Abstract
Yellow fever virus (YFV) causes a clinical syndrome of acute hemorrhagic hepatitis. YFV transmission involves non-human primates (NHP), mosquitoes and humans. By late 2016, Brazil experienced the largest YFV outbreak of the last 100 years, with 2050 human confirmed cases, with 681 cases ending in death and 764 confirmed epizootic cases in NHP. Among affected areas, Bahia state in Northeastern was the only region with no autochthonous human cases. By using next generation sequence approach, we investigated the molecular epidemiology of YFV in NHP in Bahia and discuss what factors might have prevented human cases. We investigated 47 YFV positive tissue samples from NHP cases to generate 8 novel YFV genomes. ML phylogenetic tree reconstructions and automated subtyping tools placed the newly generated genomes within the South American genotype I (SA I). Our analysis revealed that the YFV genomes from Bahia formed two distinct well-supported phylogenetic clusters that emerged most likely of an introduction from Minas Gerais and Espírito Santo states. Vegetation coverage analysis performed shows predominantly low to medium vegetation coverage in Bahia state. Together, our findings support the hypothesis of two independent YFV SA-I introductions. We also highlighted the effectiveness of the actions taken by epidemiological surveillance team of the state to prevented human cases.
Author summary
Between 2016 and 2018, Brazil witnessed the largest YFV outbreak since years, with 2.154 cases and 745 deaths. Here we report the introduction of the virus in the state of Bahia suggesting two independent introductions from Minas Gerais and Espirito Santo. We also highlight the actions taken by epidemiological surveillance division that may have prevent human infections.
Introduction
Yellow fever virus (YFV) is an enveloped, single-stranded RNA virus, with an approximately 11,000 base pair genome, belonging to the Flaviviridae family. It causes a clinical syndrome of acute hemorrhagic hepatitis, called Yellow Fever (YF) disease, that shows clinical features ranging from mild febrile illness to hemorrhage. The term "yellow" comes from jaundice observed in patients with severe liver disease [1–3]. In the Americas, YFV transmission occurs via two distinct cycles: sylvatic and urban. The sylvatic (or jungle) cycle involves transmission between non-human primates (NHP) and sylvatic mosquitoes; humans are infected through the bites of infected sylvatic mosquitoes while visiting or working in forested regions. The urban (or domestic) transmission cycle involves viremic humans who were infected by the virus in the forest regions or through the intermediate cycle, which then returns to an urban area. Those humans develop significant viremia to infect urban mosquitoes which can transmit the virus to other humans in urban settings [4–5]. The main vectors of sylvatic cycle are the Culicidae of genus Haemagogus and Sabethes, which are of strictly wild habits [6–7]. On the other hand, Aedes aegypti and Ae. albopictus are responsible for outbreaks in urban and peri-urban areas [8–9]. In Brazil, YFV has been reported beyond the limits of Amazon region, occurring throughout the country in cycles of 7 to 14 years [10]. Considering this, Brazilian health authorities adopted surveillance based on seasonality [11]. Between 2016 and 2018, Brazil witnessed the largest YFV outbreak since 1980, with 778 cases and 262 deaths during the 2016–2017 seasonal period and 1376 cases and 483 deaths during the 2017–2018 seasonal period [12]. The outbreak has brought national and international concern due to the dispersion of the epidemic in areas where vaccination coverage is limited [13], and due to its potential for international spread [14]. Genomic and epidemiological analysis of the first epidemic wave of the outbreak in Minas Gerais (2016–2017) revealed that human cases resulted from sylvatic transmission [7]. Yet, following the discovery of a pool of Ae. albopictus mosquitoes positive for yellow fever virus, the re-emergence of urban yellow fever has greatly concerned public health authorities [5, 11, 15]. Bahia is the largest state of the Brazilian northeast region, bordering the state of Minas Gerais, the epicentre of the 2016–2017 YFV outbreak in Brazil. Between July 2017 and June 2018, the epidemiological surveillance division reported 58 human YF suspected cases in Bahia, from which laboratory tests confirmed all cases were imported, with no autochthonous confirmed cases in the state. Regarding epizootic cases in NHPs, through June 2017, 624 NHP suspected cases were reported, of which 52 were confirmed by YFV RT-PCR. These cases were widely distributed throughout 28 municipalities in Bahia state, including the capital city, Salvador [16]. In the view of the recent YFV epizootic in Bahia and given the lack of vaccination in the state, it is surprising that no human cases have been reported so far. Considering the seasonality of the virus transmission in the country and the release of epidemiological data reports, from July 2017 to June 2018, 846 NHP epizootic cases were reported, and none was confirmed by laboratory tests. Also, in the period between July 2018 and June 2019, 268 NHP epizootic suspected cases have been reported in 61 municipalities in Bahia state, none was confirmed for YFV infection [16].
