Summary
Introduction
Invasive meningococcal disease (IMD) is one of the most severe vaccine-preventable disease not yet under control. In Italy, although different anti-meningococcal vaccines are available, their offer among regions is heterogeneous. The aim of this study is to describe the epidemiology of IMD in Italy based on analysis of national surveillance data for 2011-2017 to optimize the vaccination strategy.
Methods
IMD surveillance data from the Italian National Health Institute were analysed. Microsoft Excel was used to present trend analysis, stratifying by age and serogroups.
Results
In Italy, during the period 2011-2017, the incidence of IMD increased from 0.25 cases/100,000 inhabitants in 2011 to 0.33 cases/100,000 in 2017. Most cases after 2012 were caused by non-B serogroups. The number of cases in subjects aged 25-64 years increased steadily after 2012 (36 cases in 2011, 79 in 2017), mostly due to non-B serogroups, representing more than 65% of cases in those aged 25+ years.
Conclusions
In the period from 2011 to 2017, the incidence of IMDs increased in Italy. The increase, probably due also to a better surveillance, highlights the importance of the disease in the adult population and the high level of circulation of non-B serogroups in particular after 2012. Our analysis supports an anti-meningococcal vaccination plan in Italy that should include the highest number of preventable serogroups and be aimed at vaccinating a wider population through a multicohort strategy.
Keywords: Invasive meningococcal diseases, Epidemiology, Surveillance system, Anti-meningococcal vaccination strategies
Introduction
Invasive Bacterial Infection (IBI) caused by Neisseria meningitidis (N. meningitidis), commonly known as Invasive Meningococcal Disease (IMD) or meningococcal disease, is one of the most severe vaccine-preventable disease [1]. N. meningitidis is a gram-negative diplococcus often detected in the human nasopharynx of asymptomatic carriers (especially adolescents). It only infects humans; there is no animal reservoir [1-5]. Occasionally, it invades normally sterile sites (e.g. bloodstream, brain and cerebrospinal fluid) causing diseases with a variety of non-specific clinical presentations, including meningitis (the most common disease caused by N. meningitidis) and sepsis [1, 6]. Disease progress is usually acute and severe, requiring comprehensive treatment in hospital, and even when the disease is diagnosed early and adequate treatment is started, between 10% and 15% of patients die, and up to 60% have long-term sequelae [1, 6-11].
EPIDEMIOLOGY OF IMD
The incidence of IMD is generally low with regional differences. The incidence ranges from less than 1 case per 100,000 in North America and Europe to 10-1000 cases per 100,000 during epidemic years in Africa [12, 13]. In 30 European Union (EU)/European Economic Area (EEA) countries, there were 3,221 confirmed cases of IMD in 2017 with 282 deaths reported [14], and the overall notification rate (NR) was 0.6 per 100,000 persons, the same as in 2016 and 2015, after a decreasing trend observed in the previous years [14, 15].
N. meningitidis disease-causing serogroups are identified according to the antigenic structure of the polysaccharide capsule; essentially six serogroups (A, B, C, W, Y and, rarely, X) are responsible for human disease [16]. The distribution of the serogroups varies worldwide and within the same region, changing rapidly due to an epidemic or slowly over time because of secular trends, the emergence of hypervirulent clones, new vaccination strategies, the changing state of population immunity, and environmental and behavioural risk factors [12, 16-18]. Moreover, capsular switch from one serogroup to another may occur [12, 18-22]. In Europe, despite a decreasing trend in serogroups B and C (in particular in countries that introduced meningococcal vaccination) and an increasing trend in serogroups W and Y, serogroup B continues to be the main cause of IMD [14, 15]. Specifically, during 2017, most of the 2,979 cases of IMD reported with a known serogroup belonged to serogroup B (51%), followed by W, C and Y (17%, 16% and 12%, respectively) [14].
Furthermore, the incidence of meningococcal disease varies according to the age group considered. In Europe, the highest incidence occurs in young children (NR of 8.2 confirmed cases per 100,000 in children less than 1 year of age, and 2.5 per 100,000 population in those aged 1-4 years), with a second disease peak among adolescents and young adults (15-24 years old; rate of 1 per 100,000 population) [14]. IMD risk is increased in crowded situations associated with mass gatherings, life in close quarters (e.g. military barracks, college dorms), or travel to hyper-endemic regions [23]. However, even a single case of meningitis, especially in children, can trigger the so-called emotional epidemiology, which evokes memories of past pandemics and makes the disease feared [24, 25].
The prevalence of each meningococcal serogroup varies according to age. In the EU/EEA countries, serogroup B caused the highest proportion of cases in all age groups less than 65 years and accounted for 70% of IMD cases in children less than 5 years of age. Serogroup C was most prominent in 25-49-year-olds, but rare among those aged less than 24 years, especially in countries that introduced a universal infant or toddler vaccination programme [14]. Serogroups W and Y were high in those aged 65 years and greater, causing 30% and 26%, respectively, of IMD cases in these age groups [13]. Moreover, a threefold increase in serogroup W was observed between 2013 and 2017 (from 0.03 to 0.10 per 100,000), most pronounced among young children and adults, probably due to the rapid epidemic expansion of a single clone from the United Kingdom to several other EU member states [26-28].
There is potential for underestimation of cases of IMD as a result of underreporting of notification involving surveillance systems [29-34]. The available data are usually derived from passive surveillance systems that provide varying estimates throughout Europe and worldwide [14, 31, 35, 36].
ANTI-MENINGOCOCCAL VACCINES AND VACCINATION STRATEGIES
Different anti-meningococcal vaccines for primary prevention (routine immunization) of IMD and in response to outbreaks (prompt reactive vaccination) are available: monovalent vaccines against serogroup A, B (protein-based vaccine [MenB]) and C (conjugate vaccine [MenC]) and quadrivalent vaccines, mainly conjugated, against serogroups A, C, W, Y (MenACWY) [37].To date, no universal vaccine against meningococcal disease exists. Vaccination strategies adopted throughout the world are heterogeneous, usually based on local epidemiologic data and environmental circumstances within a region or country. Considering the rapid and severe clinical evolution of IMD, relative ease of transmission, and unpredictability of IMD outbreaks and epidemiology, protection can best be achieved by initiating proactive rather than reactive vaccination strategies [38].
