Abstract
Despite significant developments and persistent efforts by scientists, cancer is one of the primary causes of human death worldwide. No form of life on Earth can survive without iron, although some species can live without oxygen. Iron presents a double‐edged sword. Excess iron is a risk for carcinogenesis, while its deficiency causes anemia, leading to oxygen shortage. Every cell is eventually destined to death, either through apoptosis or necrosis. Regulated necrosis is recognized in distinct forms. Ferroptosis is defined as catalytic Fe(II)‐dependent regulated necrosis accompanied by lipid peroxidation. The main observation was necrosis of fibrosarcoma cells through inhibition of cystine/glutamate antiporter with erastin, which reduced intracellular cysteine and, thus, glutathione levels. Our current understanding of ferroptosis is relative abundance of iron (catalytic Fe[II]) in comparison with sulfur (sulfhydryls). Thus, either excess iron or sulfur deficiency causes ferroptosis. Cell proliferation inevitably requires iron for DNA synthesis and energy production. Carcinogenesis is a process toward iron addiction with ferroptosis resistance. Conversely, ferroptosis is associated with aging and neurodegeneration. Ferroptosis of immune cells during infection is advantageous for infectious agents, whereas ferroptosis resistance incubates carcinogenic soil as excess iron. Cancer cells are rich in catalytic Fe(II). Directing established cancer cells to ferroptosis is a novel strategy for discovering cancer therapies. Appropriate iron regulation could be a tactic to reduce and delay carcinogenesis.
Keywords: aging, carcinogenesis, ferroptosis, infection, iron
Ferroptosis is defined as catalytic Fe(II)‐dependent regulated necrosis accompanied by lipid peroxidation. Carcinogenesis is a process to establish iron addiction with ferroptosis resistance. Directing established cancer cells to ferroptosis is a novel strategy to discover new cancer therapies.
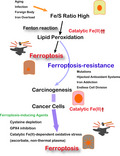
1. FERROPTOSIS
Cell death is inevitable for all forms of life on Earth, and can be either physiological or pathological. In the 1990s, there were only two major forms of cell death recognized, apoptosis and necrosis. 1 Apoptosis is regarded as an active process, with cells consuming their own energy for the activation of the caspase system to digest all sorts of macromolecules prior to death, 2 whereas necrosis has been viewed as a passive process, overwhelmed by any etiology of toxic levels, including hypoxia, inflammation, physical agents and chemical species. Over the past few decades, researchers noticed that there are various forms of regulated necrosis, including necroptosis and pyroptosis. At present, there are at least 12 distinct types of regulated cell death, one of which is ferroptosis. 2 The term “regulated” indicates that the cell death is programmed through certain intracellular signaling and takes at least a few hours.
The term ferroptosis was first coined in 2012, when Dixon et al reported a non–apoptotic regulated cell death when they treated HT1020 fibrosarcoma cells with erastin, an inhibitor of xCT (cystine/glutamate antiporter; SLC7A11), through the screening of anti–cancer agents. 3 The word ferroptosis, consisting of the two syllables, ferro (divalent ionic iron) and ptosis (falling off), is defined as catalytic Fe(II)‐dependent regulated necrosis concomitant with lipid peroxidation. 4 The discovery of ferroptosis was acclaimed by researchers of various different fields, and this rookie of regulated necrosis obtained a position not only in cancer cell death but also in stroke, 5 neurogenerative diseases, 6 cardiomyopathy 7 and even traumatic brain injury. 8 This naming was appropriate in that it reflects the major pathology of this necrotic cell death, as described later, and that pathology in eukaryotes is, indeed, full of ferroptosis.
The essence of ferroptosis is associated with an increase in the ratio of Fe (iron) to S (sulfur) over time (Figure 1). Iron is a major player in oxidative stress 9 and S or sulfhydryls (‐SH) in peptides (eg. glutathione) and proteins (eg. thioredoxin) provide the major resistance against oxidative stress. 10 Iron, if present as catalytic Fe(II), can initiate Fenton reaction, 11 even in vivo. 12
Figure 1.