In this study, by using the genomic sequence approach, we investigate the molecular epidemiology of YFV in NHP in Bahia and discuss what factors might be acting to prevent virus transmissions to humans [16].
Materials and methods
Ethical statement for biological data
This study is part of the arboviral genomic surveillance efforts of the ZiBRA project (www.zibraproject.org) which is supported by the Pan American World Health Organization (PAHO) and the Brazilian Ministry of Health (MoH). Ethical approval for tested samples was obtained from Ethics Review Committee from PAHO under reference number 2016-08-0029. Samples were provided for research and surveillance purposes within the terms of Resolution 510/2016 of CONEP (National Ethical Committee for Research, Ministry of Health, Brazil).
Population of study
We conducted molecular diagnostics and genome sequencing on 47 YFV positive tissue samples from dead NHPs collected in 24 municipalities in the state of Bahia, Northeast Brazil. Samples were delivered to the Central Public Health Laboratory (LACEN/Bahia) to perform differential diagnosis of rabies and also for collection of viscera samples to investigate YFV infection. Sample data as NHP species, municipality of collection and collection date are summarized in Table 1.
Table 1. Epidemiological data associated with each isolate processed/sequenced in this study.
| ID | Sample Type | Host | Municipality | Collection Date |
|---|---|---|---|---|
| RJ250 | Liver | Callithrix genus | São Miguel das Matas | 12/03/2017 |
| RJ251* | Liver | Callithrix genus | Cordeiros | 10/03/2017 |
| RJ252 | Liver | Callithrix genus | São Felipe | 10/03/2017 |
| RJ253 | Liver | Callithrix genus | Salvador | 14/03/2017 |
| RJ254 | Liver | Callithrix genus | Feira de Santana | 10/03/2017 |
| RJ255 | Liver | Callithrix genus | Camaçari | 14/03/2017 |
| RJ256 | Liver | Callithrix genus | Alagoinhas | 14/03/2017 |
| RJ257 | Liver | Callithrix genus | Catu | 15/03/2017 |
| RJ258* | Liver | no data available | Pedrão | 15/03/2017 |
| RJ259* | Liver | Callithrix genus | Salvador | 15/03/2017 |
| RJ260* | Liver | Alouatta genus | Santa Rita de Cassia | 13/03/2017 |
| RJ261* | Liver | Callithrix genus | Camacari | 16/03/2017 |
| RJ262 | Liver | Callithrix genus | Camacari | 16/03/2017 |
| RJ263 | Liver | Callithrix genus | Camacari | 16/03/2017 |
| RJ264 | Liver | Callithrix genus | Camacari | 16/03/2017 |
| RJ265 | Liver | Callithrix genus | Salvador | 16/03/2017 |
| RJ266 | Liver | Callithrix genus | Ituberá | 14/03/2017 |
| RJ267 | Liver | Callithrix genus | Salvador | 19/03/2017 |
| RJ268 | Liver | Callithrix genus | Feira De Santana | 17/03/2017 |
| RJ269* | Liver | Alouatta genus | Cordeiros | 17/03/2017 |
| RJ270 | Liver | Callithrix penicillata | Saúde | 17/03/2017 |
| RJ271 | Liver | Callithrix jacchus | Esplanada | 21/03/2017 |
| RJ272 | Liver | Callithrix jacchus | Biritinga | 22/03/2017 |
| RJ273 | Liver | Callithrix penicillata | Barrocas | 05/04/2017 |
| RJ274* | Liver | Callithrix penicillata | Ichu | 05/04/2017 |
| RJ275 | Liver | Callithrix jacchus | Lauro de Freitas | 04/04/2017 |
| RJ276 | Liver | Callithrix jacchus | Paulo Afonso | 05/04/2017 |
| RJ277 | Liver | Callithrix penicillata | Salvador | 06/04/2017 |
| RJ278 | Liver | Callithrix penicillata | Candeias | 06/04/2017 |
| RJ279 | Liver | Callithrix jacchus | Mata De São Joao | 06/04/2017 |
| RJ280 | Liver | Callithrix jacchus | Salvador | 05/04/2017 |
| RJ281 | Liver | Callithrix jacchus | Salvador | 05/04/2017 |
| RJ282 | Liver | Callithrix jacchus | Salvador | 06/04/2017 |
| RJ283 | Liver | Callithrix penicillata | Salvador | 06/04/2017 |
| RJ284 | Liver | Callithrix jacchus | Salvador | 06/04/2017 |
| RJ285 | Liver | Callithrix jacchus | Salvador | 06/04/2017 |
| RJ286 | Liver | Callithrix penicillata | Camaçari | 07/04/2017 |
| RJ287 | Liver | Callithrix penicillata | Feira de Santana | 06/04/2017 |
| RJ288 | Liver | Callithrix penicillata | Riachão do Jacuipe | 07/04/2017 |
| RJ289 | Liver | Callithrix jacchus | São Goncalo dos Campos | 07/04/2017 |
| RJ290 | Liver | Callithrix jacchus | São Goncalo dos Campos | 07/04/2017 |
| RJ291 | Liver | Callithrix penicillata | Riachao do Jacuipe | 07/04/2017 |
| RJ292* | Liver | Callithrix penicillata | Feira de Santana | 07/04/2017 |
