Supplemental Digital Content is available in the text.
Keywords: coronavirus; inflammation; interleukin-1; interleukin-6; pneumonia, viral; sepsis
Objectives:
The causative agent for coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2, appears exceptional in its virulence and immunopathology. In some patients, the resulting hyperinflammation resembles a cytokine release syndrome. Our knowledge of the immunopathogenesis of coronavirus disease 2019 is evolving and anti-cytokine therapies are under active investigation. This narrative review summarizes existing knowledge of the immune response to coronavirus infection and highlights the current and potential future roles of therapeutic strategies to combat the hyperinflammatory response of patients with coronavirus disease 2019.
Data Sources:
Relevant and up-to-date literature, media reports, and author experiences were included from Medline, national newspapers, and public clinical trial databases.
Study Selection:
The authors selected studies for inclusion by consensus.
Data Extraction:
The authors reviewed each study and selected approrpriate data for inclusion through consensus.
Data Synthesis:
Hyperinflammation, reminiscent of cytokine release syndromes such as macrophage activation syndrome and hemophagocytic lymphohistiocytosis, appears to drive outcomes among adults with severe coronavirus disease 2019. Cytokines, particularly interleukin-1 and interleukin-6, appear to contribute importantly to such systemic hyperinflammation. Ongoing clinical trials will determine the efficacy and safety of anti-cytokine therapies in coronavirus disease 2019. In the interim, anti-cytokine therapies may provide a treatment option for adults with severe coronavirus disease 2019 unresponsive to standard critical care management, including ventilation.
Conclusions:
This review provides an overview of the current understanding of the immunopathogenesis of coronavirus disease 2019 in adults and proposes treatment considerations for anti-cytokine therapy use in adults with severe disease.
The coronavirus disease 2019 (COVID-19) pandemic has brought the manifold consequences of inflammation into sharp focus for the medical and lay communities (1). While our understanding of the pathobiology of COVID-19 remains incomplete, one hypothesis proposes that the most severe complications of infection with this virulent virus arise from overzealous innate immune responses, akin to other viral sepsis syndromes (2, 3). Although we have just begun to approach rigorous and data-driven understanding of this complex disease, clinical management decisions depend on the most recent published evidence, despite its incomplete and nascent nature. This review lays out the current state of knowledge regarding the role of cytokine biology in COVID-19 and how this background informs the potential use of anti-cytokine therapies to combat complications of severe COVID-19.
IMMUNOPATHOLOGY OF SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2
The causative agent of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), enters the upper respiratory tract primarily through airborne droplets (4, 5). Ubiquitous expression of angiotensin-converting enzyme 2 (ACE2), the cellular receptor for SARS-CoV-2, provides a fertile cellular environment for SARS-CoV-2 replication in the nose, throat, and lungs (6–8). The receptor-binding domain of the SARS-CoV-2 spike protein possesses greater affinity for ACE2 than that of SARS-CoV-1, the causative agent of the 2002–2003 SARS pandemic (9, 10).
After approximately 1 week of mild fever, cough, fatigue, anorexia, and myalgia, a subset of infected individuals develops dyspnea and hypoxemia that herald progression to severe COVID-19 with potential for rapid decompensation and acute respiratory distress syndrome (ARDS). This complication, particularly when it requires mechanical ventilation, often proves fatal (11–14). Other tissues that express ACE2, such as the heart (15, 16), have proven vulnerable to SARS-CoV-2 and SARS-CoV-1 (17–20).
Progression to severe COVID-19 coincides with increasing levels of inflammatory biomarkers (Fig. 1). Anecdotal experience suggests that patients decompensate more suddenly and rapidly than expected based upon experience with other viral pneumonias. In comparison to other coronaviruses and respiratory viruses, SARS-CoV-2 induces a weak type I, II, and III interferon response and strong activation of the interleukin (IL)–1/IL-6 pathway (21–24). SARS-CoV-1 and Middle East respiratory syndrome coronavirus produce interferon antagonist proteins that may explain the impaired interferon response observed in SARS-CoV-2 (25). SARS-CoV-2 may activate directly pro-inflammatory pathways through tumor necrosis factor α-converting enzyme (TACE or a disintegrin and metalloproteinase 17, ADAM17) and loss of ACE2’s counter-regulatory function (26, 27).
Figure 1.
