Abstract
Background:
Cytomegalovirus (CMV) results in significant morbidity and mortality following hematopoietic cell transplantation (HCT). Establishing the cost and clinical impact are imperative to selecting appropriate CMV preventative strategies.
Methods:
This is a retrospective cohort study of consecutive patients undergoing their first allogeneic HCT between January 1, 2009 and December 31, 2013. Detailed clinical and institutional cost data were obtained from the start of conditioning through one-year post-transplant. Baseline characteristics, resource utilization, costs and outcomes were compared between patients with and without clinically significant CMV infection (csCMVi).
Results:
One-hundred seventy out of 388 (44%) patients developed csCMVi within one-year following HCT. Within the first post-transplant year, patients with csCMVi had significantly longer transplant lengths of stay (mean 91.7 vs. 78.3 days, p<0.0001) and more frequent and prolonged hospitalizations (mean 2.4 vs. 1.7 admissions, p<0.0001; mean 39.1 vs. 31.5 inpatient days, p=0.001) without significantly more admissions to the intensive care unit (28.2% vs. 21.6%, p=0.408). Use of granulocyte-colony stimulating factor was higher in patients with csCMVi (73.5% vs 54.1%, p=0.0001) though no significant differences were demonstrated in mean platelet or red blood cell transfusions. Total costs were also higher in patients with csCMVi (mean cost difference $45,811 (95% CI $26,385 - $67,544). However, the incidence of graft-versus-host disease (GVHD) and select infectious complications was not significantly different between the groups. There were no significant differences in one- and five-year post-transplant overall survival (OS) nor non-relapse mortality (NRM) between those with or without csCMVi, though relapse of underlying disease was significantly lower in the csCMVi group.
Conclusions:
Allogeneic HCT patients with csCMVi had significantly greater medical resource utilization and costs than those without. However, clinical outcomes including GVHD, infections and mortality were similar in both groups. Future study is needed to determine the cost effectiveness of CMV preventative modalities.
Keywords: Cytomegalovirus, Hematopoietic Cell Transplantation, Costs
INTRODUCTION
Cytomegalovirus (CMV), a member of the Herpesviridae family of viruses, has been associated with significant morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT). Direct effects of CMV include end-organ disease involving almost any organ system (e.g., pneumonitis, colitis and retinitis), while potential indirect effects of CMV include increases in bacterial and fungal co-infections1,2 and graft versus host disease (GVHD).3 Further, positive CMV serostatus (in donors and recipients) and early CMV reactivation have been associated with poor transplant outcomes including higher non-relapse mortality (NRM) and lower overall survival (OS).4–7 Late-onset CMV infection, occurring greater than 100 days post-transplant, has also been associated with poor outcomes including an increased risk of death.8,9
In the absence of CMV-directed prophylaxis, reactivation occurs in 70–80% of seropositive recipients following allogeneic HCT, and primary infection can occur in at least 15% of CMV seronegative recipients with seropositive donors.10 A pre-emptive strategy has been employed most commonly in lieu of universal prophylaxis to reduce exposure to CMV-directed antivirals and their associated toxicities including renal dysfunction and myelosuppression.11 However, this strategy remains imperfect as antiviral exposure/toxicity occur alongside the potential for emergence of drug-resistant CMV, the latter reported in up to 14.5% of high risk HCT recipients during pre-emptive therapy.12
Identification of novel antiviral medications and other strategies (e.g., vaccination and adoptive transfer of CMV specific T-cells) for the prevention of CMV in transplant is paramount. Notable in this realm is the recent United States Food and Drug Administration approval of letermovir, an anti-CMV therapy that works via a unique mechanism targeting the viral terminase complex. This viral enzyme is involved late in the viral replication process and is unique to the virus, circumventing many of the issues of cross-resistance and toxicities associated with current CMV therapies.13 In a phase three clinical trial utilizing letermovir for primary prophylaxis in 495 CMV seropositive adult allogeneic HCT recipients, letermovir met its primary efficacy endpoint with significantly fewer patients developing clinically significant CMV infection (csCMVi) through week 24, defined as CMV viremia necessitating preemptive therapy or CMV disease.14 Patients in the letermovir arm also had lower all-cause mortality through week 24 though this did not achieve statistical significance through week 48. Further, letermovir was well-tolerated with no significant increase in adverse effects such as myelosuppression or nephrotoxicity encountered with traditional CMV antiviral therapies.
To design new CMV preventative approaches, observational studies are needed to determine the economic burden of CMV infection. The primary objective of this study is to estimate incremental medical resource use and costs associated with csCMVi through one-year following allogeneic HCT. Secondary objectives include an evaluation of the impact of csCMVi on HCT outcomes including non-CMV infections, GVHD as well as underlying disease relapse, NRM and OS at one and five years following transplant.
PATIENTS AND METHODS
Study Patients
This retrospective cohort study included all consecutive patients undergoing their first allogeneic HCT at Duke University Medical Center between January 1, 2009 and December 31, 2013 allowing for follow-up data up to five years post-transplantation. Patients were excluded from the study cohort if they had received the investigational agent brincidofovir post-transplant (N=8), utilized syngeneic donors (N=3), developed CMV viremia and/or disease within two months preceding allogeneic HCT (N=3) or underwent sequential organ transplant followed by HCT (N=1, lung-HCT transplant). The study focused on costs from the initiation of the transplant conditioning regimen through one year post-transplant as beyond this time point, the majority of care is typically transferred back to local centers, and costs may not be accurately captured; however, transplant outcomes including survival were followed for up to five years. This study was approved by the Institutional Review Board of Duke University Health System (DUHS).
Data Extraction
Demographic and clinical data were abstracted from multiple sources including a prospectively maintained Duke Adult Blood and Marrow Transplant database, an institutional tool called Duke Enterprise Data Unified Content Explorer and manual review of electronic medical records. Institutional cost accounting data were provided by DUHS and represented all hospitalizations, emergency department visits, clinic visits, treatments, laboratory tests and procedures performed within the DUHS, inclusive of inpatient and outpatient settings.
Antimicrobial Prophylaxis
Throughout the study period, a preemptive strategy was employed for CMV management in allogeneic HCT recipients. Patients were monitored via plasma quantitative CMV polymerase chain reaction (PCR) using a DUHS laboratory-developed test incorporating the artus CMV PCR kit (Qiagen, Hilden, Germany) for amplification of a specific region of the CMV genome. Extraction of CMV DNA was performed on the MagNA Pure Compact (Roche), and amplification and detection were performed on the Lightcycler (LC) (Roche, Indianapolis, IN). Plasma CMV PCR evaluation occurred at least weekly beginning the first week post-transplant through a minimum of Day +100. Monitoring beyond Day +100 was continued at providers’ discretion in patients receiving ongoing immunosuppression. CMV directed antiviral therapy (e.g., ganciclovir, foscarnet or valganciclovir) was recommended in patients with: (1) evidence of or concern for CMV disease; or (2) when the plasma CMV DNA PCR exceeded a designated threshold value. The recommended threshold value was greater than 250 copies/milliliter (mL) through April 2014 with modification thereafter to greater than 450 international units (IU)/mL following an update to the plasma CMV DNA PCR reporting. The minimum suggested duration for induction therapy was two weeks followed by an additional two weeks of maintenance therapy. Patients not receiving CMV-directed therapies were otherwise maintained on herpes virus prophylaxis with acyclovir. Recommended bacterial prophylaxis was ciprofloxacin through a minimum period of engraftment. Fungal prophylactic regimens included either fluconazole, voriconazole or posaconazole through at least Day +100. Pneumocystis prophylaxis consisted of sulfamethoxazole/trimethoprim during conditioning through Day −2 with resumption following engraftment or Day +30.
Clinical Definitions
Clinically significant CMV infection (csCMVi) was defined as CMV viremia for which preemptive therapy was applied or CMV disease consistent with the definition applied in recent CMV randomized controlled trials.14 Assessment for CMV disease was based on standardized definitions proposed by Llungman, et al.15 Delineation of a second or subsequent episode of csCMVi required a minimum of a two-week period following completion of CMV-directed therapy. CMV viral resistance was determined via a CMV genotype demonstrating the presence of a UL97 or UL54 mutation confirmed in previous studies to confer antiviral resistance through marker transfer experiments.16 Bacteremia was defined as a recognized pathogen from one or more blood cultures. More than one episode of bacteremia was recorded in the same patient only if it occurred after a minimum of two weeks from the previous positive blood culture in patients receiving directed therapy. Common skin commensals, as defined by the National Healthcare Safety Network,17 were designated causes of bacteremia only if isolated from two or more blood specimens drawn on separate occasions meeting criteria that blood from at least two separate blood draws was collected on the same or consecutive calendar days, and from two separate blood draw sites. Proven and probable invasive fungal infections were based on modified criteria proposed by the European Organization for Research and Treatment of Cancer/Mycoses Study Group.18 These definitions were further modified to allow for inclusion of PCR testing from bronchoalveolar lavage specimens to identify probable Pneumocystis jirovecii pulmonary infections. Acute and chronic graft versus host disease were scored based on standardized criteria.19–21
Cost Assignment
We obtained detailed institutional cost accounting data representing direct and indirect (i.e., overhead) costs for all hospitalizations, emergency department visits, outpatient visits, medications, laboratory tests and procedures performed within the DUHS from 2009 to 2014. Investigation of both the DUHS cost data and use of anti-CMV medications documented in medical charts revealed that costs for inpatient use of anti-CMV treatments and outpatient administration of CMV immune globulin, cidofovir and intravenous immune globulin (IVIG) were well captured in the cost data. However, outpatient costs associated with foscarnet, ganciclovir and valganciclovir were incomplete because patients sometimes obtained these medications from pharmacies outside the DUHS. To account for costs of outpatient use of these medications, we applied unit costs derived from the DUHS data to medication dosing and duration information obtained from direct chart review, assuming full adherence.