| RJ293 | Liver | Callithrix penicillata | Feira de Santana | 07/04/2017 |
| RJ294 | Liver | Callithrix jacchus | Salvador | 09/04/2017 |
| RJ295 | Liver | Callithrix penicillata | Irara | 07/04/2017 |
| RJ296 | Liver | Callithrix jacchus | Entre Rios | 05/04/2017 |
ID = Project identifier; Sample Type = Sample type tested; Host = Host species; State: BA = Bahia; Municipality = municipality of sample collection. Collection Date = Date of sample collection. Complete genomes recovered are highlighted with an asterisk.
Viral RNA isolation and sample processing
Viral RNA was isolated from liver tissue samples using Qiagen Viral RNA Minikit following manufacturer instructions and including additional steps to avoid contamination as previously described by Faria et al, 2018 [7]. The first step consisted of homogenization of liver tissue using TissueLyser LT equipment (Qiagen). A ~2 mm diameter of tissue was cut using a disposable scalpel and added to a 2 mL Eppendorf tube containing a 5 mm stainless steel bead (Qiagen). 560 μl AVL lysis buffer (Qiagen) was added to each tube and the sample was homogenised for 5 min at 50 Hz followed by a 10 min incubation at room temperature. Samples were centrifuged at 1,200g for 2 min to pellet cellular material, and 500 μL of supernatant was transferred to a new tube containing 500 μL of 100% EtOH. RNA extraction was subsequently completed using kit. To avoid contamination between samples due to the high number of virions, regular glove changes were conducted and parafilm was used to seal the gap between collection tubes and QIAamp Mini columns (Qiagen) during centrifugation.
Real-time quantitative PCR
YFV reverse transcription quantitative real-time PCR (RT-qPCR) was performed on 47 samples using the Superscript III Platinum One-Step RT-qPCR System (Invitrogen) on a StepOnePlus Real-Time PCR machine (Applied Biosystems). The conserved YFV 5’ non-coding region was targeted using the primers YFall15F (5’ to 3’: GCTAATTGAGGTGYATTGGTCTGC), YFall103R (5’ to 3’: CTGCTAATCGCTCAAMGAACG) and the probe YFall41 (5’ to 3’: FAM- ATCGAGTTGCTAGGCAATAAACAC-BHQ), based on the previously described Domingo’s assay [17]. Thermocycler conditions consisted of reverse transcription at 45ºC for 15 min, denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10s, and annealing and extension at 60°C for 40s. To check if RNA isolation was efficient, we used RNase P as an endogenous positive control. Assays for RNase P used the primers RNaseP-F (5’ to 3’: AGATTTGGACCTGCGAGCG), RNaseP-R (GAGCGGCTGTCTCCACAAGT), and a probe (FAM- TTCTGACCTGAAGGCTCTGCGCG-BHQ1).
cDNA synthesis, library preparation and sequencing for MinION
cDNA was reverse transcribed from viral RNA using the Protoscript II First Strand Sequencing kit (NEB) with random hexamer priming. Multiplex PCR was conducted using Q5 High Fidelity Hot-Start DNA Polymerase (New England Biolabs) and the 500bp sequencing primer scheme as previously described [7]. All samples were subjected to 35 cycles of PCR using the thermocycling conditions and reaction conditions described in Faria et al, 2018 [7]. PCR products were purified using a 1x Ampure XP bead clean up and concentrations were measured using a Qubit dsDNA High Sensitivity kit on a Qubit 3.0 fluorimeter (ThermoFisher). Library preparation for the ONT MinION was conducted using Ligation Sequencing 1D (SQK-LSK108) and Native Barcoding kit (EXP-NBD103) according to the manufacturer’s instructions, but with the changes detailed in [18]. Amplified DNA and appropriate negative controls were sequenced in barcoded multiplexes of 6–12 samples per MinION run using FLO-MIN106 flow cells. Sequencing was performed without basecalling for 48 hours using MinKNOW. Consensus sequences for each barcoded sample were generated following previously published methods [18]. Raw files were basecalled using Albacore, demultiplexed and trimmed using Porechop, and then mapped with bwa to a reference genome (GenBank Accession No. JF912190). Nanopolish variant calling was applied to the assembly to detect single nucleotide variants to the reference genome. Consensus sequences were generated; non-overlapped primer binding sites, and sites for which coverage was <20X were replaced with ambiguity code N.