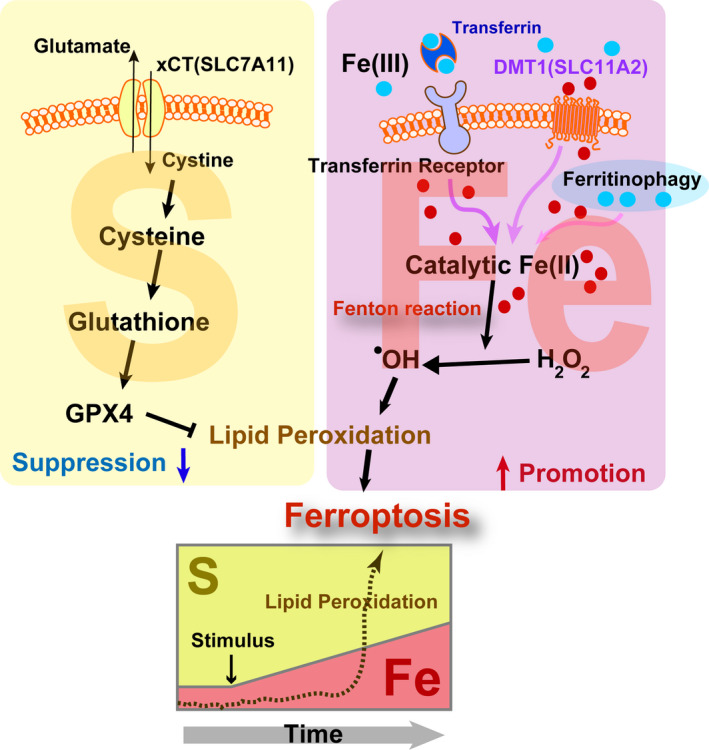
Ferroptosis as an imbalance between catalytic Fe(II) and sulfhydryls (S). Ferroptosis is defined as catalytic Fe(II)‐dependent regulated necrosis with lipid peroxidation. Blue filled circle, Fe(III); red filled circle, Fe(II); DMT1, divalent metal transporter 1; GPX4, glutathione peroxidase 4; xCT, cystine‐glutamate antiporter. Refer to the text for details
2. IRON METABOLISM
Space started to expand with the Big Bang 13.8 Ga and is still expanding. 13 Earth was born 4.6 billion years ago. 14 The first life on Earth appeared 3.8 billion years ago. 15 Although most of space consists of H (73.4%) and He (24.0%) as elements, it is true that there is a substantial amount of iron (0.109%) as well. 16 This is because Earth has been struck by a large number of meteors, consisting almost purely of iron. 17
It has been established geologically that the primordial sea was acidic, containing a high concentration of Fe(II). 18 The adult human body contains as much as 2.5‐4 g of iron, which is by far the highest amount among the heavy metals. 19 Independent life may be defined as an existence of persistent electron flow with self‐replication. Iron has been used as a medium for electron flow in life forms on Earth because no life on Earth can survive without iron. The Great Oxygen Event started with the evolution of cyanobacteria 2 billon years ago. 20 Fe(II) in the ocean reacted with a small amount of oxygen, and became ores at the bottom of the sea. This was followed by the sulfur era (euxinic ocean) for 1 billion of years, where iron and sulfur revealed an affinity to each other. 18 Finally, from approximately 1 billion years ago, the atmospheric oxygen concentration significantly increased and a variety of evolutional processes were started. Thus, the order of appearance of the elements is Fe, S and O for the living creatures on Earth (Figure 2).
Figure 2.
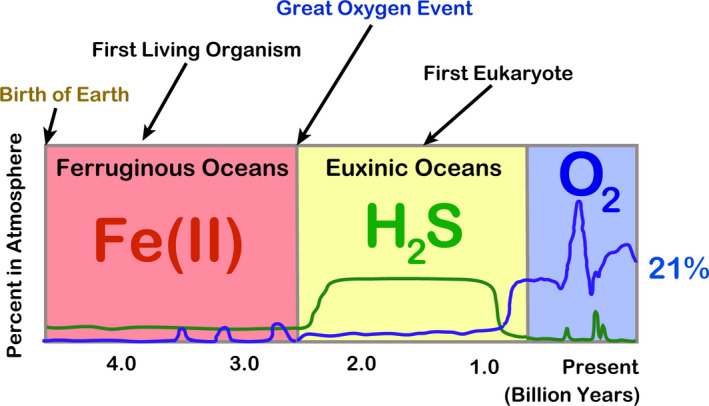
Key elements in evolution. Refer to the text and Olson and Straub (2016) 18 for details
The transferrin system was established during the 1970 and 1980s, 21 but it was in the late 1990s that iron transporters started to be cloned. 22 In mammals, iron is absorbed through the gastrointestinal system, specifically by duodenal mucosal cells with DMT1 (SLC11A2). 23 Iron solubility is significantly increased by acidic mucus of the stomach immediately prior to the duodenum, where it is thereafter neutralized with bile and pancreatic juice at its second portion. At neutral pH, Fe(III) is nearly insoluble to water and Fe(II) is much more soluble; this is enhanced at acidic pH. 9 Thus, iron is always reduced to Fe(II) when crossing any biomembrane via specific transporters. Iron is circulated extracellularly by transferrin and stored in cytosolic ferritin as Fe(III), presumably because Fe(III) is safer as a non–catalyst to biomolecules in comparison with Fe(II). Figure 3 summarizes the current understanding of iron metabolism, which is finely regulated through multilayered distinct mechanisms by transcriptional (eg. HIF‐1), 24 posttranscriptional (eg. IRP‐1/2), ubiquitin‐proteasomal (eg. FBXL‐5) 25 , 26 as well as hormonal (eg. hepcidin) 27 mechanisms. Of note, there is no active pathway discovered thus far to dump or excrete excess iron outside the body in higher organisms. This is probably because iron has been extremely precious for life during the entire evolution. Thus, the only active strategies for us to decrease iron are by phlebotomy or by intake of iron chelators of redox‐inactive small molecules (eg. desferal). 28 How catalytic Fe(II) is carried safely through cytosol via chaperone systems (eg. PCBP‐1/2) is a current hot topic. 29
Figure 3.
