Abstract
Background:
Periprosthetic joint infection (PJI) is a devastating complication following total hip replacement. The direct anterior approach for total hip replacement is becoming increasingly popular. However, little is known about the success rate of treatment with debridement, antibiotics, and implant retention (DAIR) using the direct anterior approach. The aim of this study was to analyze the effectiveness of DAIR using this approach and identify patient and surgical factors that influence the results.
Methods:
Seventy-four patients (75 hips) in whom DAIR had been performed were identified from the records of the weekly multidisciplinary infection meeting and the laboratory information management systems. In 4% (3 hips), modular components were exchanged. To consider competing risks (death), we used competing risk models.
Results:
The competing risk analysis showed a successful outcome after DAIR of 82% at 4 years of follow-up; this rate was 89% at 4 years follow-up when excluding patients managed with gentamicin beads. The sensitivity analysis revealed that obesity (body mass index [BMI] of ≥30 kg/m2), use of gentamicin beads, and an erythrocyte sedimentation rate (ESR) of >40 mm/hr increased the risk of failure.
Conclusions:
DAIR using the direct anterior approach without the routine exchange of modular components offers a success rate that is comparable with other approaches for eradicating acute PJI following primary hip arthroplasty.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Periprosthetic joint infection (PJI) is a devastating complication following total hip replacement1,2. Patients with such an infection will often undergo surgical treatment. Depending on the duration of the infection, condition of the bone and soft tissue, fixation of the components, type of microorganism, and general condition of the patient, debridement, antibiotics, and implant retention (DAIR) can be the treatment of choice1-4. According to a recent systematic review5, the pooled success rate of DAIR following total hip replacement is 72% and this success rate has been increasing since 2000.
The direct anterior approach for total hip replacement is becoming increasingly popular. However, little is known about the success rate of DAIR with the direct anterior approach. Since the skin incision for the direct anterior approach is close to the groin area, the microorganisms causing PJI may be different from those causing such an infection with other approaches. Ilchmann et al. showed that the spectrum of microorganisms causing PJI is different between the direct anterior and transgluteal approaches. Gram-negative bacilli were found only in the direct anterior approach group, and the fraction of patients with a polymicrobial infection was higher in that group6. It is therefore important to evaluate the success rate of DAIR with this approach. The aim of the current study was to analyze the effectiveness of DAIR using the direct anterior approach and identify patient and surgical factors that influence the results.
Materials and Methods
The study was approved by our institutional ethics committee (T17-112), and we complied with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting. This was an observational cohort study of 74 patients (75 hips) managed with DAIR for PJI following primary total hip replacement between 2009 and 2017 at the Haga Hospital, a high-volume teaching hospital in The Hague, the Netherlands. In the same period, 5,512 primary total hip replacements were performed, almost all (>97%) through the direct anterior approach. Inclusion criteria were a primary total hip replacement, direct anterior approach, DAIR procedure, and PJI. Exclusion criteria were a PJI following a revision total hip replacement or hemiarthroplasty, direct lateral or posterolateral approach, 1-stage revision, 2-stage revision, and Girdlestone procedure without prior DAIR. In the study period, 2009 through 2017, there were nine 2-stage revisions, one 1-stage revision, and two Girdlestone procedures for PJI following primary total hip replacement without prior DAIR. Accordingly, DAIR was the standard of care during the study period. DAIR was considered for patients with suspected PJI in the early postoperative period or suspected hematogenous (late) infection with symptoms for <4 weeks as slightly modified from the Zimmerli criteria2. The diagnosis of PJI was made according to the following major MSIS (Musculoskeletal Infection Society) criteria2,4,7—2 or more positive cultures, presence of a sinus tract, or presence of intraoperative pus—or we made such a diagnosis during our routine weekly multidisciplinary infection meeting of medical microbiologists and orthopaedic surgeons. During these meetings, the minor MSIS criteria were also considered: elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), elevated synovial fluid white blood-cell (WBC) count or positive change on leukocyte esterase test strip, elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%), positive histological analyses of periprosthetic tissue, or a single positive culture2,4,7.