The time period for the cost analysis began on the start date of the pre-transplant conditioning regimen and continued through one-year following the date of transplant. Two concerns arose. First, observed one-year costs would be underestimated for patients lost to follow-up. Second, to the extent that patients with csCMVi may be more likely to die (and incur no additional costs henceforth), csCMVi could inappropriately be interpreted as being cost-saving. To address these concerns, we calculated both actual costs as well as imputed costs to represent costs that would have been incurred had patients survived with complete one-year follow-up. To impute costs, we first computed mean daily costs for each day between Day 0 and Day +365 by csCMVi status using data from all patients up to their date of death/loss to follow-up. For patients who died or were lost to follow-up before Day +365, day- and csCMVi-specific mean costs were applied to remaining days through Day +365. Costs were summed and reported for three periods: from the start of conditioning to Day −1, Day 0 through Day +100 and Day +101 through Day +365. All costs were updated to 2018 U.S. dollars using the Consumer Price Index for Medical Care.22
Statistical analyses
Descriptive statistics were used to report baseline demographics, clinical characteristics, outcomes, medical resource use and costs. Percentages and numbers were reported for categorical variables. Means, standard deviations, medians and 25th and 75th percentiles were reported for continuous variables. In comparisons of baseline characteristics between patients with and without csCMVi, Chi-square tests were used for categorical variables, and Wilcoxon rank-sum tests were used for continuous variables.
Because medical costs are typically right-skewed, we applied nonparametric bootstrapping using the bias-corrected percentile method (2.5th and 97.5th percentiles) with 10,000 bootstrap replications to calculate the confidence intervals (CI) for differences in mean costs between patients with versus without csCMVi. Confidence intervals that exclude zero indicate statistically significant differences between unadjusted mean costs. To account for differences in baseline characteristics between patients with versus without csCMVi, we applied generalized linear models. For comparisons of medical resource use, the models were specified with negative binomial error distributions and log links; for comparisons of medical costs, models were specified with gamma error distributions and log links. Baseline covariates included age at transplant, gender, race, Karnofsky Performance Score (KPS),23 disease, conditioning regimen, stem cell source and GVHD prophylaxis including T-cell depletion. Age and KPS were modeled as linear, continuous variables, and all other covariates were modeled as categorical dummy variables.
OS was defined as time to death from the day of transplant from any cause with surviving patients censored on the date of last follow-up and presented using the Kaplan Meier estimator. Comparisons of OS at one and five years between csCMVi and non-csCMVi patients and between patients with peak CMV DNA PCR values < 1000 copies/mL or ≥ 1000 copies/mL were based on log-rank tests. NRM was defined as death without evidence of disease relapse and examined using cumulative incidence estimates to account for competing risks. Grey’s test was used to compare cumulative incidence curves between the groups at one and five years. All statistical analyses were conducted using SAS (SAS Institute, Version 9.4).
RESULTS
The cohort consisted of 388 adult allogeneic HCT recipients including 170 (43.8%) patients with csCMVi and 218 (56.2%) patients without csCMVi (Table 1). Eighteen patients were lost to follow-up during the first post-transplant year. Acute leukemia was the leading indication for allogeneic HCT across the cohort. Patients who developed csCMVi were more likely to have received a non-myeloablative conditioning regimen (90/170 [52.9%] vs 93/218 [42.7%], p=0.044) and T-cell depletion in the form of either anti-thymocyte globulin or alemtuzumab (91/170 [53.5%] vs 124/218 [43.1%], p=0.068). CMV seropositive recipients accounted for the vast majority (149/170 [87.6%]) of csCMVi cases with 149 of the 215 (69.3%) CMV seropositive recipients developing csCMVi (regardless of donor status). Twelve of the 57 (21.1%) CMV seronegative recipients who had seropositive donors developed csCMVi, and three of the 91 (3.3%) seronegative recipients with seronegative donors developed csCMVi.
Table 1.
Baseline Patient Characteristics
| Characteristic | All patients (N=388) | With csCMVi (N=170) | Without csCMVi (N=218) | P-valuea |
|---|---|---|---|---|
| Age (median) | 51 | 52 | 51 | 0.477 |
| Gender, female, no. (%) | 157 (40.5) | 75 (44.1) | 82 (37.6) | 0.195 |
| Race, no. (%) | 0.568 | |||
| Caucasian | 323 (83.2) | 138 (81.2) | 185 (84.9) | |
| African American | 58 (14.9) | 28 (16.5) | 30 (13.8) | |
| Other | 7 (1.8) | 4 (2.4) | 3 (1.4) | |
| Ethnicity (Non-Hispanic), no. (%) | 383 (98.7) | 167 (98.2) | 216 (99.1) | 0.463 |
| Karnofsky Performance Status | ||||
| Median | 90 | 85 | 90 | 0.146 |
| 100, no. (%) | 50 (12.9) | 23 (13.5) | 27 (12.4) | |
| 90, no. (%) | 151 (38.9) | 62 (36.5) | 89 (40.8) | |
| 80, no. (%) | 99 (25.5) | 33 (19.4) | 66 (30.3) | |
| <=70, no. (%) | 88 (22.7) | 52 (30.6) | 36 (16.5) | |
| Disease, no. (%) | 0.531 | |||
| Acute leukemia (AML+ALL) | 183 (47.2) | 82 (48.2) | 101 (46.3) | |
| Lymphoma (HL+NHL) | 81 (20.9) | 39 (22.9) | 42 (19.3) | |
| MDS/MPN | 61 (15.7) | 22 (12.9) | 39 (17.9) | |
| Other | 63 (16.2) | 27 (15.9) | 36 (16.5) | |
| Myeloablative Conditioning, no. (%) | 205 (52.8) | 80 (47.1) | 125 (57.3) | 0.044 |
| HLA Matching/Donor Type, no. (%) | 0.839 | |||
| MUD | 175 (45.1) | 73 (42.9) | 102 (46.8) | |
| MRD | 107 (27.6) | 47 (27.6) | 60 (27.5) | |
| MMUD | 71 (18.3) | 34 (20.0) | 37 (17.0) | |
| MMRD | 35 (9.0) | 16 (9.4) | 19 (8.7) | |
| Haploidentical Donor, no. (%) | 29 (7.5) | 14 (8.2) | 15 (6.9) | 0.615 |
| Stem Cell Source, no. (%) | 0.126 | |||
| Peripheral blood | 295 (76.0) | 131 (77.1) | 164 (75.2) | |
| Bone marrow | 27 (7.0) | 7 (4.1) | 20 (9.2) | |
| Cord blood | 66 (17.0) | 32 (18.8) | 34 (15.6) | |
| GVHD prophylaxis, no. (%) | 0.080 | |||
| MTX/CNI alone | 118 (30.4) | 40 (23.5) | 78 (35.8) | |
| MMF/CNI alone | 80 (20.6) | 37 (21.8) | 43 (19.7) | |
| Alemtuzumab | 155 (39.9) | 79 (46.5) | 76 (34.9) | |
| ATG | 30 (7.7) | 12 (7.1) | 18 (8.3) | |
| Other | 5 (1.3) | 2 (1.2) | 3 (1.4) | . |
| CMV status, no. (%)b | <0.0001 | |||
| Donor Pos, Recipient Pos | 109 (28.1) | 76 (44.7) | 33 (15.1) | |
| Donor Pos, Recipient Neg | 57 (14.7) | 12 (7.1) | 45 (20.6) | |
| Donor Neg, Recipient Pos | 106 (27.3) | 73 (42.9) | 33 (15.1) | |
| Donor Neg, Recipient Neg | 91 (23.5) | 3 (1.8) | 88 (40.4) | |
| Indeterminate/Unknown | 25 (6.4) | 6 (3.5) | 19 (8.7) |
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; CMV, cytomegalovirus; CNI, calcineurin inhibitor; csCMVi, clinically significant cytomegalovirus infection; GVHD; graft-versus-host disease; HL, Hodgkin’s lymphoma; HLA, human leukocyte antigen; MDS, myelodysplastic syndrome; MMF, mycophenolate; MMRD, mismatched related donor; MMUD, mismatched unrelated donor; MPN, myeloproliferative neoplasms; MRD, matched related donor; MTX, methotrexate; MUD, matched unrelated donor; NHL, Non-Hodgkin’s lymphoma.
