Abstract
We undertook an early phase II study of mixed 19‐peptide cancer vaccine monotherapy for 14 advanced metastatic triple‐negative breast cancer (mTNBC) patients refractory to systemic chemotherapy to develop a new type of cancer vaccine. The treatment protocol consisted of a weekly vaccination for 6 weeks, and there were no severe adverse events related to the vaccination throughout the trial. Increase of peptide‐specific IgG against the vaccinated human leukocyte antigen‐matched peptides, but not against the nonmatched peptides, was positively correlated with overall survival (OS) (P < .01). The median OS was 11.5 or 24.4 months in all 14 patients or the 10 patients who completed the vaccination. The patients with lower C‐reactive protein levels or 3 or fewer systemic chemotherapies were favorable candidates for this treatment. Advancement of this therapy to the next stage of study could be warranted based on the safety and immune boosting determined herein (clinical trial registration number: UMIN000014616).
Keywords: advanced metastatic triple‐negative breast cancer, early phase II trial, immunotherapy, mixed‐peptide vaccine
Immune boosting specific to the vaccinated human leukocyte antigen‐matched peptides at the cellular and humoral levels was observed in the vast majority of patients with refractory metastatic triple‐negative breast cancer, who completed all 6 vaccinations.
![]()
Abbreviations
- AE
adverse event
- CI
confidence interval
- CRP
C‐reactive protein
- CRPC
castration‐resistant prostate cancer
- CypB
cyclophilin B
- EGFR
epidermal growth factor receptor
- ELISPOT
enzyme‐linked immunospot
- FIU
fluorescence intensity units
- HLA
human leukocyte antigen
- HNRPL
heterogeneous nuclear ribonucleoprotein L
- HR
hazard ratio
- IFN
interferon
- IHC
immunohistochemistry
- Lck
p56lck
- MRP3
multidrug resistance‐associated protein 3
- mTNBC
advanced metastatic TNBC
- OS
overall survival
- PAP
prostatic acid phosphatase
- PD‐1
programmed death‐1
- PD‐L1
programmed death ligand‐1
- PFS
progression‐free survival
- PPV
personalized peptide vaccine
- PS
performance status
- PSA
prostate‐specific antigen
- PSMA
prostate‐specific membrane antigen
- PTHrP
parathyroid hormone‐related peptide
- SART3
squamous cell carcinoma antigen 3
- TAA
tumor‐associated antigen
- TNBC
triple‐negative breast cancer
- UBE2V
ubiquitin‐conjugated enzyme variant Kua
- ULN
upper limit of normal
- WHSC2
Wolf‐Hirschhorn syndrome critical region 2
1. INTRODUCTION
The incidence of TNBC, the most aggressive type of breast cancer with shorter OS, is approximately 15%‐20% of all breast cancers. 1 , 2 , 3 There are few or no remarkable therapeutic improvements for TNBC, particularly for patients with mTNBC. 1 , 2 , 3 , 4 , 5 Although immune activation is often observed in TNBC, 6 , 7 anti‐PD‐1 Ab monotherapy failed to achieve a clinical benefit in patients with advanced mTNBC refractory to 1‐2 systemic chemotherapies; the median OS following the monotherapy was 9.9 months. 4 An interim analysis showed that anti‐PD‐L1 Ab also did not improve the OS of advanced mTNBC patients who had no systemic chemotherapy, although it prolonged PFS (IMpassion130 phase III study). 5 Therefore, there is a need for new immunotherapy approaches not involving these 2 Abs. Peptide‐based therapeutic vaccines, including the PPVs developed by our group, might be a promising approach. 8 , 9 , 10 , 11 , 12 , 13 However, none of these vaccine trials provided sufficient clinical benefits to warrant approval for advanced cancer patients. In part, this might have been because the immune boosting levels by vaccines consisting of only a few vaccinated peptides were too weak to provide sufficient clinical benefits. Next, therefore, we developed a cancer vaccine consisting of a mixture of 20 peptides for more rapid and potent immune augmentation. 14 The 20 peptides were encoded by 12 different TAAs, most of which are expressed on a wide variety of cancers. 8 , 9 , 10 , 11 , 12 , 13 , 14 Cytotoxic T lymphocyte epitopes of these 20 peptides were restricted to HLA‐A2 (7 peptides), HLA‐A24 (8 peptides), HLA‐A3 super type (‐A3, ‐A11, ‐A31, or ‐A33) (6 peptides), or HLA‐A26 (2 peptides) of HLA‐class A molecules, providing coverage to the vast majority of patients who have different HLA alleles. 8 , 9 , 10 , 11 , 12 , 13 , 14 Moreover, peptide‐specific IgG and CTL activities were detectable in prevaccination cancer patients, including advanced mTNBC patients, suggesting the presence of memory T and B cells. 8 , 9 , 10 , 11 , 12 , 13 , 14 A previous phase I study on the use of this mixed 20‐peptide vaccine for CRPC showed that the vaccine was safe and feasible, and achieved rapid and potent immune responses without changes in immunosuppressive cell subsets. 14
Subsequently, we undertook an early phase II study of a mixed 19‐peptide vaccine monotherapy for advanced mTNBC patients (registration number: UMIN000014616). Details of the protocol are given in Document S1. This mixed peptide vaccine consisted of 19 peptides coded by 11 different TAAs (Table S1); the PSMA‐derived peptide from the mixed 20‐peptide vaccine for CRPC was excluded, as PSMA was previously shown to be an unsuitable molecular target for peptide‐based immunotherapy for breast cancers. 13
2. MATERIALS AND METHODS
2.1. Patient population
Eligible patients for this early phase II study were aged 20 years or older with histologically confirmed TNBC and had an ECOG PS of 0 or 1, life expectancy of at least 12 weeks, and adequate bone marrow function (white blood cell count 2500/mm3, lymphocyte count 1000/mm3, hemoglobin 8 g/dL, platelets 100 000/mm3), hepatic function (total bilirubin 1.5× ULN, transaminase 2× ULN), and renal function (serum creatinine 2× ULN). Exclusion criteria included acute infection, history of severe allergic reactions, pulmonary, cardiac, or other systemic diseases, or other inappropriate conditions for enrollment as judged by clinicians. Details of protocols were given in Document S1.