Patients were identified from the records of the weekly multidisciplinary infection meeting and the laboratory information management systems containing information on cultures and consultations from the medical microbiologists. The mean age (and standard deviation [SD]) of the included patients was 69 ±10 years (Table I). There were 41 females (42 hips) and 33 males (33 hips). The mean body mass index (BMI) was 30 kg/m2 (SD, 5.3 kg/m2). Fourteen patients were smokers, and 37 consumed some amount of alcohol every week. Twenty patients had diabetes mellitus, and 4 had chronic kidney failure. Thirty-four patients used anticoagulants, and 5 used immunosuppressive medications. There were 55 uncemented, 16 cemented, and 4 hybrid total hip replacements. The underlying joint disease was osteoarthritis in 62 hips, osteonecrosis in 7, and fracture in 6.
TABLE I.
Demographics
| Patient Death | Implant Failure | Implant Success | All Outcomes | |
| Hips (no.) | 10 | 12 | 53 | 75 |
| Age* (yr) | 68 ± 12 | 70 ± 11 | 70 ± 10 | 69 ± 10 |
| Follow-up* (yr) | 2.4 ± 2.5 | 0.6 ± 0.6 | 3.6 ± 1.6 | 3 ± 2 |
| BMI* (kg/m2) | 32 ± 8.3 | 31 ± 5.2 | 29 ± 4.4 | 30 ± 5.3 |
| Female:male (no. of hips) | 4:6 | 9:3 | 29:24 | 42:33 |
| Smoker:nonsmoker (no. of hips) | 2:8 | 4:8 | 8:45 | 14:61 |
| Alcohol:no alcohol (no. of hips) | 4:6 | 6:6 | 27:26 | 37:38 |
| Diabetes mellitus:no diabetes mellitus (no. of hips) | 2:8 | 4:8 | 14:39 | 20:55 |
| Kidney failure:no kidney failure (no. of hips) | 3:7 | 0:12 | 1:52 | 4:71 |
The values are given as the mean and standard deviation.
The DAIR procedure consisted of removing all infected tissue, obtaining tissue specimens for microbiology testing, and irrigating the wound with 6 to 12 L of saline solution using pulse-lavage equipment. The hip was dislocated to allow thorough debridement. Empirical intravenous antibiotic treatment was started intraoperatively shortly after the tissue cultures were obtained. The treating surgeon decided intraoperatively to retain or exchange the modular components. The components were retained if the exposure precluded their exchange, if excessive force (which risked loosening the femoral stem) was needed, or if component removal would cause substantial damage to the trunnion or the surrounding soft tissue, muscles, or bone. In addition, ceramic liners were not exchanged because their removal might have led to cup damage or liner fragmentation, possibly creating infected foreign bodies. No wound drains were used. Gentamicin beads were used through July 2013 but not later. Antibiotic treatment consisted of 2 weeks of intravenous antibiotic administration followed by 10 weeks of oral antibiotic administration, for a total of 12 weeks of treatment2. The duration of intravenous antibiotic administration could be prolonged if needed given the clinical situation. The empirical therapy was adjusted when the causative microorganism was identified, and rifampicin was added if the causative microorganism was sensitive to it. Second and subsequent DAIR procedures were indicated mainly if wound discharge persisted after 7 to 10 days, if the general condition of the patient deteriorated, or if there was a lack of improvement in infection parameters.
Failure of treatment with DAIR was defined as follows4,7-9: (1) removal of the hip prosthesis as part of a 1-stage, 2-stage, or Girdlestone procedure; or (2) failure to cure the infection, leading to antibiotic suppression therapy and/or a chronic sinus tract because the patient’s comorbidities precluded removal of the hip prosthesis.
We checked the Dutch Arthroplasty Register (LROI) to determine whether revision for infection or DAIR had been performed at other hospitals for the patients in our cohort.
