Abstract
OBJECTIVES
The purpose of this study is to investigate the effectiveness of the high-frequency rotational test for discrimination of patients with decompensated from those with compensated Meniere’s disease.
MATERIALS and METHODS
Patients with unilateral Meniere’s disease were divided into two groups (compensated and decompensated), based on the presence of clinically significant positional nystagmus as a determinant of the compensation status. All patients and subjects underwent pure tone audiometry, video nystagmography, and the vestibular autorotation test (VAT). The gain, phase, and asymmetry values of VAT were evaluated to range between 2 and 6 Hz.
RESULTS
Phase values of horizontal vestibulo-ocular reflex (VOR) at 2.0, 2.3, and 2.7 Hz were significantly higher in the decompensated group (p<0.05). There was no significant difference in gain values, phase values, of vertical VOR and horizontal asymmetry values.
CONCLUSION
Our study confirmed that horizontal phase values were determined as sensitive markers in VAT to discriminate decompensated from compensated Meniere’s disease.
Keywords: Endolymphatic hydrops, labyrinth diseases, vestibular diseases
INTRODUCTION
The vestibulo-ocular reflex (VOR) is one of the main balance-protection mechanisms, which stabilizes visual images on the retina to ensure unblurred vision during horizontal and vertical head movements, especially at frequencies >2 Hertz (Hz). There is no other mechanism that could detect head rotations at these high frequencies. To enable this function, VOR produces compensatory eye movements in the direction opposite to that of the head movement. When an acute vertigo attack occurs, labyrinthine inputs are assumed to cease suddenly. Then, compensation commences to develop for static condition initially and dynamic conditions thereafter. However, during the compensation period, symptoms of decompensated labyrinthine disorder may persist and continue to disturb the patient even after resolution of the vertigo attack. This condition may be observed in Meniere’s disease, vestibular neuritis, or labyrinthitis [1, 2]. The symptoms are mostly motion-provoked dizziness and disequilibrium during this period. A detailed neuro-otological examination can reveal subtle findings that may be overlooked during the course of the disease. If there is a strong right–left asymmetry in the VOR as in an acute vertigo attack, this condition presents with spontaneous nystagmus [3]. Later, the spontaneous nystagmus disappears, and head-shaking nystagmus may be seen. When a minimal difference remains (minimal VOR deficiency or even no VOR deficiency), only obvious or subtle positional nystagmus persists. This stage is a transitional period between statically compensated disease and dynamically decompensated disease. Examining eye movements in the positional test and caloric test is helpful in recognizing this decompensated stage with positional nystagmus and unilateral weakness [4]. Being able to recognize a decompensated disease is of importance because these patients need medical treatment and vestibular rehabilitation exercise.
It is well known that the caloric test yields low-frequency stimulation for the labyrinth. Daily head movements are at much higher frequencies, and so the caloric test results may not be consistent with symptomatology. The vestibular autorotation test (VAT) is a testing technique investigating the head and eye movements at high frequencies (2 to 6 Hz). Both the vertical and horizontal VOR responses can be detected in this method. Eye movements are recorded by electro-oculography. Relative movement of the eyes in relation to the orbit is called the response, and this may be shown as sinusoidal curves that are the expressions of harmonic components of complex movements. There are three parameters to investigate in the test: gain, phase, and asymmetry. The relationship between the amplitude of eye and head displacements is called gain of the system. In other words, eye velocity divided by the head velocity amplitude represents the gain. Another parameter is the phases of head and eye movements that reflect the degree of synchrony of these two movements. This can be assumed to be the latency of the reflex. The last parameter is asymmetry, which compares eye movements toward right and left sides [3, 4].
The reliability and repeatability of VAT have been tested in several studies. Corvera et al. [5] examined a healthy control group twice at a 1-week interval and another group in a single session. They found no significant difference between inter-individual and inter-session results. Blatt et al. [6] investigated intra-rater and inter-rater reliability of VAT and found a good level of intra-rater reliability for the gain parameter. Inter-rater reliability was good to excellent for gain, phase, and asymmetry parameters at frequencies <3.9 Hz. However, reported drawbacks were a significant difference in reliability for phase and asymmetry at frequencies >4.3 Hz and significant difficulty in completing the test procedure at frequencies >3.9 Hz. VAT findings in decompensated patients are not yet fully known. The aim of this study was to investigate the effectiveness of VAT for the discrimination of decompensated patients from compensated patients diagnosed with unilateral Meniere’s disease.