2.2. Study design and treatment
The mixed 19‐peptide vaccine was designed to induce CTL against 19 peptides derived from 11 different TAAs, including SART3, CypB, WHSC2, UBE2V, HNRPL, Lck and MRP3, PSA, PAP, EGFR, and PTHrP, as reported previously. 10 , 11 , 12 , 13 , 14 , 15
To ensure the background of the newly designed protocol, the expression levels of the 11 vaccine antigens that code these 19 peptides were examined by IHC staining of primary breast cancer (n = 20, including 5 TNBC) and metastatic breast cancer tissues (n = 20, including 5 TNBC). Detailed methods including the Abs used for IHC were previously described. 13 , 16 , 17 , 18 , 19 , 20 As a result, PTHrP, HNRPL, WHSC2, and SART3 antigens were expressed in all breast cancers tested. We found that CypB, UBE2V, EGFR, Lck, and MRP were expressed in 70%, 60%, 50%, 10%, and 0% of primary tumors, and 100%, 100%, 30%, 10%, and 10% of metastatic tissue, respectively. In contrast, neither PSA nor PAP was expressed in any breast cancers tested. It was reported that Lck, PSA, PAP, and MRP3 were expressed in breast cancer tissues, although the frequency of expression was lower than that of other TAAs, in the previously reported manuscripts. 13 , 20 , 21 , 22 , 23 , 24 Peptide‐specific IgG Abs against 15 of 19 peptides were detectable in the prevaccination plasma of the vast majority (more than 80%) of the 40 patients, and those against the remaining 4 peptides (HNRPL‐140, MRP3‐1293, PTHrp‐102, and Lck449) were also detectable in 50%‐80% of these patients. These results were partly reported previously. 13 Information on the 20 peptides, including the PSMA‐624 peptide, which was not used in this study because it was previously found to be an unsuitable molecular target for a breast cancer vaccine, 13 is shown in Table S1. Patients received the mixed 19 peptides (19 mg/1 mL containing 1 mg of each peptide) emulsified with incomplete Freund’s adjuvant (Montanide ISA‐51VG; Seppic) s.c. at the abdominal regions on days 1, 8, 15, 22, 29, and 36. The protocol was completed on day 43. This was an early phase II study of the mixed 19‐peptide monotherapy with a primary end‐point of safety and secondary end‐points of PFS and peptide‐specific immune induction. Accordingly, the following treatments were prohibited throughout the clinical study (maximum 43 days): steroid hormone (20 mg prednisone/d or more), systemic chemotherapy, immunotherapy, radiation therapy, or any new drugs for clinical study. After the study period, there were no restricted treatments. The follow‐up study (computed tomography scan, tumor markers) was carried out at least every 6 months for as long as possible.
For the assessment of immune responses, peripheral blood was collected at pretreatment and day 43. The HLA‐matched peptide‐specific IgG levels in the plasma were measured using a Luminex system. Prevaccination peptide‐specific IgG levels with a cut‐off level of 10 FIU were taken as detectable levels of IgG as reported previously. 9 , 10 , 11 , 12 , 13 Patients were considered to have a positive IgG response when the postvaccination IgG level against each of the peptides after the 6 vaccinations was 2 times higher than the prevaccination level, as reported previously. 10 , 11 , 12 , 13 They were also considered to have a positive response when the total sum of IgG levels against all 20 peptides tested or the HLA‐matched peptides after the 6 vaccinations was 2 times higher than that at prevaccination.
The HLA‐matched peptide‐specific CTL responses to the vaccine peptides were evaluated by IFN‐γ ELISPOT assay (MBL), using PBMCs, which were separated by density gradient centrifugation with Ficoll‐Paque Plus from peripheral blood (30 mL) before and after vaccination, and stored frozen until analysis. After thawing, PBMCs (1 × 105 cells/well) were incubated in 96‐well U‐bottomed plates with 100 µL medium (OpTmizer T Cell Expansion SFM) containing 10% FBS, 1% l‐glutamine (Life Technologies), interleukin‐2, and a mixture of 19 vaccinated peptides (3 µg/mL each) for 6 days. The cultured cells were harvested and tested for their ability to produce IFN‐γ in response to either the corresponding peptides or HLA‐matched negative control peptides (HLA‐A2, HLA‐A24, HLA‐A3 supertype, and for HLA‐A26‐matched HIV‐derived sequence). The cells (1 × 105 cells/well) were cultured in triplicate for 18 hours at 37°C with the C1R cells transfected with each type of HLA (1 × 104 cells/well) loaded with specific or control peptides (3 µg/mL) in a 96‐well ELISPOT plate coated with antihuman IFN‐γ Ab. After washing, the spots were developed with biotin‐conjugated antihuman IFN‐γ Ab, streptavidin‐alkaline phosphatase and BCIP(5‐bromo‐4‐chloro‐3‐indolyl‐phosphate)/NBT(nitro blue tetrazolium) (BCIP/NBT) substrate, according to the manufacturer’s instructions (MBL), and then counted using an ELISPOT reader (ImmunoSpot S5 Versa Analyzer; Cellular Technology). When the spot numbers in response to the specific peptides were significantly higher than those in response to the control peptides (P < .05 by Student’s t test with the triplicate samples), antigen‐specific CTL responses were shown as the differences between them (means of the triplicate samples). If the spot numbers in response to at least 1 HLA‐matched peptide in the postvaccination PBMC were more than 2‐fold higher than those in the prevaccination PBMC, the changes were considered to be significant as reported previously. 14
Positive CTL responses after the 6 vaccinations were defined as a more than 25‐spot increase in the total HLA‐matched peptide‐specific IFN‐γ spots. The CEF peptide pool (MABTECH) was used as a control peptide set for measurement of peptide‐specific CTL activity, as reported previously. 9 , 10 , 11 , 12 This pool consists of 23 HLA‐class I‐restricted peptides from human influenza virus, cytomegalovirus, and Epstein‐Barr virus.