Statistics
We considered death to be a competing risk because it precludes revision surgery and recurrence of the infection10,11. To consider competing risks (death), we used competing risk models from the mstate package in the R statistical software environment (R Foundation for Statistical Computing)12. To allow comparison with the literature, we also report the Kaplan-Meier estimate, although it is not valid in the setting of competing risks. Sensitivity analyses were performed on patient and surgical factors using Cox regression. According to the AQUILA (Assessment of Quality in Lower Limb Arthroplasty) checklist, we considered results for the competing risk (Kaplan-Meier) estimate to be valid when at least 20 hips remained in the analysis (i.e., were considered “at risk”)13,14.
Results
The mean follow-up (and SD) after DAIR was 3 ± 2 years (range, 0.13 to 7.1 years). The short follow-up (0.13 year) was caused by patients reaching an end point: implant failure or death. Failures occurred after a mean (and SD) of 0.6 ± 0.6 year (range, 0.13 to 2 years). One DAIR was performed in 45 hips; 2 DAIRs, in 18; 3 DAIRS, in 7 (5 in patients with gentamicin beads); and 4 DAIRs, in 5 (4 in patients with gentamicin beads). Two or more DAIRs were more frequent in patients managed with gentamicin beads, resulting in a mean of 2.9 DAIRs per hip compared with a mean of 1.3 DAIRs per hip in patients managed without gentamicin beads. The mean duration (and SD) between total hip arthroplasty and DAIR was 35 ± 91 days (range, 4 to 792 days). The majority of cases occurred in the early postoperative period: 73 (of 75 hips) were treated with DAIR within 3 months after total hip arthroplasty. There were 2 late (hematogenous) cases with symptoms at <4 weeks postoperatively. In total, there were 12 (of 74) patients in whom the DAIR procedure failed: 10 patients were managed with removal of the hip prosthesis, 1 patient received suppression antibiotic treatment, and 1 patient had a chronic sinus tract. Ten patients died during the study period. Table I shows the patient demographics according to success or failure of DAIR. In 1 case, modular components were exchanged during the first DAIR and, in 2 cases, during the second DAIR. The microorganisms identified from tissue cultures with DAIR are shown in Table II. In 28 (37%) of the 75 cases, there was a polymicrobial infection.
TABLE II.
Microorganisms Identified from Tissue Cultures with DAIR*
| Microorganism | Hips (no.) |
| Polymicrobial | 28 |
| Monomicrobial | 47 |
| Staphylococcus aureus | 32 |
| Coagulase-negative staphylococci (n = 23) | |
| S. epidermidis | 15 |
| S. lugdunensis | 5 |
| S. capitis | 1 |
| S. haemolyticus | 1 |
| S. vitulinus | 1 |
| Pseudomonas aeruginosa | 8 |
| Enterococcus faecalis | 13 |
| E. faecium | 1 |
| Enterobacteriaceae (n = 23) | |
| Enterobacter cloacae | 9 |
| Proteus mirabilis | 6 |
| Escherichia coli | 4 |
| Klebsiella pneumoniae | 2 |
| Serratia marcescens | 1 |
| Citrobacter freundii | 1 |
| Granulicatella adiacens | 2 |
| Streptococci (n = 6) | |
| Group B Streptococcus | 4 |
| Streptococcus milleri | 1 |
| Group C Streptococcus | 1 |
| Corynebacterium spp. | 4 |
| Cutibacterium acnes | 1 |
| Acinebacter baumannii | 1 |
The numbers add up to more than 75 because, in 28 (37%) of the cases, the infection was polymicrobial.