The Italian National Immunization Prevention Plan (Piano Nazionale Prevenzione Vaccinale [PNPV]) recommends different meningococcal vaccines with different age schedules. [39]. In particular, MenB vaccination is recommended for all infants, followed by one dose of MenC conjugate vaccine (or the conjugate MenACWY vaccine, according to regional evaluation) in the 13th-15th month and by the conjugate MenACWY vaccine at 12-18 years, in previously vaccinated or unvaccinated adolescents [39]. Moreover, the PNPV recommends anti-meningococcal vaccination regardless of age for people at an increased risk of developing the disease, such as those with some pathological conditions (e.g. hemoglobinopathies, asplenia, congenital or acquired immunodepression, type 1 diabetes, etc.), and their caregivers, and for all travellers to countries in the sub-Saharan belt or on a pilgrimage to Makkah al-Mukarramah [39]. At regional level, these recommendations are implemented as minimal offer, with the possibility of adding more cohorts, creating a heterogeneous offer.
AIM
The aim of the study is to describe and reinterpret as a whole the Italian epidemiological IMD data from 2011 to 2017. This will help to evaluate serogroup- and age-specific trends in order to provide a clear analysis that can be helpful to develop evidence based future Italian IMD prevention strategies.
Methods
DATA SOURCES
Data from Italian surveillance reports on vaccine-preventable invasive bacterial diseases (VP-IBDs) provided by the National Institute of Health (Istituto Superiore di Sanità [ISS]) for 2011-2017 were analysed [40, 41].
ITALIAN SURVEILLANCE OF IMD
In line with other EU/EEA countries [14], VP-IBDs caused by N. meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae are included in an enhanced national surveillance system. In Italy, it is coordinated by the ISS and has been active since 2007 [42]. This system requires that all forms of IBI from pathogens for which there is a vaccine available are reported by clinicians and laboratory staff in hospitals to local health authorities, regions and, finally, to the ISS [42]. Moreover, isolates are sent for further microbiological and molecular analysis to Regional Reference Laboratories or to the National Reference Laboratory at the ISS [42].
The surveillance system collects demographic characteristics of each patient (personal information such as name, surname and date of birth, city of residence, nationality, presence of risk factors, potential hospitalization, outcome and sequelae, state of vaccination, etc.) and data regarding the agent causing the disease (e.g. species, serotype/serogroup, etc.) using microbiological or molecular methods [42]. Microbiological characterization also includes an assessment of antibiotic sensitivity, important for detecting the circulation of antibiotic-resistant strains used in therapy and chemoprophylaxis. In addition, emerging and virulent strains can be highlighted by molecular typing, giving the opportunity to reconstruct the transmission chain in case of outbreaks [43].
DATA SELECTION AND ANALYSIS
Data from the latest consolidated IMD surveillance reports were used [40, 41]. It was decided not to use the surveillance data from 2018 because they are not consolidated yet. Data on notified cases of IMD for 2011-2017 are originally disaggregated in different reports. The data were reorganized and grouped to be analysed and critically interpreted. Based on data availability, stratifications by age group (0-12 months, 1-4, 5-9, 10-14, 15-24, 25-64, > 64 years) and serogroup were carried out. Microsoft Excel 2013 was used for trend analysis.
Results
In Italy, during the study period, the overall NR trend for meningococcal disease increased from 0.25 cases per 100,000 persons in 2011 to 0.33 in 2017 (Fig. 1) [40, 41].
Fig. 1.

Notification rate of cases of invasive meningococcal disease per 100,000 population by year, 2011-2017.
IMD BY SEROGROUP
Looking at the overall number of cases by serogroup during the period analysed, it was not possible to distinguish a homogeneous trend for serogroup B, whereas the overall number of cases caused by ACWY serogroups increased during the period (Fig. 2). Of the total number of cases of IMD notified, the percentage of cases without a notified serogroup decreased from 2013 when these cases where 56 (32.6% of the total) to 37 (16.3%) in 2016 and 19 (9.6%) in 2017 (Supplementary Tab. I).
Fig. 2.
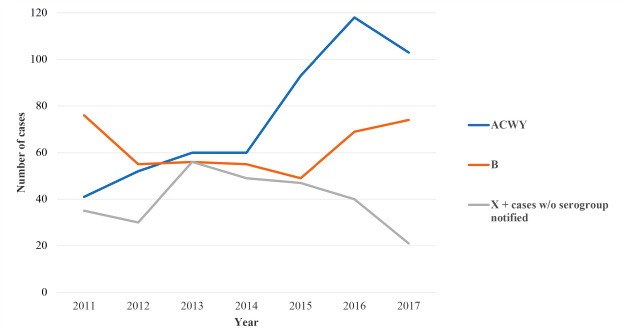
Absolute number of cases for serogroup B versus ACWY versus X + unnotified serogroup, 2011-2017.
Looking at the individual serogroups, serogroups B and C were the most prevalent meningococci in Italy (Fig. 3). Serogroup B showed a decreasing trend between 2011 and 2015, before increasing again over the last 2 years, representing the absolute and relative majority of IMD cases in 2017. An increase in serogroup C was reported in 2015 and 2016, causing more than 40% of cases and becoming the most frequent serogroup each year. Serogroups W and Y also increased over the years, representing almost 7% and 19% of typed cases in 2017, respectively. During the analysis period, six cases of IMD caused by serogroup X were reported. Detailed information on cases of IMD is given in Supplementary Table I.
Fig. 3.
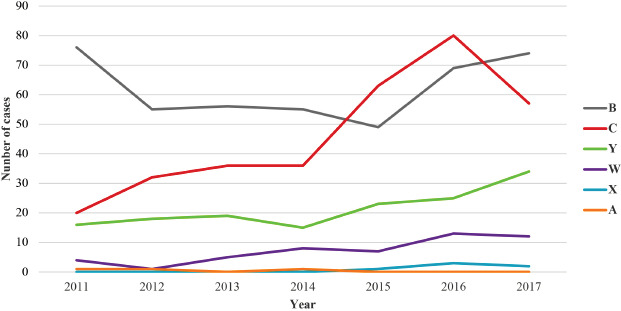
Trends for the absolute number of cases for each typed serogroup, 2011-2017.