P-values based on Chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
25 patients who had CMV status as ‘Indeterminate/Unknown’ include 18 patients with donor CMV negative and recipient indeterminate status and 7 patients with donor CMV positive and recipient CMV indeterminate status
Further details regarding the csCMVi episodes are shown in Table 2. Multiple episodes (≥ 1, range 2 to 5 episodes) were common, occurring in 77/170 (45.3%) of patients developing csCMVi. The first episode of csCMVi occurred a median of 33 days post-transplant (range four to 320 days) with 160 (94.1%) beginning prior to Day +100 and 10 (5.9%) beginning beyond Day +100. When comparing all 289 csCMVi episodes, 196 (67.8%) occurred prior to Day +100 and 93 (32.2%) occurred beyond Day +100. CMV disease occurred in 36/170 (21.2%) of patients with csCMVi and generally occurred later post-transplant (median onset 71.5 days [range 19–337 days]) and most commonly affected the gastrointestinal tract (25/36 [69.4%]). Repeated episodes of CMV disease were uncommon and documented in only one patient (1/36 [2.8%]) though disease involvement of more than one site was seen (5/36 [13.4%]). The most common antiviral therapies applied were ganciclovir (127/170 [74.7%]) followed by foscarnet (117/170 [68.8%]). Supplementary therapies such as IVIG and CMV immune globulin were utilized in 16.5% (28/170) of patients. Median total duration of CMV antiviral therapy across the one-year study period was 49 days (range 1–299 days). Figure 1 further demonstrates antiviral duration across csCMVi episodes. Genotypic assessment for antiviral resistance to CMV was performed in 29/170 (17.1%) patients with csCMVi and confirmed mutations predicting resistance were identified in 7/170 (4.1%).
Table 2.
Characteristics of Clinically Significant Cytomegalovirus Infection
| Characteristic | N=170 |
|---|---|
| csCMVi episodes | |
| csCMVi episodes per HCT recipient, median (IQI) | 1 (1–2) |
| 1, no. (%) | 170 (100) |
| 2, no. (%) | 77 (45.3) |
| 3, no. (%) | 28 (16.5) |
| ≥ 4, no. (%) | 16 (9.4) |
| Time to first episode, median days (IQI) | 33 (26–47) |
| CMV disease | |
| Any CMV disease, no. (%) | 36 (21.2) |
| >1 episode, no. (%)a | 1 (0.6) |
| >1 site, no. (%)b | 5 (2.9) |
| Time to first CMV disease, median days (IQI) | 71.5 (46–164) |
| CMV disease site, no. (%) | |
| Gastrointestinal | 25 (14.7) |
| Proven upper GI | 11 (6.5) |
| Probable upper GI | 5 (2.9) |
| Proven lower GI | 6 (3.5) |
| Probable lower GI | 7 (4.1) |
| Pulmonary | 10 (5.9) |
| Proven | 3 (1.8) |
| Probable | 7 (4.1) |
| Central nervous system | 2 (1.2) |
| Proven | 0 |
| Probable | 2 (1.2) |
| Antivirals applied (all episodes), no. (%)c | |
| Ganciclovir | 127 (74.7) |
| Foscarnet | 117 (68.8) |
| Valganciclovir | 72 (42.4) |
| Cidofovir | 6 (3.5) |
| Additional CMV-directed therapies | |
| Intravenous immunoglobulin | |
| Total patients receiving, no. (%) | 19 (11.2) |
| Number of doses, mean (SD) | 7.5 (6.1) |
| CMV immunoglobulin | |
| Total patients receiving, no. (%) | 9 (5.3) |
| Number of doses, mean (SD) | 3.4 (2.3) |
| Antiviral resistance, no. (%) | 7 (4.1) |
csCMVi, clinically significant cytomegalovirus infection; CMV, cytomegalovirus; GI, gastrointestinal; HCT, hematopoietic cell transplant; IQI, 25%−75% interquartile index; SD, standard deviation.
2 episodes of proven gastritis.
More than one site of involvement included: 2 patients with proven upper and lower GI disease, 2 patients with proven upper and probable lower GI disease and 1 patient with probable upper GI disease and proven pneumonia.
One patient died before receiving antiviral therapy.
Figure 1. Duration of Cytomegalovirus-Directed Antiviral Therapy.
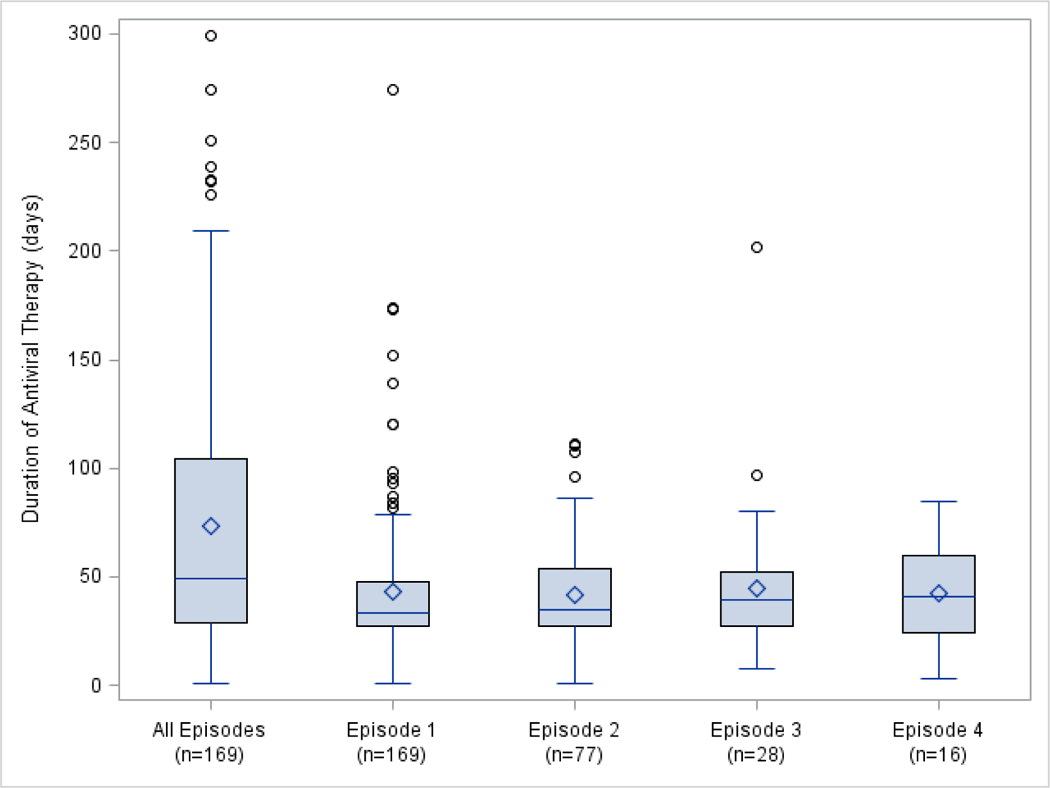
Bar in boxplot represents median antiviral duration. Diamond represents mean antiviral duration. Upper and lower limit of boxplot represent 75th and 25th percentiles, respectively. Whiskers represent values within 1.5*interquartile range and circles represent outliers. Boxplot for ‘all episodes’ represents cumulative duration of antiviral therapy.
Medical resource use during the study period, including blood, other support products and hospitalizations, is detailed in Table 3. There was a trend toward greater use of red blood cell (RBC) and platelet transfusions in patients with csCMVi (mean RBC transfusions 15.5 vs. 13.1, p=0.059; mean platelet transfusions 16.2 vs. 13.7, p=0.112) and these patients were significantly more likely to receive granulocyte colony stimulating factor (73.5% vs. 54.1%, p=0.0001). Further, patients with csCMVi had more frequent and prolonged hospitalizations throughout the study period. (2.4 vs. 1.7 mean admissions, p<0.0001; 39.1 vs. 31.5 mean inpatient days, p=0.001). However, there were no significant differences in ICU admissions. Mean total transplant length of stay, defined as the time between Day 0 and when patients were discharged to home after the acute peri-HCT care period, was also longer in patients with versus without csCMVi (mean 91.7 days +/− 31.5 vs. 78.3 days +/− 30.7, p<0.0001). The overall incidence of acute and chronic GVHD and infectious complications consisting of bacteremia and proven and probable invasive fungal infections was not significantly different between the two groups (Table 3).