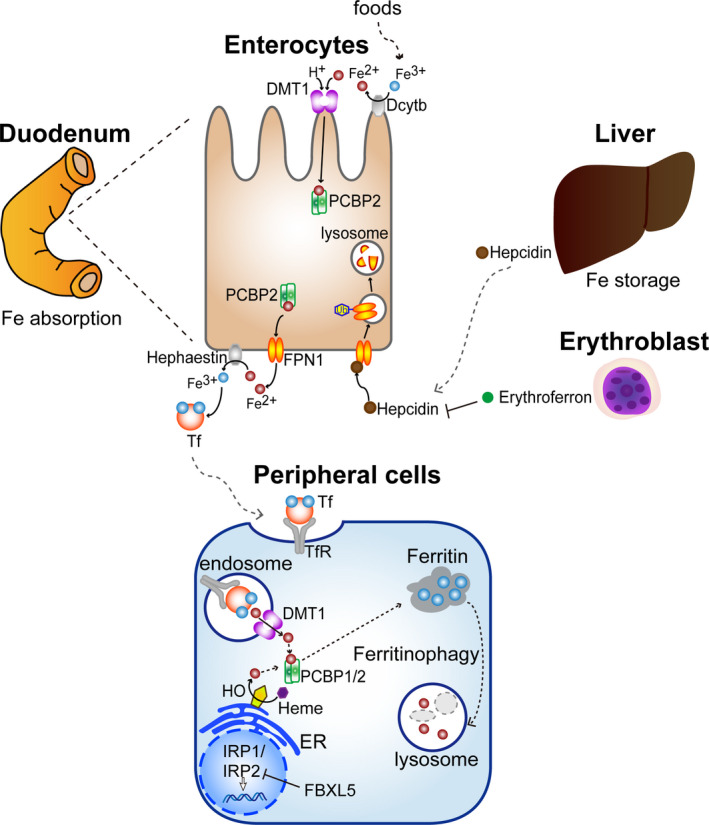
Iron metabolism in vertebrates. DMT1, divalent metal transporter 1, also as SLC11A2; FBXL, F box and leucine‐rich repeat; FPN1, ferroportin 1, also as SLC40A1; IRP, iron regulatory protein; PCBP2, poly(rC)‐binding protein 2; Tf, transferrin; TfR, transferrin receptor 1. Refer to the text for details
3. FERROPTOSIS IN INFECTION
In Japan, tuberculosis, a bacterial infectious disease, had been the top cause of human mortality until the discovery and use of antibiotics in the 1950s. Intriguingly, ferroptosis is a major mechanism of necrosis in Mycobacterium tuberculosis infection. 30 Furthermore, T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. 31 Bacteria require iron to proliferate. Thus, ferroptosis is a feast for bacterial infections. Obviously, infection is an area to be investigated more deeply from the standpoint of ferroptosis, considering the current pandemic of coronavirus disease 2019. 32
4. FERROPTOSIS IN AGING
Iron is an essential nutrient for humans. We believe that iron is the origin of life on Earth, as discussed. Iron has been extremely important in evolution. We do not have any active pathway to remove iron from our body except for physiological peeling off of surface/luminal cells or hemorrhage. Indeed, as low as 1 mg of iron out of 4 g is lost per day through peeling off, and 1 mg of iron is absorbed per day through the duodenal mucosa, which thus maintains a semi‐closed system for iron metabolism. 33 Accordingly, iron would be in excess with aging. There are several reasons for this: (i) the metabolic rate decreases with aging, 34 , 35 leading to lowered necessity for iron, namely, deficiency in molecules using iron as cofactors or working on iron metabolism; (ii) amounts of hemoglobin, which contains as much as 60% total iron, decrease with aging; and (iii) menopause causes relative iron overload in women.
Accordingly, stored iron is increased all over the body during aging. It is conceivable that the excess iron induces deleterious effects on cellular functions, eventually leading to cell death. Iron store in humans, as evaluated by serum ferritin, is increased during aging, 36 especially in elderly individuals with high neocortical amyloid‐β load, 37 and, as found in a recent prospective study, in those with increased mortality. 38 Furthermore, reports suggest the association of neurodegenerative diseases, which present numerous similarities to aging, with ferroptosis. 39 , 40 It is of note that the remaining cells in radiation‐induced senescence reveal impaired ferritinophagy and inhibition of ferroptosis in in vitro experiments 41 (Figure 4).
Figure 4.
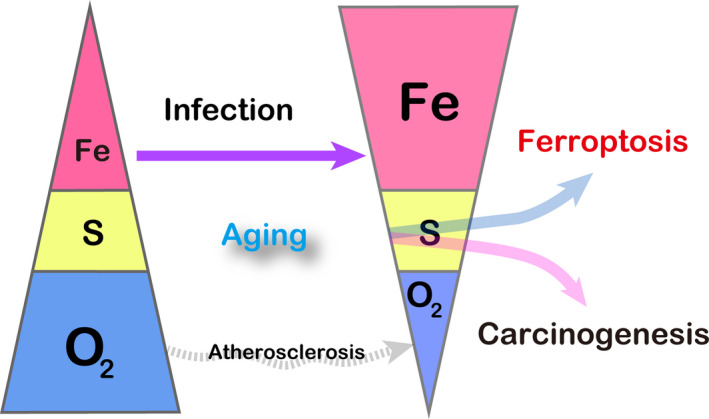
Alteration of the key elements during aging and infection. Note that this figure is conceptual. Refer to the text for details
5. FERROPTOSIS IN CARCINOGENESIS
Iron presents a double‐edged sword in any higher species. Its deficiency leads to iron‐deficiency anemia, whereas iron excess is a risk for carcinogenesis. There are three distinct levels of data on the association of iron excess and carcinogenesis: (i) human data on specific diseases; (ii) human general epidemiological data; and (iii) data from animal studies. 12 , 42
The rationale for the association of iron excess with high cancer risk is that iron excess confers an environment for the selection of stronger cells against oxidative stress, more precisely, ferroptosis‐resistant cells. We can easily imagine that excess iron causes a Fenton reaction (Fe[II] + H2O2 ‐> Fe[III] +.OH + OH−) because H2O2 is rather abundant both in the intracellular and extracellular milieu. 43 , 44 , 45 Therefore, ferroptosis is an appropriate term, which should have been established earlier.