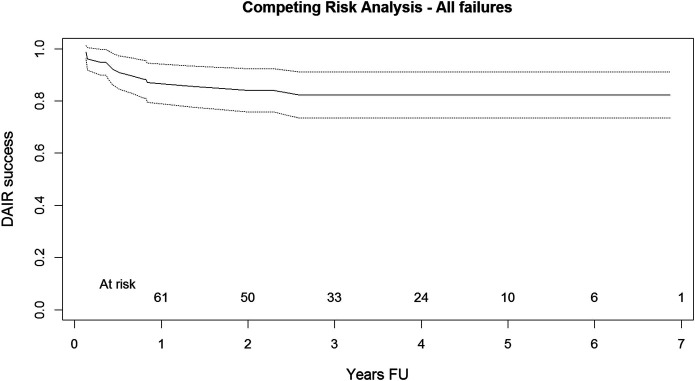
The competing risk analysis showed a rate of successful outcomes after DAIR of 82% (95% confidence interval [CI], 77% to 94%) at 4 years of follow-up, with at least 20 hips at risk through 4 years of follow-up (Fig. 1). The results of the Kaplan-Meier analysis were similar, with a successful outcome after DAIR in 81% (95% CI, 72% to 91%) at 4 years of follow-up. For patients in our cohort, the Dutch Arthroplasty Register did not identify any revisions for infection or DAIRs performed at other hospitals that we were unaware of previously. There were no cases of instability or dislocation of the components after the DAIR procedures.
Fig. 1.
Graph showing the competing risk analysis (including the upper and lower limits of the 95% CI) for freedom from failure (i.e., success of DAIR) for the follow-up period of the study. At risk = the number of hips remaining in the analysis (at risk) for each year of follow-up. FU = follow-up.
The results of the sensitivity analysis on patient and surgical factors are shown in Table III. Obese patients (BMI of ≥30 kg/m2) had a 4.1-fold (95% CI, 1.2 to 14-fold) greater risk that the DAIR procedure would fail compared with nonobese patients (BMI of <30 kg/m2). The use of gentamicin beads was associated with a 3.7-fold (95% CI, 1.2 to 12-fold) greater risk of failure of the DAIR procedure. In patients who had an ESR of >40 mm/hr, the DAIR procedure was 4.7-fold (95% CI, 1.0 to 21-fold) more likely to fail compared with patients who had an ESR of ≤40 mm/hr prior to DAIR.
TABLE III.
Sensitivity Analysis on Patient and Surgical Factors*
| Hazard Ratio (95% CI) | ||
| Crude | Adjusted | |
| Sex, male (vs. female) | 0.4 (0.1 to 1.5) | 0.6 (0.1 to 2.8) |
| Age (per year increase) | 1.00 (0.95 to 1.07) | 1.01 (0.93 to 1.08) |
| BMI (kg/m2)† | ||
| >25 (vs. ≤25) | 2.6 (0.3 to 20) | 1.8 (0.2 to 15) |
| >30 (vs. ≤30) | 4.1 (1.2 to 14) | 5.3 (1.0 to 27) |
| >35 (vs. ≤35) | 2.0 (0.5 to 7.4) | 1.5 (0.2 to 12) |
| As continuous variable ‡ | 1.1 (1 to 1.2) | 1.1 (0.95 to 1.3) |
| Smoking, yes (vs. no) | 2.3 (0.7 to 7.8) | 2.1 (0.4 to 11) |
| Alcohol, yes (vs. no) | 1.0 (0.3 to 3.1) | 2.4 (0.5 to 12) |
| Diabetes mellitus, yes (vs .no) | 1.5 (0.5 to 5.0) | 1.2 (0.2 to 6.2) |
| Anticoagulants, yes (vs. no) | 1.7 (0.5 to 5.3) | 1.9 (0.4 to 8.5) |
| ASA III-IV (vs. I-II) | 1.8 (0.5 to 5.9) | 2.5 (0.6 to 11) |
| Cemented (vs. uncemented) | 1.8 (0.5 to 5.8) | 1.5 (0.3 to 7.8) |
| Cemented (vs. hybrid) | 1.1 (0.1 to 10) | NA |
| Gentamicin beads, yes (vs. no) | 3.7 (1.2 to 12) | |
| Polymicrobial, yes (vs. no) | 0.6 (0.2 to 2.1) | 0.3 (0.1 to 2.4) |
| Staphylococcus aureus, yes (vs. no) | 0.6 (0.2 to 2.1) | 0.3 (0.1 to 2.3) |
| Staphylococci, yes (vs. no) | 0.4 (0.1 to 1.1) | 0.3 (0.1 to 1.4) |
| CNS, yes (vs. no) | 0.5 (0.1 to 2.1) | 0.8 (0.1 to 3.9) |
| Pseudomonas, yes (vs. no) | 0.8 (0.1 to 6.0) | NA |
| Enterococcus, yes (vs. no) | 1.5 (0.4 to 5.7) | 0.8 (0.1 to 6.6) |
| Enterobacteriaceae, yes (vs. no) | 0.4 (0.1 to 2.6) | NA |
| Streptococcus, yes (vs. no) | 1.6 (0.4 to 7.4) | 2.7 (0.5 to 14) |
| CRP (mg/L) | ||
| >20 (vs. ≤20) | 4.9 (0.6 to 37) | 2.8 (0.3 to 23) |
| >50 (vs. ≤50) | 1.3 (0.4 to 3.9) | 1.7 (0.4 to 7.4) |
| ESR (mm/hr), >40 (vs. ≤40) | 4.7 (1.0 to 21) | 5.8 (0.7 to 48) |
