Abstract
The tumor microenvironment favors the growth and expansion of cancer cells. Many cell types are involved in the tumor microenvironment such as inflammatory cells, fibroblasts, nerves, and vascular endothelial cells. These stromal cells contribute to tumor growth by releasing various molecules to either directly activate the growth signaling in cancer cells or remodel surrounding areas. This review introduces recent advances in findings on the interactions within the tumor microenvironment such as in cancer‐associated fibroblasts (CAFs), immune cells, and endothelial cells, in particular those established in mouse gastric cancer models. In mice, myofibroblasts in the gastric stroma secrete R‐spondin and support normal gastric stem cells. Most CAFs promote tumor growth in a paracrine manner, but CAF population appears to be heterogeneous in terms of their function and origin, and include both tumor‐promoting and tumor‐restraining populations. Among immune cell populations, tumor‐associated macrophages, including M1 and M2 macrophages, and myeloid‐derived suppressor cells (MDSCs), are reported to directly or indirectly promote gastric tumorigenesis by secreting soluble factors or modulating immune responses. Endothelial cells or blood vessels not only fuel tumors with nutrients, but also interact with cancer stem cells and immune cells by secreting chemokines or cytokines, and act as a cancer niche. Understanding these interactions within the tumor microenvironment would contribute to unraveling new therapeutic targets.
Keywords: CAFs, endothelial cells, gastric cancer, gastrin, TAMs
Gastric tumor microenvironment: Cancer‐associated fibroblasts, endothelial cells, gastrin‐expressing cells, and various immune cells including macrophages, MDSCs, and ILC2s serve as tumor‐promoting niche in gastric cancers. There are numerous crosstalks between tumor cells and surrounding stromal cell types, which contribute to tumor development derived from gastric stem cells.
1. INTRODUCTION
Cancer development is accompanied by a prominent desmoplastic reaction involving various stromal cell types and immune cells in the local microenvironment. Immune cells are primarily recruited from the bone marrow, although some are expanded from local resident immune cells. The origin of other stromal cells, such as fibroblasts, endothelial cells, and nerves, remains controversial. However, it is likely that most of these cell types are expanded or transformed from normal cells in the original organ, while a few may originate from bone marrow‐derived cells. 1 , 2 , 3 All these components constitute a tumor microenvironment that favors the growth and expansion of cancer cells. Cells in the tumor microenvironment fuel and stimulate other cell types in a paracrine fashion, creating an environment that allows tumor cells to escape from host immune surveillance and become more resistant to cancer therapy. Thus, a detailed and precise understanding of interactions within the tumor microenvironment is critical to establishing new cancer therapies. In this review, we focus on the role of tumor‐associated cells in gastric cancers. We give particular consideration to findings validated in mouse models.
2. CANCER‐ASSOCIATED FIBROBLASTS
Within the tumor microenvironment, cancer‐associated fibroblasts (CAFs) are a dominant stromal component and contribute in many ways to tumor progression. In earlier studies using preclinical models, CAFs were identified as large spindle‐like stromal cells in solid tumors with abundant connective tissue (as in pancreatic cancer). 1 Normal stroma in non‐neoplastic tissues contains a small number of fibroblasts. CAFs differ in being characterized by abundant expression of α‐smooth muscle actin (α‐SMA), a marker of activated fibroblasts. 3
Genomic analyses have identified genetic or epigenetic alterations in human and mouse CAFs, compared with non‐cancer fibroblasts, in several cancer types. 4 , 5 , 6 Tumor cells, as well as other stromal components such as immune cells, probably contribute to these gene modifications in activated CAFs through tumor‐stroma interaction. Activated CAFs can produce abundant soluble molecules, including basic fibroblast growth factor (bFGF), members of the vascular endothelial growth factor (VEGF) family, platelet‐derived growth factor (PDGF), ligands of epidermal growth factor receptor (EGFR), interleukins, and TGF‐β. These molecules, in turn, regulate tumor growth and inflammatory responses via direct cell‐to‐cell contact or in a paracrine manner. Although many products secreted from CAFs were once thought to be tumor‐promoting, recent studies have revealed that CAFs may also have an inhibitory effect on tumor progression. 7 , 8 Emerging analyses at the single‐cell level have clearly demonstrated that CAFs are largely heterogeneous, and consist of multiple molecular subsets with different effects on tumor progression. 9
Gastric cancers, in particular undifferentiated gastric cancers, often exhibit excessive fibrosis with massive infiltration of CAFs. A study using a mouse model of inflammation‐associated gastric cancer demonstrated that mouse CAFs promote gastric cancer cell growth and progression via secretion of IL‐6, CXCL12, Wnt5a, Gremlin‐1, etc. 10 To be more specific, EGF, FGF, IL‐6, CXCL12, and PDGF can directly promote tumor cell proliferation, 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 EGF, PDGF, IL‐1β, IL‐6, IL‐8, and PGE2 can promote tumor invasion and metastasis, 10 , 11 , 17 and TGF‐β, IL‐6, FGF, TNF‐α, and IL‐1β can promote epithelial‐mesenchymal transition (EMT) 10 , 11 , 17 , 19 (Figure 1). Our group has previously reported for a chemically induced mouse model of gastric cancer that CAFs are the primary source of IL‐6 and that knockout of IL‐6 inhibits gastric tumor growth. 18 IL‐6 production and secretion from CAFs are regulated in part by an autocrine loop mediated by IL‐1 signaling or miR‐149‐dependent transcriptional modulation. IL‐6 activates STAT signaling in tumor cells, leading to the promotion of tumor growth and metastasis. 15 , 16 , 17 In addition, TGF‐β and Lumican/FAK signaling may play a role in the crosstalk between gastric cancers and CAFs. 11 , 19 Furthermore, CXCL12 produced by myofibroblasts and endothelial cells promotes gastric carcinogenesis through the CXCL12/CXCR4 pathway. 20 Therefore, targeting CAF‐mediated crosstalk would be beneficial in the treatment of gastric cancers (Table 1).
Figure 1.
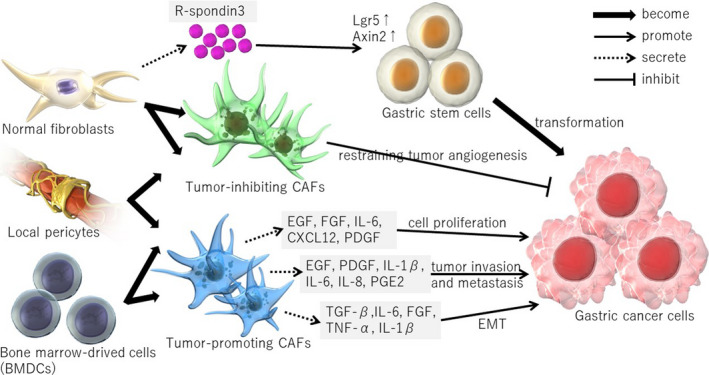
The interplay between cancer‐associated fibroblasts (CAFs) and gastric cancer. CAFs regulate tumor growth and inflammatory responses by secreting various molecules. Although most CAFs have been thought to promote tumor growth, a subset of CAFs may also have an inhibitory effect on tumor progression. Normal fibroblasts, a likely origin of CAFs, support gastric (cancer) stem cells via R‐spondin3 secretion
Table 1.