The safety profile was assessed throughout the study by monitoring for adverse events (according to the NCI Common Terminology Criteria for Adverse Events version 4.0), chemical laboratory tests, vital signs, and physical examinations. Progression‐free survival was defined as the time in days from the first vaccination until objective disease progression based on the RECIST 1.1 criteria. Overall survival was calculated as the time in months from the date of the first vaccination to death.
2.3. Ethical considerations
The protocol was approved by the Ethical Committee of Kurume University (Document S2). It was registered in the UMIN Clinical Trials Registry (UMIN000014616). The study was in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice guidelines, and it was undertaken in an outpatient setting. Written informed consent to participate in the clinical trial and to use their data for research and publication purposes was obtained from all individual participants before their inclusion in the study.
2.4. Statistical design and analyses
The Student’s t test and the χ2 test were used to compare quantitative and categorical variables among safety profiles and immune responses to the treatment, respectively. Progression‐free survival and OS data for each arm were analyzed using the Kaplan‐Meier method. The log‐rank test was used for comparison of the survival curves, and Cox proportional hazard analysis was used for estimation of HRs. The CIs reported were 95%. The Cox proportional hazards regression model was used for univariate and multivariate analyses to identify factors that had a significant impact on survival. All baseline parameters in the survival and proportional hazards regression analysis were analyzed as dichotomous variables using median or cut‐off values. Statistical analyses were carried out using SAS software version 9.1 (SAS Institute) with a 2‐sided significance level of 5%. The data that support the findings of this study are available from the corresponding author upon reasonable request.
3. RESULTS
3.1. Baseline characteristics and adverse events
Between November 2014 and November 2017, 14 patients with advanced mTNBC refractory to systemic chemotherapies were enrolled in this study. Baseline demographic and clinical characteristics of the 14 participants, including age, PS, stages at the first diagnosis, histology, hormone receptors, tumor sites, HLA types, regimen numbers of systemic chemotherapies, prevaccination CRP, neutrophil ratio, lymphocyte ratio, PFS, and OS from the first vaccination days are given in Table 1. In order to better understand the risk factors for rapid progression, these 14 patients were subdivided into the 10 patients who completed the 6 weekly vaccinations and the 4 patients who could not complete the entire 6‐week protocol due to rapid disease progression (Table 2). The groups showed difference in prior systemic chemotherapy regimens (≥3; 9 of 10 vs 1 of 4, P = .04) or the median OS from the first vaccination (24.0 or 1.4 M, P < .01), respectively.
TABLE 1.
Baseline demographic and clinical characteristics of 14 participants with advanced metastatic triple‐negative breast cancer
| Case # | Age (y) | PS | Clinical status (TNM stage) at first diagnosis | Histopathology | HR status a %ER/PR/HER‐2 | Main tumor sites | Total chemotherapy regimens (postrecurrence/advanced) b | HLA class IA molecule | CRP | %neutro | %lympho | Vaccination times c | PFS (ms) | OS (ms) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 0 | T2N0M0: IIA | IDC (scirrhous) | <10/<10/0 | Lung, liver, LNs | 2 (2) | 2/33 | 0.96 | 44.4 | 45.9 | 6 | 1.6 | 12.4 |
| 2 | 54 | 1 | T1N0M0: I | IDC (scirrhous) | <10/<10/0 | Lung, kidney, brain | 3 (1) | 26 | 0.06 | 74.0 | 17.0 | 6 | Not reached | 50.3 |
| 3 | 63 | 0 | T2N1M0; IIB | IDC (scirrhous) | <1/<1/0 | LNs, PM | 2 (1) | 24/26 | 0.19 | 67.9 | 25.4 | 6 | 18.9 | 47.4 |
| 4 | 35 | 1 | T4aN3aM1: IV | IDC (unknown) | <10/<10/0 | PT, liver, LNs | 4 (4) | 2/26 | 0.44 | 37.9 | 44.3 | 4 | 0.9 | 1.4 |
| 5 | 82 | 1 | T1N3aM0IIIc | IDC (scirrhous) | <10/<10/<10 | PT, LNs | 4 (4) | 24/26 | 0.84 | 57.0 | 26.2 | 6 | 1.4 | 7.7 |
| 6 | 46 | 0 | T1N1M0:IIA | IDC (unknown) | <10/<10/1+ | Lung, LNs | 4 (3) | 11/24 | 0.01 | 57.5 | 33.5 | 6 | 7.1 | 42.1 |
| 7 | 58 | 0 | T4cN0M0IIIB | IDC (solid) | 0/0/0 | PT, LNs, bones | 1 (1) | 33/31 | 0.09 | 53.2 | 39.3 | 6 | 38.9 | 37.8 |
| 8 | 55 | 0 | T4bN1M0: IIIB | IDC (scirrhous) | <10/<10/<10 | LNs, bones | 4 (2) | 2/24 | 0.21 | 46.2 | 48.4 | 6 | 1.4 | 28.6 |
| 9 | 41 | 0 | T3N3cM1:IV | IDC (scirrhous) | <10/ <10/<10 | PT, LNs, bones | 2 (2) | 24/33 | 0.03 | 49.7 | 34.6 | 6 | 15.6 | 19.3 |
| 10 | 60 | 1 | T1N1M0:IIA | IDC (solid) | <10/<10/0 | PT, lung, LNs | 11 (10) | 11/33 | 0.06 | 68.5 | 24.5 | 4 | 1.0 | 1.0 |
| 11 | 60 | 1 | T2N1M0:IIB | IDC (unknown) | 0/0/2+(FISH:1.4) | Lung, LNs | 4 (3) | 24 | 11.66 | 70.0 | 22.5 | 6 | 1.4 | 2.3 |
| 12 | 44 | 0 | T1N0M0:I | IDC (special type) | 0/0/1+ | PT, LNs | 3 (2) | 2/26 | 0.79 | 71.0 | 15.5 | 3 | 0.5 | 6.0 |
| 13 | 73 | 1 | T1N1M0:IIA | IDC (solid) | <10/<10/1+ | Lung, PM | 7 (6) | 2/24 | 1.51 | 51.0 | 40.0 | 6 | 4.6 | 10.7 |
| 14 | 80 | 1 | T4bN1M0:IIIB: | IDC (unknown) | <10/<10/2+(FISH:1.1) | Lung, liver | 5 (5) | 2/24 | 1.45 | 65.4 | 24.1 | 5 | 1.2 | 1.5 |
Abbreviations: %lympho, lymphocyte ratio; %neutro, neutrophil ratio; CRP, c‐reactive protein; HLA, human leukocyte antigen; HR, hormonal receptor; IDC, invasive ductal carcinoma; LN, lymph node; OS, overall survival; PFS, progression‐free survival; PM, pleural membrane; PS, performance status; PT, primary tumor.