| Leukocytes (×109/L) >10 (vs. ≤10) | 1.2 (0.4 to 3.8) | 1.5 (0.3 to 6.5) |
| DAIR (wk) | ||
| >2 (vs. ≤2) | 0.9 (0.2 to 3.1) | 0.7 (0.1 to 3.9) |
| >4 (vs. ≤4) | 2.0 (0.6 to 6.5) | 3.0 (0.7 to 13) |
| >6 (vs. ≤6) | 2.5 (0.7 to 9.4) | 3.1 (0.6 to 16) |
| >8 (vs. ≤8) | 2.3 (0.5 to 10) | 3.8 (0.7 to 19) |
| Osteoarthritis, yes (vs. no) | 2.1 (0.6 to 7.7) | 1.0 (0.1 to 8.2) |
Hazard ratio from Cox regression analysis. NA = too few cases to allow reliable analysis. Adjusted = adjusted for the use of gentamicin beads. All cases with gentamicin beads were excluded to represent the current practice of not using gentamicin beads during DAIR procedures. Immunosuppressive medication, yes (vs. no) was not estimable because there were only 5 patients on such medication. Factors given in boldface type were found to increase the risk of failure. ASA = American Society of Anesthesiologists.
The lowest BMI was 20 kg/m2, so there were no underweight cases.
BMI was used as a continuous variable: for every unit increase in BMI, the hazard ratio of infection increased by 1.1.
Excluding patients with gentamicin beads, the competing risk analysis showed a successful outcome after DAIR of 89% (95% CI, 81% to 97%) at 4 years of follow-up. The full sensitivity analysis was redone, excluding patients in whom gentamicin beads were used, to represent the current practice of not using gentamicin beads (Table III). When we excluded patients with gentamicin beads, obese patients had a 5.3-fold (95% CI, 1.0 to 27-fold) greater risk that the DAIR procedure would fail compared with nonobese patients. An ESR of >40 mm/hr was no longer associated with a high risk of failure, although the number of patients may have been too low to detect such a risk.
Discussion
The results of this study show a successful outcome after DAIR with the direct anterior approach in 82% of patients at midterm follow-up. The success rate of DAIR with the direct anterior approach was 89% at midterm follow-up when excluding patients in whom gentamicin beads had been used. We are not aware of any other studies evaluating the outcome after DAIR with the direct anterior approach. However, our results are comparable with those of a recent systematic review of other surgical approaches showing that the pooled success of DAIR was around 80% for studies published between 2011 and 20155. Of particular interest is the very low rate of component exchange in our study of 4% and the lack of component instability or dislocation after DAIR. Despite this very low rate of component exchange, the success rate of DAIR was 82% or better, suggesting that routine exchange of modular components may not be necessary for DAIR in total hip replacement with the direct anterior approach. Indeed, Sendi et al. previously stated that exchange of modular components for DAIR in total hip replacement was based on empirical reasoning only9. On the contrary, Grammatopoulos et al. showed that exchange of modular components was of benefit, particularly in cases of late PJI, and improved the 10-year survival15. Therefore, further studies, preferably randomized controlled trials, are needed to address the question of whether modular components should be routinely exchanged during DAIR for total hip replacement.