MATERIALS AND METHODS
The study was approved by the Clinical Research Ethics Committee of Gülhane Military Medicine Academy (approval date and number: 03/03/2015/132). All procedures regarding patient care were handled in accordance with The Universal Declaration of Human Rights (www.un.org/en/universal-declaration-human-rights/). Consent was obtained from all the patients and subjects.
The patients included in the study were those diagnosed with definite and probable Meniere’s disease according to the 2015 classification proposed by the Classification Committee of the Bárány Society, the Japan Society for Equilibrium Research, the European Academy of Otology and Neurotology, the Equilibrium Committee of the American Academy of Otolaryngology-Head and Neck Surgery, and the Korean Balance Society.[7] Patients with symptomatology of vertigo and/or dizziness were assessed with a detailed history, neuro-otological examination, pure tone audiometry, VAT, static positional test, caloric testing, and magnetic resonance imaging. The patients were not experiencing a vertigo attack during the evaluation. For pure tone average, thresholds at 0.5, 1, and 2 kHz were considered.
Active head movements at high frequencies were recorded using VAT equipment and software (VAT Version 3.0, Western Systems Research, Inc., Pasadena, CA, USA). Horizontal and vertical eye movements were recorded using a pair of electro-oculographic electrodes placed on each outer canthus and another pair of electrodes above and below the left eye after cleaning the skin. The head band containing an electronystagmography (ENG) amplifier and an angular velocity sensor were fitted snugly on the head. During the test, the patient was asked to fix the eyes on a target at a distance of 120 cm in front and start to move the head synchronously with the sound of the computer. Head rotations were performed in horizontal and vertical planes. Velocity was 0.5–0.9 Hz in the first 6 seconds, and it gradually increased from 1 to 6 Hz in the next 12 seconds. Then, gain, phase, and asymmetry values were measured at the frequencies of 2.0, 2.3, 2.7, 3.1, 3.5, 4.3, 4.7, and 5.1 Hz. The test procedure was repeated three times in each plane. The coherence level was set to 0.6. Gain, phase, and asymmetry values for eye movements in the horizontal plane and gain and phase values for eye movements in the vertical plane were then calculated. Gain was defined as eye velocity amplitude divided by head velocity amplitude. Phase was defined as the position of the eye (in degrees) relative to the position of the head. Asymmetry referred to deviation of the eyes from the midline in horizontal head rotation.
For spontaneous nystagmus evaluation, caloric testing, static positional tests, the eye movement recording and caloric testing, video goggles and videonystagmography (VNG) software and equipment, and a cold/warm irrigation system (CHARTR, ICS Medical, Schaumburg/IL, USA) were used. A stricter rule than is routinely applied in our laboratory was selected to define unilateral weakness [8]. This was a 25% weaker response on one side compared to the other. Directional preponderance was accepted to be a condition in which the mean-peak slow-phase velocity of the nystagmus beating toward one side was >30% than the mean-peak slow-phase velocity of the nystagmus beating toward the opposite side.
The study included 51 patients with Meniere’s disease, comprising 32 males and 19 females with a mean age of 41.4, years (range, 19–72 years). Definite Meniere’s disease was diagnosed in 51 patients according to the 2015 classification proposed by the Classification Committee of the Bárány Society, the Japan Society for Equilibrium Research, the European Academy of Otology and Neurotology, the Equilibrium Committee of the American Academy of Otolaryngology-Head and Neck Surgery, and the Korean Balance Society. All the patients had unilateral caloric weakness (>25%) in one ear, and 20 patients also had directional preponderance >30% toward the unaffected ear. A control group was formed of 26 healthy volunteers comprising 16 males and 10 females with a mean age of 34.2 years (range, 27–55 years). The control group subjects had no complaint of hearing loss or vertigo.
The patients with Meniere’s disease were separated into two groups based on the development of dynamic compensation (compensated Meniere’s disease group and decompensated Meniere’s disease group). If slow-phase velocity of a nystagmus observed in the static positional test was >6 degrees/second, the patient was accepted as dynamically decompensated (21 patients). Those patients with a slow phase of a nystagmus <6 degrees/second were included in the compensated group (30 patients). The threshold value of 6 degrees/second was determined according to Barber’s abnormal positional nystagmus criteria [9]. The decompensated Meniere’s disease patients had active symptoms such as motion-provoked vertigo or head movement intolerance during the positional tests. Benign paroxysmal positional vertigo was ruled out by the absence of crescendo and decrescendo phases of nystagmus and the absence of reversed nystagmus that are characteristic features of posterior canal benign paroxysmal positional vertigo. None of the patients had spontaneous nystagmus, which meant that all the patients were statically compensated.
The mean values of gain, phase, and asymmetry from the compensated and decompensated Meniere’s disease groups were plotted against the control group data and the normative data of the VAT equipment.