Function of each soluble molecules in gastric cancer microenvironment
| Soluble molecules | Secreting cells | Target cells | Function | References |
|---|---|---|---|---|
| IL‐1 | CAFs, macrophages | Immune cells | Induce the recruitment and activation of inflammatory cells | (13, 43, 44) |
| CAFs | Cancer cells | Promote tumor invasion and metastasis and EMT | (10, 13) | |
| M1 macrophages | MDSCs | Activate and recruit MDSCs | (27, 28, 29, 31 | |
| Macrophages | parietal cells | Propagate the inflammatory response and inhibit gastric acid secretion | (46 | |
| Local mucosa | Induce stepwise spontaneous gastric inflammation, metaplasia, dysplasia, and carcinoma | (14, 119 | ||
| IL‐4 | TH2 cells, basophils,ILC2 | M2 macrophages | M2 polarization of macrophages | (14, 26, 32, 49 |
| IL‐6 | CAFs | Cancer cells | Promote tumor cell proliferation, tumor invasion and metastasis, and EMT | (10, 14, 15, 16, 17, 18 |
| CAFs | Immune cells | Induce inflammation | (13 | |
| Macrophages | Induce the recruitment and activation of inflammatory cells | (43, 44 | ||
| IL‐8 | CAFs | Cancer cells | Promote tumor invasion and metastasis | (17, 119 |
| CAFs | Immune cells | Induce inflammation | (13, 119 | |
| M1 macrophages | MDSCs | Activate and recruit MDSCs | (27, 28, 29 | |
| macrophages | Induce the recruitment and activation of inflammatory cells | (44 | ||
| IL‐10 |
M2 macrophages, other inflammatory cell types, mucosal cells |
Unclear | (31, 35, 46 | |
| regulatory macrophages (Mregs) | Counter‐regulate the M1 response | (49 | ||
| IL‐11 | Parietal cells | gp130 | Activate JAK‐STAT signaling pathways, induce atrophic gastritis | (14 |
| IL‐12 | M1 macrophage | TH1 cells | Amplify a type 1 immune response | (26 |
| IL‐13 | TH2 cells, basophils, ILC2 | M2 macrophages | M2 polarization of macrophages | (32 |
| IL‐17 | Th17 | Induce gastritis and oxyntic atrophy | (14 | |
| IL‐22 | CAFs | Cancer cells | Promote gastric cancer cell invasion | (14 |
| IL‐33 | gastric mucosal cells | ILC2, TH2 | Activate ILC2 and Th2 immunocytes, and thus initiate a Th2 cytokine response | (33 |
| IFN‐γ | Th1 cells | Suppresses inflammation and induces gastritis and oxyntic atrophy | (14 | |
| M1 macrophages | (31 | |||
| Angiostatic activities and chemoattracts anti‐tumoral lymphocytes | (119 | |||
| TNF‐α | CAFs | Immune cells | Induce inflammation | (13 |
| CAFs | Cancer cells | Promote EMT | (10 | |
| M1 macrophages | MDSCs | Activate and recruit MDSCs | (27, 28, 29, 31 | |
|
Macrophages, other inflammatory cell types, mucosal cells |
Induce the recruitment and activation of inflammatory cells | (44, 46 | ||
| macrophages | Epithelial cells | Promote Wnt signaling, contributes to gastric tumorigenesis | (120 | |
| Wnt5a | MSC‐derived CAF | Cancer cells | Promote tumor growth | (10 |
| PGE2 | CAFs | Cancer cells | Promote tumor invasion and metastasis | (13 |
| Gastric mucosal cells | Macrophages | Recruit macrophage to gastric tumors, and promote the Wnt/β‐catenin signaling activity, which thus contributes to gastric cancer | (47, 120 | |
| COX‐2 | Cancer cells | promote tumor growth by inhibiting apoptosis, promote cell proliferation and stimulating angiogenesis within cancer cells | (119 | |
| NOS | Macrophages | Epithelial cells | Causes methylation of genes associated with tumor suppression | (31 |
| MDSCs | T cells | Suppress host immunity | (55, 58 | |
| ROS | MDSCs | T cells | Suppress host immunity | (55, 58 |
| Arg‐1 | MDSCs | T cells | Suppress host immunity | (55, 58 |
| PD‐L1 | MDSCs | T cells | Suppress host immunity | (55, 58 |
| MMPs | CAFs | Cancer cells | Inhibit tumor growth, invasion, and metastasis | (13 |
| CAFs | Inflammatory cells | Participant in reconstruction of the tumor microenvironment | (13 | |
| Epithelial cells | M1 macrophage | Suppress M1 macrophage polarization, exerts a restrictive role on H. pylori‐induced gastric injury and the development of premalignant lesions | (50 | |
| TGF‐β | CAFs | Cancer cells | Promote EMT | (10, 11, 13, 19 |
| Cancer cells | Fibroblasts and cancer cells | Stimulate collagen synthesis in both fibroblasts and cancer cells, which leads to diffuse fibrosis in the case scirrhous GC | (119 | |
| M2 macrophage | Unclear | (35 | ||
| EGF | CAFs | Cancer cells | Promote tumor cell proliferation and tumor invasion and metastasis | (13, 17 |
| HGF | CAFs | Cancer cells | Affect the proliferation and migration of cancer cells | (11, 13 |
| VEGF | CAFs | Cancer cells | Affect the proliferation and migration of cancer cells | (11, 13 |
| FGF | CAFs | Cancer cells | Promote tumor cell proliferation and EMT | (11 |
| PDGF | CAFs | Cancer cells | Promote tumor cell proliferation and tumor invasion and metastasis | (11, 13 |
| CXCR4 | endothelial cells | Cancer cells | Aggressive tumor behavior, such as tumor invasion, metastasis, and poor differentiation | (119 |
| CXCL9 | M1 macrophage | TH1 cells | Amplify a type 1 immune responses | (26 |
| CXCL10 | M1 macrophage | TH1 cells | Amplify a type 1 immune responses | (26 |
| CXCL12 | CAFs | Cancer cells | Promote tumor cell proliferation | (10, 12, 20 |
| STAT‐3 | Cancer cells | Dendritic cells | Increase the capacity of tumors to evade the immune system by inhibiting the maturation of dendritic cells, thereby suppressing the immune response | (119 |
| Gremlin‐1 | MSC‐derived CAF | Cancer cells | Promote tumor growth | (10 |
| R‐spondin3 | Myofibroblasts | Gastric stem cells | Lead to an increase in proliferation of Wnt‐responsive Axin2+Lgr5− stem cells and finally gastric gland hyperplasia | (21, 22 |
Given the heterogeneous nature of CAFs, it is likely that CAFs originate from various cells. Numerous publications have shown that CAFs originate from local normal fibroblasts, bone marrow mesenchymal stem cells (MSCs), or local pericytes. 2 Recent studies have shown that normal gastric myofibroblasts secrete R‐spondin3 and contribute to stem cell activity, at least in the gastric antrum. 21 Antral glands are maintained by long‐lived stem cells residing at the gland base, some of which are characterized by the expression of Lgr5 or Axin2. During mucosal inflammation and regeneration, R‐spondin3‐expressing myofibroblasts expand and promote proliferation and stem cell function in Axin2+ stem cells. 22 Therefore, normal myofibroblasts act as an important component of the gastric stem cell niche, and likely give rise to CAFs that activate stem cells in cancer tissues via R‐spondin signaling (Figure 1). Studies using bone marrow transplantation in mice have shown that MSCs display a remarkable feature called tumor‐specific tropism, in which they actively migrate to tumor sites. 10 , 23 In fact, in a mouse model of gastric cancer, at least 20% of CAFs were estimated to arise from bone marrow‐derived MSCs. 10 Notably, the presence of bone marrow‐derived cells in the tumor mesenchyme was confirmed in human gastric adenocarcinomas and rectal adenomas in patients who developed tumors following bone marrow transplantation. 24 In addition to bone marrow‐derived MSCs, a local MSC‐like cell type expressing Gremlin‐1 has been identified in the gastrointestinal mucosa. 25 Whether CAFs are indeed derived from Gremlin‐1+ local MSCs remains uncertain. However, lineage tracing studies in mice will elucidate more details of the cellular origin of CAFs in future.