Table 3.
Resource Utilization and Clinical Outcomes
| Parameter | All Patients (N=388) | With csCMVi (N=170) | Without csCMVi (N=218) | Unadjusted p-valuea,b | Adjusted p-valuea,c |
|---|---|---|---|---|---|
| Transfusion/Support Products | |||||
| Red blood cells | |||||
| Mean units (SD) | 14.1 (14.5) | 15.5 (14.8) | 13.1 (14.2) | 0.077 | 0.059 |
| Median units (IQI) | 9 (5–19.5) | 11.5 (6–21) | 9 (4–17) | ||
| Platelets | |||||
| Mean units (SD) | 14.8 (20.4) | 16.2 (21.6) | 13.7 (19.3) | 0.179 | 0.112 |
| Median units (IQI) | 7.5 (3–19) | 8 (3–22) | 7 (3–17) | ||
| GCSF, no. (%) | 243 (62.6) | 125 (73.5) | 118 (54.1) | 0.0001 | 0.0001 |
| Hospitalizationsd | |||||
| Any inpatient admission, no. (%) | 358 (92.3) | 162 (95.3) | 196 (89.9) | 0.055 | 0.024 |
| Inpatient admissions | |||||
| Mean number (SD) | 2.0 (1.5) | 2.4 (1.6) | 1.7 (1.4) | <0.0001 | <0.0001 |
| Median number (IQI) | 2 (1–3) | 2 (1–3) | 1 (1–2) | ||
| Inpatient days | |||||
| Mean days (SD) | 34.8 (26.5) | 39.1 (27.5) | 31.5 (25.3) | 0.024 | 0.001 |
| Median days (IQI) | 32 (17–45) | 35 (24–49) | 29 (12–40) | ||
| Intensive care unit stay, no. (%) | 95 (24.5) | 48 (28.2) | 47 (21.6) | 0.131 | 0.408 |
| Transplant Length of Stay | |||||
| Mean days (SD) | 84.2 (31.7) | 91.7 (31.5) | 78.3 (30.7) | <0.0001 | <0.0001 |
| Median days (IQI) | 85 (65–95.5) | 89 (75–103) | 83.5 (61–92) | ||
| Graft-versus-host Disease | |||||
| Acute GVHD, no. (%) | 252 (64.9) | 115 (67.6) | 137 (62.8) | 0.326 | 0.755 |
| Grade of GVHD | |||||
| Grade 2 – 4 | 238 (61.3) | 112 (65.9) | 126 (57.8) | 0.106 | 0.270 |
| Grade 3 – 4 | 107 (27.6) | 55 (32.4) | 52 (23.9) | 0.065 | 0.204 |
| Site of GVHD | |||||
| Skin | 186 (47.9) | 83 (48.8) | 103 (47.3) | 0.758 | 0.644 |
| Liver | 17 (4.4) | 6 (3.5) | 11 (5.0) | 0.472 | 0.587 |
| Upper GI | 126 (32.5) | 58 (34.1) | 68 (31.2) | 0.542 | 0.672 |
| Lower GI | 130 (33.5) | 65 (38.2) | 65 (29.8) | 0.083 | 0.063 |
| Chronic GVHD, no. (%) | 129 (33.2) | 64 (37.6) | 65 (29.8) | 0.106 | 0.122 |
| Grade of GVHD | |||||
| Grade 1 | 41 (10.57) | 27 (15.9) | 14 (6.4) | 0.004 | 0.003 |
| Grade 2 | 67 (17.27) | 24 (14.1) | 43 (19.7) | 0.150 | 0.124 |
| Grade 3 | 21 (5.41) | 13 (7.6) | 8 (3.7) | 0.093 | 0.134e |
| Site of GVHD | |||||
| Skin | 86 (22.2) | 42 (24.7) | 44 (20.2) | 0.289 | 0.320 |
| Mouth | 29 (7.5) | 15 (8.8) | 14 (6.4) | 0.375 | 0.195 |
| Eye | 21 (5.4) | 11 (6.5) | 10 (4.6) | 0.419 | 0.341 |
| GI | 49 (12.6) | 26 (15.3) | 23 (10.6) | 0.166 | 0.385e |
| Liver | 18 (4.6) | 7 (4.1) | 11 (5.0) | 0.667 | 0.898e |
| Lung | 7 (1.8) | 1 (0.6) | 6 (2.8) | 0.150 | NA |
| Joint | 4 (1.0) | 2 (1.2) | 2 (0.9) | 0.803 | NA |
| Genital | 1 (0.3) | 1 (0.6) | 0 (0.0) | 0.978 | NA |
| Renal | 1 (0.3) | 1 (0.6) | 0 (0.0) | 0.978 | NA |
| Infections | |||||
| Bacteremia | |||||
| Any bacteremia, no. (%) | 124 (32) | 59 (34.7) | 65 (29.8) | 0.307 | 0.202 |
| Onset of bacteremia | |||||
| Median transplant day (IQI) | 22 (7.5–92) | 27 (8–146) | 16 (7–85) | ||
| Invasive fungal infections | |||||
| IFI, no. (%) | 34 (8.8)) | 20 (11.7) | 14 (6.4) | 0.069 | 0.117 |
| Proven infection | 21 (5.4) | 12 (7.1) | 9 (4.1) | ||
| Probable infection | 13 (3.4) | 8 (4.7) | 5 (2.3) | ||
| Onset of IFI | |||||
| Mean transplant day (SD) | 142.3 (118) | 159.3 (108.5) | 117.9 (130.5) | 0.401 | 0.062 |
| Median transplant day (IQI) | 136 (24–266) | 150.5 (48.5–282.5) | 54 (12–210) | ||
csCMVi, clinically significant cytomegalovirus infection; GCSF, granulocyte colony stimulating factor; GI, gastrointestinal; GVHD, graft-versus-host disease; IFI, invasive fungal infection, IQI, 25%−75% interquartile index; NA, not applicable; SD, standard deviation.
P-values based on generalized linear models with negative binomial distributions and log links.
Independent variable included CMV status only.
P-values adjusted for baseline demographic and clinical variables reported in Table 1.
From start of conditioning through first post-transplant year
P-value based on model including all baseline covariates excluding graft-versus-host disease prophylaxis.
The one- and five-year OS for the cohort was 56.7% and 35.8% with no significant difference between those with and without csCMVi (one-year OS p=0.807; five-year OS p=0.880, Figure 2). Similarly, there was no significant difference in one- and five-year NRM (one-year NRM p=0.221; five-year NRM p=0.132, Figure 3). However, patients with csCMVi had an overall lower cumulative incidence of disease relapse at one- and five-years post-transplant (one-year disease relapse 4.7% csCMVi vs. 11.5% without csCMVi, p=0.019; five-year disease relapse 5.9% csCMVi vs. 13.8% without csCMVi, p=0.012, Figure 4). When comparing patients with csCMVi based on peak CMV DNA PCR values < 1000 copies/mL versus ≥ 1000 copies/mL across episodes there was no significant difference in OS (one-year OS p=0.942; five-year OS p=0.101, Figure S1) or disease relapse (one-year disease relapse p=0.126; five-year disease relapse p=0.319, Figure S2) between these two groups. However, five-year NRM was significantly higher in those with peak CMV DNA PCR values ≥ 1000 copies/mL (one-year NRM p=0.343; five-year NRM, p=0.024, Figure S3).
Figure 2.
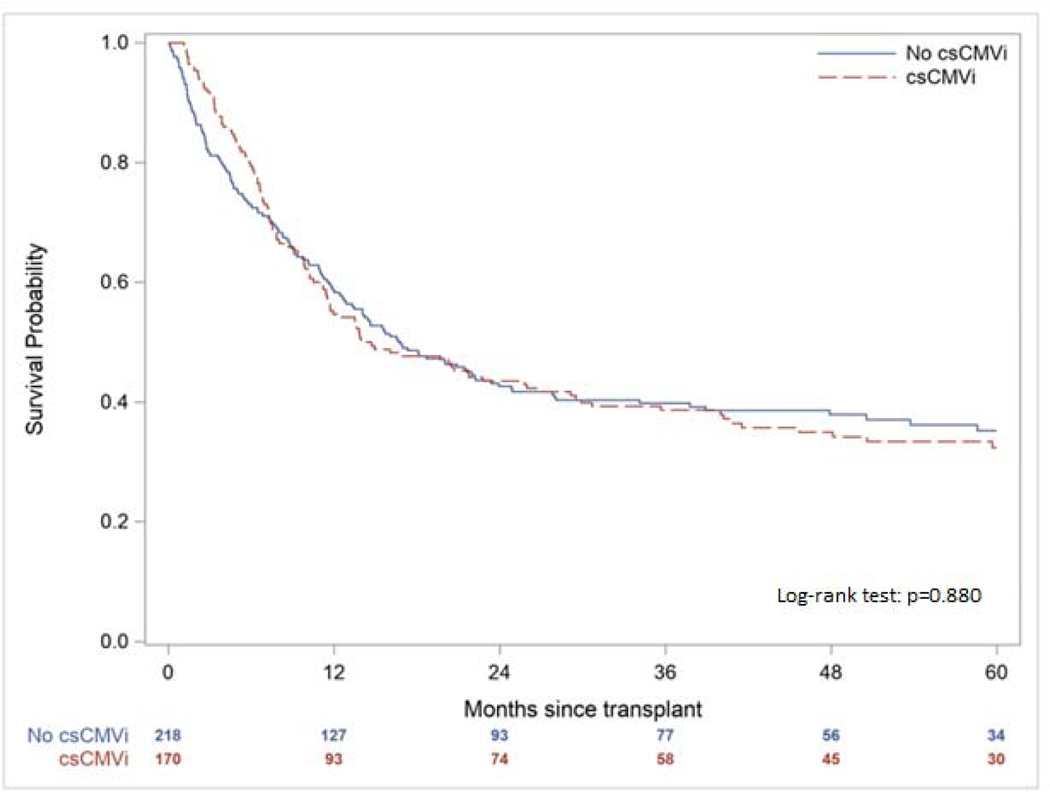
Overall Survival According to the Presence of Clinically Significant Cytomegalovirus Infection (csCMVi)
Figure 3.