Sensitivity, specificity, positive predictive value, and negative predictive values of the VAT device were calculated in the study.
Statistical Analysis
Data obtained in the study were analyzed statistically using the The Statistical Package for the Social Sciences (SPSS) for Windows version 22.0 software (IBM Corp.; Armonk, NY, USA). Conformity of the variable to normal distribution was evaluated with the Kolmogorov–Smirnov goodness-of-fit test, and the results were normal. Continuous variables were evaluated using the Kruskal–Wallis test or analysis of variance (ANOVA). Comparisons between the groups in terms of VAT parameters were made using the Mann–Whitney U test. Normal and abnormal values were compared using the chi-squared test.
RESULTS
There was no significant difference in average air conduction and bone conduction thresholds between the compensated (20±20.25 dB and 14±18.71 dB, and 22±17.22 dB and 17±15.56 dB for right and left ears, respectively) and decompensated groups (20±21.98 dB and 15±15.7 dB for right ears and 21±26.11 dB and 15±15.24 dB for right and left ears, respectively; chi-squared test: p>0.05).
All the patients had caloric weakness, regardless of the decompensated or compensated status. The comparison of the percentage of unilateral weakness and the percentage of directional preponderance did not yield a significant difference between the compensated and decompensated Meniere’s disease groups (48% vs 62% for canal paresis and 18% vs 47% for directional preponderance, respectively; Mann–Whitney U test, p>0.05). Data on direction and slow-phase velocity of positional nystagmus, the degree of unilateral caloric weakness, and directional preponderance are given in Table 1.
Table 1.
Slow-phase velocity of right- and left-beating nystagmus, degree of caloric weakness, and directional preponderance by groups
| Groups | Lesion Site | The Number of Patients and Slow-Phase Velocity of Positional Nystagmus by Direction (n, Mean±SD*) | Caloric Weakness (%) Mean±SD* | Directional Preponderance (%) Mean±SD* | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Right | Left | Right beating nystagmus | Left-beating nystagmus | Right | Left | Right | Left | |
| Decompensated | 12 | 9 | 10 patients (9.44°±6.80) | 11 patients (9.96°±2.42) | 60.08±24.76 | 64.66±13.42 | 38.5±17.84 | 53±36.7 |
|
| ||||||||
| Compensated | 11 | 19 | 12 patients (2.85°±0.56) | 5 patients (2.43°±0.36) | 45.72±20.84 | 49.42±22.85 | 22.53±8.44 | 15.1±8.71 |
Standard Deviation
The tests showed normal age and gender distributions in both groups (For age: Kolmogorov–Smirnov test, p=0.624 compensated group, p=0.472 decompensated group. For gender: Kolmogorov– Smirnov test; p=1.0; 19 males, 11 females in compensated group; 13 males, 8 females in decompensated group; 16 males, 10 females in control group; chi-squared test, p>0.05).
The ANOVA or Kruskal–Wallis tests were used to compare gain, phase, and asymmetry in horizontal VOR, and gain and phase in vertical VOR. There was no significant difference in horizontal gain at all frequencies tested between the two groups (p>0.05) (Figure 1, Table 2).
Figure 1.
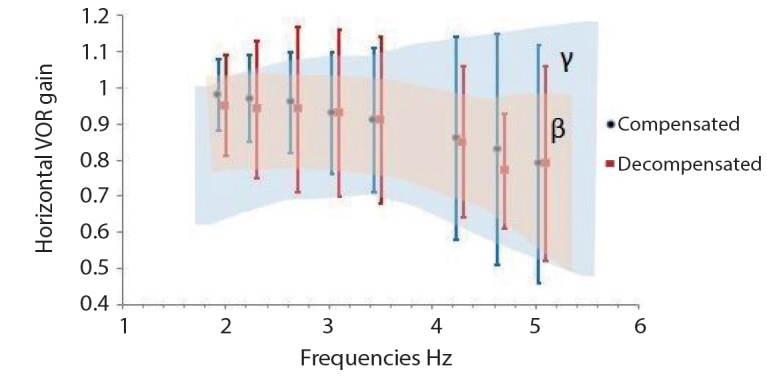
Horizontal VOR Gain (γ, normative value range of VAT device; β, control group value range).
Table 2.