IMDS BY AGE
The age-specific incidence rate was higher in children less than 1 year of age (NR of at least 3.20 confirmed cases per 100,000 population per year in 2013), followed by 1-4-year-olds, with another smaller peak in 15-24-year-olds in 2011, 2012 and 2015-2017 (Tab. I). In the age group 25-64 years, an increase in the absolute number of cases was seen achieving a peak in 2016 with 83 cases and 79 in 2017.
Tab. I.
Absolute number, percentage of cases of invasive meningococcal disease and incidence rate (per 100,000 population) of IMD by age group and year, Italy, 2011-2017.
| Year | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||||||||||||||
| Age group | N | % | Incidence | N | % | Incidence | N | % | Incidence | N | % | Incidence | N | % | Incidence | N | % | Incidence | N | % | Incidence |
| 0-12 months | 18 | 11.8% | 3.24 | 17 | 12.4% | 3.20 | 21 | 12.2% | 4.01 | 21 | 12.8% | 4.13 | 22 | 11.7% | 4.43 | 22 | 9.7% | 4.59 | 15 | 7.6% | 3.21 |
| 1-4 years | 23 | 15.1% | 1.00 | 25 | 18.3% | 1.13 | 27 | 15.7% | 1.22 | 25 | 15.2% | 1.13 | 18 | 9.5% | 0.83 | 22 | 9.7% | 1.05 | 19 | 9.6% | 0.93 |
| 5-9 years | 19 | 12.5% | 0.67 | 13 | 9.5% | 0.47 | 11 | 6.4% | 0.39 | 11 | 6.7% | 0.38 | 7 | 3.7% | 0.24 | 16 | 7.1% | 0.56 | 13 | 6.5% | 0.46 |
| 10-14 years | 11 | 7.2% | 0.39 | 8 | 5.8% | 0.29 | 13 | 7.6% | 0.46 | 15 | 9.1% | 0.53 | 10 | 5.3% | 0.35 | 9 | 4.0% | 0.32 | 12 | 6.1% | 0.42 |
| 15-24 years | 32 | 21.1% | 0.53 | 22 | 16.1% | 0.37 | 26 | 15.1% | 0.44 | 18 | 10.9% | 0.30 | 39 | 20.6% | 0.66 | 51 | 22.5% | 0.86 | 35 | 17.7% | 0.59 |
| 25-64 years | 36 | 23.7% | 0.11 | 34 | 24.8% | 0.10 | 51 | 29.6% | 0.16 | 53 | 32.3% | 0.16 | 68 | 36.0% | 0.20 | 83 | 36.6% | 0.25 | 79 | 39.9% | 0.24 |
| > 64 years | 13 | 8.6% | 0.11 | 18 | 13.1% | 0.15 | 23 | 13.4% | 0.18 | 21 | 12.8% | 0.16 | 25 | 13.2% | 0.19 | 24 | 10.6% | 0.18 | 25 | 12.6% | 0.18 |
| Total | 152 | 100% | 0.25 | 137 | 100% | 0.23 | 172 | 100% | 0.29 | 164 | 100% | 0.27 | 189 | 100% | 0.31 | 227 | 100% | 0.37 | 198 | 100% | 0.33 |
IMD BY AGE GROUPS AND SEROGROUPS
Serogroup B had the highest prevalence in the paediatric population less than 5 years of age, nevertheless serogroups W and Y cases increased over the period (Fig. 4). In the age-groups 5-9 and 10-14 years there was a decrease in cases by serogroup B compared with the other age groups. In the same age-group a relative increase in the number of cases from serogroups C, W and Y was observed. In adolescents (15-24 years), most of the cases were caused by serogroups B and C in the study period (with a serogroup C outbreak recorded in 2015/2016 and a fluctuating trend of serogroup B), while serogroup Y increased reaching 27% of the overall typed cases in 2017. In older age groups (25+ years), cases of IMD caused by non-B serogroups were predominant (except for 2011); among these, most cases were attributed to serogroup C, representing at least 44% of the total during the last 5 years. In adults and the elderly (25+ years), a substantial prevalence of serogroups Y and W was also registered (28% in 2011, 23% in 2012, 23% in 2013, 21% in 2014, 25% in 2015, 13% in 2016, 25% in 2017).
Fig. 4.
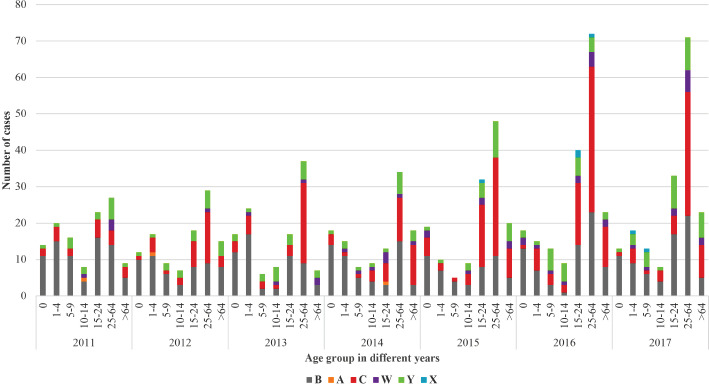
Number of cases of invasive meningococcal disease by age groups and serogroups, 2011-2017.
Focusing on the period from 2015 to 2017 (Fig. 5, Supplementary Fig. 1 and 2), in those aged 0-12 months, the prevalence of serogroup B was always more than 55% of the typed serogroups, increasing over time (58% of cases in 2015, 72% in 2016 and 85% in 2017). Among those aged 1-4 years, serogroup B decreased from 70% in 2015 to 50% in 2017, serogroup C increased in 2016 to 40%, returning in 2017 to the level seen in 2011-2014, whereas almost 17% of the typed cases during 2017 were serogroup Y. Among those aged 15-24 years, serogroup C was the most frequently typed in 2015 and in 2016, whereas in 2017, serogroup B prevailed, representing more than half of the serogroups typed (52%), followed by serogroup Y (27%). In adults and the elderly, serogroup C was the predominant serogroup notified each year (56% and 40% of cases in 2015, 56% and 48% in 2016, 48% and 39% in 2017, respectively), followed by serogroups B, Y and W.