Phylogenetic analysis
YFV sequence dataset was created by adding the eight novel genomes generated in this study to a reference dataset compiled by Faria et al. (2018) [7] and publicly available at https://github.com/arbospread/YFV-monitoring. Sequences were aligned using MAFFT [19] and manually edited using AliView [20]. Maximum likelihood (ML) phylogenies were performed in IQ-TREE program under a GTR + Γ4 nucleotide substitution model. [21]. Prior to the tree reconstruction, ModelFinder application [22], as implemented in IQ-TREE, was used to select the best-fitted substitution model for the analysed dataset. Time-scaled phylogenetic trees were inferred with the sequences clustering in the current YFV outbreak clade. Temporal signal was assessed in TempEst [23] and the BEAST/BEAGLE package [24] was used for the Bayesian phylogenetic analysis. Trees were estimated under the SRD06 substitution model [25], the uncorrelated lognormal distributed relaxed molecular clock [26] and the nonparametric Bayesian Skygrid coalescent model [27]. Monte Carlo Markov chains (MCMC) were run for 100 million generations and the chain convergence and adequate effective sample size (ESS) were diagnosed in Tracer software [28]. Maximum clade credibility (MCC) trees were summarized using the TreeAnnotator tool and visualized in Figtree v1.4.4 (https://github.com/rambaut/figtree).
Results
Sample Collection, RT-qPCR screening and sequencing
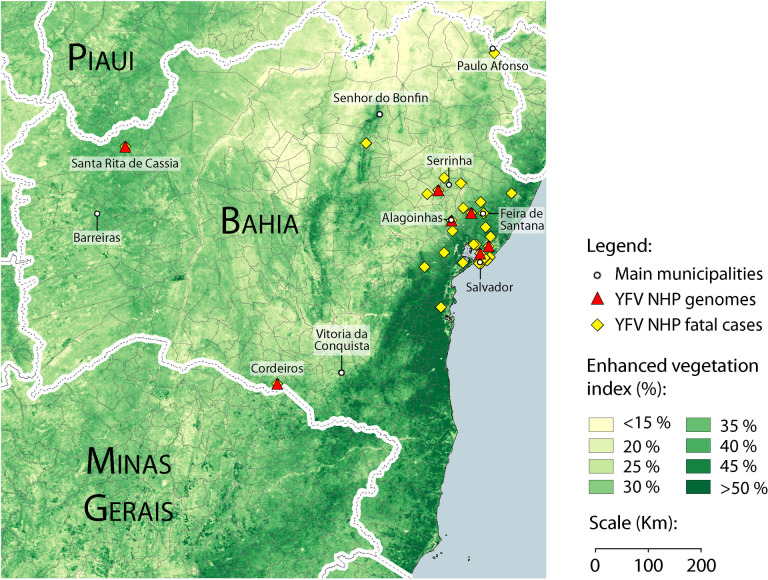
We conducted molecular screening of YFV on liver fragments of non-human primates collected in 24 municipalities of the Bahia state, northeastern Brazil. Most of these municipalities are concentrated in the eastern region of the state, in the vicinity of the cities of Salvador and Feira de Santana (Fig 1). However, some samples were collected in more distant regions, especially those collected in the northwest of the state, in the city of Santa Rita de Cássia. Additionally, four samples are from the southern city of Vitória da Conquista. Among the tested samples, non-human primates from the Callitrichidae family (n = 44) predominated (Callithrix genus: Callithrix jacchus, n = 14; Callithrix penicillata n = 13), but we also included non-human primates from the Atelidae family (Alouatta genus n = 2) (Table 1). Due to the deteriorated conditions of the primates, 17 Callithrix could only be identified at the species level by the surveillance team. YFV RT-qPCR tests results, expressed as cycle threshold values (Ct), ranged from 7.59 to 36.81 (median = 31.31) (Table 1). The majority of samples had Ct > 30.0, all from Callithrix genus.
Fig 1. Geographical distribution of YFV NHP cases in Bahia state and vegetation coverage analysis.