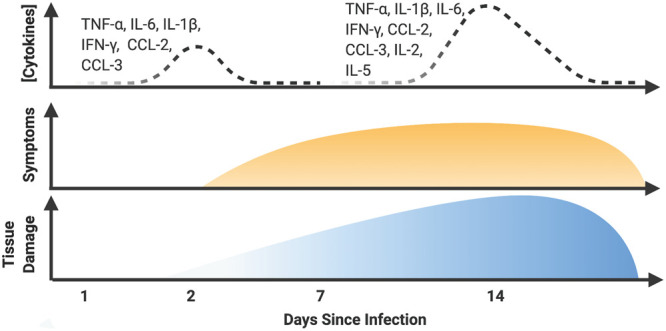
Infiltration of key immune cells and activity of key cytokines in coronavirus disease 2019 (COVID-19). This hypothetical diagram portrays how the second wave of inflammatory activity may be a major determinant of outcome in COVID-19. This second wave features both innate and adaptive cytokines. This diffuse cytokine release syndrome damages not only the lungs but also the heart, kidneys, and other organs. Identification of patients at risk for a cytokine release syndrome and prompt treatment with direct and selective inhibitors of the inflammasome, interleukin (IL)–6, or IL-1 (β or α) may prevent severe organ damage. IFN-γ = interferon gamma, TNF-α = tumor necrosis factor-α.
The exuberant IL-1/IL-6 response to SARS-CoV-2 appears to contribute importantly to patient symptomology and outcomes (Fig. 2). In the lung, coronavirus infection of type II alveolar epithelial cells activates the inflammasome, a multiprotein complex that produces mature IL-1β (28), as well as mature IL-18 (another pro-inflammatory cytokine) and N-terminal gasdermin-D (the pore-forming protein that permits release of IL-1β from cells) (29–31). After maturation, IL-1β induces chemokine secretion and adhesion molecule expression (32, 33). IL-1β amplifies the inflammatory response by inducing endothelial cell and vascular smooth muscle cell secretion of IL-6, which can activate a broad array of cell types (34, 35). An auto-induction amplification loop whereby IL-1β induces IL-1β secretion perpetuates these actions (36–38).
Figure 2.
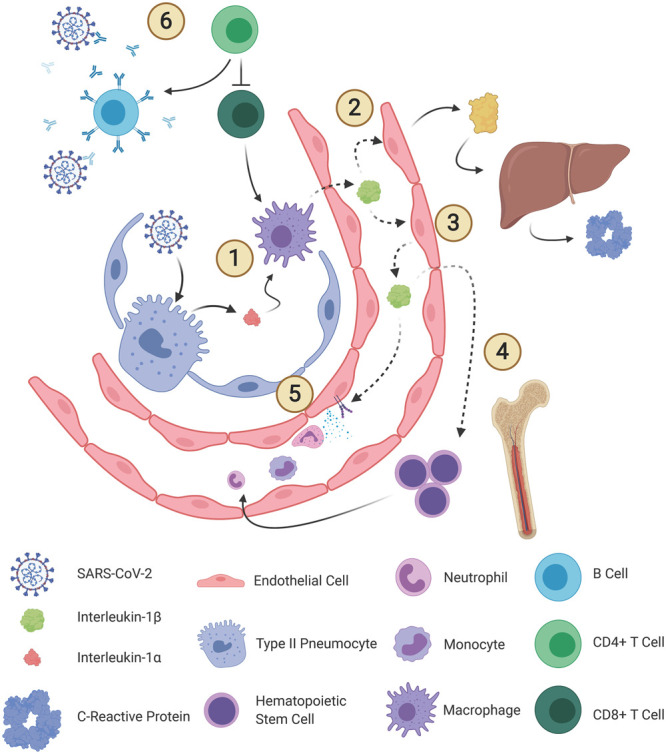
Roles of interleukin (IL)–1β in coronavirus disease 2019. IL-1β is a primordial pro-inflammatory cytokine that plays multiple roles in innate immunity (1). After detecting damage-associated molecular patterns released from type 2 pneumocytes, sentinel immune cells, such as alveolar macrophages, activate IL-1β through the inflammasome (1). IL-1β then exerts pleiotropic paracrine and endocrine effects. IL-1β promotes secretion of IL-6 (2) as well as IL-1β (3) from endothelial cells. IL-6 initiates hepatic production of acute phase reactants, among many inflammatory actions. IL-1β initiates hematopoietic progenitor cell proliferation (4) and facilitates infiltration of neutrophils and monocytes by upregulating adhesion molecule expression and chemokine secretion (5). Exhausted CD4+ T cells can fail to execute antibody-mediated viral clearance, which allows a second, more powerful, and destructive wave of cytokine activity (6). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immune evasion tactics also impair the immune response. CCL = chemokine ligand.