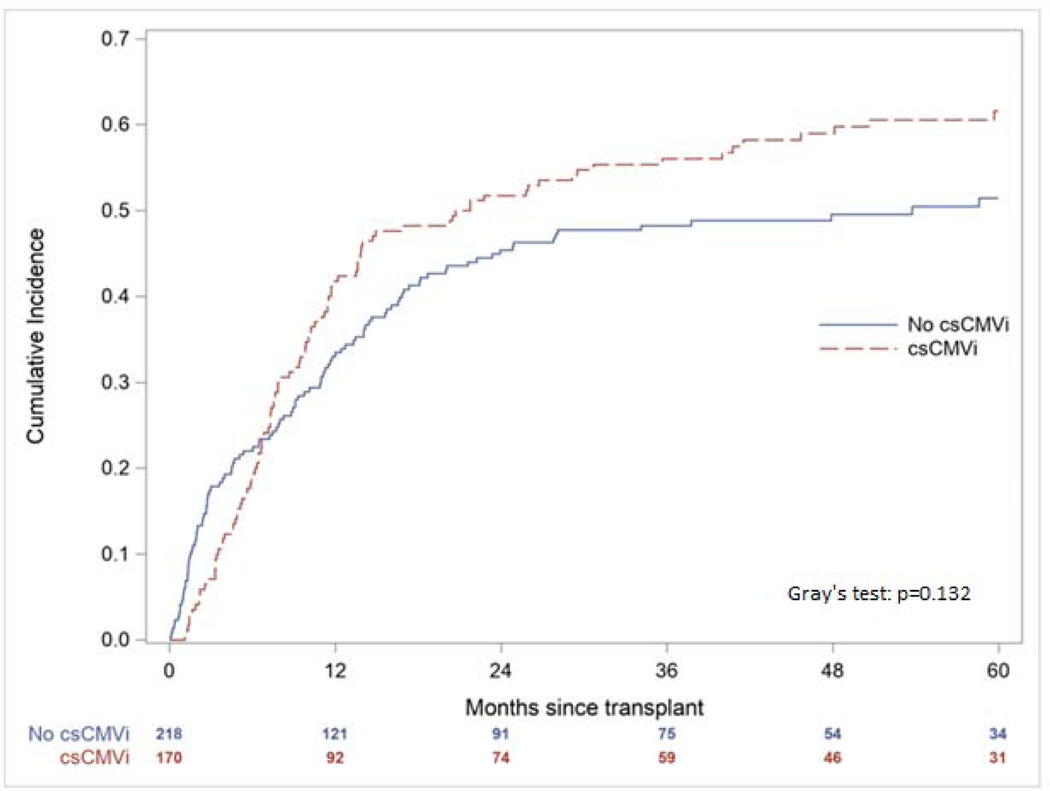
Cumulative Incidence of Non-Relapse Mortality According to the Presence of Clinically Significant Cytomegalovirus Infection (csCMVi)
Figure 4.
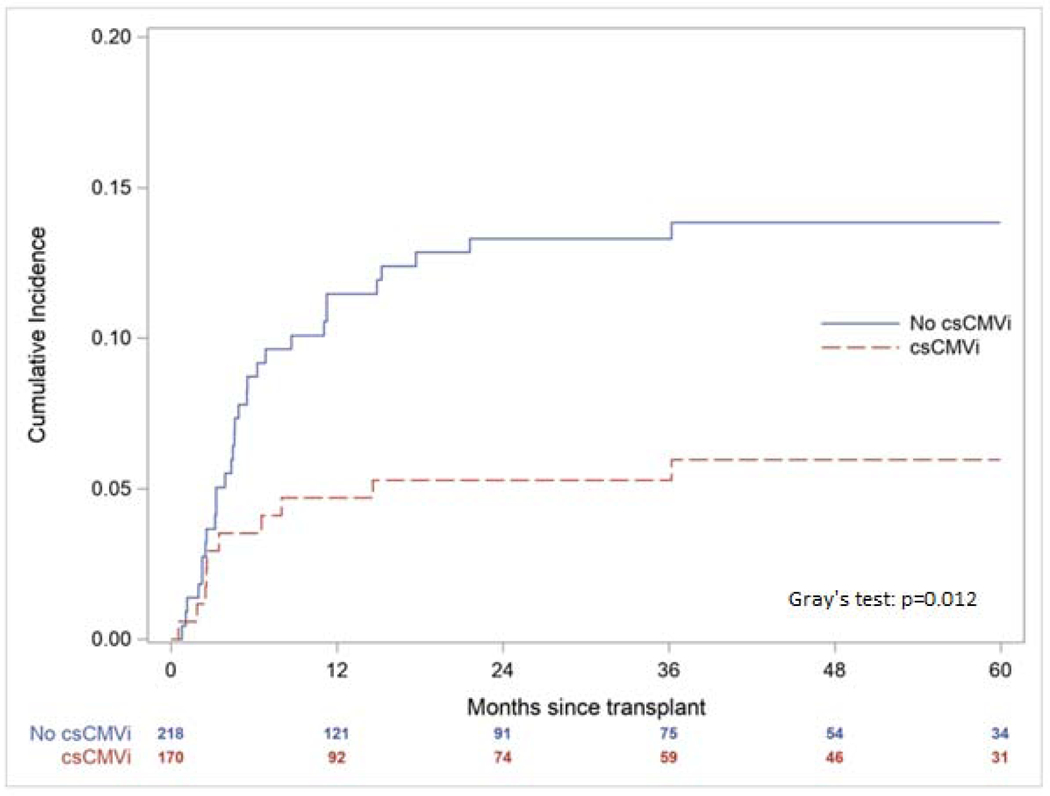
Cumulative Incidence of Hematologic Disease Relapse According to the Presence of Clinically Significant Cytomegalovirus Infection (csCMVi)
Actual and imputed costs are detailed in Tables 4a and 4b. Mean total actual and imputed one-year costs of allogeneic HCT for the entire cohort were $180,517 and $203,995, respectively. Total actual and imputed one-year costs were significantly higher in those with csCMVi versus those without csCMVi (cost difference $45,811 [95% CI $26,385 - $67,544] and $54,030 [95% CI $32,121 - $76,250] respectively). Further evaluation of the impact of multiple episodes of csCMVi is demonstrated in Table 4b wherein patients with more than one episode of csCMVi incurred higher actual and imputed costs across the study period than patients with one episode of csCMVi (cost difference $51,038 [95% CI $22,683 - $84,435] and $37,590 [95% CI $5,007 - $74,052] respectively). Evaluation of the impact of peak viral load attained on costs in those with csCMVi demonstrated no differences in costs associated with a CMV DNA PCR value < 1000 copies/mL versus ≥ 1000 copies/mL (data not shown). A separate analysis of actual and imputed costs across the same time periods in patients with a travel time less than or equal to one hour as opposed to greater than one hour from the medical center also did not demonstrate significant differences (data not shown).
Table 4a.