Sampling locations of YFV genome sequences generated in this study are shown as red triangles. Main municipalities are represented as white circles. Yellow diamonds show all epizootic cases tested on the study. Green color gradient shows vegetation index of Bahia state.
Genome sequencing and phylogenetic analysis
Genomes were generated using a previously validated MinION portable YFV sequencing protocol [7]. Briefly, positive samples were enriched by performing a multiplex PCR, using two sets of overlapping primers to amplify the YFV complete genome. Samples with amplicon yield from 4.0 ng/uL and above were sequenced. Due to high Ct values and, consequently, low viral load, it was possible to recover eight complete genomes, with genome coverage (20x) similar for all samples (median = 99%; S1 Table). ML phylogenetic tree reconstructions and automated subtyping tools (Yellow Fever Virus Typingtool available at www.genomedetective.com/app/typingtool/yellowfevervirus/) [7] placed the newly generated genomes within the South American genotype I, among the sequences of the current Brazilian outbreak (Fig 1).
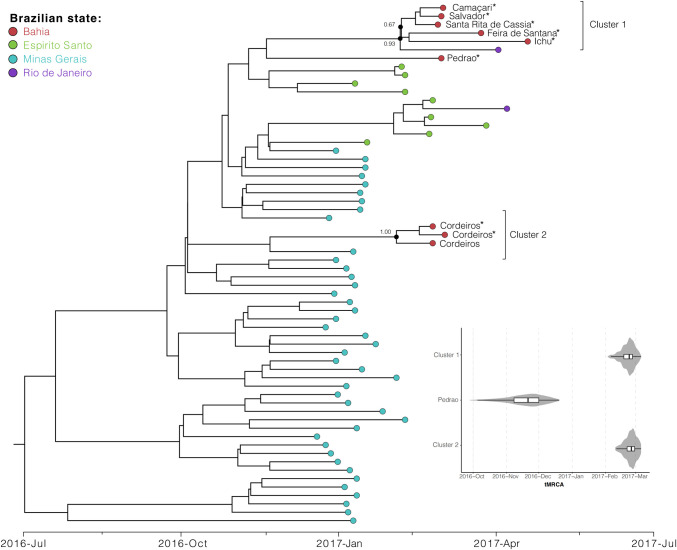
Our Bayesian dated phylogenetic analysis revealed that the YFV genomes isolated from NHP infected in Bahia formed two distinct well-supported phylogenetic clusters. Cluster 1 is composed by sequences isolated from infected NHP found in the municipalities of Camaçari, Salvador, Feira de Santana and Ichu, which are separated of a maximum great-circle distance of 156 km. Cluster 1 is also composed of a sequence isolated in the city of Santa Rita de Cassia, which is at a minimal distance of 585 km (great-circle distance) apart from the other cities in the same cluster. A single sequence from the municipality of Pedrao is located basally to Cluster 1, however with small support to form a clade with those sequences. Within Cluster 1, was also observed a YFV genome isolated in Rio de Janeiro state and basally to the whole cluster, sequences from Espirito Santo state are located. This, suggests that Cluster 1 has emerged from an introduction from one of these two states, however, only future studies with higher sampling coverage from Bahia state could provide more information about the origins and transmission dynamics of YFV in northeast Brazil. In contrast, our phylogenetic analysis shows that Cluster 2 (pp = 1.00), composed by three sequences isolated from NHPs sampled in the city of Cordeiros, suggests that Minas Gerais state is the origin of this cluster. Cordeiros is located further south in the Bahia state and close to the Minas Gerais state borders. The time of the most recent common ancestor of both clusters (Fig 2) is late February 2017 (95% Bayesian credible interval: February to March 2017).
Fig 2. Bayesian and molecular phylogenetic of the YFV NHP cases in Bahia.
Bayesian dated phylogenetic analysis revealed that the YFV genomes isolated from NHP infected in Bahia formed two distinct well-supported phylogenetic clusters. Cluster 1 (red colored superior sequences; posterior probability, PP = 0.93) emerged most likely from an introduction from Minas Gerais state (red colored inferior sequences PP = 0.67), however an origin in Espírito Santo state is also possible. Cluster 2 (PP = 1.00) originated from an introduction from Minas Gerais (PP = 1.00) composed by three sequences isolated from NHPs sampled in the city of Cordeiros, located further south in the Bahia state and close to the Minas Gerais state borders. Violin plots showing estimated posterior distributions of the time of the most recent common ancestor (tMRCA). The time of the most recent common ancestor of both clusters is late February 2017 (95% Bayesian credible interval: February to March 2017).