Horizontal VOR gain by groups
| Horizontal VOR Gain at Frequencies Mean±Standard Deviation | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.0 Hz | 2.3 Hz | 2.7 Hz | 3.1 Hz | 3.5 Hz | 4.3 Hz | 4.7 Hz | 5.1 Hz | |
| Groups | ||||||||
| Decompensated Meniere group | 0.95±0.14 | 0.94±0.19 | 0.94±0.23 | 0.93±0.23 | 0.91±0.23 | 0.85±0.21 | 0.77±0.16 | 0.79±0.27 |
| Compensated Meniere group | 0.98±0.1 | 0.97±0.12 | 0.96±0.14 | 0.93±0.17 | 0.91±0.2 | 0.86±0.28 | 0.83±0.32 | 0.79±0.33 |
| Normative values of VAT device | 0.91±0.06 | 0.93±0.08 | 0.95±0.09 | 0.96±0.09 | 0.96±0.08 | 0.96±0.09 | 0.94±0.09 | 0.92±0.1 |
| Control group | 0.95±0.12 | 0.95±0.12 | 0.95±0.12 | 0.94±0.12 | 0.93±0.12 | 0.87±0.14 | 0.84±0.18 | 0.8±0.22 |
VOR: vestibulo-ocular reflex
Phase values in horizontal VOR at 2.0, 2.3, and 2.7 Hz were significantly higher in the decompensated group (p<0.05) (Figure 2, Table 3).
Figure 2.
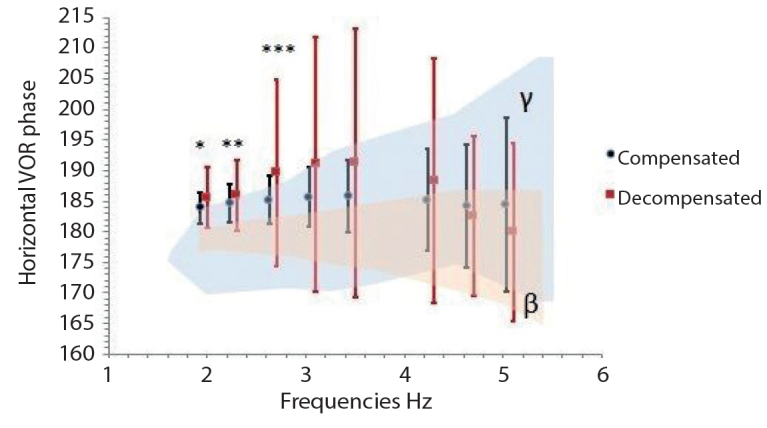
Horizontal VOR Phase (γ, normative value range of VAT device; β, control group value range, * 2.0 Hz, p=0.013, ** 2.3 Hz, p=0.038, *** 2.7 Hz, p=0.025).
Table 3.
Horizontal VOR Phase by Groups
| Horizontal VOR Phase at Frequencies Mean±Standard Deviation | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.0 Hz | 2.3 Hz | 2.7 Hz | 3.1 Hz | 3.5 Hz | 4.3 Hz | 4.7 Hz | 5.1 Hz | |
| Decompensated Meniere group | 185.97±4.97 | 187.13±5.77 | 187.77±15.31 | 187.72±20.8 | 186.85±21.93 | 184.93±20.02 | 184.15±13.08 | 182.72±14.59 |
| Compensated Meniere group | 183.84±2.53 | 185.07±3.16 | 185.54±4.02 | 185.61±4.84 | 185.72±5.88 | 185.49±8.32 | 184.25±10.08 | 184.02±14.15 |
| Normative values of VAT device | 185.8±3.6 | 186.4±3.8 | 187.2±4.4 | 188.1±5.6 | 188.9±6.2 | 189.7±6.2 | 191.7±9.4 | 193.5±12.4 |
| Control group | 179.9±1.84 | 179.83±2.36 | 179.86±3.13 | 180.34±4.01 | 180.91±5.12 | 181.24±7.56 | 180.74±8.69 | 181.96±10.02 |
VOR: vestibulo-ocular reflex
Horizontal asymmetry values of all groups were around zero, with small differences at all frequencies. Differences between the groups were not statistically significant (p>0.05).
In respect of the gain in the vertical plane, the height in gain at all frequencies in the decompensated group failed to produce a significant difference compared to the compensated group (p>0.05) (Figure 3, Table 4). Differences in the vertical VOR phase values between the groups were not statistically significant (p>0.05) (Figure 4, Table 5).
Figure 3.