IL-1β expression and activity in patients with COVID-19 exceed that of healthy controls (39, 40). IL-6 and C-reactive protein, both downstream of IL-1β, can serve as biomarkers of IL-1 activity. Patients with severe COVID-19 exhibit greater elevations in IL-6 and C-reactive protein than those with moderate COVID-19 (41, 42). Higher IL-6 levels predict both ARDS occurrence and death in adults with COVID-19 (43). While IL-6 concentrations begin to rise approximately 2 weeks after illness onset in COVID-19 nonsurvivors, they remain stable in COVID-19 survivors (44). This innate immune response has been compared to chimeric antigen receptor T cell-induced cytokine release syndrome, secondary hemophagocytic lymphohistiocytosis, and macrophage activation syndrome (45).
An intriguing finding among patients with COVID-19 is early depletion and late functional exhaustion of CD4+ T cells (40, 44, 46–48), potentially due to direct viral infection via CD147 (49–51) or migration of T cells to the lungs. Since CD4+ T cells regulate the innate immune response, their depletion may promote a second wave of cytokine release and pulmonary immune cell infiltration (52). Indeed, lower lymphocyte and interferon-γ expressing CD4+ T cell counts portend worse outcomes in COVID-19 (44, 48). Helper (CD3+CD4+), suppressor (CD3+CD8+), and regulatory (CD3+CD4+CD25+CD127low+) T cell counts may be lower in severe COVID-19 cases than in moderate cases (39, 42). Concentrations of IL-10, an anti-inflammatory cytokine, also are higher in severe COVID-19 cases, likely representing a counter-regulatory response (39). The mechanisms of inflammation resolution in COVID-19 warrant further research (53).
IL-1 BLOCKERS IN COVID-19
Pharmacology of IL-1 Blockers
Three distinct pharmacologic options can interrupt IL-1 activity (Fig. 3) (54).
Figure 3.
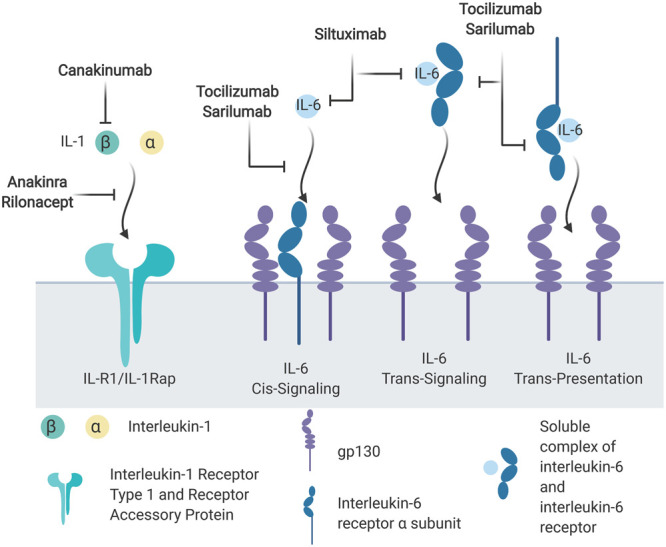
Pharmacologic interleukin (IL)–1 and IL-6 inhibitors. IL-1β and IL-1α initiate intracellular signaling by binding the IL-1 receptor (IL-1R) type 1, which recruits the IL-1 receptor accessory protein (IL-1Rap). IL-6 binds to the IL-6 receptor α as well as two glycoprotein (gp) 130 receptors. Three possible scenarios lead to IL-6 signaling. IL-6 binds cell surface IL-6 receptor and gp130 in cis signaling. Trans signaling occurs when soluble IL-6/IL-6 receptor complex binds membrane-associated gp130. Last, plasmacytoid dendritic cells can present IL-6/IL-6 receptor to gp130 on T helper cell (Th) 17 cells.
Although anakinra, canakinumab, and rilonacept are approved for subcutaneous administration (Table 1; and Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A262), the IV route may be preferred in critically ill patients based upon experience with anakinra in patients with severe sepsis and hemophagocytic lymphohistiocytosis syndrome (55). Canakinumab and rilonacept have longer half-lives that allow for expanded dosing intervals, but the shorter half-life of anakinra allows for more rapid clearance of the drug upon discontinuation (56, 57).
Table 1.