Ferric nitrilotriacetate (Fe‐NTA)‐induced renal carcinogenesis is an interesting model in mice and rats in that: (i) it demonstrated that repeated Fenton reaction, indeed, generated high‐grade carcinoma 46 , 47 ; (ii) genetic alterations of the induced renal cancers are quite similar to those of human cancers in general (eg. homozygous deletion of Cdkn2a/2b and amplification of c‐Met) 48 , 49 , 50 ; (iii) the acute phase of this model provides a reproducible timecourse, which has been used to identify optimal molecular markers for oxidative stress (eg. 8‐hyderoxy‐2’‐deoxyguanosine and 4‐hydroxy‐2‐nonenal) 51 , 52 , 53 ; and (iv) this model further opened up a novel research area, called oxygenomics. 54 , 55 , 56 Indeed, acute renal tubular necrosis induced by Fe‐NTA is ferroptosis. Although this is a kind of exaggerated direct model, catalytic Fe(II) overwhelms sulfur antioxidants, consistent with the definition of ferroptosis. 57 Of note, conditional knockout of glutathione peroxidase 4 produces renal tubular ferroptosis. 58 Thus, at least some of the carcinogenesis is a process toward iron addiction with ferroptosis‐resistance.
Recently, several lines of research revealed that cigarette smoke induces ferroptosis in bronchial epithelial cells. 59 , 60 These data suggest that every carcinogenesis has to be reconsidered from the viewpoint of iron excess (Figures 4 and 5), and iron removal may be a smart strategy for cancer prevention, as in the case of asbestos‐induced mesothelial carcinogenesis. 61
Figure 5.
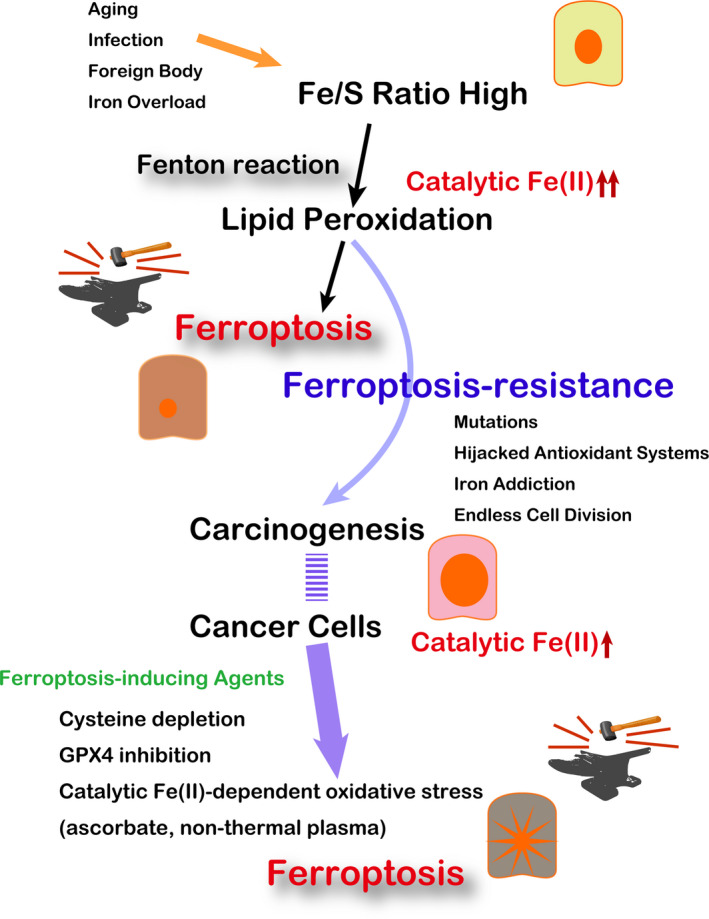
Significance of ferroptosis in carcinogenesis and tumor biology. GPX4, glutathione peroxidase 4. Refer to the text for details
6. FERROPTOSIS IN TUMOR BIOLOGY
It has been established that neoplastic cells harbor higher levels of catalytic Fe(II) in comparison with their non–tumorous counterparts. 62 , 63 , 64 This is associated with the discovery of ferroptosis when Dixon et al used erastin on H‐ras‐mutated fibrosarcoma cells. 3 Indeed, Ras oncogene and iron metabolism are closely linked. 65 Recently developed fluorescent turn‐on probes for catalytic Fe(II) are helpful for this purpose. 66
Abundance of high catalytic Fe(II) indicates a high level of oxidative stress. This has been recognized for a long time as “persistent oxidative stress” in cancer. 67 Indeed, many cytotoxic chemotherapeutic cancer drugs, such as doxorubicin 68 and bleomycin, 69 use this characteristic of cancer cells for killing them by further increasing oxidative stress. The cancer cell death induced by these agents is presumed to be ferroptosis, and ferroptosis has obtained a central position in drug discovery, 70 based on the iron dependence of cancer cells.