Costs Within the First Year of Allogeneic Hematopoietic Cell Transplantation
| All Patients (N=388) | Patients with csCMVi (N=170) | Patients without csCMVi (N=218) | Cost Difference (95% CI) | Mean Cost Ratio (unadjusted p-value)a,b | Mean Cost Ratio (adjusted p-value)a,c | |
|---|---|---|---|---|---|---|
| TOTAL COSTS (ACTUAL)d | ||||||
| Tx start to D0e | N=388 | N=170 | N=218 | |||
| Mean (SD) | 23,683 (15,423) | 24,200 (17,093) | 23,280 (14,010) | 920 (−2,715 to 4,002) | 1.040 (0.561) | 1.074 (0.143) |
| Median (IQI) | 20,488 (11,580–32,548) | 19,957 (10,948–34,715) | 21,195 (12,074–31,117) | |||
| Maximum | 130,258 | 130,258 | 74,757 | |||
| D0 to D100 | N=388 | N=170 | N=218 | |||
| Mean (SD) | 120,156 (75,615) | 131,176 (75,635) | 111,561 (74,648) | 19,615 (4,296 to 34,125) | 1.176 (0.012) | 1.173 (0.004) |
| Median (IQI) | 107,127 (66,686–156,599) | 116,827 (73,907–168,452) | 100,108 (61,780–143,872) | |||
| Maximum | 518,903 | 419,250 | 518,903 | |||
| D101 to D365 | N=328 | N=154 | N=174 | |||
| Mean (SD) | 43,388 (63,213) | 56,166 (69,178) | 32,078 (55,196) | 24,088 (11,490 to 38,284) | 1.750 (<0.001) | 1.727 (0.001) |
| Median (IQI) | 16,044 (4,009–62,328) | 25,259 (8,223–79,294) | 6,360 (2,886–39,378) | |||
| Maximum | 384,446 | 374,691 | 384,446 | |||
| D0 to D365 | N=388 | N=170 | N=218 | |||
| Mean (SD) | 156,834 (97,535) | 182,056 (96,888) | 137,165 (93,637) | 44,891 (26,126 to 65,266) | 1.327 (<0.0001) | 1.349 (<0.0001) |
| Median (IQI) | 136,877 (87,986–201,169) | 164,004 (113,376–236,289) | 114,841 (76,598–172,914) | |||
| Maximum | 573,064 | 573,064 | 533,683 | |||
| Tx start to D365 | N=388 | N=170 | N=218 | |||
| Mean (SD) | 180,517 (104,749) | 206,256 (103,171) | 160,445 (101,758) | 45,811 (26,385 to 67,544) | 1.286 (<0.0001) | 1.316 (<0.0001) |
| Median (IQI) | 162,154 (106,014–231,170) | 190,438 (131,010–265,363) | 146,392 (89,314–196,965) | |||
| Maximum | 606,680 | 606,680 | 591,894 | |||
| TOTAL COSTS (IMPUTED)d | ||||||
| Tx start to D0e | N=388 | N=170 | N=218 | |||
| Mean (SD) | 23,683 (15,423) | 24,200 (17,093) | 23,280 (14,010) | 920 (−2,715 to 4,002) | 1.040 (0.561) | 1.074 (0.143) |
| Median (IQI) | 20,488 (11,580–32,548) | 19,957 (10,948–34,715) | 21,195 (12,074–31,117) | |||
| Maximum | 130,258 | 130,258 | 74,757 | |||
| D0 to D100 | N=388 | N=170 | N=218 | |||
| Mean (SD) | 124,885 (76,730) | 134,074 (76,465) | 117,719 (76,346) | 16,355 (32 to 30,814) | 1.139 (0.040) | 1.132 (0.026) |
| Median (IQI) | 112,190 (69,809–163,625) | 126,076 (76,511–170,900) | 106,844 (65,956–153,568) | |||
| Maximum | 523,838 | 419,250 | 523,838 | |||
| D101 to D365 | N=388 | N=170 | N=218 | |||
| Mean (SD) | 55,428 (61,831) | 76,079 (69,116) | 39,324 (50,032) | 36,755 (24,084 to 48,445) | 1.935 (<0.0001) | 1.887 (<0.0001) |
| Median (IQI) | 39,324 (8,468–76,079) | 71,807 (20,671–88,521) | 33,785 (4,866–39,378) | |||
| Maximum | 384,446 | 374,899 | 384,446 | |||
| D0 to D365 | N=388 | N=170 | N=218 | |||
| Mean (SD) | 180,313 (103,760) | 210,153 (103,784) | 157,043 (97,828) | 53,110 (32,005 to 73,454) | 1.338 (<0.0001) | 1.346 (<0.0001) |
| Median (IQI) | 159,500 (108,901–231,451) | 196,741 (139,355–273,785) | 136,297 (89,097–204,216) | |||
| Maximum | 573,272 | 573,272 | 563,162 | |||
| Tx start to D365 | N=388 | N=170 | N=218 | |||
| Mean (SD) | 203,995 (110,437) | 234,352 (109,161) | 180,323 (105,764) | 54,030 (32,121 to 76,250) | 1.300 (<0.0001) | 1.318 (<0.0001) |
| Median (IQI) | 184,365 (124,650–254,238) | 224,881 (156,627–303,504) | 160,841 (108,102–227,824) | |||
| Maximum | 629,533 | 606,888 | 629,533 | |||
| INPATIENT COSTS (ACTUAL)d | N=388 | N=170 | N=218 | |||
| Mean (SD) | 125,692 (100,187) | 138,029 (96,828) | 116,071 (101,916) | 21,958 (2,373 to 41,900) | 1.189 (0.273) | 1.330 (0.069) |
| Median (IQI) | 117,609 (51,336–175,831) | 131,958 (64,209–189,114) | 102,874 (46,456–158,113) | |||
| Maximum | 585,274 | 432,322 | 585,274 | |||
| OUTPATIENT COSTS (ACTUAL)d | N=388 | N=170 | N=218 | |||
| Mean (SD) | 54,824 (46,404) | 68,227 (47,174) | 44,373 (43,090) | 23,854 (15,044 to 33,237) | 1.538 (0.001) | 1.571 (0.001) |
| Median (IQI) | 41,352 (20,237–79,089) | 53,906 (32,777–101,806) | 30,907 (15,310–67,450) | |||
| Maximum | 343,934 | 228,325 | 343,934 | |||
| INPATIENT COSTS (IMPUTED)d | N=388 | N=170 | N=218 | |||
| Mean (SD) | 140,731 (104,640) | 156,794 (102,272) | 128,205 (104,986) | 28,589 (8,301 to 48,625) | 1.223 (0.165) | 1.344 (0.039) |
| Median (IQI) | 130,375 (61,812–191,571) | 145,948 (84,962–209,882) | 115,778 (50,595–177,739) | |||
| Maximum | 611,138 | 460,580 | 611,138 | |||
| OUTPATIENT COSTS (IMPUTED)d | N=388 | N=170 | N=218 | |||
| Mean (SD) | 63,264 (45,164) | 77,558 (45,279) | 52,118 (41,907) | 25,441 (16,934 to 34,301) | 1.488 (<0.0001) | 1.466 (<0.0001) |
| Median (IQI) | 49,947 (31,153–87,094) | 65,411 (43,829–109,173) | 38,216 (24,143–72,156) | |||
| Maximum | 343,934 | 241,322 | 343,934 | |||
Table 4b.