3. IMMUNE CELLS
3.1. Macrophages
In the tumor microenvironment, macrophages or monocytes are known as tumor‐associated macrophages (TAMs) and are among the most abundant immune cells. The degree of TAM infiltration in tumor tissues is positively correlated with poor prognosis in various cancers. It is thought that TAMs promote cancer progression by secreting various factors, including inflammatory cytokines, growth factors, and proteolytic enzymes. In addition, TAMs interact with other stromal components and often suppress the host immune response, resulting in immune escape and the subsequent growth of tumor cells.
Macrophages are classified into 2 main types: M1 and M2. M1 macrophages have a pro‐inflammatory role and produce various cytokines (Figure 2). M1 macrophages, through their expression of cytokines and chemokines such as IL‐12, CXCL9, and CXCL10, drive the polarization and recruitment of TH1 cells, thereby amplifying a type 1 response. 26 In addition, other previous reports have suggested that M1 macrophages secrete IL‐1β, TNF‐α, and IL‐8, to activate and recruit MDSCs. 27 , 28 , 29 , 30 Furthermore, activated macrophages are known to produce nitric oxide synthase (NOS) and/or reactive oxygen species (ROS) that causes epigenetic changes in the gastric epithelial cells. 31
Figure 2.
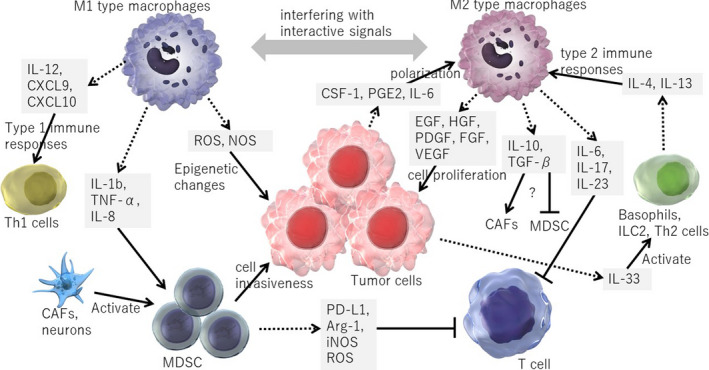
The interplay between tumor‐associated macrophages (TAMs) and gastric cancer. TAMs and tumor cells stimulate each other by secreting several cytokines and growth factors. M1 and M2 macrophages can be reprogrammed into each other, leading the immunological shift within the tumor microenvironment. Myeloid‐derived suppressor cells (MDSCs) suppress host immunity by inhibiting T‐cell responses, and have pro‐tumorigenic effects
Conversely, M2 polarization of macrophages are driven by TH2 cells, basophils, and type 2 innate lymphoid cells (ILC2s) through their secretion of IL‐4, IL‐13, or IL‐33 26 , 32 (Figure 2). IL‐33 is also expressed by a subset of epithelial cells and could be released upon epithelial injury. 33 , 34 In tumor tissues, the majority of macrophages are polarized to M2. A previous study using peritoneal macrophage showed that M2 macrophages produce anti‐inflammatory cytokines, including TGF‐β and IL‐10. 35 Nevertheless, the cellular targets of molecules secreted by M2 macrophages remain largely unclear. Presumably, TGF‐β is likely to contribute to tumor progression by activating CAFs and promoting fibrosis in gastric cancer models. 10 , 36 IL‐10 may trigger activation of the T‐cell–inhibitory receptor PD‐L1 on TAMs, which favors the inhibition of tumor‐specific T‐cell immunity, 26 therefore contributing to the general suppression of anti‐tumor activities in the tumor environment. TAMs can also promote angiogenesis and contribute to remodeling of the tumor microenvironment.
There is a direct crosstalk between tumor cells and TAMs. Tumor cells secrete several cytokines and growth factors that induce M2 polarization in TAMs, such as colony stimulating factor 1 (CSF‐1), PGE2, and IL‐6. TAMs, in turn, can directly stimulate tumor cell growth by secreting EGF, HGF, PDGF, FGF, and VEGF 37 , 38 (Figure 2). M2 macrophages can be reprogrammed into M1 macrophages by interfering with these interactive signals, leading to the shift in immune microenvironment. 39 , 40
In mouse models of gastritis and gastric cancers, there is a marked infiltration of macrophages. These macrophages are recruited by epithelium‐derived chemokines and cytokines. 41 , 42 , 43 , 44 , 45 , 46 They produce pro‐inflammatory cytokines such as TNF‐α, and stimulate tumor growth, in part through Wnt activation. 45 , 47 Indeed, depletion of macrophages in these mouse models suppresses epithelial proliferation and tumorigenesis. 44 , 46 , 47 In this context, the polarization of macrophages is altered by factors that include MMP‐7, IL‐33, and EGF signaling. 33 , 48 , 49 M1 and M2 macrophages appear to make distinct contributions to the crosstalk between macrophages and epithelial cells. 46 , 50 Macrophages, in addition to directly stimulating epithelial growth, probably interact with other stromal components and contribute to angiogenesis or tumor immunity. 51 Future studies are needed to clarify the precise role of macrophages in gastric tumors.