Fig. 5.
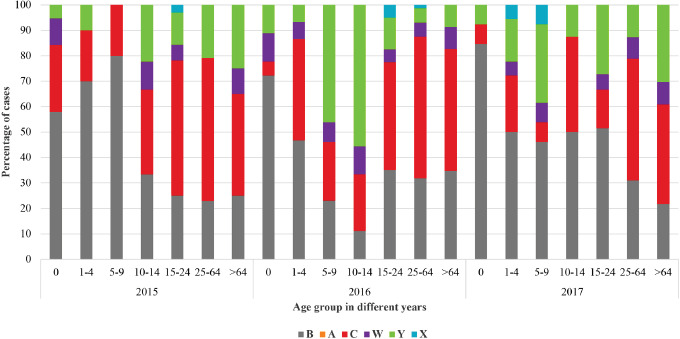
Proportion of cases by serogroups and age groups, Italy, 2015-2017.
Discussion
INCIDENCE AND SURVEILLANCE OF IMD IN ITALY
Italy has shown a lower incidence of IMD compared with the EU/EEA countries overall (NR, 0.6 per 100,000 population during the lasts 3 years) [14]. Nevertheless, in the period 2011-2017, the incidence of IMD has increased from 0.25 cases per 100,000 inhabitants to 0.33 [40, 41]. One reason that could explain the increase in IMDs is the peak of cases recorded in Tuscany in 2015 and 2016, especially in adolescents and young adults [40, 43-45] and in Liguria in 2016 and 2017 [40, 41]. Moreover, there have also been improvements in the laboratory diagnosis confirmation system, both at regional and national level [33]. For example, in Tuscany during 2015, real-time polymerase chain reaction (PCR), a more sensitive, rapid and accurate laboratory diagnostic test [33], was made available to all hospitals by a regional law [46], reducing the underestimation of IMD [29] and consequently improving the accuracy of the surveillance system and differentiating IBIs [29, 33, 47]. These methods contributed to a reduction in the percentage of cases of IBI with an unidentified cause [40] and, specifically, of IMD, for which the number of cases without a notified serogroup decreased during the study period [40]. This was confirmed by a data comparison between the number of cases of IBD identified from Italian Hospital Discharge Records and those notified to the Surveillance System reported from 2007 to 2016, which showed increasing concordance between the two institutional information systems for VP-IBD [30]. However, despite the improvements, the surveillance system still fails to identify every case of IMD and underreporting is still present [30, 40, 41].
IMD SEROGROUPS
In Italy, during 2011-2017, serogroup B was overall the predominant N. meningitidis serogroup, as observed in other EU/EEA countries [14-16]. Nevertheless, the overall number of cases caused by non-B serogroups, increased during the study period. In particular, an increase in cases of IMD related to serogroups Y and W was described [40, 41, 48], whereas serogroup C was almost s from 2012 to 2014, increasing in 2015 and 2016 [40, 43-45]. This epidemiological evidence, as well as the possibility of capsular switch from one serogroup to another [12, 18-22], should lead the national decision makers to boost the use of the MenACWY vaccine instead of MenC in the next PNPV, as already done in Emilia-Romagna [49], Apulia [50] and Sicily [51, 52] and recently implemented in other countries [53, 54], to ensure more comprehensive protection.
IMD BY AGE GROUPS
The age distribution of IMD cases in Italy is similar to the general distribution within the EU/EEA countries [14], with the highest peak incidence in infants and young children and a second peak among adolescents and young adults [40, 41]. Nevertheless, during the study period, the number of cases of IMD notified annually for the 25-64-year-old age group highlights the importance of the disease in the adult population. Moreover, during the last 3 years analysed (2015-2017), an overall increase in the absolute number of cases in adults was seen, especially caused by non-B serogroups. Thus, an effective immunization strategy with a vaccine covering a higher range of serogroups should be implemented in a wider population.
Conclusions
In Italy, since 2011 there has been an increasing number of cases and increased attention given to meningococcal disease [40, 41], although underreporting to the national surveillance system and underestimation of the number of cases of IMD is still present [29, 30]. The data showed that serogroup B is still the most relevant causative agent, but other vaccine-preventable serogroups (e.g. serogroups C, Y and W) have almost reached the same importance [40, 41]. Moreover, although IMD is often considered to be a disease affecting children, the number of cases among older age groups is high and increasing [40, 41].
It is essential to continue to enhance the surveillance systems, improving complete reporting of data to adequately monitor the epidemiology of IMD and to design the most effective public health action plan to tackle the disease. For example, after the peak in the number of cases of serogroup C in 2015, [40, 43-45] the Tuscany Region carried out extraordinary public health measures, including active free-of-charge offer of the MenACWY vaccine to all teenagers (up to 20 years of age) and adults (up to 45 years) living in the areas at greatest risk and an extra dose of MenC conjugate vaccine at 6 years of age [46, 55]. Indeed, during the outbreak, cases of IMD were reported in previously vaccinated children and adults. Most of these cases were in individuals who were vaccinated more than 2 years before developing the disease, indicating a rapid loss of protection that suggested the implementation of a booster dose [56].
A vaccination strategy against meningococcal disease should protect against all possible pathogenetic N. meningitidis serogroups. Indeed the Italian PNPV has included the possibility to switch to quadrivalent vaccination instead of monovalent vaccine against serogroup C with the aim of enhancing coverage in adolescents and high-risk adults [39]. As a result, some Italian regions, such as Emilia-Romagna [49], Sicily [51] and Apulia [50], have switched to quadrivalent vaccination and added an additional age cohort to their calendar. Furthermore, consensus among several national scientific societies has led to the development of the “Calendario per la Vita 2019” in which they suggest switching to quadrivalent vaccination, the addition of another age cohort with a boost between 6 and 9 years old and a broader range of high-risk adults compared with the PNPV [39, 57]. Nevertheless, looking at the epidemiologic data, those more than 24 years of age are an important target population [40, 41] that is not fully addressed with the PNPV. In our opinion a possible strategy to consider is a boost (or a first anti-meningococcal vaccination) during the 10-year periodic recall for vaccination against diphtheria, tetanus, and pertussis already in use [39]. Further analysis should be done to better understand the best preventive strategy.