The sensitivity analysis revealed that obesity, use of gentamicin beads, and an ESR of >40 mm/hr increased the risk of failure. Only obesity remained a risk factor for DAIR failure when patients with gentamicin beads were excluded to better represent the current practice of not using gentamicin beads. Patient-related factors (such as obesity) may also explain some of the variation in the success of DAIR5.
A study by Kuiper et al. suggested that the use of gentamicin beads was associated with a higher failure rate of the DAIR procedure8. Our results confirm those findings: patients managed with gentamicin beads had a 3.7-fold greater risk of DAIR failure compared with those not managed with gentamicin beads. Since gentamicin beads have to be explanted at some point, there were more DAIR procedures performed in individuals managed with gentamicin beads (mean, 2.9 DAIRs per hip) compared with individuals managed without gentamicin beads (mean, 1.3 DAIRs per hip).
Other authors have reported that the microorganism causing the infection could influence the success of DAIR: coagulase-negative Staphylococcus (CNS)8 or Staphylococcus aureus7. We found no effect of the type of microorganism on the outcome of DAIR. Also, polymicrobial infections did not seem to influence the outcome given the number of patients available. It should be mentioned, however, that our cohort may not have been sufficiently large to detect a difference. Moreover, the use of rifampicin may have contributed to our overall high success rate of DAIR with the direct anterior approach16-18.
We should consider some limitations. As noted above, a sample size of 75 hips may have been too small to detect all relevant patient and surgical factors influencing the outcome. Nevertheless, we were able to identify obesity, use of gentamicin beads, and an ESR of >40 mm/hr as associated with a higher risk of failure of DAIR with the direct anterior approach.
Additionally, our criteria for not exchanging modular components may be considered less than robust. However, a landmark paper by Zimmerli et al. that described a treatment algorithm for patients with PJI did not mention component exchange as part of the treatment2. Another limitation of our study may be the midterm follow-up. Longer follow-up and replication of our results by others are necessary to either confirm or refute our results.
The strengths of our study include the cross-checking with the Dutch Arthroplasty Register: for patients in our cohort, no revisions for infections or DAIRs were performed at other hospitals that we were unaware of previously. Ten patients died during the study period, so a competing risk analysis was needed. In our study, the results from this analysis were similar to those from the Kaplan-Meier analysis: 82% success (95% CI , 77% to 94%) at 4 years of follow-up for the competing risk analysis compared with 81% (95% CI, 72% to 91%) at 4 years for the Kaplan-Meier analysis.
In conclusion, DAIR through the direct anterior approach without the routine exchange of modular components offers a success rate comparable with other approaches for eradicating acute PJI following primary hip arthroplasty. Obesity, use of gentamicin beads, and an ESR of >40 mm/hr increase the risk of failure.
Footnotes
Investigation performed at the Haga Hospital, The Hague, the Netherlands
Disclosure: The authors indicated that no external funding was received for any aspect of this work. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A160).