Potential Anti-Cytokine Therapies for Coronavirus Disease 2019
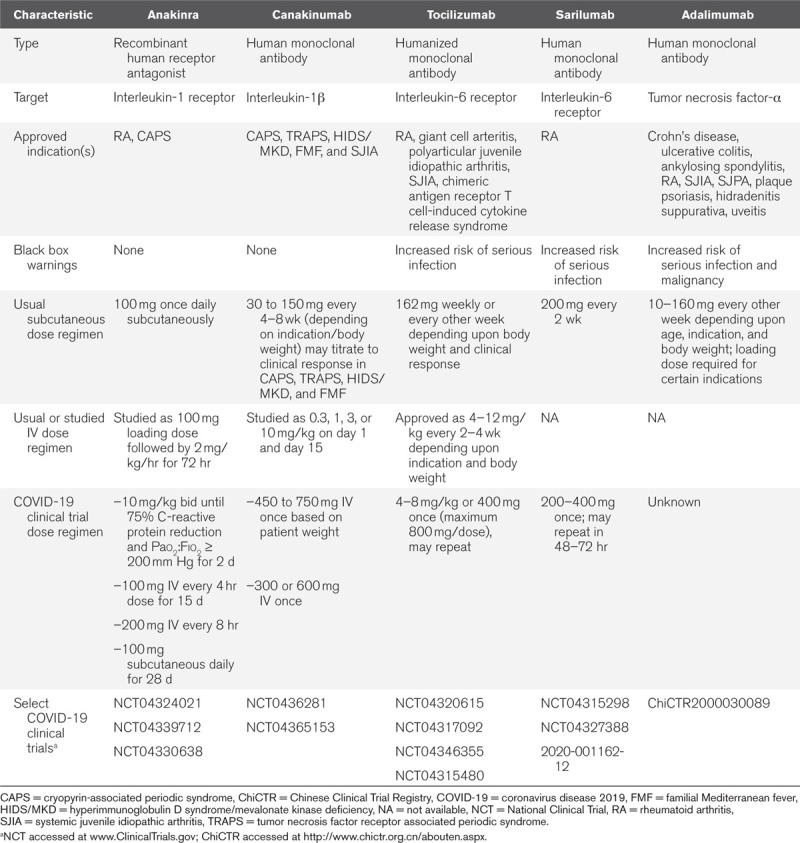
Organ dysfunction occurs commonly in critically ill adults with COVID-19. Anakinra and rilonacept have been used in patients with end-stage renal disease on hemodialysis in patients without COVID-19 (58, 59). Renal dysfunction has minimal effects on the pharmacokinetics of monoclonal antibodies such as canakinumab (60). Dose adjustments are not recommended for anakinra, rilonacept, or canakinumab in patients with hepatic dysfunction. None of the IL-1 blockers appear to cause hepatic toxicity.
Efficacy and Safety of IL-1 Blockade
Evidence to support the efficacy of IL-1 blockade in COVID-19 includes two case series and extrapolation of human and experimental studies of other cytokine release syndromes, including notable data from severe sepsis clinical trials. Several randomized clinical trials with anakinra and canakinumab are underway (Table 1; and Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A262). Cavalli et al (61) reported that 21 of 29 patients (72%) with COVID-19 who received IV anakinra (5 mg/kg bid for a median of 9 d) experienced clinical improvement, defined as a 75% or greater reduction in serum C-reactive protein concentration and a Pao2:Fio2 ratio of greater than 200 mm Hg for 48 hours and 26 of the 29 survived (90%) through day 21 (only 56% of a historical control group survived to day 21). A series of eight patients who received IV anakinra (200 mg every 8 hr for 7 d or 300 mg daily for 7 d followed by 100 mg once daily), showed no significant changes in C-reactive protein concentration, but the Pao2:Fio2 ratio increased (62).At the end of follow-up, three patients were dead, four remained on mechanical ventilation, and one patient who did not require ICU admission was discharged alive. Similarly, a case series of five patients with COVID-19 reported that C-reactive protein and temperature declined while Pao2:Fio2 ratio increased after anakinra 100 mg IV every 8 hours and a retrospective analysis of 10 patients who received canakinumab 300 mg subcutaneously demonstrated a more rapid reduction in C-reactive protein levels and a faster improvement in Pao2:Fio2 ratio when compared to a historical control group (63, 64).
A separate case series reported the efficacy and safety of anakinra 100 mg bid for 72 hours followed by 100 mg daily for 7 days (100 mg daily for 72 hr followed by 100 mg every other day for 7 d if creatinine clearance < 30 mL/min or on dialysis) in patients hospitalized with COVID-19 who did not require ICU admission compared to a historical control group (65). After multivariate adjustment, anakinra-treated patients had a significantly lower risk of death or mechanical ventilation than usual care historical controls (hazard ratio, 0.22; 95% CI, 0.10–0.49). Anakinra significantly reduced C-reactive protein within 48–72 hours.
Two landmark clinical trials investigated the efficacy and safety of anakinra in patients with severe sepsis not due to SARS-CoV-2 (66–68). The isolated organisms were most frequently bacteria and fungi. All participants received background antimicrobial therapy. While anakinra did not reduce mortality in these studies, this short-term (up to 72 hr), high-dose anakinra regimens did not increase the risk of bacterial superinfection, a concern in patients with COVID-19. A post hoc analysis found that anakinra reduced mortality in the subgroup of patients with hepatobiliary dysfunction and disseminated intravascular coagulation, two features consistent with macrophage activation syndrome (69). Anakinra appears effective in patients with hemophagocytic lymphohistiocytosis syndrome and in animals with chimeric antigen receptor T cell-induced cytokine release syndrome (55, 70, 71). Anakinra also demonstrates safety and efficacy in a range of acute inflammatory cardiovascular conditions (72).