Here we introduce two unconventional methods to enhance oxidative stress explicitly in cancer cells: (i) pharmacological level of ascorbate infusion; 71 and (ii) non–thermal atmospheric pressure plasma (NTP) or low‐temperature plasma 72 , 73 (Figure 5). L‐ascorbate, also known as vitamin C, is an essential reductant and cofactor in many enzymes. However, ascorbate works as a pro–oxidant in the presence of abundant catalytic Fe(II) to generate hydroxyl radicals. Thus, clinical trials are in progress to use a pharmacological level (~mmol/L) of ascorbate to kill cancer cells in addition to a standard radio‐chemotherapy in advanced stage cancer patients, including glioblastoma multiforme and non–small‐cell lung cancer. 64 For this strategy, maintenance of a high constant level of ascorbate is important with a continuous infusion. The safety of ascorbate is well established. 64
Plasma is the 4th state in physics after solid, liquid and gas. Plasma is an ionized condition with the highest energy among the four states. Space is full of plasma, and thunder and aurora are the natural plasma on Earth. It has been thought for a long time that extremely high temperature is necessary to transform gas into plasma, which has been practically used for fine lithotomy of transistor board structure since the 1970s. With the development of electronic techniques to regulate the high voltage and current, generation of NTP became possible in the late 1990s. 73 , 74
Thereafter, many researchers started to use NTP for medical applications, including cancer therapy. We demonstrated that NTP can provide oxidative stress precisely to anywhere, with the major responsible species being hydroxyl radicals. 75 We compared the biological effects of NTP between malignant mesothelioma cells and fibroblasts, and found that malignant mesothelioma cells are significantly more susceptible to NTP than fibroblasts with dependence on intracellular catalytic Fe(II) (Figure 6), whereas the two cell lines were equally susceptible to cisplatin. The cell death caused by NTP in malignant mesothelioma cells was ferroptosis. 63 Its biological effects may involve simultaneous degradation of ferritin core and reduction of Fe(III) to Fe(II). 76 The drawback of NTP is that it only reaches areas close to the surface areas, such as 1 mm in depth. Thus, treatment for cancer dissemination in somatic cavities and for killing the remaining cancer cells at the surgical margin may be excellent targets for the use of NTP. Indeed, some clinical trials are in progress. Furthermore, NTP‐exposed liquids (medium or infusion) have similar effects against neoplastic cells, which has also been studied intensively recently (Figure 6). 77
Figure 6.
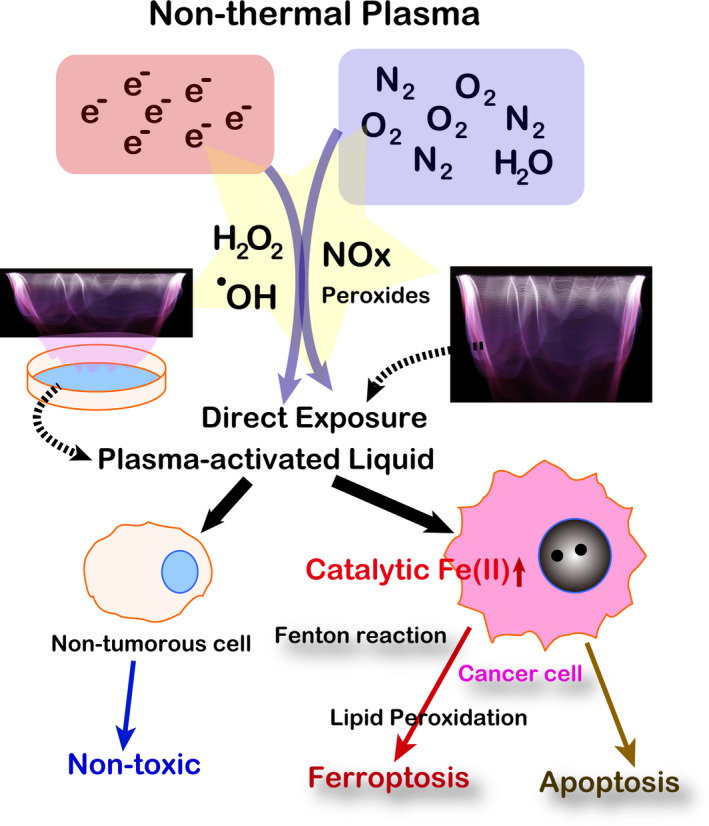
Non–thermal plasma as a ferroptosis inducer in cancer cells. Refer to the text for details
7. CONCLUSION
There are many modifiers and signaling pathways involved in ferroptosis, such as BAP1 and p53. 70 Ferritinophagy is one of the important processes for ferroptosis 78 (Figures 1 and 3). miRNA is also associated with ferroptosis. 79 We recently found that carbonic anhydrase 9 confers ferroptosis resistance to mesothelioma cells. 80 Ferroptosis is a fundamental process for understanding carcinogenesis and tumor biology because all living creatures on Earth depend heavily on iron to survive. Based on the awakening of the ferrous (Fe[II]) in ferroptosis, we now start to recognize that cancer is an inevitable side effect of using iron and oxygen.