References
- 1.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014. April;27(2):302-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004. October 14;351(16):1645-54. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012. July 18;94(14):e104. [DOI] [PubMed] [Google Scholar]
- 4.Slullitel PA, Oñativia JI, Buttaro MA, Sánchez ML, Comba F, Zanotti G, Piccaluga F. State-of-the-art diagnosis and surgical treatment of acute peri-prosthetic joint infection following primary total hip arthroplasty. EFORT Open Rev. 2018. July 17;3(7):434-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang SJ, Ting J, Simpson AHRW, Gaston P. Outcomes following debridement, antibiotics and implant retention in the management of periprosthetic infections of the hip: a review of cohort studies. Bone Joint J. 2017. November;99-B(11):1458-66. [DOI] [PubMed] [Google Scholar]
- 6.Ilchmann T, Zimmerli W, Bolliger L, Graber P, Clauss M. Risk of infection in primary, elective total hip arthroplasty with direct anterior approach or lateral transgluteal approach: a prospective cohort study of 1104 hips. BMC Musculoskelet Disord. 2016. November 14;17(1):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009. June;63(6):1264-71. Epub 2009 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuiper JW, Vos SJ, Saouti R, Vergroesen DA, Graat HC, Debets-Ossenkopp YJ, Peters EJ, Nolte PA. Prosthetic joint-associated infections treated with DAIR (debridement, antibiotics, irrigation, and retention): analysis of risk factors and local antibiotic carriers in 91 patients. Acta Orthop. 2013. August;84(4):380-6. Epub 2013 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sendi P, Lötscher PO, Kessler B, Graber P, Zimmerli W, Clauss M. Debridement and implant retention in the management of hip periprosthetic joint infection: outcomes following guided and rapid treatment at a single centre. Bone Joint J. 2017. March;99-B(3):330-6. [DOI] [PubMed] [Google Scholar]
- 10.Keurentjes JC, Fiocco M, Schreurs BW, Pijls BG, Nouta KA, Nelissen RG. Revision surgery is overestimated in hip replacement. Bone Joint Res. 2012. October 1;1(10):258-62. Epub 2013 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nouta KA, Pijls BG, Fiocco M, Keurentjes JC, Nelissen RG. How to deal with lost to follow-up in total knee arthroplasty : a new method based on the competing risks approach. Int Orthop. 2014. May;38(5):953-9. Epub 2013 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010. September;99(3):261-74. Epub 2010 Mar 15. [DOI] [PubMed] [Google Scholar]
- 13.Cowan JB, Mlynarek RA, Nelissen RG, Pijls BG, Gagnier JJ. Evaluation of quality of lower limb arthroplasty observational studies using the Assessment of Quality in Lower Limb Arthroplasty (AQUILA) checklist. J Arthroplasty. 2015. September;30(9):1513-7. Epub 2015 Mar 31. [DOI] [PubMed] [Google Scholar]
- 14.Pijls BG, Dekkers OM, Middeldorp S, Valstar ER, van der Heide HJ, Van der Linden-Van der Zwaag HM, Nelissen RG. AQUILA: assessment of quality in lower limb arthroplasty. An expert Delphi consensus for total knee and total hip arthroplasty. BMC Musculoskelet Disord. 2011. July 22;12:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grammatopoulos G, Kendrick B, McNally M, Athanasou NA, Atkins B, McLardy-Smith P, Taylor A, Gundle R. Outcome following debridement, antibiotics, and implant retention in hip periprosthetic joint infection-an 18-year experience. J Arthroplasty. 2017. July;32(7):2248-55. Epub 2017 Mar 6. [DOI] [PubMed] [Google Scholar]
- 16.Lesens O, Ferry T, Forestier E, Botelho-Nevers E, Pavese P, Piet E, Pereira B, Montbarbon E, Boyer B, Lustig S, Descamps S; Auvergne-Rhône-Alpes Bone and Joint Infections Study Group. Should we expand the indications for the DAIR (debridement, antibiotic therapy, and implant retention) procedure for Staphylococcus aureus prosthetic joint infections? A multicenter retrospective study. Eur J Clin Microbiol Infect Dis. 2018. October;37(10):1949-56. Epub 2018 Aug 7. [DOI] [PubMed] [Google Scholar]
- 17.Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodríguez-Pardo D, Horcajada JP, Benito N, Bahamonde A, Granados A, del Toro MD, Cobo J, Riera M, Ramos A, Jover-Sáenz A, Ariza J; REIPI Group for the Study of Prosthetic Infection. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013. January;56(2):182-94. Epub 2012 Aug 31. [DOI] [PubMed] [Google Scholar]
- 18.Scheper H, van Hooven D, van de Sande M, van der Wal R, van der Beek M, Visser L, de Boer M, Nelissen R. Outcome of acute staphylococcal prosthetic joint infection treated with debridement, implant retention and antimicrobial treatment with short duration of rifampicin. J Infect. 2018. May;76(5):498-500. Epub 2018 Feb 1. [DOI] [PubMed] [Google Scholar]