The emergency of Covid-19 pandemic is catalysing everyone’s attention and preventive measures, such as vaccinations, for other infections are at risk of being overshadowed. But when community life and the vivacity of interhuman relationships will resume fully, we will need to be prepared for the recirculation of meningococci and other pathogens in the population. Therefore, recommended vaccinations should continue to be administered and the recommendation to ensure a wider and adequate vaccination coverage for meningococcal disease appears even more important.
IMD is a rare but severe vaccine-preventable disease that has garnered great public attention due to its seriousness and unpredictability as well as the long-term impact of sequelae. The key role of public health is to go beyond the emotional epidemiology and have a broader view of the disease and its consequences, as well as monitor serogroups, trends and outbreaks and strengthen methodological evidence-based tools for decision-making processes, public health policies, planning of health care services and intervention measures, including immunization.
Figures and tables
Supplementary Materials
Tab. SI.
Cases of invasive meningococcal disease according to serogroup and year, Italy, 2011-2017.
| Year | Overall (2011-17) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||||||||||
| N | % Out of the total typed | N | % Out of the total typed | N | % Out of the total typed | N | % Out of the total typed | N | % Out of the total typed | N | % Out of the total typed | N | % Out of the total typed | N | % Out of the total typed | |
| Serogroup | ||||||||||||||||
| A | 1 | 0.9% | 1 | 0.9% | 0 | 0.0% | 1 | 0.9% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 3 | 0.3% |
| B | 76 | 65.0% | 55 | 51.4% | 56 | 48.3% | 55 | 47.8% | 49 | 34.3% | 69 | 36.3% | 74 | 41.3% | 434 | 44.9% |
| C | 20 | 17.1% | 32 | 29.9% | 36 | 31.0% | 36 | 31.3% | 63 | 44.1% | 80 | 42.1% | 57 | 31.9% | 324 | 33.5% |
| W | 4 | 3.4% | 1 | 0.9% | 5 | 4.3% | 8 | 7.0% | 7 | 4.9% | 13 | 6.8% | 12 | 6.7% | 50 | 5.2% |
| X | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 0.7% | 3 | 1.6% | 2 | 1.1% | 6 | 0.6% |
| Y | 16 | 13.7% | 18 | 16.8% | 19 | 16.4% | 15 | 13.0% | 23 | 16.1% | 25 | 13.2% | 34 | 19.0% | 150 | 15.5% |
| IMD with serogroup notified | 117 | 107 | 116 | 115 | 143 | 190 | 179 | 967 | ||||||||
| IMD without serogroup notified | 35 | 30 | 56 | 49 | 46 | 37 | 19 | 272 | ||||||||
| Total IMD notified | 152 | 137 | 172 | 164 | 189 | 227 | 198 | 1,239 | ||||||||
| % of the total IMD with serogroup notified | 77.0% | 78.1% | 67.4% | 70.1% | 75.7% | 83.7% | 90.4% | 78.0% | ||||||||
IMD: invasive meningococcal disease.
Fig. S1.
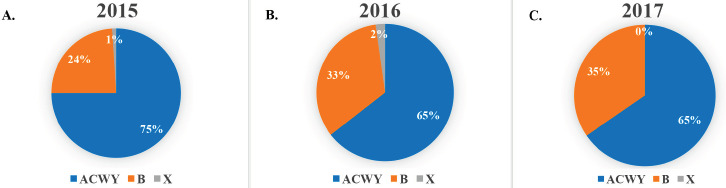
Proportion of cases in subjects ≥ 15 years divided for serogroup B versus ACWY versus X, 2015-2017.
Fig. S2.
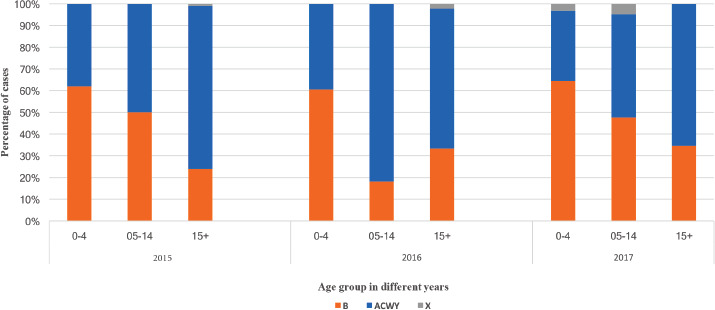
Proportion of cases for serogroup B versus ACWY versus X and age groups, 2015-2017.
Acknowledgements
The authors would like to thank Dr. Andrea Barbara who provided medical writing services and Edra Spa for the revision by a native English speaker.
Funding sources: Sanofi Pasteur Italia funded all costs associated with the development and the publishing of the present manuscript.
Footnotes
Conflicts of interest statement
SI, LB, SP and GCL work for Sanofi Pasteur Italia. The other authors declare no conflict of interest.
Authors’ contributions
Conceptualization: SI, LB, AT, CA, PB, PC, MC, SE, GG, GI, PLL, FV, SP and GCL; methodology: SI, LB and AT; acquisition of data: SI, LB, AT and GCL; formal analysis: SI, LB and AT; interpretation of data: SI, LB, AT, CA, PB, PC, MC, SE, GG, GI, PLL, FV, SP and GCL; writing - original draft: SI, LB, AT and SP; writing - review and editing: SI, LB, AT, CA, PB, PC, MC, SE, GG, GI, PLL, FV, SP and GCL; supervision: GCL; project administration: GCL. All authors have read and agreed to the submitted version of the manuscript.
