perhaps could be affected by neuromodulator use as well, as 50% (4 of 8) of the patients with infection-related amputation were taking neuromodulator medication preoperatively. Furthermore, patients with infection-related amputation commonly deal with pain in the extremity for a longer period than patients with acute traumatic amputation, perhaps resulting in the centralization of pain sensations and a higher likelihood of residual symptoms.
At the time of the latest follow-up, 85% (17 of 20) of the patients with a lower-extremity amputation and 40% (2 of 5) of the patients with an upper-extremity amputation reported daily prosthetic use. This is comparable with the findings of prior work by the senior author regarding TMR for all indications, which demonstrated an 80% prosthesis wear rate at 3.4 months postoperatively44. Furthermore, in the current study, we found a 36% rate of self-reported unemployment due to disability. To our knowledge, this measure has not previously been reported in the literature.
We hypothesize that TMR has the associated advantages of decreased muscle atrophy, improved nerve ingrowth, less muscle denervation, and greater neuroplasticity. The nerve ingrowth from the residual amputated nerve into the motor nerve results in less muscle denervation and thus less muscle atrophy. In turn, this leads to greater residual muscle bulk and improved prosthetic fit. The underlying principle of TMR is that the amputated nerve now has somewhere to go and something to do, ultimately taking advantage of neuroplasticity. We propose that all of these phenomena combine to give success to TMR.
There were several limitations to this study. We have developed our institutional protocol on the basis of the experience of our team members and patients over time. The patient population is heterogeneous and relatively small. Patient-reported data were analyzed at the latest follow-up and not tracked longitudinally to examine the effects of specific medication changes on pain. Rehabilitative efforts were not standardized for all patients. Further traumatic and surgical details were unavailable. Additionally, there was no control group or second arm of the study to which to compare outcomes.
In summary, our early data suggest that TMR for orthopaedic trauma amputees was associated with low overall pain scores at 2-year follow-up, decreased overall opioid and neuromodulator medication use, and an overall high rate of daily prosthetic use.
Footnotes
Investigation performed at The Ohio State University Wexner Medical Center, Columbus, Ohio
Disclosure: The authors indicated that no external funding was received for any aspect of this work. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A154).
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008. March;89(3):422-9. [DOI] [PubMed] [Google Scholar]
- 2.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983. November;17(3):243-56. [DOI] [PubMed] [Google Scholar]
- 3.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985. March;21(3):267-78. [DOI] [PubMed] [Google Scholar]
- 4.Pierce RO, Jr, Kernek CB, Ambrose TA., 2nd The plight of the traumatic amputee. Orthopedics. 1993. July;16(7):793-7. [DOI] [PubMed] [Google Scholar]
- 5.Ducic I, Mesbahi AN, Attinger CE, Graw K. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg. 2008. March;121(3):908-14; discussion 915-7. [DOI] [PubMed] [Google Scholar]
- 6.Harris AM, Althausen PL, Kellam J, Bosse MJ, Castillo R; Lower Extremity Assessment Project (LEAP) Study Group. Complications following limb-threatening lower extremity trauma. J Orthop Trauma. 2009. January;23(1):1-6. [DOI] [PubMed] [Google Scholar]
- 7.Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. 2013. August;154(8):1274-80. Epub 2013 Apr 19. [DOI] [PubMed] [Google Scholar]
- 8.Elbert T, Rockstroh B. Reorganization of human cerebral cortex: the range of changes following use and injury. Neuroscientist. 2004. April;10(2):129-41. [DOI] [PubMed] [Google Scholar]
- 9.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995. June 8;375(6531):482-4. [DOI] [PubMed] [Google Scholar]
- 10.Preissler S, Feiler J, Dietrich C, Hofmann GO, Miltner WHR, Weiss T. Gray matter changes following limb amputation with high and low intensities of phantom limb pain. Cereb Cortex. 2013. May;23(5):1038-48. Epub 2012 Apr 17. [DOI] [PubMed] [Google Scholar]
- 11.Giummarra MJ, Moseley GL. Phantom limb pain and bodily awareness: current concepts and future directions. Curr Opin Anaesthesiol. 2011. October;24(5):524-31. [DOI] [PubMed] [Google Scholar]
- 12.Montoya P, Ritter K, Huse E, Larbig W, Braun C, Töpfner S, Lutzenberger W, Grodd W, Flor H, Birbaumer N. The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. Eur J Neurosci. 1998. March;10(3):1095-102. [DOI] [PubMed] [Google Scholar]
- 13.Nikolajsen L, Staehelin Jensen T. Phantom limb pain. Curr Rev Pain. 2000;4(2):166-70. [DOI] [PubMed] [Google Scholar]
- 14.Hertel R, Strebel N, Ganz R. Amputation versus reconstruction in traumatic defects of the leg: outcome and costs. J Orthop Trauma. 1996;10(4):223-9. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed A, Bhatnagar S, Mishra S, Khurana D, Joshi S, Ahmad SM. Prevalence of phantom limb pain, stump pain, and phantom limb sensation among the amputated cancer patients in India: a prospective, observational study. Indian J Palliat Care. 2017. Jan-Mar;23(1):24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlen PL, Wall PD, Nadvorna H, Steinbach T. Phantom limbs and related phenomena in recent traumatic amputations. Neurology. 1978. March;28(3):211-7. [DOI] [PubMed] [Google Scholar]
- 17.Kuiken TA, Childress DS, Rymer WZ. The hyper-reinnervation of rat skeletal muscle. Brain Res. 1995. April 3;676(1):113-23. [DOI] [PubMed] [Google Scholar]