IL-6 BLOCKERS IN COVID-19
Pharmacology of IL-6 Blockers
IL-6’s three distinct signaling pathways render targeting its activity complex (Fig. 3) and thus the three approved IL-6 monoclonal antibodies differ in their pharmacological effects on the IL-6 pathway (Table 1; and Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A262) (35, 73, 74).
Tocilizumab should be given IV, as the cytokine release syndrome appears to accelerate tocilizumab clearance (75). Sarilumab and siltuximab have delayed onset of action when administered subcutaneously (76, 77). Renal impairment dose adjustments are not required for any of the IL-6 blockers. The prescribing information for tocilizumab, sarilumab, and siltuximab recommend treatment interruptions or discontinuations for elevated hepatic transaminase concentrations or decreases in neutrophil or platelet counts.
Potential serious adverse effects of tocilizumab and sarilumab include elevations of hepatic transaminases, increases in serum cholesterol and triglyceride concentrations, and opportunistic infection. Tocilizumab increased low-density lipoprotein cholesterol levels by 11% and triglyceride levels by 14% at 4 weeks in adults with rheumatoid arthritis (78). Two case reports illustrate the potential for tocilizumab to contribute to acute pancreatitis (79). The long half-life of these agents (30–40 hr and 8–10 d for IV tocilizumab and subcutaneous sarilumab, respectively) may constitute a disadvantage if adverse effects do occur. IL-6 blockade restores cytochrome P450 activity; therefore, doses of concomitant therapies with narrow therapeutic windows metabolized by cytochrome P450 substrates require close monitoring. Because IL-6 concentrations increase after tocilizumab treatment due to circulation of the cytokine-antibody complex, IL-6 cannot serve as a treatment response biomarker (80).
Efficacy and Safety of IL-6 Blockade
There are several ongoing studies with the different IL-6 receptor blockers in patients with COVID-19 (Table 1; and Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A262). Two trials have reported preliminary or interim results. One announcement reported that tocilizumab met its primary outcome of death or need for mechanical ventilation in a randomized trial of 129 patients with moderate-severe COVID-19. Detailed results of this clinical trial remain unreported (81). A press release indicates that an ongoing sarilumab adaptive clinical trial will proceed with enrollment of only severe or critical patients with COVID-19 based upon analysis of initial results (81). Tocilizumab has an approved indication for chimeric antigen receptor T cell-induced cytokine release syndrome (75). There are no studies of IL-6 blockers in patients with severe sepsis due to non-COVID-19 causes.
Observational studies suggest that tocilizumab rapidly decreases fever, reduces systemic inflammation over 5–7 days and associates with improved indices of oxygenation within 48–72 hours, and decreased risk of intubation or mortality (80, 82–87). For example, a case series of 20 patients who received tocilizumab for COVID-19 reported that C-reactive protein concentration decreased to less than 2 mg/L within 5 days, oxygen requirements decreased, and all patients survived to hospital discharge (82). Additionally, variation in background antiviral and immunosuppressive therapies across studies and across patients within studies further argues for cautious interpretation of these results. Nevertheless, clinicians must make treatment decisions using clinical judgment and these limited data until completion and full reporting of COVID-19 clinical trials.
POTENTIAL ROLE OF OTHER ANTI-CYTOKINE THERAPIES IN COVID-19
SARS-CoV-1, and presumably SARS-CoV-2, activate TACE/ADAM17 during the process of gaining host cell entry and can increase circulating tumor necrosis factor-α levels (26, 27, 88). The commercially available tumor necrosis factor-α inhibitors include adalimumab (human monoclonal antibody), etanercept (fusion protein), infliximab (chimeric monoclonal antibody), golimumab (human monoclonal antibody), and certolizumab pegol (humanized fragment antigen binding fragment) (Table 1). Like IL-1 blockade, tumor necrosis factor-α antagonism does not appear to increase the risk of secondary infection in patients with sepsis receiving background antimicrobial therapy (89, 90). One ongoing clinical trial is investigating tumor necrosis factor blockade and others have called for research on this therapeutic in COVID-19 (91). Biosimilar tumor necrosis factor-α blockers have become available (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A262).