3.2. Myeloid‐derived suppressor cells
Myeloid‐derived suppressor cells (MDSCs) comprise a population of myeloid progenitor and immature myeloid cells, and are regulatory immune cells associated with sites of chronic inflammation and cancer that suppress CD8+ T‐cell function by their expression of PD‐L1 and CTLA‐4. Therefore, targeted in vivo depletion of the granulocytic MDSC subset is sufficient to induce the intratumoral accumulation of endogenous CD8+ T cells and tumor cell apoptosis in certain cancer models. 52 , 53 Treatment with immune checkpoint inhibitors blocks the suppressive effects of MDSCs on CD8+ T cells, causing tumor regression in various mouse models of cancers. 54 MDSCs are also endowed with the ability to suppress host immunity through Arg‐1, iNOS, and ROS, like macrophages. It was reported that MDSCs also have direct pro‐tumorigenic effects. 55
MDSCs are characterized by strong dual expression of CD11b and Gr‐1. Increased myeloid CD11b+Gr‐1+ cells are particularly associated with inflammation‐associated cancers. In the context of inflammation and cancer, the spleen becomes an important source of myeloid cells in animal models and human patients. 56 , 57 In mouse models of gastrointestinal (GI) and pancreatic cancers, MDSCs are activated and recruited to local tissues by sustained expression of inflammatory cytokines such as IL‐1β, TNF‐α, DKK‐1, and IL‐8 27 , 28 , 29 (Figure 2). Chemokine signaling, modulated by CXCR4 or CXCR2 receptors, also mediates the activity of MDSCs. 53 , 58 , 59 Other tumor microenvironmental components, such as CAFs or neurons, also contribute to differentiation and activation of MDSCs. 10 , 58 While MDSCs might have somewhat beneficial effects on regulating insulin tolerance in diabetes patients and maternal‐fetal tolerance in pregnancy, a large body of cancer research works has suggested that MDSCs promote tumor progression in numerous cancer models. 60 Therefore, targeting MDSCs might be a novel approach for stimulating host immunity against tumor cells and subsequent tumor regression.
3.3. Lymphocytes
Tumor‐infiltrating lymphocytes (TILs) consist of T cells, B cells, and natural killer (NK) cells, and T‐cell‐mediated adaptive immunity is considered to play a major role in anti‐tumor immunity. The subsets of T cells are represented by CD8+ cytotoxic T cells, CD4+ T helper cells, FOXP3+ regulatory T cells, memory T cells, and NK cells. These lymphocytes can infiltrate stroma and tumor cells to modulate the host immune response against tumor cells, while upregulated PD‐L1 or CTLA‐4 expression suppresses these anti‐tumor immune responses in certain tumor microenvironment.
Nevertheless, evidence to the role of lymphocytes in mouse gastric cancer models remains quite limited. 61 Mohammed and colleagues demonstrated the number of infiltrating lymphocytes in the Tff1‐knockout gastric tissues that develop chronic inflammation and gastric neoplasms at the antropyloric gastric regions. In this context, loss of TFF1 causes decreased IL‐17 expression in CD8+ T cells, which resulted in loss of cytotoxic function by T‐cell–mediated tumor immunity. 62 O'Reilly and colleagues showed that NF‐κB1 deficiency resulted in aberrant JAK‐STAT signaling, which dysregulated the expression of effectors of inflammation, antigen presentation, and immune checkpoints. They also demonstrated that gastric PD‐L1 upregulation was driven by aberrant STAT1 activation, and this provides a functional link between loss of NF‐κB1, aberrant STAT1 activation, increased PD‐L1 expression, and GC development. 63
In human GCs, high densities of T lymphocytes in tumor tissues are associated with favorable survival, while PD‐L1 expression correlates with shortened overall survival. 64 There are also T regulatory cells (Tregs) as a component of TIL, which are able to inhibit the immune response mediated by CD4+ and CD8+ T cells, by suppressing T‐cell proliferation, antigen presentation, and cytokine production. 65 Recent genome sequencing studies have revealed that high levels of PD‐L1 expression and favorable response to immune checkpoint inhibitors are associated with Epstein‐Barr virus (EBV) and microsatellite instability (MSI) tumor molecular subtypes of GC. 66 However, mouse models that recapitulate such GC subtypes in human have not been well established. To understand the precise mechanism in TIL‐mediated tumor immunity in GCs, generation and analysis of new mouse models with high PD‐L1/CTLA‐1 expression would be needed in the future.
4. ENDOTHELIAL CELLS
Angiogenesis is a process by which cancers supply the tumor microenvironment with nutrients and oxygen, thereby contributing to tumor growth. 67 Multiple drugs that target angiogenesis‐related molecules, such as VEGF, are available in clinics and are effective in many cancers. Endothelial and vascular blood vessel cells have a function beyond supplying nutrition to tumor tissues; they also act as cancer niche cells and create a cancer‐promoting environment. 68 For example, endothelial cells can stimulate and activate colorectal cancer stem cells in a paracrine or juxtacrine fashion by mediating Notch and Akt signaling. 69 , 70 Similarly, lymphatic endothelial cells can activate metastatic signatures 71 , 72 and immunoregulatory function 73 in gastric cancers via secretion of soluble factors, suggesting a paracrine tumor‐promoting function of endothelial cell lineage in gastric cancers.
Our group had previously identified a unique perivascular niche that supports normal and preneoplastic stem cells in the mouse stomach. 20 , 74 , 75 Long‐lived gastric stem cells exist at the proliferative zone of each gastric gland, which is called the isthmus, and maintain the mucosal homeostasis. Stem cells are supported by their local stem cell niches, which consist of various stromal, immune, and even epithelial cell types. 76 , 77 Given that previous studies have suggested that inflammatory CXCL12/CXCR4 signaling has an important role in gastritis and gastric cancer, 10 , 12 , 78 we examined the expression pattern of CXCL12 and its receptor CXCR4 in the stomach using fluorescent reporter mice. Interestingly, each molecule is expressed in specific cell types. CXCL12 is expressed in stromal cells, in close proximity to isthmus stem cells. Immunofluorescence and FACS analysis has confirmed that these gastric CXCL12+ cells are indeed a subset of CD31+ endothelial cells. 20 , 74 In contrast, CXCR4 expression was also observed in cells adjacent to CXCL12+ endothelium, including type‐2 innate lymphoid cells (ILC2s) and isthmus stem/progenitor cells (Figure 3).
Figure 3.