References
- [1].Centres for Disease Control and Prevention (CDC). Meningococcal disease. Hamborsky J, Kroger A, Wolfe C, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington, DC: Public Health Foundation; 2015, pp. 231-246. [Google Scholar]
- [2].Soriano-Gabarró M, Wolter J, Hogea C, Vyse A. Carriage of Neisseria meningitidis in Europe: a review of studies undertaken in the region. Expert Rev Anti Infect Ther 2011;9:761-74. https://doi.org/10.1586/eri.11.89 10.1586/eri.11.89 [DOI] [PubMed] [Google Scholar]
- [3].Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:853-61. https://doi.org/10.1016/S1473-3099(10)70251-6 10.1016/S1473-3099(10)70251-6 [DOI] [PubMed] [Google Scholar]
- [4].Peterson ME, Li Y, Shanks H, et al. Serogroup-specific meningococcal carriage by age group: a systematic review and meta-analysis. BMJ Open 2019;9:e024343 https://doi.org/10.1136/bmjopen-2018-024343 10.1136/bmjopen-2018-024343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gasparini R, Comanducci M, Amicizia D, et al. Molecular and serological diversity of Neisseria meningitidis carrier strains isolated from Italian students aged 14 to 22 years. J Clin Microbiol 2014;52:1901-10. https://doi.org/10.1128/JCM.03584-13 10.1128/JCM.03584-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Manchanda V, Gupta S, Bhalla P. Meningococcal disease: history, epidemiology, pathogenesis, clinical manifestations, diagnosis, antimicrobial susceptibility and prevention. Indian J Med Microbiol 2006;24:7-19. https://doi.org/10.4103/0255-0857.19888 10.4103/0255-0857.19888 [DOI] [PubMed] [Google Scholar]
- [7].Borg J, Christie D, Coen PG, Booy R, Viner RM. Outcomes of meningococcal disease in adolescence: prospective, matched- cohort study. Pediatrics 2009;123:e502-9. https://doi.org/10.1542/peds.2008-0581 10.1542/peds.2008-0581 [DOI] [PubMed] [Google Scholar]
- [8].Buysse CM, Vermunt LC, Raat H, Hazelzet JA, Hop WC, Utens EM, Joosten KF. Surviving meningococcal septic shock in childhood: long-term overall outcome and the effect on health-related quality of life. Crit Care 2010;14:R124 https://doi.org/10.1186/cc9087 10.1186/cc9087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang B, Haji Ali Afzali H, Marshall H. The inpatient costs and hospital service use associated with invasive meningococcal dis- ease in South Australian children. Vaccine 2014;32:4791-8. https://doi.org/10.1016/j.vaccine.2014.05.069 10.1016/j.vaccine.2014.05.069 [DOI] [PubMed] [Google Scholar]
- [10].Sadarangani M, Scheifele DW, Halperin SA, Vaudry W, Le Saux N, Tsang R, Bettinger JA, investigators of the Canadian Immunization Monitoring Program, ACTive (IMPACT) Outcomes of invasive meningococcal disease in adults and children in Canada between 2002 and 2011: a prospective cohort study. Clin Infect Dis 2015;60:e27-35. https://doi.org/10.1093/cid/civ028 10.1093/cid/civ028 [DOI] [PubMed] [Google Scholar]
- [11].Olbrich KJ, Müller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther 2018;7:421-38. https://doi.org/10.1007/s40121-018-0213-2 10.1007/s40121-018-0213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MAP. The changing and dynamic epidemiology of meningococcal disease. Vaccine 2012;30:B26-36. https://doi.org/10.1016/j.vaccine.2011.12.032 10.1016/j.vaccine.2011.12.032 [DOI] [PubMed] [Google Scholar]
- [13].Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, Echaniz-Aviles G, Hakawi A, Kamiya H, Karachaliou A, Lucidarme J, Meiring S, Mironov K, Sáfadi MAP, Shao Z, Smith V, Steffen R, Stenmark B, Taha M-K, Trotter C, Vázquez JA, Zhu B. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines 2019;18:15-30. https://doi.org/10.1080/14760584.2019.1557520 10.1080/14760584.2019.1557520 [DOI] [PubMed] [Google Scholar]
- [14].European Centre for Disease Prevention and Control (ECDC). Invasive meningococcal disease. Annual Epidemiological Report for 2017. Stockholm: ECDC; 2019. [Google Scholar]
- [15].Whittaker R, Dias JG, Ramliden M, Ködmön C, Economopoulou A, Beer N, Pastore Celentano L. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004-2014. Vaccine 2017;35:2034-41. https://doi.org/10.1016/j.vaccine.2017.03.007 10.1016/j.vaccine.2017.03.007 [DOI] [PubMed] [Google Scholar]
- [16].Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009;27:B51-63. https://doi.org/10.1016/j.vaccine.2009.04.063 10.1016/j.vaccine.2009.04.063 [DOI] [PubMed] [Google Scholar]
- [17].Kriz P, Wieffer H, Holl K, Rosenlund M, Budhia S, Vyse A. Changing epidemiology of meningococcal disease in Europe from the mid-20th to the early 21st century. Expert Rev Vaccines 2011;10:1477-86. https://doi.org/10.1586/erv.11.117 10.1586/erv.11.117 [DOI] [PubMed] [Google Scholar]
- [18].Harrison LH, Shutt KA, Schmink SE, Marsh JW, Harcourt BH, Wang X, Whitney AM, Stephens DS, Cohn AA, Messonnier NE, Mayer LW. Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era - United States, 2000-2005. J Infect Dis 2010;201:1208-24. https://doi.org/10.1086/651505 10.1086/651505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tzeng YL, Thomas J, Stephens DS. Regulation of capsule in Neisseria meningitidis. Crit Rev Microbiol 2016;42:759-72. https://doi.org/10.3109/1040841X.2015.1022507 10.3109/1040841X.2015.1022507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stefanelli P, Fazio C, Neri A, Sofia T, Mastrantonio P. First report of capsule replacement among electrophoretic type 37 Neisseria meningitidis strains in Italy. J Clin Microbiol 2003;41:5783-6. https://doi.org/10.1128/JCM.41.12.5783-5786.2003 10.1128/JCM.41.12.5783-5786.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Swartley JS, Marfin AA, Edupuganti S, Liu LJ, Cieslaku P, Perkins B, Wenger JD, Stephens DS. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA 1997;94:271-6. https://doi.org/10.1073/pnas.94.1.271 10.1073/pnas.94.1.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stefanelli P, Fazio C, Vacca P, Palmieri A, Ambrosio L, Neri A, Piana A, Castiglia P, Argiolas F, Santus S, Masala L, Steri G, Riccardo F, Iannazzo S, Maraglino FP, D’Amario C, Rezza G. An outbreak of severe invasive meningococcal disease due to a capsular switched Neisseria meningitidis hypervirulent strain B:cc11. Clin Microbiol Infect 2019;25:111.e1-4. https://doi.org/10.1016/j.cmi.2018.07.014 10.1016/j.cmi.2018.07.014 [DOI] [PubMed] [Google Scholar]
- [23].Centers for Disease Control and Prevention (CDC). Meningococcal risk factors 2019. Available at; https://www.cdc.gov/meningococcal/about/risk-factors.html. Accessed on March 23, 2020.