Glucocorticoids, given their broad-spectrum immune-suppressing effects, have seen wide use in patients with severe COVID-19 for the treatment of cytokine release syndrome and ARDS. Although the Surviving Sepsis Campaign suggests use of short courses of glucocorticoids for moderate-severe ARDS related to COVID-19, evidence supporting their benefit is lacking in this population and concerns that their use may prolong viral shedding persist (92, 93). Preliminary results from the Randomised Evaluation of COVID-19 Therapy trial reported that dexamethasone 6 mg daily for up to 10 days significantly reduced all-cause mortality in patients hospitalized for COVID-19. Subgroup analysis suggested significantly greater reductions in mortality for dexamethasone in patients receiving invasive mechanical ventilation (29.0% vs 40.7%; p < 0.001) and those receiving oxygen without invasive mechanical ventilation (21.5% vs 25.0%; p = 0.002). All-cause mortality rates for dexamethasone in patients not receiving invasive mechanical ventilation or oxygen was 17.0% versus 13.2% for usual care.
SECONDARY AND OPPORTUNISTIC INFECTIONS IN COVID-19
In the advanced stages of COVID-19 disease, immune exhaustion and inhibition of usual defense mechanisms such as ciliary clearance can favor bacterial superinfection as in other severe viral pneumonitides. Anti-cytokine treatments may impair further host defenses and/or delay the recognition of infection. The absolute risk associated with short-term treatment may be acceptable in patients with life-threatening cytokine release syndrome.
IL-1 blockade has a favorable safety profile as demonstrated by the minimal increase in risk of fatal and lack of opportunistic infection associated with long-term canakinumab treatment (94). Chronic IL-6 and tumor necrosis factor-α blockade predispose to opportunistic infection, and all IL-6 and tumor necrosis factor blockers carry a black box warning for serious infection. In patients with bacterial or fungal severe sepsis on background antimicrobial therapy, short-term use of IL-1 or tumor necrosis factor-α blockers does not increase the risk of infection. Clinicians should consider testing for latent tuberculosis, hepatitis B, and hepatitis C during hospital admission in patients who receive an IL-6 or tumor necrosis factor-α blocker.
THERAPEUTIC CONSIDERATIONS FOR ANTI-CYTOKINE THERAPIES IN COVID-19
Current treatment for severe COVID-19 includes supportive respiratory and hemodynamic care. No agent has received approval from the U.S. Food and Drug Administration for the treatment of severe COVID-19, but randomized trials of many therapeutic candidates are ongoing (95). While the optimal COVID-19 treatment would be an effective antiviral intervention, the high mortality of hospitalized patients with COVID-19 complications mandate adjunctive therapies as well (96).
Important unanswered questions include the target population for use (including the severity of COVID-19, age, comorbidities, the underlying immunologic profile [i.e., chemokine concentrations, immune cell function, inflammation resolution, and anti-inflammatory mediators], the presence of chronic or acute organ dysfunction), the optimal time to initiate therapy (asymptomatic, mild, or severe), the optimal dose and duration (related to the disease severity), the optimal biomarkers and clinical indicators of response, the use of concomitant agents (some of which may have immunemodulating effects), and the prevalence and risk factors for safety concerns. The heterogeneity of the sepsis syndrome poses a further barrier to implementation of anti-cytokine therapies in COVID-19 (97).
Indeed, improving outcomes in the heterogeneous population of adults with severe sepsis remains a challenge (98, 99). The specific risks and benefits of each anti-cytokine agent must be thoughtfully considered within the context of particular patients and diverse populations. Furthermore, clinicians and investigators should continue to explore strategies beyond cytokine blockade, such as immune stimulation with checkpoint inhibitors to promote viral clearance given the profound lymphopenia prevalent among patients with severe COVID-19, although these agents have their own toxicities.
Both the National Institutes of Health and the Surviving Sepsis Campaign concluded that current evidence is insufficient to issue recommendations related to the use of anti-cytokine therapies in COVID-19 (92, 100). Several institutions have made their own treatment protocols available publicly (101–104). Research is needed to identify patient subgroups with differential therapeutic responses to anti-cytokine therapies (69, 97).
Anti-cytokine therapies may offer an important treatment option in COVID-19. Of considerable concern, SARS-CoV-2 may cycle through the population, and we must prepare for recurring waves of involvement. Such resurgence may well occur before the development and testing of a vaccine. Furthermore, even if a vaccine were available, one cannot assume that COVID-19 will not mutate rendering a vaccination approach incompletely protective. Last, the elderly and those with cardiometabolic risk factors who have high susceptibility to severe COVID-19 generally mount weaker responses to vaccination than younger individuals.
CONCLUSIONS
Our understanding of the immunopathogenesis of COVID-19 in adults has evolved rapidly. For COVID-19 adults with a cytokine release syndrome clinical picture, clinicians must currently rely on anecdotes and observational studies to guide treatment decisions regarding anti-cytokine therapies. Prospective randomized trials evaluating a number of different anti-cytokine therapies in adults with COVID-19 are underway. New evidence will continue to inform clinicians about the role for anti-cytokine therapy in critically ill adults with COVID-19.