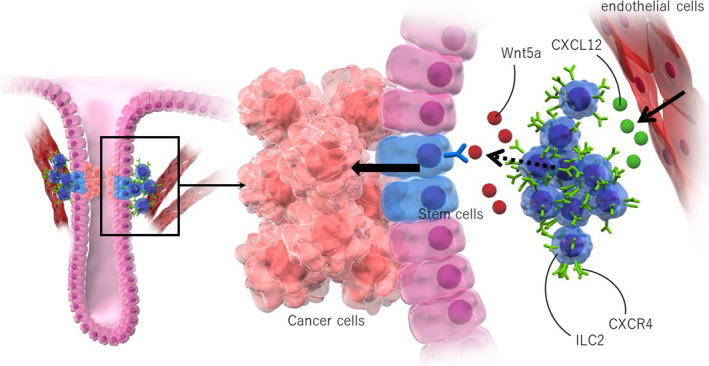
Endothelial cancer niches promote gastric cancer progression. CXCL12 is expressed in stromal endothelial cells, in close proximity to gastric isthmus stem cells, while CXCR4+ type 2 innate lymphoid cells (ILC2s) are recruited by CXCL12+ endothelial cells and enriched in this region. Isthmus stem cells become more proliferative with the expansion of CXCL12+ endothelial cells and CXCR4+ ILC2s
In models of mouse gastritis and gastric cancer, isthmus stem cells are increased and become more proliferative with the expansion of CXCL12+ endothelial cells and CXCR4+ ILC2s. Gastric cancer development in mice is inhibited by genetic deletion of CXCL12, pharmacological inhibition of CXCR4, and ablation of ILC2s, indicating that the CXCL12/CXCR4‐dependent stem cell niche has a significant contribution to gastric carcinogenesis. 20 , 74 Mechanistically, gastric ILC2s express high levels of Wnt5a, one of the non‐canonical Wnt ligands (Figure 3). They also activate RhoA signaling in gastric stem cells, the change of which is often observed in human gastric cancers. 79 , 80 , 81 These findings again highlight the importance of the endothelial perivascular niche in regulating tissue stem cells and carcinogenesis in multiple organs. 82 , 83 , 84 , 85
Furthermore, it has been suggested that the perivascular network is important in the metastasis of various cancers, including gastric cancer. 71 , 86 A potential mechanism of metastatic promotion by vascular endothelial cells is the activation of tumor cells by locally invaded vessels or endothelial cells, which induces EMT and allows the tumor cells to enter systemic circulation more efficiently. In addition, circulating tumor cells or tumor‐derived soluble factors may change vascular formation and other features of distant organs in a way that creates a favorable microenvironment for cancer cells. This microenvironment is known as the pre‐metastatic niche. Indeed, CXCL12/CXCR4 signaling contributes to the development of the pre‐metastatic niche and disruption of CXCL12/CXCR4 signaling is effective in inhibiting metastasis. 83 , 87 , 88 , 89
Recent scRNA‐seq studies have determined that tumor endothelial cells are heterogeneous at a molecular level, and that the various subtypes contribute to angiogenesis and tumor progression and respond to targeting therapies in different ways. 90 , 91 , 92 Detailed subtyping and a deeper understanding of this heterogeneity would require an extensive functional validation study, but would be helpful in establishing new therapeutic strategies beyond VEGF targeting. Disrupting the interaction between compromised tumor endothelial cell types and cancer cells may be a promising approach to controlling the tumor microenvironment.
5. GASTRIN
The peptide hormone gastrin is produced by G cells in the gastric antrum. It has been suggested that gastrin, in addition to having a physiological role in regulating acid secretion, may help to promote gastric cell proliferation and carcinogenesis. More details are provided in a separate review. 93 Here, we briefly review the role of amidated gastrin in stem cell regulation and cancer development.
Transgenic mice with hypergastrinemia (INS‐GAS mice) show increased epithelial proliferation and develop metaplasia and cancer in the proximal stomach, suggesting that gastrin has a proliferative effect in this part of the stomach. 94 The development of gastric tumors in INS‐GAS mice is significantly inhibited by an antagonist of CCK2R, a gastrin receptor, indicating that proliferation and carcinogenesis occur through the gastrin/CCK2R pathway. 95 Nevertheless, gastrin knockout (GAS‐KO) mice, in which gastric tumor development is, in theory, inhibited, often develop spontaneous gastric tumors, particularly in the distal section of the stomach. 96 In fact, studies using hypergastrinemic INS‐GAS mice and GAS‐KO mice demonstrate that gastrin has different effects on proximal and distal gastric carcinogenesis; gastrin promotes proximal tumor development but inhibits distal tumor development 97 , 98 (Figure 4).
Figure 4.
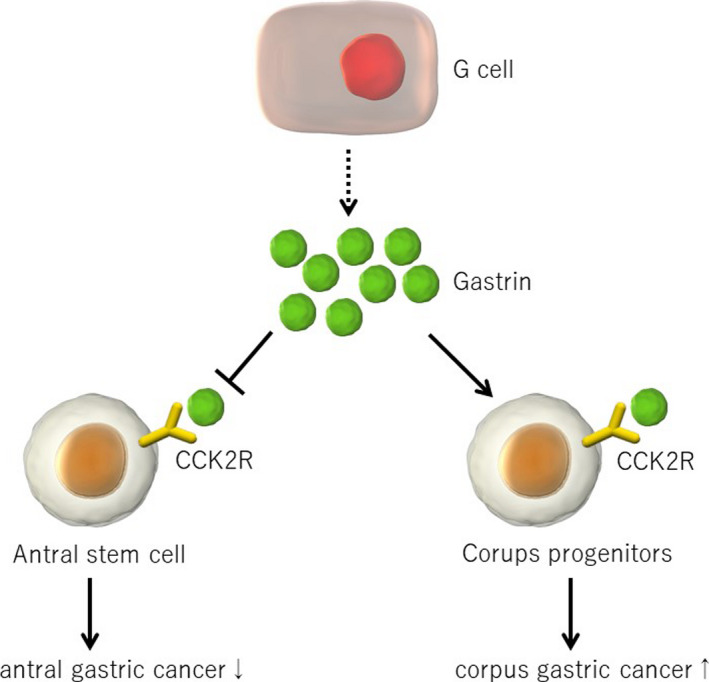
Gastrin’s effects on the development of stomach cancer. Gastrin receptor Cckbr is expressed in gastric stem and progenitors in both the proximal and distal stomach. While gastrin promotes proliferation of Cckbr+ cells in the proximal stomach, gastrin/Cck2r suppresses proliferation and stem cell function in the distal stomach
In situ hybridization imaging and lineage tracing studies in mice have revealed the specific site of CCK2R expression in murine stomachs. 99 In the proximal stomach, CCK2R is strongly expressed in mature parietal and enterochromaffin‐like (ECL) cells, consistent with its role in acid secretion. In addition, a subset of proliferating progenitors in the neck zone of the gastric corpus glands also express CCK2R. These cells can expand and supply daughter cells in response to hypergastrinemia, which is likely to have been through activation of the ERK pathway in progenitor cells. 100 , 101 Therefore, the tumor‐promoting effects of gastrin in the proximal stomach probably occur via stimulation of CCK2R‐expressing proliferating progenitors in the isthmus‐neck region. Given that the serum gastrin level is usually elevated in patients with long‐term proton pump inhibitor (PPI) use, increased gastric cancer risk in such patients might be affected by activation of the gastrin/CCK2R pathway. 102
Conversely, CCK2R has been found to be expressed in rare cells residing near the base of the antral and cardiac glands. 99 , 103 These basal CCK2R+ cells generally do not overlap with Lgr5+ basal stem cells, but show robust proliferation and act as long‐lived stem cells. 104 CCK2R+ stem cells normally divide asymmetrically and supply daughter cells within the gland. Interestingly, gastrin suppresses the stem cell activity of CCK2R+ cells. In gastrin‐deficient conditions, CCK2R+ stem cells divide more rigorously and more symmetrically. This increase in symmetric stem cell division results in robust accumulation of gene mutations during carcinogenesis, 105 which could account for the promotion of antral carcinogenesis by gastrin deficiency. Thus, these data support the idea that the proximal and distal portions of the stomach are really 2 distinct organs, based, in large part, on differences in the regulatory role of gastrin, and highlight the importance of the gastrin‐dependent hormonal niche within the gastric tumor microenvironment.