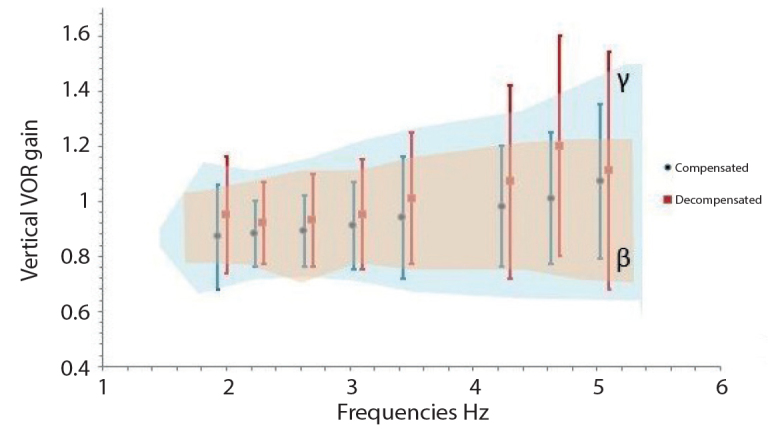
Vertical VOR Gain (γ, normative value range of VAT device; β, control group value range).
Table 4.
Vertical VOR gain by groups
| Vertical VOR Gain at Frequencies Mean±Standard Deviation | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.0 Hz | 2.3 Hz | 2.7 Hz | 3.1 Hz | 3.5 Hz | 4.3 Hz | 4.7 Hz | 5.1 Hz | |
| Groups | ||||||||
| Decompensated Meniere group | 0.91±0.21 | 0.9±0.15 | 0.89±0.17 | 0.91±0.2 | 0.94±0.24 | 1.03±0.35 | 1.05±0.4 | 1.09±0.43 |
| Compensated Meniere group | 0.86±0.19 | 0.91±0.12 | 0.92±0.13 | 0.95±0.16 | 0.98±0.22 | 0.99±0.22 | 0.99±0.24 | 1±0.28 |
| Normative values of VAT device | 0.93±0.22 | 0.96±0.18 | 0.98±0.2 | 1±0.24 | 1.02±0.28 | 1.02±0.32 | 1.04±0.4 | 1.11±0.42 |
| Control group | 0.92±0.12 | 0.93±0.14 | 0.92±0.18 | 0.95±0.16 | 0.96±0.18 | 0.98±0.21 | 0.97±0.23 | 0.97±0.23 |
VOR: vestibulo-ocular reflex
Figure 4.
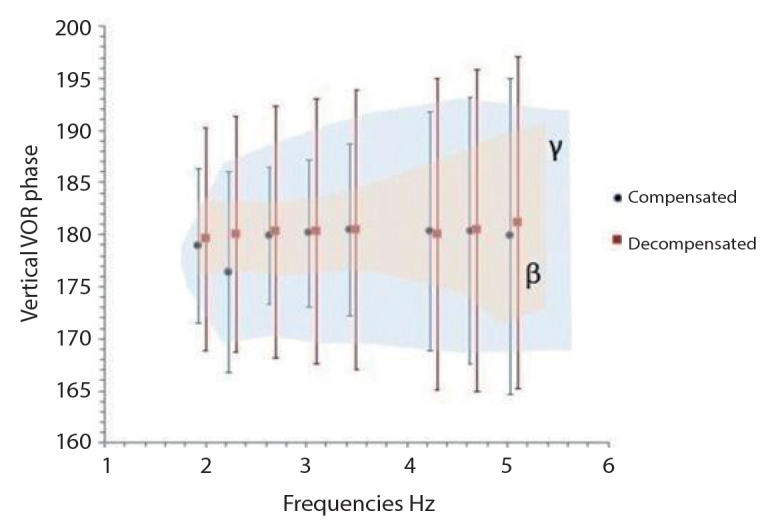
Vertical VOR Phase (γ, normative value range of VAT device; β, control group value range).
Table 5.
Vertical VOR phase by groups
| Vertical VOR Phase at Frequencies Mean±Standard Deviation | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.0 Hz | 2.3 Hz | 2.7 Hz | 3.1 Hz | 3.5 Hz | 4.3 Hz | 4.7 Hz | 5.1 Hz | |
| Groups | ||||||||
| Decompensated Meniere group | 179.55±10.7 | 180.05±11.29 | 180.28±12.1 | 180.3±12.71 | 180.42±13.38 | 179.99±14.94 | 180.38±15.43 | 181.12±15.91 |
| Compensated Meniere group | 178.94±7.39 | 176.4±9.67 | 179.88±6.55 | 180.1±7.12 | 180.39±8.23 | 180.32±11.41 | 180.32±12.77 | 179.83±15.2 |
| Normative values of VAT device | 194.5±10.9 | 195.6±11.2 | 196.7±12 | 198±13.6 | 199.1±14.6 | 199.8±15.8 | 200.4±15 | 199.6±15.2 |
| Control group | 179.9±3.49 | 179.83±3.5 | 179.86±3.64 | 180.34±3.79 | 180.91±4.32 | 181.2±7 | 180.74±9.15 | 181.96±8.92 |
VOR: vestibulo-ocular reflex
When all the Meniere patients were evaluated as a single group without distinction of decompensated/compensated, VAT showed an abnormal finding in 29 of 51 patients (56.8%). Thus, VAT sensitivity was 56.8%. In 4 of 26 healthy volunteers (15.3%), VAT showed an abnormal finding, and specificity was 84.6%. The positive predictive value was 87.87%, and the negative predictive value was 50%. When the decompensated Meniere disease group was evaluated alone, sensitivity was determined at 76.1%, specificity at 84.6%, the positive predictive value at 80%, and the negative predictive value at 81.4%.