Discussion
In the last two decades, YFV transmissions have been recorded beyond the limits of the considered endemic area (Amazon region). Human cases and/or NHP epizootic cases occurring in Bahia, Minas Gerais, São Paulo, Paraná and Rio Grande do Sul characterize a recurrent expansion of the viral circulation area in the eastern and south directions of the country, where the virus had not been registered for decades [29].
One of the most significant events in the history of YF was recorded in the 2017/2018 period: the spread of the virus reached the Brazilian east coast, in the region of the Atlantic Forest biome, which houses a wide diversity of non-human primates and potential wild vectors [7, 13, 15].
This recent YFV outbreak has been favored by changes in environmental, eco-social and human behavior [30]. Although Bahia state did not report autochthonous human cases, a large number of epizootic cases in NHP have been reported in that state [16].
Here we reported the generation of novel YFV sequences recovered from NHP from the northeast region of Brazil not covered before by other studies. Our phylogenetic analysis has shown the simultaneous introduction of YFV into two distinct sites in the Bahia state. Considering the distance between the locations, these introductions are consistent with the hypothesis that animals may have been transported by humans as some events of primate’s traffic by human activity has already been reported by surveillance teams within Bahia state (data not published). Possas and colleagues [31] discussed that NHPs are not responsible for the rapid spread of YFV observed in the last outbreak in Brazil, once the migration habits of NHP species in Brazilian Southeastern forests show evidences that they do not use the ground and deforested areas to migrate.
According to our vegetation coverage analysis, Bahia state shows predominantly low to medium vegetation coverage (Fig 1). In addition to our knowledge about mosquitoes’ speed mobility and the time between the two introductions we suggest that the occurrence of cases in distant areas was not caused by NHP natural migration nor by mosquitoes spread from an initial point [7].
Factors like (i) high density of vectors and primary hosts, (ii) the presence of susceptible individuals and (iii) low vaccine coverage areas play an important role in the emergence of a YFV outbreak. Over the last decades YF cases have been controlled in Brazil through the efforts of the epidemiological surveillance teams that acts in a preventive way, through investigation and deployment of vector barriers in places considered at risk of transmission, avoiding the spread of the virus to other regions and also the re-establishment of urban transmission [16].
Upon the resurgence of YF in southeastern, Bahia state has intensified the vaccine campaign and it was widely distributed to population in municipalities bordering states with YFV circulation or where there was NHP YFV detection.
According to the vaccination recommendation area, the municipalities covered by intensification of campaign in 2018 were Camaçari, Candeias, Itaparica, Lauro de Freitas, Mata de São João, Salvador, São Francisco do Conde and Vera Cruz. Still according to Brazilian surveillance epidemiological reports, between July 2017 and June 2018, the state reported 378 cases of NHP YFV, still lower than the Southeastern states of Sao Paulo (2,119 reported cases), Minas Gerais (n = 1,600) and Rio de Janeiro (n = 622). [32].
The 2016–2018 YFV outbreak in Brazil has shown human cases with deaths in the south-eastern states, especially Minas Gerais, where the outbreak began and has expanded. Considering the proximity between the state of Minas Gerais and the state of Bahia, together with the wide circulation of the vector, the occurrence of NHP infected by the YFV and low vaccine coverage in Bahia state, we wondered why Bahia did not present the occurrence of human cases.
The best hypothesis to explain this fact lies in two main aspects: the first is related to the type of NHP infected by the virus, predominantly Callithrichidae family primates, which, as described by other studies, have a lower viral replication ability, reducing its transmission through the sylvatic cycle [5, 33–34].
The goal of monitoring epizootic cases among NHP is to provide an early warning of risk of YFV transmission to humans, the second fact is based on the actions of epidemiological surveillance teams (municipal and of the state) that together carried out several activities to reduce virus transmission. Beyond vaccination campaign, vector blockade with insecticide (heavy and costly ultra-low volume application) which is already part of the strategies used by the epidemiological surveillance service in Brazil and was intensified in the state of Bahia. The use of agrochemicals in public health is standardized by the WHO as a complementary tool for vector control and it was adopted by the Health Surveillance Secretariat of the Ministry of Health in Brazil [35]. In addition, the investigation of suspicious cases in a timely manner was crucial to the prevention of urban yellow fever favored by Ae. Aegypti vector. The integrated actions promoted a blockade in the transmission chain of 28 municipalities with laboratory proven NHP YFV circulation, so that there would be no record of a human case with autochthonous transmission in Bahia between December/2016 and June/2019. During the monitoring period (July/2018 to June/2019), 216 NHP cases were reported in 61 municipalities. Considering this post-outbreak epidemiological data, when compared with the previous monitoring period (July/2017 to June/2018), with a record of 846 cases, a reduction of 291% compared to the current period. Thus, the coordinated actions of epidemiological surveillance team in Bahia state were the main factor for preventing human cases during the YFV epidemics which affected other states in Brazil. Vector surveillance and control are essential for the prevention and control of vector borne diseases and, in YF epidemics, the identification of Ae. aegypti and other Ae. species helps to inform authorities the level of risk of an urban outbreak [35]. In Bahia state, epidemiological surveillance actions such as the use of vaccine blocking strategies and vector control possibly contributed to the success in preventing autochthonous human YFV cases during the 2016–2018 epidemics in Brazil and should be taken as an example of coordinated management for future vector borne diseases outbreaks.