CONFLICTS OF INTERESTS
The authors have no conflicts of interests to disclose.
ACKNOWLEDGMENTS
This work was supported, in part, by JST CREST (Grant Number JPMJCR19H4), a JSPS Kakenhi (Grant Number JP17H04064, JP19H05462, JP20H05502 and 20K07309) and Research Grant of the Princess Takamatsu Cancer Research Fund.
Toyokuni S, Yanatori I, Kong Y, Zheng H, Motooka Y, Jiang L. Ferroptosis at the crossroads of infection, aging and cancer. Cancer Sci. 2020;111:2665–2671. 10.1111/cas.14496
REFERENCES
- 1. Toyokuni S. Reactive oxygen species‐induced molecular damage and its applicaton in pathology. Pathol Int. 1999;49:91‐102. [DOI] [PubMed] [Google Scholar]
- 2. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell. 2012;149:1060‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeGregorio‐Rocasolano N, Marti‐Sistac O, Gasull T. Deciphering the iron side of stroke: neurodegeneration at the crossroads between iron dyshomeostasis, excitotoxicity, and ferroptosis. Front Neurosci. 2019;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med. 2019;133:221‐233. [DOI] [PubMed] [Google Scholar]
- 7. Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672‐2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kenny EM, Fidan E, Yang Q, et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit Care Med. 2019;47:410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koppenol WH, Hider RH. Iron and redox cycling. do's and don'ts. Free Radic Biol Med. 2019;133:3‐10. [DOI] [PubMed] [Google Scholar]
- 10. Toyokuni S, Ito F, Yamashita K, Okazaki Y, Akatsuka S. Iron and thiol redox signaling in cancer: an exquisite balance to escape ferroptosis. Free Radic Biol Med. 2017;108:610‐626. [DOI] [PubMed] [Google Scholar]
- 11. Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc. 1894;65:899‐910. [Google Scholar]
- 12. Toyokuni S. The origin and future of oxidative stress pathology: From the recognition of carcinogenesis as an iron addiction with ferroptosis‐resistance to non–thermal plasma therapy. Pathol Int. 2016;66:245‐259. [DOI] [PubMed] [Google Scholar]
- 13. Peplow M. Planck snaps infant Universe. Nature. 2013;495:417‐418. [DOI] [PubMed] [Google Scholar]
- 14. Dalrymple GB. The age of the Earth in the twentieth century: a problem (mostly) solved. Geol Soc Spec Publ. 2001;190:205‐221. [Google Scholar]
- 15. Lanier KA, Williams LD. The origin of life: models and data. J Mol Evol. 2017;84:85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnett D. Supernovae and nucleosynthesis. Princeton, New Jersey: Princeton University Press; 1996. [Google Scholar]
- 17. Jarosewich E. Chemical‐analyses of meteorites ‐ a compilation of stony and iron meteorite analyses. Meteoritics. 1990;25:323‐337. [Google Scholar]
- 18. Olson KR, Straub KD. The role of hydrogen sulfide in evolution and the evolution of hydrogen sulfide in metabolism and signaling. Physiology. 2016;31:60‐72. [DOI] [PubMed] [Google Scholar]
- 19. Ganz T. Erythropoietic regulators of iron metabolism. Free Radic Biol Med. 2019;133:69‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knoll AH, Nowak MA. The timetable of evolution. Sci Adv. 2017;3:e1603076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med. 2019;133:46‐54. [DOI] [PubMed] [Google Scholar]
- 22. Gunshin H, Mackenzie B, Berger U, et al. Cloning and characterization of a mammalian proton‐coupled metal‐ion transporter. Nature. 1997;388:482‐488. [DOI] [PubMed] [Google Scholar]
- 23. Yanatori I, Kishi F. DMT1 and iron transport. Free Radic Biol Med. 2019;133:55‐63. [DOI] [PubMed] [Google Scholar]
- 24. Hirota K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia‐inducible factors (HIFs). Free Radic Biol Med. 2019;133:118‐129. [DOI] [PubMed] [Google Scholar]
- 25. Moroishi T, Nishiyama M, Takeda Y, Iwai K, Nakayama KI. The FBXL5‐IRP2 axis is integral to control of iron metabolism in vivo. Cell Metab. 2011;14:339‐351. [DOI] [PubMed] [Google Scholar]
- 26. Iwai K. Regulation of cellular iron metabolism: Iron‐dependent degradation of IRP by SCF(FBXL5) ubiquitin ligase. Free Radic Biol Med. 2019;133:64‐68. [DOI] [PubMed] [Google Scholar]
- 27. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mobarra N, Shanaki M, Ehteram H, et al. A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Oncol Stem Cell Res. 2016;10:239‐247. [PMC free article] [PubMed] [Google Scholar]
- 29. Yanatori I, Richardson DR, Toyokuni S, Kishi F. The iron chaperone poly(rC)‐binding protein 2 forms a metabolon with the heme oxygenase 1/cytochrome P450 reductase complex for heme catabolism and iron transfer. J Biol Chem. 2017;292:13205‐13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amaral EP, Costa DL, Namasivayam S, et al. A major role for ferroptosis in Mycobacterium tuberculosis‐induced cell death and tissue necrosis. J Exp Med. 2019;216:556‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harigae H, Hino K, Toyokuni S. Iron as soul of life on earth revisited: from chemical reaction, ferroptosis to therapeutics. Free Radic Biol Med. 2019;133:1‐2. [DOI] [PubMed] [Google Scholar]