Estrogen receptor (ER) and progesterone (PR) status were assessed by immunohistochemistry (IHC); human epidermal growth factor receptor‐2 (HER‐2) status was assessed by either FISH or IHC. The cut‐off for ER positivity and PR positivity, depending on the institute for primary surgery, was 1% or 10% positive tumor cells with nuclear staining.
Regimen numbers for postrecurrence cases (n = 10) or inoperable advanced cases (n = 4).
Patients of cases 4, 10, 12, and 14 had not completed 6 vaccinations.
TABLE 2.
Characteristics of patients with advanced metastatic triple‐negative breast cancer, grouped according to completion of 6 weeks of 19‐peptide vaccine monotherapy
| All (n = 14) | Completed (n = 10) | Did not complete (n = 4) | P value | |
|---|---|---|---|---|
| Age | ||||
| Median (range) | 55 (35‐82) | 55 (40‐82) | 52 (35‐80) | .91 a |
| HLA type | ||||
| A24 | 6 | 3 | 3 | NA |
| A2 | 8 | 7 | 1 | |
| A3 supertype | 5 | 4 | 1 | |
| A26 | 5 | 3 | 2 | |
| Performance status | ||||
| 0 | 7 | 6 | 1 | .56 b |
| 1 | 7 | 4 | 3 | |
| Systemic chemotherapy regimens c | ||||
| 1 | 3 | 3 | 0 | .11 b |
| 2 | 4 | 3 | 1 | |
| 3 | 3 | 3 | 0 | |
| 4 or more | 4 | 1 | 3 | |
| Lymphocytes (%) | ||||
| Median (range) | 29.9 (15.5‐48.4) | 34.1 (17.0‐48.4) | 24.3 (15.5‐44.3) | .41 a |
| Neutrophils (%) | ||||
| Median (range) | 57.3 (37.9‐74.0) | 55.1 (44.0‐74.0) | 67 (32.9‐71.0) | .69 a |
| CRP | ||||
| Median (range) | 0.3 (0.01‐11.7) | 0.2 (0.01‐11.7) | 0.6 (0.06‐1.45) | .47 a |
| OS from first vaccination (mo) | ||||
| Median (95% CI) | 11.5 (1.5‐42.0) | 24.0 (2.3‐not reached) | 1.4 (1.0‐6.0) | <.01 c , d |
| OS from first diagnosis (mo) | ||||
| Median (95% CI) | 80.0 (25.1‐179.7) | 80.0 (20.5‐164.6) | 106.6 (13.0‐193.8) | .52 d |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; HLA, human leukocyte antigen; NA, not available; OS, overall survival.
Student’s t test.
Fisher’s exact test.
Regimen numbers for postrecurrence cases (n = 10) or inoperable advanced cases (n = 4).
Log‐rank test.
Adverse events during the treatment are summarized in Table S2. The most common AEs (occurring in more than 25% of patients) were injection site reactions (9 patients) and lymphocytopenia (4 patients). Adverse events of grade 3 occurred in 5 patients (2 patients with γ‐glutamyltransferase increase, 2 with aspartate aminotransferase increase, and 1 with pleural effusion). There was no grade 4 AEs. Grade 5 events were observed in 2 patients with AEs not otherwise specified. According to the assessment by an independent safety evaluation committee in this trial, all of these AEs of grade 3 or 5 were related to cancer progression or the combination chemotherapies; the injection site reactions were related to the vaccination.
3.2. Immune responses
Prevaccination peptide‐specific IgG levels to each of the 20 peptides—the 19 peptides included in the vaccine and the nonvaccinated PSMA‐derived peptide—were measured using a Luminex system with a cut‐off level of 10 FIU taken as a detectable level of IgG, as reported previously. 9 , 10 , 11 , 12 , 13 , 14 The positive rate of Ab in the patients’ plasma against the 20 peptides or HLA‐A‐matched peptides ranged from 36% to 100% or 17% to 100%, respectively (Table S3). The IgG levels against HLA‐A‐matched peptides for each patient are bolded in Table S3. The total sum of IgG levels to the 20 peptides or HLA‐A‐matched peptides in each patient ranged from 53 to 14 482 or 53 to 9691 FIU, respectively. Postvaccination peptide‐specific IgG levels were measured at the end of clinical study (day 43) in plasma from the 10 patients who completed all 6 vaccinations. Immune responses were considered positive when the postvaccination IgG level was 2 times higher than the prevaccination titer. 8 , 9 , 10 , 11 , 12 Postvaccination IgG levels showing at least a 2‐fold increase compared to their prevaccination counterparts are highlighted in red in the table. The percentage of patients showing positive IgG responses against 1 or more than 1 peptide ranged from 0% to 90% (Table S3). Immunoglobulin G responses against at least 1 HLA‐matched or non‐HLA‐matched peptide were positive in the majority of the patients tested (9 or 8 of 10 patients tested, respectively). The sum of postvaccination IgG levels to all 20 peptides or HLA‐A‐matched peptides in each of the 10 patients who completed all 6 vaccinations ranged from 180 to 74 943 or 77 to 71 669 FIU, respectively.
Cytotoxic T lymphocyte activity against the HLA‐matched peptides vaccinated in prevaccination PBMCs was measured in the 10 patients who completed all 6 vaccinations, and it was greatly suppressed, with no IFNγ spots in 9 patients and 19 IFNγ spots in the remaining patient. That against CEF peptide pools was also suppressed, with no IFNγ spots in 7 patients and more than 50 spots in the remaining 3 patients (Table S4).