- 18.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004. December;28(3):245-53. [DOI] [PubMed] [Google Scholar]
- 19.Kim PS, Ko JH, O’Shaughnessy KK, Kuiken TA, Pohlmeyer EA, Dumanian GA. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg Am. 2012. August;37(8):1609-16. Epub 2012 Jul 4. [DOI] [PubMed] [Google Scholar]
- 20.Valerio IL, Dumanian GA, Jordan SW, Mioton LM, Bowen JB, West JM, Porter K, Ko JH, Souza JM, Potter BK. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019. March;228(3):217-26. Epub 2019 Jan 8. [DOI] [PubMed] [Google Scholar]
- 21.Hehr JD, Bowen B, Alexander JH, Mioton LM, Jordan SW, West JM, Scharschmidt T, Mayerson JL, Dumanian GA, Valerio IL. Targeted reinnervation in the amputee patient updates for multiple cohorts on pain outcomes and narcotic use trends. J Am Coll Surg. 2018. October;227(4)(Suppl 1):S212. [Google Scholar]
- 22.O’Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield K, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J Bone Joint Surg Am. 2008. February;90(2):393-400. [DOI] [PubMed] [Google Scholar]
- 23.Bowen JB, Wee CE, Kalik J, Valerio IL. Targeted muscle reinnervation to improve pain, prosthetic tolerance, and bioprosthetic outcomes in the amputee. Adv Wound Care (New Rochelle). 2017. August 1;6(8):261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheesborough JE, Souza JM, Dumanian GA, Bueno RA., Jr Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand (N Y). 2014. June;9(2):253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumanian GA, Potter BK, Mioton LM, Ko JH, Cheesborough JE, Souza JM, Ertl WJ, Tintle SM, Nanos GP, Valerio IL, Kuiken TA, Apkarian AV, Porter K, Jordan SW. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019. August;270(2):238-46. [DOI] [PubMed] [Google Scholar]
- 26.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010. July;150(1):173-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revicki DA, Chen WH, Harnam N, Cook KF, Amtmann D, Callahan LF, Jensen MP, Keefe FJ. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009. November;146(1-2):158-69. Epub 2009 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askew RL, Cook KF, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol. 2016. May;73:103-11. Epub 2016 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014. October;472(10):2984-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gart MS, Souza JM, Dumanian GA. Targeted muscle reinnervation in the upper extremity amputee: a technical roadmap. J Hand Surg Am. 2015. September;40(9):1877-88. [DOI] [PubMed] [Google Scholar]
- 31.Agnew SP, Schultz AE, Dumanian GA, Kuiken TA. Targeted reinnervation in the transfemoral amputee: a preliminary study of surgical technique. Plast Reconstr Surg. 2012. January;129(1):187-94. [DOI] [PubMed] [Google Scholar]
- 32.Bowen JB, Ruter D, Wee C, West J, Valerio IL. Targeted muscle reinnervation technique in below-knee amputation. Plast Reconstr Surg. 2019. January;143(1):309-12. [DOI] [PubMed] [Google Scholar]
- 33.Kuiken TA, Barlow AK, Hargrove L, Dumanian GA. Targeted muscle reinnervation for the upper and lower extremity. Tech Orthop. 2017. June;32(2):109-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahuja V, Thapa D, Ghai B. Strategies for prevention of lower limb post-amputation pain: a clinical narrative review. J Anaesthesiol Clin Pharmacol. 2018. Oct-Dec;34(4):439-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Jong R, Shysh AJ. Development of a multimodal analgesia protocol for perioperative acute pain management for lower limb amputation. Pain Res Manag. 2018. June 3;2018:5237040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kent ML, Hsia HJ, Van de Ven TJ, Buchheit TE. Perioperative pain management strategies for amputation: a topical review. Pain Med. 2017. March 1;18(3):504-19. [DOI] [PubMed] [Google Scholar]
- 37.Raichle KA, Osborne TL, Jensen MP, Ehde DM, Smith DG, Robinson LR. Preoperative state anxiety, acute postoperative pain, and analgesic use in persons undergoing lower limb amputation. Clin J Pain. 2015. August;31(8):699-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanley MA, Jensen MP, Smith DG, Ehde DM, Edwards WT, Robinson LR. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain. 2007. February;8(2):102-9. Epub 2006 Sep 1. [DOI] [PubMed] [Google Scholar]
- 39.Nikolajsen L, Ilkjaer S, Krøner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain. 1997. September;72(3):393-405. [DOI] [PubMed] [Google Scholar]
- 40.Roullet S, Nouette-Gaulain K, Biais M, Bernard N, Bénard A, Revel P, Capdevila X, Sztark F. Preoperative opioid consumption increases morphine requirement after leg amputation. Can J Anaesth. 2009. December;56(12):908-13. Epub 2009 Nov 12. [DOI] [PubMed] [Google Scholar]
- 41.Yan PZ, Butler PM, Kurowski D, Perloff MD. Beyond neuropathic pain: gabapentin use in cancer pain and perioperative pain. Clin J Pain. 2014. July;30(7):613-29. [DOI] [PubMed] [Google Scholar]
- 42.Bone M, Critchley P, Buggy DJ. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Reg Anesth Pain Med. 2002. Sep-Oct;27(5):481-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith DG, Ehde DM, Hanley MA, Campbell KM, Jensen MP, Hoffman AJ, Awan AB, Czerniecki JM, Robinson LR. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. J Rehabil Res Dev. 2005. Sep-Oct 42(5):645-54. [DOI] [PubMed] [Google Scholar]
- 44.Alexander JH, Jordan SW, West JM, Compston A, Fugitt J, Bowen JB, Dumanian GA, Pollock R, Mayerson JL, Scharschmidt TJ, Valerio IL. Targeted muscle reinnervation in oncologic amputees: early experience of a novel institutional protocol. J Surg Oncol. 2019. September;120(3):348-58. Epub 2019 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]