DISCUSSION
In routine practice, one of the main purposes of a neuro-otologist is to discriminate patients with dynamically decompensated disease from those with statically compensated disease, for whom medical treatment and/or vestibular rehabilitation would be necessary instead of attack-preventing treatment. These patients with a decompensated disease have disturbing symptoms in routine head movements. Therefore, it would also be beneficial to find some additional findings underlying these disturbing symptoms, such as dizziness or short-duration vertigo. Patients with a decompensated vestibular lesion typically show direction-fixed positional nystagmus that usually arises from side-to-side input difference. When planning this study, instead of mixing patients with various peripheral diseases, it was decided to include only patients with Meniere’s disease to form a homogenous group. Moreover, Meniere’s disease is a disease in which the insult fluctuates from time to time.[10] Therefore, decompensated and compensated stages of the disease may be observed one after another at a certain period of time. Bearing this in mind, it was aimed to test the efficacy of VAT in terms of identifying patients with a decompensated disease.
After dividing the Meniere’s disease patients into decompensated and compensated groups and making investigations using VAT, the results showed that the decompensated group had lower horizontal gain and a higher horizontal phase lead, which is a typical sign of a peripheral vestibular disease, even though all the values were within normal limits. [11] Low vertical gain and high vertical phase (phase lead) are also signs of peripheral vestibular disease. Interestingly, the horizontal phase values of both the compensated and decompensated groups were higher than the normative data. The phase values in horizontal VOR at 2.0, 2.3, and 2.7 Hz were significantly higher in the decompensated group. Interestingly, the frequencies where the phase was leading were not high frequencies but medium frequencies. This shows that the patients make corrective movements to ensure fixation at these frequencies. In the decompensated group, the horizontal asymmetry value was the furthest from zero, but the differences between the groups in the asymmetry values were not statistically significant. All these findings imply that the decompensated group still suffered from some findings of the peripheral disease during the compensation period.
No significant difference was determined between the groups in the vertical tests. This could be attributed to the short distance through the vertical course of the eyes during up and down movements in vertical tests. This short distance may not be enough to produce a pathological finding such as a corrective saccade that causes phase lead.
The efficacy of high-frequency rotational tests in discriminating decompensated Meniere’s patients from compensated ones has not been studied to date, to the best of our knowledge. Using another device, the head auto rotation test (HART), Hirvonen et al. [12] investigated gain, phase and asymmetry data at 1 to 5 Hz in two groups of Meniere disease: a conservatively treated group and a gentamicin/surgically (labyrhinthectomy, vestibular neurectomy) treated group. Lower horizontal gain was observed in the gentamicin/surgically treated group compared to the conservatively treated group. Both groups had a lower gain than the control group. Interestingly, a higher phase lag was obtained from control subjects compared to the conservatively treated group. Both Meniere groups had more asymmetry. In 50% of the conservatively treated group and 72% of the gentamicin/surgically treated group, some kind of abnormality was determined in HART. A decreased gain at 2 and 3 Hz correlated with caloric weakness. HART was found to be more effective than the caloric test in revealing abnormalities in the conservatively treated group. Hirvonen et al. [3] found a lesser gain at 1 to 5 Hz in patients with vestibular schwannoma than in a control group tested using HART equipment. Hirvonen et al. [13] studied patients after vestibular schwannoma removal using the same equipment and “retinal image velocity (RIV) parameter” at 1 to 5 Hz, which is a combination of gain and phase and can be defined as the difference between eye and head velocities. A larger RIV was found in the patients compared to the control group at 1 to 4 Hz. These results imply that the caloric test and VAT investigate different points of vestibular function. Therefore, rather than viewing them as rivals, benefit should be taken from both as complementary methods.