Supplementary Material
Footnotes
Dr. Buckley is receiving research funding from the American College of Clinical Pharmacy Foundation and the National Institutes of Health (K23HL150311). Dr. Van Tassell is receiving research support from Novartis, Regeneron, and Kiniksa for research related to coronavirus disease 2019. Dr. Abbate is receiving research support from Novartis, Olatec, and Swedish Orphan Biovitrum. Dr. Devlin is receiving research funding from the National Institute of Aging; National Heart, Lung, and Blood Institute; and the Canadian Institute of Health Research; he is on the editorial board of Critical Care Medicine. Dr. Libby receives funding support from the National Heart, Lung, and Blood Institute (R01HL080472 and 1R01HL134892); the American Heart Association (18CSA34080399); and the RRM Charitable Fund. Dr. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech. Dr. Libby is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM Therapeutics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech. Dr. Libby serves on the board of XBiotech. Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis. Dr. Libby has a financial interest in Xbiotech, a company developing therapeutic human antibodies. Dr. Libby’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Mehta P, McAuley DF, Brown M, et al. Correspondence COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 6736:19–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 3.Darden DB, Hawkins RB, Larson SD, et al. The clinical presentation and immunology of viral pneumonia and implications for management of coronavirus disease 2019. Crit Care Explor. 2020; 2:e0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anfinrud P, Stadnytskyi V, Bax CE, et al. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med. 2020; 382:2061–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020; 382:1564–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamming I, Timens W, Bulthuis ML. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004; 203:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020; 367:1444–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor article SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020; 581:221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020; 17:613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8:420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020; 323:1775–1776 [DOI] [PubMed] [Google Scholar]
- 13.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020; 323:1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020; 323:1488–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicin L, Abplanalp WT, Mellentin H, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020; 41:1804–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular Res. 2020; 116:1097–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020; 5:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac Involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5:819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020; 22:911–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020; 181:1036–1045.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020; 55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011; 117:3720–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong EZ, Chan YFZ, Leong WY, et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020; 27:879–882.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017; 39:529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008; 105:7809–7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010; 84:1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bochner BS, Landy SD, Plaut M, et al. Interleukin 1 production by human lung tissue. I. Identification and characterization. J Immunol. 1987; 139:2297–2302 [PubMed] [Google Scholar]
- 29.Zalinger ZB, Elliott R, Weiss SR. Role of the inflammasome-related cytokines Il-1 and Il-18 during infection with murine coronavirus. J Neurovirol. 2017; 23:845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen IY, Moriyama M, Chang MF, et al. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019; 10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM, et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015; 485:330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplanski G, Farnarier C, Kaplanski S, et al. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994; 84:4242–4248 [PubMed] [Google Scholar]
- 33.Bevilacqua MP, Pober JS, Mendrick DL, et al. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987; 84:9238–9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loppnow H, Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990; 85:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heink S, Yogev N, Garbers C, et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol. 2017; 18:74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner SJ, Auger KR, Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987; 139:1911–1917 [PubMed] [Google Scholar]
- 37.Warner SJ, Auger KR, Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987; 165:1316–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinarello CA, Ikejima T, Warner SJ, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987; 139:1902–1910 [PubMed] [Google Scholar]
- 39.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020; 130:2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen W, Su W, Tang H, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020; 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46:846–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016; 6:664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020; 17:533–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020; 17:541–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Xu W, Hu G, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 20201–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016; 19:181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narayanan K, Huang C, Lokugamage K, et al. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J Virol. 2008; 82:4471–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010; 84:1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panigrahy D, Gilligan MM, Huang S, et al. Inflammation resolution: A dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020; 39:337–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: From bench to bedside. Biodrugs. 2018; 32:111–118 [DOI] [PubMed] [Google Scholar]
- 55.Mehta P, Cron RQ, Hartwell J, et al. Silencing the cytokine storm: The use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020; 2:e358-e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alten R, Gram H, Joosten LA, et al. The human anti-IL-1 beta monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Res Ther. 2008; 10:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noe A, Howard C, Thuren T, et al. Pharmacokinetic and pharmacodynamic characteristics of single-dose Canakinumab in patients with type 2 diabetes mellitus. Clin Ther. 2014; 36:1625–1637 [DOI] [PubMed] [Google Scholar]