Conclusion
Together, our findings support the hypothesis of two independent YFV SA-I introductions and highlighted the possible contribution of the actions taken by epidemiological surveillance team of the state of Bahia on prevention of human cases.
Supporting information
Sequences were mapped against FJ912190 reference genome. Numbers correspond quantity of reads mapped on reference genome. Ct = RT-qPCR Cycle threshold value. Accession number correspond to Genbank database accession number.
(DOCX)
Acknowledgments
The authors are grateful to Pan American Health Organization/Brazil, to all personnel from Central Public Health Laboratory of Bahia (SVS/MS) that coordinated surveillance and performed sample collection and clinical records, to Flaviviruses Reference Laboratory of Oswaldo Cruz Institute from Rio de Janeiro, for performing YFV diagnostics and Epidemiological Surveillance Department of Bahia state for management of surveillance data. We also thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for national and abroad scholarships and the Department of Surveillance of Transmissible Diseases—Brazilian Ministry of Health (DEVIT/MS) for sampling authorization and laboratory access support.
Data Availability
All data are fully available. New sequences have been deposited in GenBank under accession numbers MN604280-MN604287.
Funding Statement
This work was supported by CNPq/MCTI and DECIT/SCTIE/MoH (440685/2016-8), CAPES (88887.130716/2016-00). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chen C, Jiang D, Ni Met al. Phylogenomic analysis unravels evolution of yellow fever virus within hosts. PLOS Neglected Tropical Diseases, 2018;12(9) e0006738 10.1371/journal.pntd.0006738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanna A, Andrieu A, Carvalho L et al. Yellow fever cases in French Guiana, evidence of an active circulation in the Guiana Shield, 2017 and 2018. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin, 2018; 23(36). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon LV, Hashmi MF, Torp KD. Yellow Fever. [Updated 2019 Sep 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 4.Huang YJS, Nuckols JT, Horne KM et al. Mutagenesis analysis of T380R mutation in the envelope protein of yellow fever virus. Virology Journal, 2014;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Possas C, Martins RM, Oliveira RL et al. Urgent call for action: avoiding spread and re-urbanisation of yellow fever in Brazil. Memórias Do Instituto Oswaldo Cruz. 2018;113:1–2. 10.1590/0074-02760170361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso JC, de Almeida MAB, dos Santos E et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, southern Brazil, 2008. Emerging Infectious Diseases. 2010;16(12):1918–1924. 10.3201/eid1612.100608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faria NR, Kraemer MUG, Hill SC et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science. 2018;361(6405):894–899. 10.1126/science.aat7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondet B. [Epidemiology of arbovirus diseases: use and value of physiologic age determination of female mosquito vectors]. Bulletin de La Société de Pathologie Exotique. 1996;89(2):155–60. French [PubMed] [Google Scholar]
- 9.Mondet B. [Yellow fever epidemiology in Brazil]. Bulletin de la Societe de pathologie exotique. 2001;94(3):260–267.French [PubMed] [Google Scholar]
- 10.Câmara FP, Gomes ALBB, Carvalho LM F et al. Dynamic behavior of sylvatic yellow fever in Brazil (1954–2008). Revista Da Sociedade Brasileira de Medicina Tropical. 2011;44:297–299. 10.1590/s0037-86822011005000024 [DOI] [PubMed] [Google Scholar]
- 11.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde. Boletim Epidemiológico: Monitoramento de Febre Amarela Brasil 2019(a), informe nº18, 9 jun 2019.
- 12.World Health Organization. Emergencies preparedness, response—Yellow fever–Brazil. 18Apr2019, 2019. Available on https://www.who.int/csr/don/18-april-2019-yellow-fever-brazil/en/.