Perez et al. [14] compared the VAT values before intratympanic gentamicin injection and after the last injection in 30 Meniere’s disease patients with definite unilateral Meniere’s disease. At the end of the treatment, vertical VOR results were monitored in the normal range, but a decrease was observed in horizontal VOR gain and phase values. The horizontal and vertical VAT results decreased significantly, especially between 2.0 and 3.7 Hz. The most common pattern in horizontal tests was normal gain/decreased phase (23%) and decreased gain/decreased phase (23%). However, in the current study, the most common pattern in the horizontal tests was decreased gain/increased phase. The most common pattern in vertical tests in the Perez et al.’s study was normal gain/normal phase, which was similar to the current study findings.
Ng et al. [11] assessed 64 patients with Meniere’s disease using VAT to evaluate high-frequency horizontal VOR function in Meniere’s disease. In all patients, the horizontal phase values at 5 and 6 Hz were found to be over 180 degrees, and the abnormal VAT response was detected in 85% of the patients. The most common pattern in the horizontal tests was decreased gain/increased phase, which was similar to the current study findings. High frequency response in that study could not be detected by conventional vestibular tests, and VAT provided additional information to other tests for VOR assessment.
O’Leary et al. [15] evaluated 10 Meniere’s patients in the acute period using VAT. The study showed that despite being in the acute period, all patients performed the test easily. Horizontal gain and phase results were found to be within the normal range. Vertical gain results were observed to be significantly higher, especially in the range between 2 and 6 Hz. They stated that the vertical phase delay decreased with increasing frequency. Consequently, Meniere’s patients were found to be more sensitive to high-frequency vertical tests in the acute period.
Murphy evaluated 120 patients with dizziness using both ENG and VAT [16]. 18 patients who could not complete the VAT were excluded from the study, and the patient completion rate of VAT was 85%. The current study completion rate in the control group was 86.6% and it was compatible with published data. According to the Murphy study, 48 of 102 patients were identified as having abnormalities in both VAT and ENG; 22 patients had abnormal ENG and normal VAT, 20 patients had normal ENG and abnormal VAT, and 12 patients had normal ENG and normal VAT results. Thus, ENG was found to be more valuable in suspected peripheral vertigo patients at priority assessment, and VAT was more valuable in patients with unidentified suspicious dizziness and head trauma.
In the current study, all the patients had caloric weakness of >25%, and 20 of them had directional preponderance of >30% toward the unaffected ear, whereas all the patients with Meniere’s had abnormal VNG results. In 56.8% of the patients, VAT findings were abnormal, and 43.2% of patients were within the normal range. When the decompensated group was evaluated separately, VAT was abnormal in 76.1% of the patients. It should be noted that all of the decompensated Meniere’s patients with VAT abnormalities had a positional nystagmus over 6 degrees/second in the static positional test. This demonstrates the importance of static positional testing.
Chen et al. [17] assessed 48 patients with peripheral vestibular pathology using VAT and the caloric test. In 36 patients (75%), VAT results were abnormal, and 33 patients (68.8%) had caloric test abnormalities, of which 28 had canal paresis, and 16 had directional preponderance. When the patients with abnormal VAT results and abnormal ENG results were combined, a pathological finding was detected in 44 (91.7%) of the total patients. It was stated that the inaccurate diagnosis could be prevented by VAT as a complementary test to the caloric test. In the current study, when all the Meniere’s patients were evaluated as a single group without distinction between decompensated and compensated, VAT was abnormal in 56.8%.
CONCLUSION
Horizontal phase values were determined as sensitive markers in VAT to discriminate decompensated from compensated Meniere’s disease. The results of this study confirmed that VNG (static, positional, caloric test) and VAT investigate vestibular function at different frequencies. The caloric test assesses low-frequency VOR, and VAT assesses high-frequency VOR. Considering the results of this study, in which all the patients had caloric weakness, VNG and VAT are not two rival tests, but two separate tests that complement each other. In the 43.2% of patients who had canal paresis in the caloric test, normal values were seen in VAT. It seems to be reasonable to conclude that it may not always be necessary to stimulate high-frequency VOR to detect vestibular pathology.
Footnotes
This study was presented at the EAONO/8th Instructional Workshop of the European Academy of Otology and NeuroOtology Including Consensus in Auditory Implants, January 18–21, 2017, Izmir, Turkey.
Ethics Committee Approval: The study was approved by the Clinical Research Ethics Committee of Gulhane Military Medicine Academy (approval date and number: 03/03/2015/132).