The vaccination induced positive CTL responses (25 or more IFNγ spots) in 5 of 10 patients, but did not affect the CTL activity against CEF (23 virus‐related peptide mix) peptides.
3.3. Clinical outcome
After the median follow‐up of 12 months, ranging from 2 to 60 months, 12 or 11 of the total 14 patients had progressed or died, respectively. Clinical responses were evaluated based on investigator‐derived assessment of disease response and progression using RECIST criteria. They consisted of 6 cases with stable disease and 8 with progressive disease at the end of the clinical trial (day 43). The median PFS was as follows: 1.5 months (95% CI, 1.0‐15.6) for all 14 patients, 5.8 months (95% CI, 1.4‐18.9) for the 10 patients who completed the clinical study, and 0.9 months (95% CI, 0.5‐1.2) for the 4 patients who failed to completed the clinical study (P < .01 among the 3 groups; P < .01 between the 10 and 4 patients) (Figure 1A). The median OS was 11.5 (95% CI, 1.5‐42.1) or 24.0 months (95% CI, 2.3‐not reached) in all 14 patients or the 10 patients who completed the clinical study, respectively (P < .01) (Figure 1B). The median OS of the 4 patients who failed to complete the clinical study was 1.4 months (95% CI, 1.0‐6.0).
FIGURE 1.
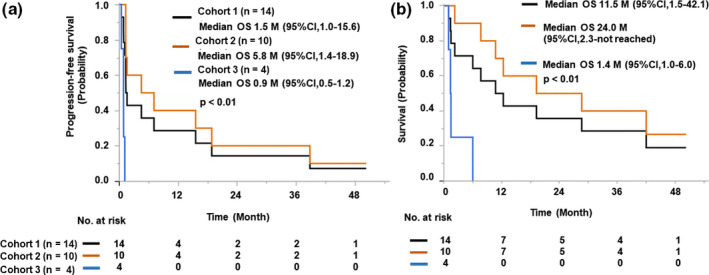
Kaplan‐Meier curves of patients with advanced metastatic triple‐negative breast cancer treated with 19‐peptide vaccine monotherapy, grouped according to treatment completion. A, Progression‐free survival. B, Overall survival (OS). The 14 patients (Cohort 1, black) entered in this study were subdivided into 10 patients (Cohort 2, orange) who completed the 6 vaccinations and 4 patients (Cohort 3, blue) who could not complete them due to rapid disease progression
3.4. Risk factors
Risk factors for rapid disease progression during the vaccination were examined as the next step in the clinical study, although only 14 patients were entered in this early phase II study. The baseline CRP level was a risk factor, as the median OS of the patients with median or higher CRP level (0.3 mg/dL) was significantly shorter than that of those with lower than median CRP (6.0 months; 95% CI, 1.4‐10.7 vs 42.1 months and 95% CI, 1.0‐not reached, respectively; HR, 0.07; P < .01) (Figure 2A).
FIGURE 2.
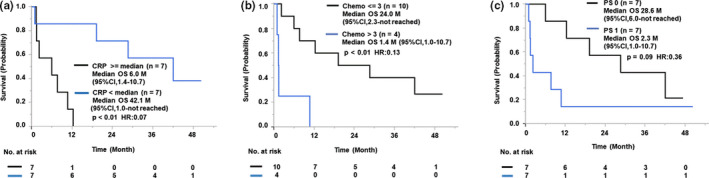
Risk factors for disease progression during treatment of patients with advanced metastatic triple‐negative breast cancer treated with 19‐peptide vaccine monotherapy. Kaplan‐Meier curves for the correlation between overall survival (OS) and prevaccination C‐reactive protein (CRP) levels (A), regimen numbers of systemic chemotherapies (Chemo) (B), and performance status (PS) (C) are shown. CI, confidence interval; HR, hazard ratio
The number of prior chemotherapy regimens was also a risk factor, as the median OS of the 4 patients with more than 3 prior chemotherapy regimens (1.4 months; 95% CI, 1.0‐10.7) was significantly shorter than that of the 10 patients with 3 or fewer prior chemotherapy regimens (24.0 months; 95% CI, 2.3‐not reached) (P < .01; HR, 0.13) (Figure 2B). In addition, PS might also become a risk factor if the number of cases were increased, as the median OS of the patients with PS1 (n = 7) was shorter than that of the patients with PS0 (n = 7) (2.3 months vs 28.6 months, P = .09 by log‐rank analysis, P = .03 by Wilcoxon analysis; HR, 0.36) (Figure 2C). Neither the prevaccination lymphocyte ratio nor the neutrophil ratio, which were previously reported to be risk factors hampering the clinical benefits of peptide‐based cancer vaccine, 10 was found to be a risk factor in this study (data not shown). Neither the prevaccination IgG level nor prevaccination CTL activity was a risk factor (data not shown).
We also examined whether the vaccination‐induced immune boosting was a favorable prognostic factor. The details of OS and peptide‐specific IgG against HLA‐matched peptides in pre‐ and postvaccination plasma in each patient are shown in Figure 3A. These results indicated that the patients who had higher net IgG titers (postvaccination IgG level minus prevaccination IgG level) seemed to have longer OS. Indeed, the median OS of the patients with the median or higher net IgG titer (not reached; 95% CI, 28.6‐not reached) was significantly longer than that of patients with less than the median titer (10.7 months; 95% CI, 2.3‐19.3; P < .01; HR, 2.22e‐10) (Figure 3B). In contrast, the net titer of peptide‐specific IgG against HLA‐non‐matched peptides did not affect the OS (data not shown). The median OS of the 5 patients with positive CTL responses (25 or more IFNγ/well spots) against the HLA‐matched peptides (42.1 months; 95% CI, 19.3‐not reached) was longer than that of the 5 patients with a negative response (10.7 months; 95% CI, 2.3‐not reached; P = .07; HR, 0.22) (Figure 3C). In contrast, the postvaccination CTL response against the CEF peptide set used as a control did not affect the OS, which was expected from the results given in Table 3.