- 13.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde. Boletim Epidemiológico: Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus Zika até a Semana Epidemiológica 6, 2016. v. 47, n. 10, p. 7, 2016. ISSN 2358-9450. Available on http://portalarquivos2.saude.gov.br/images/pdf/2016/marco/23/2016-007—Dengue-SE-6-publica—-o.pdf
- 14.Barrett ADT. The reemergence of yellow fever. Science 2018;361(6405):847–848. 10.1126/science.aau8225 [DOI] [PubMed] [Google Scholar]
- 15.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde. Boletim Epidemiológico: Monitoramento dos casos de arboviroses urbanas transmitidas pelo Aedes (dengue, chikungunya e Zika) até a Semana Epidemiológica 12 de 2019(b) e Levantamento Rápido de Índices para Aedes aegypti (LIRAa). v. 50, nº 13, ABRIL 2019.
- 16.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde. Diretoria de Vigilância Epidemiológica. Superintendência de Vigilância em Saúde. Boletim Epidemiológico de Febre Amarela, Bahia, 2019(c). Período de Monitoramento Jul/2018 –Jun/2019.
- 17.Domingo C, Patel P, Yillah J et al. Advanced Yellow Fever Virus Genome Detection in Point-of-Care Facilities and Reference Laboratories. Journal of Clinical Microbiology, 2012;50(12):4054–4060. 10.1128/JCM.01799-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quick J, Grubaugh ND, Pullan ST, Claro IM, Smith AD, Gangavarapu K, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nature protocols. 2017;12:1261–1276. 10.1038/nprot.2017.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in bioinformatics. 2019;20:1160–1166. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics (Oxford, England). 2014;30:3276–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen LT, Schmidt HA, von Haeseler A et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution.2015;32(1):268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalyaanamoorthy S, Minh BQ, Wong TKF et al. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14(6):587–589. 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rambaut A, Lam TT, Carvalho LM et al. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evolution. 2016;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suchard MA, Lemey P, Baele G et al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution. 2018;4(1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro B, Rambaut A, Drummond AJ.Choosing Appropriate Substitution Models for the Phylogenetic Analysis of Protein-Coding Sequences. Molecular Biology and Evolution. 2005;23(1):7–9. 10.1093/molbev/msj021 [DOI] [PubMed] [Google Scholar]
- 26.Drummond AJ, Ho SYW, Phillips MJ et al. Relaxed Phylogenetics and Dating with Confidence. PLOS Biology. 2006;4(5):e88 10.1371/journal.pbio.0040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill MS, Lemey P, Faria NR et al. Improving Bayesian Population Dynamics Inference: A Coalescent-Based Model for Multiple Loci. Molecular Biology and Evolution. 2012;30(3):713–724. 10.1093/molbev/mss265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambaut A, Drummond AJ, Xie D et al. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Systematic Biology. 2018;67(5):901–904. 10.1093/sysbio/syy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.BRASIL, Ministério da Saúde, Secretaria de Vigilância em Saúde. Boletim Epidemiológico: Emergência epidemiológica de febre amarela no Brasil, no período de dezembro de 2016 a julho de 2017. v. 48, nº 28, 2017.
- 30.Possas C, Lourenço-de-Oliveira R, Tauil PL et al. Yellow fever outbreak in Brazil: the puzzle of rapid viral spread and challenges for immunisation. Memórias Do Instituto Oswaldo Cruz. 2018;113(10):e180278 10.1590/0074-02760180278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Possas C, Antunes AMS, Mendes FML et al. Emerging and Resurgent Arboviral Diseases: Global Vaccine Patent Landscape and the Case for Immunome. In: Singh H., Keswani C., Singh S. (eds) Intellectual Property Issues in Microbiology. Springer, Singapore: 2019. [Google Scholar]
- 32.BRASIL, Ministério da Saúde, Secretaria de Vigilância em Saúde. Boletim Epidemiológico: Monitoramento do Período Sazonal da Febre Amarela. Brasil– 2017/2018. Informe nº 27, 2017/2018.
- 33.Mares-Guia MAM, Horta MA, Romano A et al. Yellow fever epizootics in non-human primates, Southeast and Northeast Brazil (2017 and 2018). Parasites Vectors 2020; 13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida MAB, Cardoso JC, dos Santos E et al. Surveillance for Yellow Fever Virus in Non-Human Primates in Southern Brazil, 2001–2011: A Tool for Prioritizing Human Populations for Vaccination. PLOS Neglected Tropical Diseases 2014;8(3):e2741 10.1371/journal.pntd.0002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. A global strategy to Eliminate Yellow fever Epidemics 2017–2026. Geneva: World Health Organization [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences were mapped against FJ912190 reference genome. Numbers correspond quantity of reads mapped on reference genome. Ct = RT-qPCR Cycle threshold value. Accession number correspond to Genbank database accession number.
(DOCX)
Data Availability Statement
All data are fully available. New sequences have been deposited in GenBank under accession numbers MN604280-MN604287.