6. CONCLUSION
The tumor microenvironment is rather complex and very different from the microenvironment of normal tissue. Inflammation appears to be a key initial step in the creation of the tumor microenvironment, and is associated with the recruitment of various immune cells and the activation of epithelial stem or immediate progenitors, which are the main origin of gastrointestinal cancers. 76 , 104 , 106 , 107 , 108 Recruitment, growth, or differentiation of CAFs and blood vessels is caused by numerous interactions between tumor cells and other stromal components. Recent studies have suggested that nerves and neurotransmitters also play a critical role within the tumor microenvironment for many cancers, including gastric cancers. 109 , 110 , 111 , 112 , 113 , 114 , 115 Some tumor stromal components may be anti‐tumorigenic, 7 , 58 , 116 but tumor cells and other stromal cells appear to acquire the ability to escape this anti‐tumorigenic host defense. Understanding the precise biology of the tumor microenvironment is of critical importance. There is a limitation in currently available mouse gastric cancer models, as most of them do not develop invasive or metastatic cancer but show modest metaplastic or dysplastic changes that have been used as an alternative endpoint. 117 However, it remains uncertain whether such a lesion is a true precursor of gastric cancers. 106 , 108 , 118 Therefore, generation of invasive gastric cancer models coincident with a proper tumor microenvironment in mice would be encouraged. Targeting pro‐tumorigenic stroma and activating an anti‐tumorigenic microenvironment could be promising new therapeutic approaches in cancer treatment.
DISCLOSURES
The authors disclose no conflicts.
ACKNOWLEDGMENTS
YH is supported by the KAKENHI Grant‐in‐Aid for Scientific Research,17K09347 and 17H05081, P‐CREATE from AMED, Inoue Science Research Award, Advanced Research and Development Programs for Medical Innovation (PRIME), Kanae Foundation of the Promotion of Medical Science, Princess Takamatsu Cancer Research Fund, Yokoyama Clinical Pharmacological Research Foundation, SENSHIN Medical Research Foundation, Kowa Life Science Foundation, Takeda Science FoundationVisionary Research Grant, Bristol Myers Squibb Research grant, and Pharmacological Research Foundation.
Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696–2707. 10.1111/cas.14521
REFERENCES
- 1. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392‐401. [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer‐associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16(5):282‐295. [DOI] [PubMed] [Google Scholar]
- 3. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582‐598. [DOI] [PubMed] [Google Scholar]
- 4. Costea DE, Hills A, Osman AH, et al. Identification of two distinct carcinoma‐associated fibroblast subtypes with differential tumor‐promoting abilities in oral squamous cell carcinoma. Cancer Res. 2013;73(13):3888‐3901. [DOI] [PubMed] [Google Scholar]
- 5. Ishimoto T, Miyake K, Nandi T, et al. Activation of transforming growth factor beta 1 signaling in gastric cancer‐associated fibroblasts increases their motility, via expression of rhomboid 5 homolog 2, and ability to induce invasiveness of gastric Cancer Cells. Gastroenterology. 2017;153(1):191‐204.e16. [DOI] [PubMed] [Google Scholar]
- 6. Berdiel‐Acer M, Sanz‐Pamplona R, Calon A, et al. Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Mol Oncol. 2014;8(7):1290‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizutani Y, Kobayashi H, Iida T, et al. Meflin‐positive cancer‐associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res. 2019;79(20):5367‐5381. [DOI] [PubMed] [Google Scholar]
- 9. Elyada E, Bolisetty M, Laise P, et al. Cross‐species single‐cell analysis of pancreatic ductal adenocarcinoma reveals antigen‐presenting cancer‐associated fibroblasts. Cancer Discov. 2019;9(8):1102‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quante M, Tu SP, Tomita H, et al. Bone marrow‐derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hasegawa T, Yashiro M, Nishii T, et al. Cancer‐associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor‐beta signaling. Int J Cancer. 2014;134(8):1785‐1795. [DOI] [PubMed] [Google Scholar]
- 12. Shibata W, Ariyama H, Westphalen CB, et al. Stromal cell‐derived factor‐1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut. 2012;62(2):192‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang L, Xu AM, Liu S, Liu W, Li TJ. Cancer‐associated fibroblasts in digestive tumors. World J Gastroenterol. 2014;20(47):17804‐17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bockerstett KA, DiPaolo RJ. Regulation of gastric carcinogenesis by inflammatory cytokines. Cell Mol Gastroenterol Hepatol. 2017;4(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu X, Tao P, Zhou Q, et al. IL‐6 secreted by cancer‐associated fibroblasts promotes epithelial‐mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8(13):20741‐20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li P, Shan JX, Chen XH, et al. Epigenetic silencing of microRNA‐149 in cancer‐associated fibroblasts mediates prostaglandin E2/interleukin‐6 signaling in the tumor microenvironment. Cell Res. 2015;25(5):588‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karakasheva TA, Lin EW, Tang Q, et al. IL‐6 Mediates cross‐talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res. 2018;78(17):4957‐4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kinoshita H, Hirata Y, Nakagawa H, et al. Interleukin‐6 mediates epithelial‐stromal interactions and promotes gastric tumorigenesis. PLoS One. 2013;8(4):e60914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang XF, Zhou Q, Yu ZJ, et al. Cancer‐associated fibroblast‐derived Lumican promotes gastric cancer progression via the integrin beta 1‐FAK signaling pathway. Int J Cancer. 2017;141(5):998‐1010. [DOI] [PubMed] [Google Scholar]
- 20. Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28(6):800‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sigal M, Logan CY, Kapalczynska M, et al. Stromal R‐spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548(7668):451‐455. [DOI] [PubMed] [Google Scholar]
- 22. Sigal M, Reines MDM, Mullerke S, et al. R‐spondin‐3 induces secretory, antimicrobial Lgr5(+) cells in the stomach. Nat Cell Biol. 2019;21(7):812‐823. [DOI] [PubMed] [Google Scholar]
- 23. von Einem JC, Peter S, Gunther C, et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells ‐ TREAT‐ME‐1 ‐ a phase I, first in human, first in class trial. Oncotarget. 2017;8(46):80156‐80166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280(43):36099‐36109. [DOI] [PubMed] [Google Scholar]
- 25. Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1–2):269‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889‐896. [DOI] [PubMed] [Google Scholar]
- 27. Sade‐Feldman M, Kanterman J, Ish‐Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor‐alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38(3):541‐554. [DOI] [PubMed] [Google Scholar]