FIGURE 3.
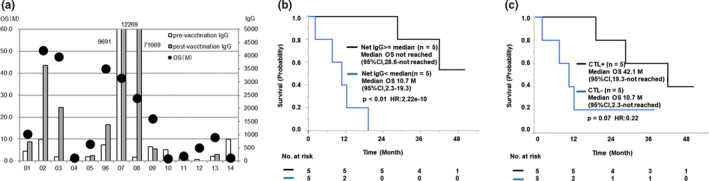
Correlation between vaccination‐induced immune boosting and overall survival (OS) in 14 patients with advanced metastatic triple‐negative breast cancer treated with 19‐peptide vaccine monotherapy. A, OS and peptide‐specific IgG against human leukocyte antigen (HLA)‐matched peptides in pre‐ and postvaccination plasma in each patient. B, Kaplan‐Meier curves for the correlation between OS and net IgG titer against the vaccinated HLA‐matched peptides. C, Kaplan‐Meier curves for the correlation between OS and positive CTL response against the HLA‐matched peptides. CI, confidence interval; HR, hazard ratio
TABLE 3.
Cox proportional hazards regression analyses for risk factors hampering clinical benefit (overall survival) of 14 patients with advanced metastatic triple‐negative breast cancer treated with 19‐peptide vaccine monotherapy
| Factor (n) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| CRP (14) | 13.46 (2.22‐260.28) | <.01 | 1.13E+9 (0.31‐5.81E+76) | .18 |
| PS (14) | 0.36 (0.10‐1.24) | .10 | 0.59 (0.02‐24.80) | .74 |
| Regimen numbers of prior systemic chemotherapy (14) | 0.24 (0.06‐0.83) | .02 | 0.13 (<0.01‐1.77) | .13 |
| IgG boosting (10) | <0.01 (0.22‐0.22) | <.01 | 2.47E‐10 (8.22E‐65‐1.54) | .08 |
| CTL boosting (10) | 0.52 (0.09‐2.85) | .43 | 0.40 (0.01‐17.92) | .60 |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; HR, hazard ratio; PS, performance status.
4. DISCUSSION
This early phase II trial of the mixed 19‐peptide vaccine monotherapy for advanced mTNBC patients refractory to systemic chemotherapy showed its safety and potent immune boosting for the majority of the patients tested. Although the median OS for 4 of 14 entered patients who failed to complete the study (a weekly vaccination for 6 weeks) due to rapid disease progression was as short as 1.4 months (95% CI, 1.0‐6.0), that of the remaining 10 patients who completed the study was 24.0 months (95% CI, 2.3‐not reached), which was longer than any other previously reported outcomes, including the OS in the recently carried out anti‐PD1 or PDL‐1 Ab studies, to our best knowledge. 4 , 5
We previously reported that the CRP, PS, and regimen numbers of prior systemic chemotherapies were unfavorable for the OS of patients receiving the PPV, by univariate analysis. 10 , 11 , 12 , 13 We also reported that vaccine‐induced immune boosting (IgG or CTL boosting) was a favorable factor for the OS of patients receiving the PPV, by univariate analysis. 10 , 11 , 12 , 13 Similar results were obtained for the 19‐mix peptide vaccines. To better understand the risk factors for the rapid progression, we used a Cox proportional hazards regression model to undertake multivariate analyses of 5 factors (CRP, PS, regimen numbers of prior systemic chemotherapy, IgG boosting, and CTL boosting) (Table 3). None of them, however, was significantly correlated with the OS—although IgG boosting (n = 10) (P = .08, HR < 0.01) and the prevaccination CRP level (n = 14) (P = .18, HR < 0.01) showed a trend of correlation—probably due to the small number of patients tested.
Assessment of HLA‐matched peptide‐specific IgG and CTL was carried out to evaluate immune responses to this multipeptide monotherapy. A significant increase in both HLA‐matched and nonmatched peptide‐specific IgG responses was observed, whereas it was not observed in the CEF peptide set (23 virus‐related peptide mix) used as a control. Notably, the median OS of the patients with the median or higher net IgG titer against the HLA‐matched peptides was significantly longer than that of patients with less than the median titer. In addition, the median net IgG titer against the HLA‐matched peptides was further increased even after the clinical study in 2 patients (#3 and #8) whose samples became available for the follow‐up IgG study. The median net IgG titers against the HLA‐matched peptides before, at the end of study, and 7 months after the study were 152, 2031, and 15 460 FIU in patient #3, who is free of recurrence after more than 5 years. The median net IgG titers against the HLA‐matched peptides before, at the end of study, and 16 months after were 138, 71 669, and 98 240 FIU in patient #8, whose OS was 28.4 months. These results suggest the presence of long‐lasting memory lymphocytes induced by the 6 vaccinations. In contrast, the net titer of peptide‐specific IgG against the HLA‐nonmatched peptide set did not affect the OS. These results suggest a causal relationship between the OS and the vaccinated HLA‐matched peptides. Similar results were obtained for the peptide‐specific CTL activity, although the correlation level was not statistically significant, probably due to the small number of patients tested. The sensitivity of the assay for CTL responses was lower compared to that for IgG responses, which was described previously. 15
In our previously reported PPV trial for advanced mTNBC patients refractory to systemic chemotherapy, we found that IgG responses against at least 1 HLA‐matched peptide were positive in 7 of 15 patients (46.6%), and the median total net sum of postvaccination IgG levels to the vaccinated HLA‐A‐matched peptides was 31 FIU. 11 In the present study using a mixed 19‐peptide vaccine, IgG responses against at least 1 HLA‐matched peptide were positive in 9 of 10 patients tested (90%), which was 2‐fold higher than the percentages of patients showing a positive response in the PPV trial, and the median total net sum of postvaccination IgG levels to the vaccinated HLA‐A‐matched peptides was 553 FIU, 18‐fold higher than the IgG levels of patients showing the IgG levels in the PPV trial. The median numbers of HLA‐matched peptides per patient were 4 and 10 in the PPV trial and 19‐peptide vaccine trial, respectively. These results indicated that the numbers of HLA‐matched peptides in this mixed 19‐peptide vaccine were 2.5‐fold higher than for the previously reported PPV, which in turn could have resulted in the more rapid and potent immune boosting with the 19‐peptide vaccine.