- [24].Duan C, Linder H, Huremović D. Societal, public, and [emotional] epidemiological aspects of a pandemic. Huremović D, ed. Psychiatry of pandemics. Cham: Springer International Publishing; 2019, pp. 45-53. https://doi.org/10.1007/978-3-030-15346-5_4 10.1007/978-3-030-15346-5_4 [DOI] [Google Scholar]
- [25].Dettori M, Arru B, Azara A, Piana A, Mariotti G, Camerada MV, Stefanelli P, Rezza G, Castiglia P. In the digital era, is community outrage a feasible proxy indicator of emotional epidemiology? The case of meningococcal disease in Sardinia, Italy. Int J Environ Res Public Health 2018;15:1-8. https://doi.org/10.3390/ijerph15071512 10.3390/ijerph15071512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970-2015): multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine 2016;34:1515-23. https://doi.org/10.1016/j.vaccine.2016.02.014 10.1016/j.vaccine.2016.02.014 [DOI] [PubMed] [Google Scholar]
- [27].Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, Ramsay ME, Borrow R. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis 2015;60:578-85. https://doi.org/10.1093/cid/ciu881 10.1093/cid/ciu881 [DOI] [PubMed] [Google Scholar]
- [28].Lucidarme J, Scott KJ, Ure R, Smith A, Lindsay D, Stenmark B, Jacobsson S, Fredlund H, Cameron JC, Smith-Palmer A, McMenamin J, Gray SJ, Campbell H, Ladhani S, Findlow J, Molling P, Borrow R. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup w strain, Scotland and Sweden, July to August 2015. Eurosurveillance 2016;21 https://doi.org/10.2807/1560-7917.ES.2016.21.45.30395 10.2807/1560-7917.ES.2016.21.45.30395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Azzari C, Nieddu F, Moriondo M, Indolfi G, Canessa C, Ricci S, Bianchi L, Serranti D, Poggi GM, Resti M. Underestimation of invasive meningococcal disease in Italy. Emerg Infect Dis 2016;22:469-75. https://doi.org/10.3201/eid2203.150928 10.3201/eid2203.150928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pezzotti P, Bellino S, Riccardo F, Lucaroni F, Cerquetti M, Pantosti A, Rezza G, Stefanelli P. Vaccine preventable invasive bacterial diseases in Italy: a comparison between the national surveillance system and recorded hospitalizations, 2007-2016. Vaccine 2019;37:41-8. https://doi.org/10.1016/j.vaccine.2018.11.047 10.1016/j.vaccine.2018.11.047 [DOI] [PubMed] [Google Scholar]
- [31].Vespa Presa J, Abalos MG, Sini de Almeida R, Cane A. Epidemiological burden of meningococcal disease in Latin America: a systematic literature review. Int J Infect Dis 2019;85:37-48. https://doi.org/10.1016/j.ijid.2019.05.006 10.1016/j.ijid.2019.05.006 [DOI] [PubMed] [Google Scholar]
- [32].Giorgi Rossi P, Mantovani J, Ferroni E, Forcina A, Stanghellini E, Curtale F, Borgia P. Incidence of bacterial meningitis (2001-2005) in Lazio, Italy: the results of an integrated surveillance system. BMC Infect Dis 2009;9 https://doi.org/10.1186/1471-2334-9-13 10.1186/1471-2334-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guiducci S, Moriondo M, Nieddu F, Ricci S, De Vitis E, Casini A, Poggi GM, Indolfi G, Resti M, Azzari C. Culture and real-time polymerase chain reaction sensitivity in the diagnosis of invasive meningococcal disease: does culture miss less severe cases? PLoS One 2019;14:e0212922 https://doi.org/10.1371/journal.pone.0212922 10.1371/journal.pone.0212922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baldovin T, Lazzari R, Cocchio S, Furlan P, Bertoncello C, Saia M, Russo F, Baldo V. Invasive meningococcal disease in the Veneto region of Italy: a capture-recapture analysis for assessing the effectiveness of an integrated surveillance system. BMJ Open 2017;7 https://doi.org/10.1136/bmjopen-2016-012478 10.1136/bmjopen-2016-012478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].European Centre for Disease Prevention and Control (ECDC). Surveillance systems overview for 2017. 2018. Available at: https://www.ecdc.europa.eu/en/publications-data/surveillance-systems-overview-2017. Accessed on January 27, 2020.
- [36].European Centre for Disease Prevention and Control (ECDC). Introduction to the Annual Epidemiological Report. 2017. Available at: https://www.ecdc.europa.eu/en/annual-epidemiological-reports/methods. Accessed on January 27, 2020.
- [37].World Health Organization. Meningococcal meningitis 2018. Available at: https://www.who.int/en/news-room/fact-sheets/detail/meningococcal-meningitis. Accessed on November 23, 2019.