- 28. Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin‐1beta induces gastric inflammation and cancer and mobilizes myeloid‐derived suppressor cells in mice. Cancer Cell. 2008;14(5):408‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asfaha S, Dubeykovskiy AN, Tomita H, et al. Mice that express human interleukin‐8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144(1):155‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serizawa T, Hirata Y, Hayakawa Y, et al. Gastric metaplasia induced by helicobacter pylori is associated with enhanced SOX9 expression via interleukin‐1 signaling. Infect Immun. 2015;84(2):562‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee K, Hwang H, Nam KT. Immune response and the tumor microenvironment: how they communicate to regulate gastric cancer. Gut and liver. 2014;8(2):131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL‐33 to IL‐13 regulates metaplasia in the mouse stomach. Gut. 2018;67(5):805‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buzzelli JN, Chalinor HV, Pavlic DI, et al. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cellular and Molecular Gastroenterology and Hepatology. 2015;1(2):203‐221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakagawa H, Suzuki N, Hirata Y, et al. Biliary epithelial injury‐induced regenerative response by IL‐33 promotes cholangiocarcinogenesis from peribiliary glands. Proc Natl Acad Sci U S A. 2017;114(19):E3806‐E3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oishi S, Takano R, Tamura S, et al. M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T‐cell proliferation. Immunology. 2016;149(3):320‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamaguchi T, Fushida S, Yamamoto Y, et al. Tumor‐associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19(4):1052‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao G, Liu L, Peek RM Jr, et al. Activation of epidermal growth factor receptor in macrophages mediates feedback inhibition of M2 polarization and gastrointestinal tumor cell growth. J Biol Chem. 2016;291(39):20462‐20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song H, Wang T, Tian L, et al. Macrophages on the peritoneum are involved in gastric cancer peritoneal metastasis. Journal of Cancer. 2019;10(22):5377‐5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Veremeyko T, Yung AWY, Anthony DC, Strekalova T, Ponomarev ED. Early growth response Gene‐2 Is essential for M1 and M2 macrophage activation and plasticity by modulation of the transcription factor CEBPbeta. Front Immunol. 2018;9:2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang F, Parayath NN, Ene CI, et al. Genetic programming of macrophages to perform anti‐tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10(1):3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Okumura T, Ericksen RE, Takaishi S, et al. K‐ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010;70(21):8435‐8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kinoshita H, Hayakawa Y, Konishi M, et al. Three types of metaplasia model through Kras activation, Pten deletion, or Cdh1 deletion in the gastric epithelium. J Pathol. 2019;247(1):35‐47. [DOI] [PubMed] [Google Scholar]
- 43. Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX‐2/mPGES‐1 transgenic mice. EMBO J. 2004;23(7):1669‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaparakis M, Walduck AK, Price JD, et al. Macrophages are mediators of gastritis in acute Helicobacter pylori infection in C57BL/6 mice. Infect Immun. 2008;76(5):2235‐2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oshima H, Hioki K, Popivanova BK, et al. Prostaglandin E(2) signaling and bacterial infection recruit tumor‐promoting macrophages to mouse gastric tumors. Gastroenterology. 2011; 140(2):596‐607.e7. [DOI] [PubMed] [Google Scholar]
- 46. Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B. Goldenring JR. Macrophages promote progression of spasmolytic polypeptide‐expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146(7):1727‐1738.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oguma K, Oshima H, Aoki M, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor‐alpha in gastric tumour cells. EMBO J. 2008;27(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eissmann MF, Dijkstra C, Jarnicki A, et al. IL‐33‐mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat Commun. 2019;10(1):2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hardbower DM, Singh K, Asim M, et al. EGFR regulates macrophage activation and function in bacterial infection. J Clin Invest. 2016;126(9):3296‐3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krakowiak MS, Noto JM, Piazuelo MB, et al. Matrix metalloproteinase 7 restrains Helicobacter pylori‐induced gastric inflammation and premalignant lesions in the stomach by altering macrophage polarization. Oncogene. 2015;34(14):1865‐1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuroda T, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein‐1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res. 2005;11(21):7629‐7636. [DOI] [PubMed] [Google Scholar]
- 52. Stromnes IM, Brockenbrough JS, Izeradjene K, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63(11):1769‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2‐expressing myeloid‐derived suppressor cells are essential to promote colitis‐associated tumorigenesis. Cancer Cell. 2013;24(5):631‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baumann T, Dunkel A, Schmid C, et al. Regulatory myeloid cells paralyze T cells through cell‐cell transfer of the metabolite methylglyoxal. Nat Immunol. 2020;21(5):555‐566. [DOI] [PubMed] [Google Scholar]
- 55. Gabrilovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cortez‐Retamozo V, Etzrodt M, Newton A, et al. Origins of tumor‐associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109(7):2491‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dubeykovskaya Z, Si Y, Chen X, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 2016;7:10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Highfill SL, Cui Y, Giles AJ, et al. Disruption of CXCR2‐mediated MDSC tumor trafficking enhances anti‐PD1 efficacy. Sci Transl Med. 2014;6(237):237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ostrand‐Rosenberg S, Fenselau C. Myeloid‐derived suppressor cells: Immune‐suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol. 2018;200(2):422‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lv J, Zhao R, Wu D, et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. Journal of hematology & oncology. 2019;12(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soutto M, Chen Z, Bhat AA, et al. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat Commun. 2019;10(1):3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O'Reilly LA, Putoczki TL, Mielke LA, et al. Loss of NF‐kappaB1 causes gastric cancer with aberrant inflammation and expression of immune checkpoint regulators in a STAT‐1‐dependent manner. Immunity. 2018;48(3):570‐83 e8. [DOI] [PubMed] [Google Scholar]
- 64. Ju X, Shen R, Huang P, et al. Predictive relevance of PD‐L1 expression with pre‐existing TILs in gastric cancer. Oncotarget. 2017;8(59):99372‐99381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Amedei A, Della Bella C, Silvestri E, Prisco D, D'Elios MM. T cells in gastric cancer: friends or foes. Clin Dev Immunol. 2012;2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD‐1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449‐1458. [DOI] [PubMed] [Google Scholar]
- 67. Weygant N, Ge Y, Westphalen CB, Ma WW, Vega KJ. Role of the Microenvironment in Gastrointestinal Tumors. Journal of oncology. 2019;2019: :1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10(2):138‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lu J, Ye X, Fan F, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged‐1. Cancer Cell. 2013;23(2):171‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang R, Bhattacharya R, Ye X, Fan F, Boulbes DR, Ellis LM. Endothelial cells promote colorectal cancer cell survival by activating the HER3‐AKT pathway in a paracrine fashion. Mol Cancer Res. 2019;17(1):20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Z, Wang Z, Li G, et al. CXCL1 from tumor‐associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin beta1/FAK/AKT signaling. Cancer Lett. 2017;28(385):28‐38. [DOI] [PubMed] [Google Scholar]
- 72. Tokumoto MW, Tanaka H, Tauchi Y, et al. Identification of tumour‐reactive lymphatic endothelial cells capable of inducing progression of gastric cancer. Br J Cancer. 2015;113(7):1046‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tokumoto M, Tanaka H, Tauchi Y, et al. Immunoregulatory function of lymphatic endothelial cells in tumor‐draining lymph nodes of human gastric cancer. Anticancer Res. 2017;37(6):2875‐2883. [DOI] [PubMed] [Google Scholar]