However, the increase of IgG for the vaccinated peptide does not guarantee the antitumor immune response because it might simply reflect the prompt baseline immune status of the patients. The evaluation of the benefit from vaccine needs the next step of clinical studies with relatively large numbers of patients for the vaccination arm and untreated patient arm.
It is important to investigate the expression of 11 antigens cording 19 peptides and presence of IgG levels against 19 peptides in prevaccination TNBC samples from 14 enrolled patients. However, the prevaccination tumor samples from 11 of 14 enrolled patients were not available for the study, primarily because these patients were introduced from hospitals other than Kurume University Hospital following long‐term treatment after the initial diagnosis. Tumor samples from the remaining 3 patients (cases #2 and #13 with primary tumors [Table 1] and case #7 case with metastatic tumor) were provided for the 11 TAA expression profile using IHC, as described under “Materials and Methods”. The results showed that HNRPL, WHSC2, SART3, CypB, PTHrP, and UBE2V antigens were expressed in the majority (more than 50%) of tumor cells from 3, 3, 3, 2, 2, and 1 sample tested, respectively. The CypB, PTHrP, UBE2V, EGFR2, Lck, MRP3, and PAP antigens were expressed in some (10%‐50%) tumor cells from 1,1, 2, 2, 2,1, and 1 sample, respectively. Prostate‐specific antigen was not expressed in any of 3 samples tested. These results were mostly expected from previously reported results (see Materials and Methods). Peptide‐specific IgG Abs against only 3 (UBE2V‐43, Lck‐488, and SART3‐734) of 19 peptides were detectable in the prevaccination plasma of the vast majority (more than 80%) of 14 enrolled patients, and those against 12 of 19 peptides were detectable in 50%‐80% of enrolled patients, as shown in Table S3. Those against the remaining 3 peptides (CypB‐129, WHSC2‐141, and Lck‐208) were 30%‐50%. These results were somewhat different from the results shown in the Materials and Methods section. Namely, the positive rates in the former group were mostly lower than those of the latter group, suggesting that prevaccination humoral immunity against the 19 peptides in the 14 enrolled patients were suppressed by prevaccination long‐term chemotherapies. Severe suppression of prevaccination cellular immunity against the 19 peptides was also observed (Table S4).
It could also be important to investigate the relationship between CTL and IgG boosting, and the correlation between OS and each of the 19 peptides. Both the CTL and IgG boosting in response to the HLA‐A matched peptides were observed in 6 of 10 patients who completed the 6 vaccinations, whereas only IgG boosting was observed in 3 of these patients. Neither CTL nor IgG boosting was observed in the remaining 1 patient. These results suggested the strong relationship between the peptide‐specific CTL and IgG boosting with higher sensitivity in IgG response, as reported previously. 15 We also studied the correlation between OS and IgG boosting against each of the 19 vaccinated peptides. As a result, the OS of the patients who showed Lck‐486 (P = .02, log‐rank test), PAP213 (P < .01), CypB‐129 (P = .02), PSA‐248 (P = .03), or UBB2V‐43 (P < .01) boosting, but not boosting of the remaining 15 peptides, was significantly longer than those showing no IgG boosting (data not shown). The results revealed that, among the 19 vaccinated peptides, these 5 peptides were significantly associated with OS. Although the underlying mechanisms remain unclear, we reported that a mAb reacting to the Lck‑486 peptide showed antitumor activity in a murine model with suppression of T regulatory cells at tumor sites. 25 The Lck antigen is pivotal for activation of T regulatory cells and PD‑1‑positive T cell activities. 26 , 27 The anti‑Lck‑486 Ab augmented by the vaccination might have promoted the antitumor activity partly through suppression of T regulatory cells at tumor sites. The relationship between OS and CTL boosting against each of the 19 vaccinated peptides was not included in the study as a mixture of HLA‐A matched peptides were used for CTL measurement.
The prevaccination CRP levels of 4 of 6 patients showing CTL boosting against the vaccinated peptides were lower than the median level (0.3 mg/dL). Those of the remaining 2 patients, 3 of 4 patients without CTL boosting, and 3 of 4 patients who could not complete the 6 vaccinations due to rapid progression were higher than the median level (0.3 mg/dL). The mean number of prevaccination chemotherapy regimens of 4 patients who could not complete the 6 vaccinations due to rapid progression was higher than that of other patients. These results suggest that higher prevaccination CRP levels and higher number of chemotherapies hampered the CTL boosting.
The most common AEs related to the vaccination were grade 1 or 2 injection site reactions. The main cause of grade 3 or higher AEs was disease progression. This could be a significant safety benefit for TNBC patients, and differed from anti‐PD‐1/PD‐L1 Abs with 10%‐20% of immune‐related AEs such as thyroid function disorder, hepatitis, and pneumonitis. 4 , 5
Immune boosting specific to the vaccinated HLA‐matched peptides at the cellular and humoral levels was observed in the vast majority of patients who completed all 6 vaccinations. Advancement to the next stage of study appears to be warranted, based on the demonstrated safety and immune boosting. At the same time, the small number of patients makes it impossible to discuss or evaluate the clinical benefits of this new type of mixed 19‐peptide vaccine monotherapy. This limitation should be addressed in the next step of the phase II clinical study by using a larger patient group while paying attention to the risk factors identified in this early phase II clinical study.
DISCLOSURES
U. Toh received remuneration from Chugai, Kyowa Kirin, Daiichi Sankyo, and Eisai. K. Itoh received research funding from Taiho Pharmaceutical Company. A. Yamada is a part‐time executive of BrightPath Biotherapeutics. and has stock in that company. The other authors have no conflicts of interest to declare.