- 34. Goyal MS, Vlassenko AG, Blazey TM, et al. Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 2017;26:353‐360.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kitazoe Y, Kishino H, Tanisawa K, Udaka K, Tanaka M. Renormalized basal metabolic rate describes the human aging process and longevity. Aging Cell. 2019;18:e12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Casale G, Bonora C, Migliavacca A, Zurita IE, de Nicola P. Serum ferritin and ageing. Age Ageing. 1981;10:119‐122. [DOI] [PubMed] [Google Scholar]
- 37. Goozee K, Chatterjee P, James I, et al. Elevated plasma ferritin in elderly individuals with high neocortical amyloid‐beta load. Mol Psychiatr. 2018;23:1807‐1812. [DOI] [PubMed] [Google Scholar]
- 38. Kadoglou NPE, Biddulph JP, Rafnsson SB, Trivella M, Nihoyannopoulos P, Demakakos P. The association of ferritin with cardiovascular and all‐cause mortality in community‐dwellers: the English longitudinal study of ageing. PLoS One. 2017;12:e0178994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maher P. Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: Implications for age‐related neurodegenerative diseases. Free Radic Biol Med. 2018;115:92‐104. [DOI] [PubMed] [Google Scholar]
- 40. Cozzi A, Orellana DI, Santambrogio P, et al. Stem cell modeling of neuroferritinopathy reveals iron as a determinant of senescence and ferroptosis during neuronal aging. Stem Cell Reports. 2019;13:832‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masaldan S, Clatworthy SAS, Gamell C, et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018;14:100‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toyokuni S. Iron‐induced carcinogenesis: the role of redox regulation. Free Radic Biol Med. 1996;20:553‐566. [DOI] [PubMed] [Google Scholar]
- 43. Toyokuni S. Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep. 2002;7:189‐197. [DOI] [PubMed] [Google Scholar]
- 44. Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem. 2014;289:8735‐8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones DP, Sies H. The redox code. Antioxid Redox Signal. 2015;23:734‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ebina Y, Okada S, Hamazaki S, Ogino F, Li JL, Midorikawa O. Nephrotoxicity and renal cell carcinoma after use of iron‐ and aluminum‐ nitrilotriacetate complexes in rats. J Natl Cancer Inst. 1986;76:107‐113. [PubMed] [Google Scholar]
- 47. Nishiyama Y, Suwa H, Okamoto K, Fukumoto M, Hiai H, Toyokuni S. Low incidence of point mutations in H‐, K‐ and N‐ras oncogenes and p53 tumor suppressor gene in renal cell carcinoma and peritoneal mesothelioma of Wistar rats induced by ferric nitrilotriacetate. Jpn J Cancer Res. 1995;86:1150‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka T, Iwasa Y, Kondo S, Hiai H, Toyokuni S. High incidence of allelic loss on chromosome 5 and inactivation of p15 INK4B and p16 INK4A tumor suppressor genes in oxystress‐induced renal cell carcinoma of rats. Oncogene. 1999;18:3793‐3797. [DOI] [PubMed] [Google Scholar]
- 49. Toyokuni S. Mysterious link between iron overload and CDKN2A/2B. J Clin Biochem Nutr. 2011;48:46‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akatsuka S, Yamashita Y, Ohara H, et al. Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS One. 2012;7:e43403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toyokuni S, Mori T, Dizdaroglu M. DNA base modifications in renal chromatin of Wistar rats treated with a renal carcinogen, ferric nitrilotriacetate. Int J Cancer. 1994;57:123‐128. [DOI] [PubMed] [Google Scholar]
- 52. Toyokuni S, Mori T, Hiai H, Dizdaroglu M. Treatment of Wistar rats with a renal carcinogen, ferric nitrilotriacetate, causes DNA‐protein cross‐linking between thymine and tyrosine in their renal chromatin. Int J Cancer. 1995;62:309‐313. [DOI] [PubMed] [Google Scholar]
- 53. Toyokuni S, Luo XP, Tanaka T, Uchida K, Hiai H, Lehotay DC. Induction of a wide range of C2–12 aldehydes and C7–12 acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic Biol Med. 1997;22:1019‐1027. [DOI] [PubMed] [Google Scholar]
- 54. Akatsuka S, Aung TT, Dutta KK, et al. Contrasting genome‐wide distribution of 8‐hydroxyguanine and acrolein‐modified adenine during oxidative stress‐induced renal carcinogenesis. Am J Pathol. 2006;169:1328‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoshihara M, Jiang L, Akatsuka S, Suyama M, Toyokuni S. Genome‐wide profiling of 8‐oxoguanine reveals its association with spatial positioning in nucleus. DNA Res. 2014;21:603‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Akatsuka S, Li GH, Kawaguchi S, et al. Augmented oxidative stress increases 8‐oxoguanine preferentially in the transcriptionally active genomic regions. Free Radic Res. 2020. https://www.tandfonline.com/doi/full/10.1080/10715762.2020.1733548. [DOI] [PubMed] [Google Scholar]