- [38].Vuocolo S, Balmer P, Gruber WC, Jansen KU, Anderson AS, Perez JL, York LJ. Vaccination strategies for the prevention of meningococcal disease. Hum Vaccines Immunother 2018;14:1203-15. https://doi.org/10.1080/21645515.2018.1451287 10.1080/21645515.2018.1451287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ministero della Salute. Piano Nazionale Prevenzione Vaccinale (PNPV) 2017-2019. Rome: Ministero della Salute; 2017. [Google Scholar]
- [40].Istituto Superiore di Sanità. Sorveglianza delle malattie batteriche invasive in Italia. Rapporto consolidato 2017. Rome: Istituto Superiore di Sanità; 2018. [Google Scholar]
- [41].Istituto Superiore di Sanità. Dati di sorveglianza delle malattie batteriche invasive aggiornati al 3 aprile 2017. Rome: Istituto Superiore di Sanità; 2017. [Google Scholar]
- [42].Istituto Superiore di Sanità. Protocollo per la sorveglianza nazionale delle malattie invasive da meningococco, pneumococco ed emofilo e delle meningiti batteriche in Italia. Rome: Istituto Superiore di Sanità; 2018. [Google Scholar]
- [43].Stefanelli P, Fazio C, Neri A, Ciammaruconi A, Balocchini E, Anselmo A, Azzari C, Rossolini GM, Vacca P, Fortunato A, Palozzi A, Fillo S, Lista F, Moriondo M, Nieddu F, Rezza G. Genome-based study of a spatio-temporal cluster of invasive meningococcal disease due to Neisseria meningitidis serogroup C, clonal complex 11. J Infect 2016;73:136-44. https://doi.org/10.1016/j.jinf.2016.05.003 10.1016/j.jinf.2016.05.003 [DOI] [PubMed] [Google Scholar]
- [44].Menichetti F, Fortunato S, Ricci A, Salani F, Ripoli A, Tascini C, Fusco FM, Mencarini J, Bartoloni A, Di Pietro M. Invasive meningococcal disease due to group C N. meningitidis ST11 (cc11): the Tuscany cluster 2015-2016. Vaccine 2018;36:5962-6. https://doi.org/10.1016/j.vaccine.2018.08.050 10.1016/j.vaccine.2018.08.050 [DOI] [PubMed] [Google Scholar]
- [45].Miglietta A, Innocenti F, Pezzotti P, Riccobono E, Moriondo M, Pecile P, Nieddu F, Rossolini GM, Azzari C, Balocchini E, Rezza G, Voller F, Stefanelli P. Carriage rates and risk factors during an outbreak of invasive meningococcal disease due to Neisseria meningitidis serogroup C ST-11 (cc11) in Tuscany, Italy: a cross-sectional study. BMC Infect Dis 2019;19:29 https://doi.org/10.1186/s12879-018-3598-3 10.1186/s12879-018-3598-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Regione Toscana. Delibera n.571 del 27-04-2015. Prevenzione della diffusione del meningococco C in Toscana. Aggiornamento calendario e direttive aprile 2015. 2015. [Google Scholar]
- [47].Heinsbroek E, Ladhani S, Gray S, Guiver M, Kaczmarski E, Borrow R, Ramsay M. Added value of PCR-testing for confirmation of invasive meningococcal disease in England. J Infect 2013;67:385-90. https://doi.org/10.1016/j.jinf.2013.06.007 10.1016/j.jinf.2013.06.007 [DOI] [PubMed] [Google Scholar]
- [48].Neri A, Pezzotti P, Fazio C, Vacca P, D’Ancona FP, Caporali MG, Stefanelli P. Epidemiological and molecular characterization of invasive meningococcal disease in Italy, 2008/09-2012/13. PLoS One 2015;10:e0139376 https://doi.org/10.1371/journal.pone.0139376 10.1371/journal.pone.0139376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Regione Emilia-Romagna. Delibera Giunta Regionale N. 427 del 05/04/2017. Approvazione del Piano Regionale di Prevenzione Vaccinale 2017;2017:1-30. [Google Scholar]
- [50].Regione Puglia. Calendario vaccinale per la vita 2017. Available at: https://www.vaccinarsinpuglia.org/assets/uploads/files/calendario-vaccinale-Puglia-2017.pdf. Accessed on January 22, 2020.
- [51].VaccinarSì in Sicilia. Nuovo calendario vaccinale siciliano. - VaccinarSì in Sicilia 2017. Available at: https://www.vaccinarsinsicilia.org/notizie/2017/11/nuovo-calendario-vaccinale-siciliano. Accessed on December 4, 2019.
- [52].Costantino C, Restivo V, Ventura G, D’Angelo C, Randazzo MA, Casuccio N, Palermo M, Casuccio A, Vitale F. Increased vaccination coverage among adolescents and young adults in the district of Palermo as a result of a public health strategy to counteract an ‘epidemic panic.’ Int J Environ Res Public Health 2018;15 https://doi.org/10.3390/ijerph15051014 10.3390/ijerph15051014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Presa J, Findlow J, Vojicic J, Williams S, Serra L. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: a review. Infect Dis Ther 2019;8:307-33. https://doi.org/10.1007/s40121-019-0254-1 10.1007/s40121-019-0254-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Knol MJ, Ruijs WLM, Antonise-Kamp L, de Melker HE, van der Ende A. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Eurosurveillance 2018;23 https://doi.org/10.2807/1560-7917.ES.2018.23.16.18-00158 10.2807/1560-7917.ES.2018.23.16.18-00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Regione Toscana. Vaccinazioni - Campagna contro il meningococco C n.d. Available at: http://www.regione.toscana.it/-/campagna-contro-il-meningococco-c. Accessed on November 23, 2019.
- [56].Pezzotti P, Miglietta A, Neri A, Fazio C, Vacca P, Voller F, Rezza G, Stefanelli P. Meningococcal C conjugate vaccine effectiveness before and during an outbreak of invasive meningococcal disease due to Neisseria meningitidis serogroup C/cc11, Tuscany, Italy. Vaccine 2018;36:4222-7. https://doi.org/10.1016/j.vaccine.2018.06.002 10.1016/j.vaccine.2018.06.002 [DOI] [PubMed] [Google Scholar]
- [57].VaccinarSì. Calendario vaccinale per la vita 2019. 2019. Available at: https://www.vaccinarsi.org/notizie/2019/07/calendario-vaccinale-per-la-vita-2019. Accessed on January 22, 2020.