- 74. Sakitani K, Hayakawa Y, Deng H, et al. CXCR4‐expressing Mist1(+) progenitors in the gastric antrum contribute to gastric cancer development. Oncotarget. 2017;8(67):111012‐111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hayakawa Y, Sethi N, Sepulveda AR, Bass AJ, Wang TC. Oesophageal adenocarcinoma and gastric cancer: should we mind the gap? Nat Rev Cancer. 2016;16(5):305‐318. [DOI] [PubMed] [Google Scholar]
- 76. Hayakawa Y, Fox JG, Wang TC. The origins of gastric cancer from gastric stem cells: Lessons from mouse models. Cell Mol Gastroenterol Hepatol. 2017;3(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hayakawa Y, Fox JG, Wang TC. Isthmus stem cells are the origins of metaplasia in the gastric corpus. Cell Mol Gastroenterol Hepatol. 2017;4(1):89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ericksen RE, Rose S, Westphalen CB, et al. Obesity accelerates Helicobacter felis‐induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut. 2014;63(3):385‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou J, Hayakawa Y, Wang TC, Bass AJ. RhoA mutations identified in diffuse gastric cancer. Cancer Cell. 2014;26(1):9‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain‐of‐function mutations of RHOA in diffuse‐type gastric carcinoma. Nat Genet. 2014;46(6):583‐587. [DOI] [PubMed] [Google Scholar]
- 81. Zhang H, Schaefer A, Wang Y, et al. Gain‐of‐function RHOA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. 2019;10(2):288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pitt LA, Tikhonova AN, Hu H, et al. CXCL12‐producing vascular endothelial niches control acute T cell leukemia maintenance. Cancer Cell. 2015;27(6):755‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110(14):E1291‐E1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mendez‐Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim HS, Won YJ, Shim JH, et al. Morphological characteristics of vasculogenic mimicry and its correlation with EphA2 expression in gastric adenocarcinoma. Sci Rep. 2019;9(1):3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang P, Hu Y, Zhou Q. The CXCL12‐CXCR4 signaling axis plays a key role in cancer metastasis and is a potential target for developing novel therapeutics against metastatic cancer. Curr Med Chem. 2019. [DOI] [PubMed] [Google Scholar]
- 88. Kim M, Koh YJ, Kim KE, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70(24):10411‐10421. [DOI] [PubMed] [Google Scholar]
- 89. Liu Y, Cao X. Characteristics and significance of the pre‐metastatic niche. Cancer Cell. 2016;30(5):668‐681. [DOI] [PubMed] [Google Scholar]
- 90. Goveia J, Rohlenova K, Taverna F, et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell. 2020;37(1):21‐36.e13 [DOI] [PubMed] [Google Scholar]
- 91. Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24(8):1277‐1289. [DOI] [PubMed] [Google Scholar]
- 92. Zhao Q, Eichten A, Parveen A, et al. Single‐cell transcriptome analyses reveal endothelial cell heterogeneity in tumors and changes following antiangiogenic treatment. Cancer Res. 2018;78(9):2370‐2382. [DOI] [PubMed] [Google Scholar]
- 93. Hayakawa Y, Chang W, Jin G, Wang TC. Gastrin and upper GI cancers. Curr Opin Pharmacol. 2016;31:31‐37. [DOI] [PubMed] [Google Scholar]
- 94. Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118(1):36‐47. [DOI] [PubMed] [Google Scholar]
- 95. Cui G, Takaishi S, Ai W, et al. Gastrin‐induced apoptosis contributes to carcinogenesis in the stomach. Lab Invest. 2006;86(10):1037‐1051. [DOI] [PubMed] [Google Scholar]
- 96. Zavros Y, Eaton KA, Kang W, et al. Chronic gastritis in the hypochlorhydric gastrin‐deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24(14):2354‐2366. [DOI] [PubMed] [Google Scholar]
- 97. Tomita H, Takaishi S, Menheniott TR, et al. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140(3):879‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Takaishi S, Tu S, Dubeykovskaya ZA, et al. Gastrin is an essential cofactor for helicobacter‐associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol. 2009;175(1):365‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hayakawa Y, Jin G, Wang H, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64(4):544‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nakajima T, Konda Y, Izumi Y, et al. Gastrin stimulates the growth of gastric pit cell precursors by inducing its own receptors. Am J Physiol Gastrointest Liver Physiol. 2002;282(2):G359‐G366. [DOI] [PubMed] [Google Scholar]
- 101. Sheng W, Malagola E, Nienhuser H, et al. Hypergastrinemia expands gastric ECL cells through CCK2R+ progenitor cells via ERK activation. Cell Mol Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Niikura R, Hayakawa Y, Hirata Y, Yamada A, Fujishiro M, Koike K. Long‐term proton pump inhibitor use is a risk factor of gastric cancer after treatment for Helicobacter pylori: a retrospective cohort analysis. Gut. 2018;67(10):1908‐1910. [DOI] [PubMed] [Google Scholar]
- 103. Lee Y, Urbanska AM, Hayakawa Y, et al. Gastrin stimulates a cholecystokinin‐2‐receptor‐expressing cardia progenitor cell and promotes progression of Barrett's‐like esophagus. Oncotarget. 2017;8(1):203‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hata M, Hayakawa Y, Koike K. Gastric Stem Cell and Cellular Origin of Cancer. Biomedicines. 2018;6(4):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chang W, Wang H, Kim W, et al. Hormonal suppression of stem cells inhibits symmetric cell division and gastric tumorigenesis. Cell Stem Cell. 2020;26(5):739‐754.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hata M, Kinoshita H, Hayakawa Y, et al. GPR30‐expressing gastric chief cells do not dedifferentiate but are eliminated via pdk‐dependent cell competition during development of metaplasia. Gastroenterology. 2020;158(6):1650‐1666.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hayakawa Y, Tsuboi M, Asfaha S, et al. BHLHA15‐positive secretory precursor cells can give rise to tumors in intestine and colon in mice. Gastroenterology. 2019;156(4):1066‐1081.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kinoshita H, Hayakawa Y, Niu Z, et al. Mature gastric chief cells are not required for the development of metaplasia. Am J Physiol Gastrointest Liver Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Renz BW, Takahashi R, Tanaka T, et al. beta2 adrenergic‐neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33(1):75‐90 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Middelhoff M, Westphalen CB, Hayakawa Y, et al. Dclk1‐expressing tuft cells: critical modulators of the intestinal niche? Am J Physiol Gastrointest Liver Physiol. 2017;313(4):G285‐G299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hayakawa Y, Wang TC. Nerves switch on angiogenic metabolism. Science. 2017;358(6361):305‐306. [DOI] [PubMed] [Google Scholar]
- 112. Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31(1):21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rabben HL, Zhao CM, Hayakawa Y, Wang TC, Chen D. Vagotomy and gastric tumorigenesis. Curr Neuropharmacol. 2016;14(8):967‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Konishi M, Hayakawa Y, Koike K. Role of Muscarinic Acetylcholine Signaling in Gastrointestinal Cancers. Biomedicines. 2019;7(3):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Renz BW, Tanaka T, Sunagawa M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8(11):1458‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hayakawa Y, Fox J, Gonda T, Worthley D, Muthupalani S, Wang T. Mouse models of gastric cancer. Cancers. 2013;5(1):92‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kinoshita H, Hayakawa Y, Koike K. Metaplasia in the stomach—precursor of gastric cancer? Int J Mol Sci. 2017;18(10):2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chung HW, Lim JB. Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J Gastroenterol. 2014;20(7):1667‐1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Oshima H, Hioki K, Popivanova BK, et al. Prostaglandin E₂ signaling and bacterial infection recruit tumor‐promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140(2):596‐607.e7. [DOI] [PubMed] [Google Scholar]