- 57. Mukaide T, Hattori Y, Misawa N, et al. Histological detection of catalytic ferrous iron with the selective turn‐on fluorescent probe RhoNox‐1 in a Fenton reaction‐based rat renal carcinogenesis model. Free Radic Res. 2014;48:990‐995. [DOI] [PubMed] [Google Scholar]
- 58. Angeli JPF, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoshida M, Minagawa S, Araya J, et al. Involvement of cigarette smoke‐induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun. 2019;10:3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Park EJ, Park YJ, Lee SJ, Lee K, Yoon C. Whole cigarette smoke condensates induce ferroptosis in human bronchial epithelial cells. Toxicol Lett. 2019;303:55‐66. [DOI] [PubMed] [Google Scholar]
- 61. Ohara Y, Chew SH, Shibata T, Okazaki Y, Yamashita K, Toyokuni S. Phlebotomy as a preventive measure for crocidolite‐induced mesothelioma in male rats. Cancer Sci. 2018;109:330‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ito F, Nishiyama T, Shi L, et al. Contrasting intra‐ and extracellular distribution of catalytic ferrous iron in ovalbumin‐induced peritonitis. Biochem Biophys Res Commun. 2016;476:600‐606. [DOI] [PubMed] [Google Scholar]
- 63. Shi L, Ito F, Wang Y, et al. Non–thermal plasma induces a stress response in mesothelioma cells resulting in increased endocytosis, lysosome biogenesis and autophagy. Free Radic Biol Med. 2017;108:904‐917. [DOI] [PubMed] [Google Scholar]
- 64. Schoenfeld JD, Sibenaller ZA, Mapuskar KA, et al. O2(−) and H2O2‐mediated disruption of fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;31:487‐500.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Finkel T, Cooper GM. Detection of a molecular‐complex between Ras proteins and transferrin receptor. Cell. 1984;36:1115‐1121. [DOI] [PubMed] [Google Scholar]
- 66. Hirayama T. Fluorescent probes for the detection of catalytic Fe(II) ion. Free Radic Biol Med. 2019;133:38‐45. [DOI] [PubMed] [Google Scholar]
- 67. Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1‐3. [DOI] [PubMed] [Google Scholar]
- 68. Benchekroun MN, Pourquier P, Schott B, Robert J. Doxorubicin‐induced lipid peroxidation and glutathione peroxidase activity in tumor cell lines selected for resistance to doxorubicin. Eur J Biochem. 1993;211:141‐146. [DOI] [PubMed] [Google Scholar]
- 69. Gutteridge J, Rowley D, Halliwell B. Superoxide‐dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin‐dependent degradation of DNA. Biochem J. 1981;199:263‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hassannia B, Vandenabeele P, Vanden BT. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830‐849. [DOI] [PubMed] [Google Scholar]
- 71. Riordan NH, Riordan HD, Meng X, Li Y, Jackson JA. Intravenous ascorbate as a tumor cytotoxic chemotherapeutic agent. Med Hypotheses. 1995;44:207‐213. [DOI] [PubMed] [Google Scholar]
- 72. Tanaka H, Mizuno M, Toyokuni S, et al. Cancer therapy using non–thermal atmospheric pressure plasma with ultra‐high electron density. Phys Plasmas. 2015;22:122004. [Google Scholar]
- 73. Tanaka H, Hori M. Medical applications of non–thermal atmospheric pressure plasma. J Clin Biochem Nutr. 2017;60:29‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kajiyama H, Utsumi F, Nakamura K, et al. Future perspective of strategic non–thermal plasma therapy for cancer treatment. J Clin Biochem Nutr. 2017;60:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Okazaki Y, Wang Y, Tanaka H, et al. Direct exposure of non–equilibrium atmospheric pressure plasma confers simultaneous oxidative and ultraviolet modifications in biomolecules. J Clin Biochem Nutr. 2014;55:207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Furuta T, Shi L, Toyokuni S. Non–thermal plasma as a simple ferroptosis inducer in cancer cells: A possible role of ferritin. Pathol Int. 2018;68:442‐443. [DOI] [PubMed] [Google Scholar]
- 77. Nakamura K, Peng Y, Utsumi F, et al. Novel intraperitoneal treatment with non–thermal plasma‐activated medium inhibits metastatic potential of ovarian cancer cells. Sci Rep. 2017;7:6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Latunde‐Dada GO. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861:1893‐1900. [DOI] [PubMed] [Google Scholar]
- 79. Xiao FJ, Zhang D, Wu Y, et al. miRNA‐17‐92 protects endothelial cells from erastin‐induced ferroptosis through targeting the A20‐ACSL4 axis. Biochem Biophys Res Commun. 2019;515:448‐454. [DOI] [PubMed] [Google Scholar]
- 80. Li Z, Jiang L, Chew SH, Hirayama T, Sekido Y, Toyokuni S. Carbonic anhydrase 9 confers resistance to ferroptosis/apoptosis in malignant mesothelioma under hypoxia. Redox Biol. 2019;26:101297. [DOI] [PMC free article] [PubMed] [Google Scholar]