Costs in Patients with One or Multiple Episodes of Clinically Significant Cytomegalovirus Infection Within the First Year of Allogeneic Hematopoietic Cell Transplantation
| All Patients with csCMVi (N=170) | One Episode of csCMVi (N=93) | Multiple Episodes of csCMVi (N=77) | Mean Cost Difference (95% CI) | Mean Cost Ratio (unadjusted p-value)a,b | Mean Cost Ratio (adjusted p-value)a,c | |
|---|---|---|---|---|---|---|
| TOTAL COSTS (ACTUAL)d | ||||||
| Tx start to Day 0e | N=170 | N=93 | N=77 | |||
| Mean (SD) | 24,200 (17,093) | 24,973 (17,673) | 23,265 (16,430) | −1,708 (−6,974 to 3,490) | 0.932 (0.500) | 0.893 (0.148) |
| Median (IQI) | 19,957 (10,948–34,715) | 20,590 (12,678–34,623) | 18,598 (8,945–34,715) | |||
| Maximum | 130,258 | 130,258 | 68,225 | |||
| D0 to D100 | N=170 | N=93 | N=77 | |||
| Mean (SD) | 131,176 (75,635) | 128,602 (78,368) | 134,285 (72,585) | 5,683 (−15,551 to 29,792) | 1.044 (0.637) | 1.050 (0.553) |
| Median (IQI) | 116,827 (73,907–168,452) | 112,930 (65,905–162,351) | 126,857 (76,511–182,858) | |||
| Maximum | 419,250 | 419,250 | 409,902 | |||
| D101 to D365 | N=154 | N=78 | N=76 | |||
| Mean (SD) | 56,166 (69,178) | 35,248 (51,163) | 77,635 (78,440) | 42,387 (22,434 to 64,881) | 2.203 (<0.0001) | 2.549 (<0.0001) |
| Median (IQI) | 25,259 (8,223–79,294) | 13,815 (4,042–49,046) | 53,083(16,838–122,479) | |||
| Maximum | 374,691 | 293,757 | 374,691 | |||
| D0 to D365 | N=170 | N=93 | N=77 | |||
| Mean (SD) | 182,056 (96,888) | 158,165 (86,399) | 210,912 (101,469) | 52,746 (24,755 to 84,190) | 1.333 (<0.001) | 1.382 (<0.0001) |
| Median (IQI) | 164,004 (113,376–236,289) | 141,506 (102,795–212,543) | 194,365 (144,507–258,350) | |||
| Maximum | 573,064 | 493,336 | 573,064 | |||
| Tx Start to D365 | N=170 | N=93 | N=77 | |||
| Mean (SD) | 206,256 (103,171) | 183,139 (95,131) | 234,177 (106,164) | 51,038 (22,683 to 84,435) | 1.279 (0.001) | 1.324 (<0.001) |
| Median (IQI) | 190,438 (131,010–265,363) | 166,807 (117,618–236,717) | 232,931 (161,847–286,777) | |||
| Maximum | 606,680 | 537,959 | 606,680 | |||
| TOTAL COSTS (IMPUTED)d | ||||||
| Tx Start to D0e | N=170 | N=93 | N=77 | |||
| Mean (SD) | 24,200 (17,093) | 24,973 (17,673) | 23,265 (16,430) | −1,708 (−6,974 to 3,490) | 0.932 (0.500) | 0.893 (0.148) |
| Median (IQI) | 19,957 (10,948–34,715) | 20,590 (12,678–34,623) | 18,598 (8,945–34,715) | |||
| Maximum | 130,258 | 130,258 | 68,225 | |||
| D0 to D100 | N=170 | N=93 | N=77 | |||
| Mean (SD) | 134,074 (76,465) | 133,851 (79,906) | 134,342 (72,610) | 491 (−20,391 to 25,557) | 1.004 (0.968) | 1.011 (0.896) |
| Median (IQI) | 126,076 (76,511–170,900) | 121,998 (79,333–162,351) | 126,857 (76,511–182,858) | |||
| Maximum | 419,250 | 419,250 | 409,902 | |||
| D101 to D365 | N=170 | N=93 | N=77 | |||
| Mean (SD) | 76,079 (69,116) | 58,502 (49,560) | 97,309 (82,550) | 38,808 (18,517 to 61,525) | 1.663 (0.001) | 1.701 (0.001) |
| Median (IQI) | 71,807 (20,671–88,521) | 66,974 (16,561–76,079) | 77,865 (28,591–158,012) | |||
| Maximum | 374,899 | 293,757 | 374,899 | |||
| D0 to D365 | N=170 | N=93 | N=77 | |||
| Mean (SD) | 210,153 (103,784) | 192,353 (96,575) | 231,651 (108,652) | 39,298 (9,734 to 73,955) | 1.204 (0.018) | 1.255 (0.005) |
| Median (IQI) | 196,741 (139,355–273,785) | 183,747 (115,795–249,447) | 216,443 (144,507–302,378) | |||
| Maximum | 573,272 | 493,336 | 573,272 | |||
| Tx start to D365 | N=170 | N=93 | N=77 | |||
| Mean (SD) | 234,352 (109,161) | 217,326 (103,789) | 254,916 (112,580) | 37,590 (5,007 to 74,052) | 1.173 (0.032) | 1.217 (0.008) |
| Median (IQI) | 224,881 (156,627–303,504) | 212,749 (148,345–275,910) | 247,446 (176,143–330,477) | |||
| Maximum | 606,888 | 537,959 | 606,888 | |||
| INPATIENT COSTS (ACTUAL)d | N=170 | N=93 | N=77 | |||
| Mean (SD) | 138,029 (96,828) | 128,843 (90,874) | 149,124 (103,072) | 20,280 (−8,108 to 53,033) | 1.157 (0.465) | 1.302 (0.211) |
| Median (IQI) | 131,958 (64,209–189,114) | 122,421 (62,878–180,642) | 139,468 (85,831–205,432) | |||
| Maximum | 432,322 | 432,322 | 416,384 | |||
| OUTPATIENT COSTS (ACTUAL)d | N=170 | N=93 | N=77 | |||
| Mean (SD) | 68,227 (47,174) | 54,295 (44,460) | 85,053 (45,071) | 30,758 (15,774 to 43,536) | 1.566 (0.002) | 1.369 (0.021) |
| Median (IQI) | 53,906 (32,777–101,806) | 38,072 (26,990–68,359) | 75,757 (48,933–114,996) | |||
| Maximum | 228,325 | 208,047 | 228,325 | |||
| INPATIENT COSTS (IMPUTED)d | N=170 | N=93 | N=77 | |||
| Mean (SD) | 156,794 (102,272) | 151,131 (97,804) | 163,634 (107,669) | 12,504 (−18,857 o 47,862) | 1.083 (0.659) | 1.193 (0.354) |
| Median (IQI) | 145,948 (84,962 – 209,882) | 143,217 (81,664 – 191,759) | 151,552 (89,538 – 213,605) | |||
| Maximum | 460,580 | 434,318 | 460,580 | |||
| OUTPATIENT COSTS (IMPUTED)d | N=170 | N=93 | N=77 | |||
| Mean (SD) | 77,558 (45,279) | 66,196 (42,572) | 91,282 (44,901) | 25,086 (10,424 to 38,072) | 1.379 (<0.001) | 1.251 (0.008) |
| Median (IQI) | 65,411 (43,829 – 109,173) | 52,353 (34,576 – 85,201) | 79,390 (51,559 – 120,041) | |||
| Maximum | 241,322 | 208,047 | 241,322 | |||
CI, confidence interval; csCMVi, clinically significant cytomegalovirus infection; D, day; IQI, 25%−75% interquartile index; SD, standard deviation; Tx, transplant.
P-values based on generalized linear models with gamma distributions and log links.
Independent variable included CMV status only.
Independent variables included CMV status and baseline demographic and clinical variables in Table 1 except HLA and donor and recipient CMV serostatus.
All costs expressed in U.S. dollars.
Cost does not include D0.
DISCUSSION
Our cohort represents the largest published single center evaluation of the costs of CMV in allogeneic HCT. This large retrospective cohort study combines detailed cost accounting data with clinical data and chart review to present several insights into the economic burden of csCMVi: (1) patients with csCMVi have higher overall costs associated with HCT, approximately $45,000 (25%) added to the cost of HCT; (2) higher costs are incurred not just in the first 100 days but persist from days 101 to 365 after HCT when patients are typically discharged home from the transplant center; (3) higher costs are due to a combination of both direct treatment costs (e.g., antiviral therapies directed against csCMVi) and associated medical care (e.g., increased hospitalizations and length of stay). Findings from this study are comparable to other single center economic assessments of the impact of CMV in allogeneic HCT. However, these studies included smaller sample sizes, shorter follow-up periods and less detailed clinical and cost data.24, 25 Jain, et al24 compared 90 patients requiring CMV therapy in the first six months following allogeneic HCT to 44 patients who did not and estimated a mean additional cost between $58,000 to $74,000 per patient based on costs of antiviral therapy and estimated daily costs of inpatient hospitalization. In a retrospective cohort of 208 allogeneic HCT recipients, Robyn, et al.25 found that recipients with greater than one CMV episode had a 25 to 30% increase in the costs associated with transplant though this evaluation was limited to inpatient costs only over a 12-month period post-transplant. In addition, while other investigators have demonstrated more substantial incremental costs of CMV in HCT recipients ($280,954 over a two-year period),26 their analysis utilized the Truven Health MarketScan® database, thereby representing paid claims as opposed to the detailed cost accounting data provided in this study. This is an important distinction as our study provides a more accurate representation of the costs required to deliver care compared to negotiated payments, for which there is likely a higher mark-up for commercial payers.
The major advantage of our single-center design is the opportunity to use a detailed cost dataset to generate these findings; at the same time, this design has limitations with regard to drawing clinical conclusions. For example, while large for a single center study, the cohort of 388 patients is only a fraction of the thousands of patients in the Center for International Blood and Marrow Transplant Research (CIBMTR) registry studies. Therefore, absence of statistically significant differences in the primary clinical findings in this study (e.g., no significant association between csCMVi and infections, GVHD, NRM and OS) may have occurred because the study was not powered to detect potential differences. However, large registry studies would not have access to the detailed patient-level cost data that is presented in the current study. Further, given the association of higher levels of CMV viremia with worse outcomes including OS and NRM27 we explored the impact of higher levels of CMV viremia (using a viral load threshold of 1000 copies/mL) in those with csCMVi. While mortality, including NRM, was higher in those viral loads ≥ 1000 copies/mL we were unable to demonstrate a significant difference in one-year OS and NRM in this group. Interestingly, a significantly lower incidence of hematologic disease relapse was seen in patients with csCMVi in this cohort. Conflicting data are available regarding this putative beneficial effect of CMV. The reduction in hematologic disease relapse is consistent with that reported in other single center studies28–34 most notably with AML, with corroboration in meta-analyses focusing specifically on the AML population.35,36 However, this benefit has not been consistently confirmed, inclusive of a retrospective query of over 9000 patients within the CIBMTR registry.4 Nonetheless, this observation necessitates close follow-up as we begin to incorporate primary prophylactic therapies such as letermovir with proven efficacy in preventing csCMVi.