Supporting information
Table S1
Table S2
Table S3
Table S4
Doc S1
Doc S2
ACKNOWLEDGMENTS
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We thank all patients and family, staff, and investigators involved in the study. We also thank Mr Teruhito Ito for scientific advisement.
Toh U, Sakurai S, Saku S, et al. Early phase II study of mixed 19‐peptide vaccine monotherapy for refractory triple‐negative breast cancer. Cancer Sci. 2020;111:2760–2769. 10.1111/cas.14510
REFERENCES
- 1. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol. 2008;26:1275‐1281. [DOI] [PubMed] [Google Scholar]
- 2. Gobbini E, Ezzalfani M, Dieras V, et al. Time trends of overall survival among metastatic breast cancer patients in the real‐life ESME cohort. Eur J Cancer. 2018;96:17‐24. [DOI] [PubMed] [Google Scholar]
- 3. Cardoso F, Spence D, Mertz S, et al. Global analysis of advanced/metastatic breast cancer: decade report (2005–2015). Breast. 2018;39:131‐138. [DOI] [PubMed] [Google Scholar]
- 4. Cortés J, Lipatov O, Im S‐A, et al. KEYNOTE‐119: phase III study of pembrolizumab (pembro) versus single‐agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann Oncol. 2019;30(suppl. 5):v859‐v860. [Google Scholar]
- 5. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med. 2018;379:2108‐2121. [DOI] [PubMed] [Google Scholar]
- 6. Lehmann BD, Jovanović B, Chen XI, et al. Refinement of triple‐negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11:e0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okabe M, Toh U, Iwakuma N, et al. Predictive factors of the tumor immunological microenvironment for long‐term follow‐up in early stage breast cancer. Cancer Sci. 2017;108:81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin‐2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bezu L, Kepp O, Cerrato G, et al. Trial watch: peptide‐based vaccines in anticancer therapy. OncoImmunology. 2018;7(12):e1511506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sasada T, Yamada A, Noguchi M, Itoh K. Personalized peptide vaccine for treatment of advanced cancer. Curr Med Chem. 2014;21:2332‐2345. [DOI] [PubMed] [Google Scholar]
- 11. Narita Y, Arakawa Y, Yamasaki F, et al. A randomized, double‐blind, phase III trial of personalized peptide vaccination for recurrent glioblastoma. Neuro Oncol. 2019;21:348‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noguchi M, Fujimoto K, Arai G, et al. Personalized peptide vaccination for castration‐resistant prostate cancer progressing after docetaxel: a randomized, double‐blind, placebo‐controlled, phase III trial. J Clin Oncol. 2019;37(15 Suppl):5033. [Google Scholar]
- 13. Takahashi R, Toh U, Iwakuma N, et al. Feasibility study of personalized peptide vaccination for metastatic recurrent triple‐negative breast cancer patients. Breast Cancer Res. 2014;16:R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noguchi M, Arai G, Matsumoto K, et al. Phase I trial of a cancer vaccine consisting of 20 mixed peptides in patients with castration‐resistant prostate cancer: dose‐related immune boosting and suppression. Cancer Immunol Immunother. 2015;64:493‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mine T, Sato Y, Noguchi M, et al. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre‐existing, peptide‐specific cellular responses. Clin Cancer Res. 2004;10:929‐937. [DOI] [PubMed] [Google Scholar]
- 16. Kibe S, Yutani S, Motoyama S, et al. Phase II study of personalized peptide vaccination for previously treated advanced colorectal cancer. Cancer Immunol Res. 2014;2:1154‐1162. [DOI] [PubMed] [Google Scholar]
- 17. Kawano K, Tsuda N, Waki K, et al. Personalized peptide vaccination for cervical cancer patients who have received prior platinum‐based chemotherapy. Cancer Sci. 2015;106:1111‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iwasa S, Yamada Y, Heike Y, et al. Phase I study of a new cancer vaccine of ten mixed peptides for advanced cancer patients. Cancer Sci. 2016;107:590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muroya D, Yutani S, Shichijo S, et al. Personalized kampo medicine facilitated both cytotoxic T lymphocyte response and clinical benefits induced by personalized peptide vaccination for advanced esophageal cancer. Evid Based Complementary Altern Med. 2016;2016:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada T, Terazaki Y, Sakamoto S, et al. Feasibility study of personalized peptide vaccination for advanced non‐small cell lung cancer patients who failed two or more treatment regimens. Int J Oncol. 2015;46:55‐62. [DOI] [PubMed] [Google Scholar]
- 21. Elsberger B, Fullerton R, Zino S, et al. Breast cancer patients' clinical outcome measures are associated with Src kinase family member expression. Br J Cancer. 2010;103:899‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Narita D, Raica M, Suciu C, et al. Immunohistochemical expression of androgen receptor and prostate‐specific antigen in breast cancer. Folia Histochem Cytobiol. 2006;44:165‐172. [PubMed] [Google Scholar]
- 23. Wang YI, Harada M, Yano H, et al. Prostatic acid phosphatase as a target molecule in specific immunotherapy for patients with non‐prostate adenocarcinoma. J Immunother. 2005;28:535‐541. [DOI] [PubMed] [Google Scholar]
- 24. Partanen L, Staaf J, Tanner M, et al. Amplification and overexpression of the ABCC3 (MRP3) gene in primary breast cancer. Gene Chromosome Canc. 2012;51:832‐840. [DOI] [PubMed] [Google Scholar]
- 25. Matsueda S, Itoh K, Shichijo S. Antitumor activity of antibody against cytotoxic T lymphocyte epitope peptide of lymphocyte‐specific protein tyrosine kinase. Cancer Sci. 2018;109:611‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balbin OA, Prensner JR, Sahu A, et al. Reconstructing targetable pathways in lung cancer by integrating diverse omics data. Nat Commun. 2013;4:2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bardhan K, Patsoukis N, Weaver J, et al. PD‐1 inhibits the TCR signaling cascade by sequestering SHP‐2 phosphatase, preventing its translocation to lipid rafts and facilitating Csk‐mediated inhibitory phosphorylation of Lck. J Immunol. 2016;196(Suppl. 1):128‐215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Doc S1
Doc S2