- 58.Nowak KL, Hung A, Ikizler TA, et al. Interleukin-1 inhibition, chronic kidney disease-mineral and bone disorder, and physical function. Clin Nephrol. 2017; 88:132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung AM, Ellis CD, Shintani A, et al. IL-1 receptor antagonist reduces inflammation in hemodialysis patients. J Am Society Nephrol. 2011; 22:437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foltz IN, Karow M, Wasserman SM. Evolution and emergence of therapeutic monoclonal antibodies: What cardiologists need to know. Circulation. 2013; 127:2222–2230 [DOI] [PubMed] [Google Scholar]
- 61.Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020; 2:e325–e331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimopoulos G, de Mast Q, Markou N, et al. Favorable anakinra responses in severe Covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020; 28:117–123.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pontali E, Volpi S, Antonucci G, et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020; 146:213–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ucciferri C, Auricchio A, Di Nicola M, et al. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020; 2:e457–ee458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: A cohort study. Lancet Rheumatol. 2020; 2:e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fisher CJ, Jr, Slotman GJ, Opal SM, et al. ; IL-1RA Sepsis Syndrome Study Group. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: A randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994; 22:12–21 [DOI] [PubMed] [Google Scholar]
- 67.Fisher CJ, Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994; 271:1836–1843 [PubMed] [Google Scholar]
- 68.Opal SM, Fisher CJ, Jr, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997; 25:1115–1124 [DOI] [PubMed] [Google Scholar]
- 69.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: Reanalysis of a prior phase III trial. Crit Care Med. 2016; 44:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018; 24:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018; 24:739–748 [DOI] [PubMed] [Google Scholar]
- 72.Abbate A, Toldo S, Marchetti C, et al. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020; 126:1260–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garbers C, Heink S, Korn T, et al. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018; 17:395–412 [DOI] [PubMed] [Google Scholar]
- 74.Cervantes-Barragan L, Züst R, Weber F, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007; 109:1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le RQ, Li L, Yuan W, et al. FDA approval summary: Tocilizumab for treatment of chimeric antigen receptor t cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018; 23:943–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Georgy A, Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther. 2013; 51:443–455 [DOI] [PubMed] [Google Scholar]
- 77.Xu C, Su Y, Paccaly A, et al. Population pharmacokinetics of sarilumab in patients with rheumatoid arthritis. Clin Pharmacokinet. 2019; 58:1455–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: A randomized controlled trial. Arthritis Rheumatol. 2020; 72:31–40 [DOI] [PubMed] [Google Scholar]
- 79.Morrison AR, Johnson JM, Ramesh M, et al. Letter to the editor: Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J Med Virol. 2020 Apr 21. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sciascia S, Aprà F, Baffa A, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020; 38:529–532 [PubMed] [Google Scholar]
- 81.Sax P. NEJM Journal Watch - HIV and ID Observations. 2020 Available at: https://blogs.jwatch.org/hiv-id-observations/index.php/leaked-remdesivir-study-information-tocilizumab-and-sarilumab-trials-and-the-hazards-of-early-covid-19-research-findings/2020/04/27/. Accessed May 13, 2020.
- 82.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020; 117:10970–10975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klopfenstein T, Zayet S, Lohse A, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020; 50:397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020; 19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020 May 5. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatology. 2020; 2:e474–e484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Potere N, Di Nisio M, Cibelli D, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyper-inflammation: A case-control study. Annals Rheumatic Dis. 2020 Jul 9. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 88.Wang W, Ye L, Ye L, et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007; 128:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995; 273:934–941 [PubMed] [Google Scholar]
- 90.Cohen J, Carlet J. INTERSEPT: An international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996; 24:1431–1440 [DOI] [PubMed] [Google Scholar]
- 91.Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020; 395:1407–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020; 395:473–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017; 377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 95.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020 May 15. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 96.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020. Jul 10. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 97.Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019; 321:2003–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Remy KE, Brakenridge SC, Francois B, et al. Immunotherapies for COVID-19: Lessons learned from sepsis. Lancet Respir Med. 2020. Apr 28. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Internal Med. 2020. Jun 30. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 100.National Institutes of Health: COVID-19 Treatment Guidelines. 2020 Available at: https://covid19treatmentguidelines.nih.gov/immune-based-therapy/. Accessed May 17, 2020. [PubMed]
- 101.Brigham and Women’s Hospital: COVID-19 Protocols. 2020 Available at: https://covidprotocols.org/. Accessed May 17, 2020.
- 102.Massachusetts General Hospital: COVID-19 Treatment Guidance. 2020 Available at: https://www.massgeneral.org/news/coronavirus/treatment-guidance/. Accessed May 17, 2020.
- 103.University of California San Francisco: UCSF Health COVID-19 Clinical Resources. 2020 Available at: https://infectioncontrol.ucsfmedicalcenter.org/coronavirus. Accessed May 17, 2020.
- 104.Association of American Medical Colleges: Coronavirus (COVID-19) Clinical Guidance Repository. 2020 Available at: https://www.aamc.org/coronavirus-covid-19-clinical-guidance-repository. Accessed May 17, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