Some clinical outcomes in this cohort may be reflective of center-specific practices such as the use of alemtuzumab (a common practice at the time of the evaluation) and may not apply to other centers. Another potential limitation of this study is the difficulty in capturing costs when patients return to the care of their local oncologist; however, the finding that costs were similar in patients who live less than an hour from the transplant center (and therefore more likely get the majority of their care at the transplant center) versus those who live more than an hour away suggests that this dataset accurately captured the majority of costs. Finally, while we were able to capture direct medical costs, not captured here are costs incurred by patients in terms of lower quality of life and delayed return to work associated with protracted durations of antiviral therapy.
In conclusion, csCMVi is associated with significant increases in costs throughout the first post-transplant year. As more data are generated on the clinical effectiveness of new CMV preventative strategies inclusive of antiviral prophylactic therapies such as letermovir, cost-effectiveness analyses will be needed to better understand their incremental value in caring for patients undergoing allogeneic HCT.
Supplementary Material
Highlights:
Cytomegalovirus following transplant has significant morbidity and mortality
Implementation of efficacious preventative strategies is imperative
Strategy selection requires an assessment of the economic impact of cytomegalovirus
ACKNOWLEDGEMENTS:
The authors would also like to thank Kelly Corbet for his assistance with data ascertainment and Nancy Henshaw, Ph.D. for her assistance in delineating molecular methods utilized for CMV plasma quantification over the study period.
FINANCIAL DISCLOSURE STATEMENT:
This work was independently conducted with support through an investigator-initiated research grant from Merck & Company to A.D.S., S.D.R, Y.L. and J.L.S.; National Institutes of Health [KL2 TRO001115-03 and 2P30AG028716-11 to A.D.S.]; American Society of Hematology [HONORS Award to V.K.G.]; and Duke University [Eugene A. Stead Student Research Scholarship to V.K.G.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer L. Saullo, Division of Infectious Diseases, Duke University Medical Center, Durham, NC, USA
Yanhong Li, Duke Clinical Research Institute, Durham, NC, USA
Julia A. Messina, Division of Infectious Diseases, Duke University Medical Center, Durham, NC, USA
Jillian Thompson, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA
Tara Dalton, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA
Vinay K. Giri, Duke University School of Medicine, Durham, NC, USA
Shelby D. Reed, Departments of Medicine and Population Health Sciences, Duke University Medical Center, Durham, NC, USA
Rachel Miller, Division of Infectious Diseases, Duke University Medical Center, Durham, NC, USA
Mitchell E. Horwitz, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA
Barbara D. Alexander, Division of Infectious Diseases, Duke University Medical Center, Durham, NC, USA
Anthony D. Sung, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA
REFERENCES:
- 1.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 2002;185:273–82. 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 2.Yong MK, Ananda-Rajah M, Cameron PU, et al. Cytomegalovirus Reactivation Is Associated with Increased Risk of Late-Onset Invasive Fungal Disease after Allogeneic Hematopoietic Stem Cell Transplantation: A Multicenter Study in the Current Era of Viral Load Monitoring. Biol Blood Marrow Transplant 2017;23:1961–7. 10.1016/j.bbmt.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Cantoni N, Hirsch HH, Khanna N, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant 2010;16:1309–14. 10.1016/j.bbmt.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 2016;127:2427–38. 10.1182/blood-2015-11-679639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramanathan M, Teira P, Battiwalla M, et al. Impact of early CMV reactivation in cord blood stem cell recipients in the current era. Bone Marrow Transplant 2016;51:1113–20. 10.1038/bmt.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood 2013;122:3359–64. 10.1182/blood-2013-05-499830. [DOI] [PubMed] [Google Scholar]
- 7.Ljungman P, Brand R, Hoek J, et al. Donor cytomegalovirus status influences the outcome of allogeneic stem cell transplant: a study by the European group for blood and marrow transplantation. Clin Infect Dis 2014;59:473–81. 10.1093/cid/ciu364. [DOI] [PubMed] [Google Scholar]
- 8.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003;101:407–14. 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 9.Rowe J, Grim SA, Peace D, et al. The significance of cytomegalovirus viremia at day 100 or more following allogeneic hematopoietic stem cell transplantation. Clin Transplant 2013;27:510–6. 10.1111/ctr.12128. [DOI] [PubMed] [Google Scholar]
- 10.Nichols WG, Boeckh M. Recent advances in the therapy and prevention of CMV infections. J Clin Virol 2000;16:25–40. 10.1016/S1386-6532(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 11.Pollack M, Heugel J, Xie H, et al. An international comparison of current strategies to prevent herpesvirus and fungal infections in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2011;17:664–73. 10.1016/j.bbmt.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014;209:557–61. 10.1093/infdis/jit475. [DOI] [PubMed] [Google Scholar]
- 13.Melendez DP, Razonable RR. Letermovir and inhibitors of the terminase complex: a promising new class of investigational antiviral drugs against human cytomegalovirus. Infect Drug Resist 2015;8:269–77. 10.2147/IDR.S79131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med 2017;377:2433–44. 10.1056/NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis 2017;64:87–91. 10.1093/cid/ciw668. [DOI] [PubMed] [Google Scholar]
- 16.Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010;23:689–712. 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Healthcare Safety Network (NHSN) Patient Safety Component Manual (January 2017). Available at: https://www.cdc.gov/nhsn/pdfs/validation/2017/pcsmanual_2017.pdf. Accessed 1 February, 2018.
- 18.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813–21. 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974;18:295–304. 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Shulman HM, Cardona DM, Greenson JK, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant 2015;21:589–603. 10.1016/j.bbmt.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389–401 e1. 10.1016/j.bbmt.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Available at: https://www.bls.gov/cpi/home.htm. Accessed 1, December 2018.
- 23.Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer In: MacLeod CM. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949: 191–205. [Google Scholar]
- 24.Jain NA, Lu K, Ito S, et al. The clinical and financial burden of pre-emptive management of cytomegalovirus disease after allogeneic stem cell transplantation-implications for preventative treatment approaches. Cytotherapy 2014;16:927–33. 10.1016/j.jcyt.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robin C, Hemery F, Dindorf C, et al. Economic burden of preemptive treatment of CMV infection after allogeneic stem cell transplantation: a retrospective study of 208 consecutive patients. BMC Infect Dis 2017;17:747 10.1186/s12879-017-2854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macalalad AR, Yang H, Zhou Z, Wu EQ, Chaudhari P, Snydman DR. Economic consequences of cytomegalovirus disease among stem cell transplant recipients. Biol Blood Marrow Transplant. 2015;21(2):S296 10.1016/j.bbmt.2014.11.469. [DOI] [Google Scholar]
- 27.Green ML, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after hematopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3): e119–27. https://doi:10.1016/S2352-3026(15)00289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011;118:1402–12. 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 29.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood 2013;122:1316–24. 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transplant 2014;20:46–52. 10.1016/j.bbmt.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takenaka K, Nishida T, Asano-Mori Y, et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant 2015;21:2008–16. 10.1016/j.bbmt.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Peric Z, Wilson J, Durakovic N, et al. Early human cytomegalovirus reactivation is associated with lower incidence of relapse of myeloproliferative disorders after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2018;53:1450–6. 10.1038/s41409-018-0172-y. [DOI] [PubMed] [Google Scholar]
- 33.Jang JE, Kim SJ, Cheong JW, et al. Early CMV replication and subsequent chronic GVHD have a significant anti-leukemic effect after allogeneic HSCT in acute myeloid leukemia. Ann Hematol 2015;94:275–82. 10.1007/s00277-014-2190-1. [DOI] [PubMed] [Google Scholar]
- 34.Koldehoff M, Ross SR, Duhrsen U, Beelen DW, Elmaagacli AH. Early CMV-replication after allogeneic stem cell transplantation is associated with a reduced relapse risk in lymphoma. Leuk Lymphoma 2017;58:822–33. 10.1080/10428194.2016.1217524. [DOI] [PubMed] [Google Scholar]
- 35.Elmaagacli AH, Koldehoff M. Cytomegalovirus replication reduces the relapse incidence in patients with acute myeloid leukemia. Blood 2016;128:456–9. 10.1182/blood-2016-04-713644. [DOI] [PubMed] [Google Scholar]
- 36.Zhang YL, Zhu Y, Xiao Q, Wang L, Liu L, Luo XH. Cytomegalovirus infection is associated with AML relapse after allo-HSCT: a meta-analysis of observational studies. Ann Hematol 2019. 10.1007/s00277-018-3585-1. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