Informed Consent: Informed consent form was received from all the patients participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.C.G., B.S., Y.H.; Design – M.C.G., B.S.; Supervision – B.S., Y.H.; Resource – M.C.G., A.C.; Materials – M.C.G.; Data Collection and/or Processing – M.C.G., A.C., V.K.Ç.; Analysis and/or Interpretation - M.C.G., B.S., Y.H.; Literature Search – M.C.G., A.C.; Writing – M.C.G., B.S.; Critical Reviews – M.C.G., B.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Flook M, Lopez-Escamez JA. Meniere’s Disease: Genetics and the Immune System. Current Otorhinolaryngology Reports. 2018;5:1–8. doi: 10.1007/s40136-018-0182-8. [DOI] [Google Scholar]
- 2.Frejo L, Martin-Sanz E, Teggi R, Trinidad G, Soto-Varela A, Santos-Perez S, et al. Extended phenotype and clinical subgroups in unilateral Meniere disease: A cross-sectional study with cluster analysis. Clin Otolaryngol. 2017;42:1172–80. doi: 10.1111/coa.12844. [DOI] [PubMed] [Google Scholar]
- 3.Hirvonen TP, Aalto H, Pyykkö I. Decreased vestibuloocular reflex gain of vestibular schwannoma patients. Auris Nasus Larynx. 2000;27:23–6. doi: 10.1016/S0385-8146(99)00044-9. [DOI] [PubMed] [Google Scholar]
- 4.Telian SA, Sheppard NT. Practical management of the balance disorder patient. Singular Publishing Group; San Diego: 1996. [Google Scholar]
- 5.Corvera J, Corvera-Behar G, Lapilover V, Ysunza A. Evaluation of the vestibular autorotation test for measuring vestibular oculomotor reflex in clinical research. Arch Med Res. 2000;31:384–7. doi: 10.1016/S0188-4409(00)00068-0. [DOI] [PubMed] [Google Scholar]
- 6.Blatt PJ, Schubert MC, Roach KE, Tusa RJ. The reliability of the vestibular autorotation test (VAT) in patients with dizziness. J Neurol Phys Ther. 2008;32:70–9. doi: 10.1097/NPT.0b013e3181733709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Menière’s disease. J Vestib Res. 2015;25:1–7. doi: 10.3233/VES-150549. [DOI] [PubMed] [Google Scholar]
- 8.Satar B, Karahatay S, Sen D, Cekin E, Birkent H. Analytic view to concordance between electrocochleography and caloric test in Meniere’s disease. Eur Arch Otorhinolaryngol. 2008;265:159–65. doi: 10.1007/s00405-007-0425-7. [DOI] [PubMed] [Google Scholar]
- 9.Barber HO, Wright G. Positional nystagmus in normals. Adv Otorhinolaryngol. 1973;19:276–83. doi: 10.1159/000393999. [DOI] [PubMed] [Google Scholar]
- 10.Hoa M, Friedman RA, Fisher LM, Derebery MJ. Prognostic implications of and audiometric evidence for hearing fluctuation in Meniere’s disease. Laryngoscope. 2015;125(Suppl 12):S1–S12. doi: 10.1002/lary.25579. [DOI] [PubMed] [Google Scholar]
- 11.Ng M, Davis LL, O’Leary DP. Autorotation test of the horizontal vestibulo-ocular reflex in Meniere’s disease. Otolaryngol Head Neck Surg. 1993;109:399–412. doi: 10.1177/019459989310900304. [DOI] [PubMed] [Google Scholar]
- 12.Hirvonen TP, Pyykkö I, Aalto H. A head autorotation test for patients with Menière’s disease. Auris Nasus Larynx. 1998;25:111–9. doi: 10.1016/S0385-8146(98)00030-3. [DOI] [PubMed] [Google Scholar]
- 13.Hirvonen TP, Aalto H, Pyykkö I, Juhola M. Increased retinal image velocity after vestibular lesion. Otolaryngol Head Neck Surg. 2000;123:766–9. doi: 10.1067/mhn.2000.111357. [DOI] [PubMed] [Google Scholar]
- 14.Perez N, Martin E, Garcia-Tapia R. Results of vestibular autorotation testing at the end of intratympanic gentamicin treatment for Meniere’s disease. Acta Otolaryngol. 2003;123:506–14. doi: 10.1080/0036554021000028086. [DOI] [PubMed] [Google Scholar]
- 15.O’Leary DP, Davis LL. Vestibular autorotation testing of Meniere’s disease. Otolaryngol Head Neck Surg. 1990;103:66–71. doi: 10.1177/019459989010300110. [DOI] [PubMed] [Google Scholar]
- 16.Murphy TP. Vestibular autorotation and electronystagmography testing in patients with dizziness. Am J Otol. 1994;15:502–5. [PubMed] [Google Scholar]
- 17.Chen TS, Song W, Lu H. Comparative study of vestibular autorotation test and caloric test for patients with peripheral vestibular disorders. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006;20:724–7. [PubMed] [Google Scholar]


