Abstract
Background:
Pertussis remains an important global public health concern, despite the presence of extensive immunization programs. Incidence and severity of pertussis are typically higher in neonates and young infants. As a strategy to protect these young infants, maternal vaccination with Tdap (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis) has been recommended in Brazil. The objective of this study was to evaluate the effects of Tdap vaccination during pregnancy on the anti-pertussis toxin (PT) IgG response in mothers and their infants at birth.
Material and Methods:
Maternal and cord blood samples were collected from vaccinated (n=243) and unvaccinated (n=75) pregnant women, at the time of delivery, from July 2015 to August 2016 in São Paulo, Brazil. Anti-PT IgG antibodies were quantified by Enzyme-Linked Immunosorbent Assay (ELISA) and geometric mean concentrations (GMC) were calculated. Relationship between timing of vaccination and antibody concentrations were evaluated.
Results:
Maternal and cord blood GMCs among the vaccinated group were 5.4 and 5.6 fold higher [66.5 International Units (IU)/mL and 89.8 IU/mL] compared to the unvaccinated group (12.4 IU/mL and 16.1 IU/mL), respectively (p<0.001). Higher anti-PT IgG GMCs were observed when vaccination occurred ≥ 60 days before delivery compared to < 60 days, suggesting that vaccination early in the third trimester may be more effective than later in pregnancy.
Conclusion:
Tdap maternal vaccination results in significantly higher anti-PT IgG in newborn infants and supports the current recommendation of the Brazilian Immunization Program.
Keywords: Pertussis, maternal vaccination, newborn infants, maternal antibodies, Tdap
1. Introduction
Pertussis is a poorly controlled vaccine preventable disease, with cases on the rise throughout the world. In Brazil, a progressive increase in pertussis cases was observed during the period of 2011-2014, with 2248, 5446, 6438 and 7689 cases, respectively. It is noteworthy that the majority of these cases occurred in children under one year of age, and that the incidence, morbidity and mortality of pertussis were higher in infants aged < 2 months [1–5].
Siblings and parents, mainly mothers, have frequently been identified as primary sources of pertussis infection for infants [6, 7]. In response, several countries have recently adopted pertussis maternal immunization practices to protect young infants, whose disease is more severe, until they can receive their own routine primary vaccination [8–11]. This measure provides protection to newborns via passive transplacental transfer, offering antibodies in the neonatal period between birth and 2 months of age [9]. The current childhood routine vaccination schedule used in Brazil includes 3 doses of the diphtheria, tetanus, and whole-cell pertussis (DTwP) + Haemophilus influenzae b + hepatitis B (DTwP-Hib-HBV) pentavalent vaccine given at 2, 4 and 6 months of age, followed by two booster doses of DTwP at 15 months and 4 years of age [12].
Even though an accepted serologic correlate of protection has not been established, high levels of antibodies to pertussis toxin (PT) and pertactin (PRN) have been associated with clinical protection against pertussis [8, 13, 14]. In Brazil, maternal immunization with Tdap vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis) was introduced in November, 2014, after the Ministry of Health recommended vaccinating women from weeks 27 to 36 of pregnancy [15]. However, in 2017, the recommendation was changed to the 20th week of pregnancy to reach as many pregnant women as possible, including those with limited access to prenatal care [16]. Most guidelines recommend vaccination in the third trimester, but the best period to vaccinate during gestation remains unknown [17–21]. Moreover, while the effect of maternal Tdap booster on placental transfer of PT-specific IgG antibodies in infants has been previously reported [19], [22–28], there is no studies on placental transfer on anti-PT IgG antibodies in Brazil.
The present study aimed to evaluate the new strategy of the Brazilian Immunization Program against pertussis, assessing the immunogenicity of Tdap vaccine during pregnancy by measuring the anti-PT IgG response in vaccinated and unvaccinated women and their respective newborn infants.
2. Material and Methods
2.1. Study population, specimen collection and processing
In this cross-sectional study, 362 pregnant women were recruited during their delivery from two different hospitals, Hospital and Maternity Interlagos and the Hospital Leonor Mendes de Barros, from July 2015 to August 2016 in Sao Paulo, Brazil. Information on demographic, socioeconomic and obstetric history, antenatal care and delivery of newborns was obtained from all pregnant women who accepted to participate. The study was approved by the Ethics Committee of the Instituto Adolfo Lutz, and a written informed consent was obtained from the participants. Vaccination status was verified with each individual’s vaccination records and confirmed with the centralized Information System from National Immunization Program (SI PNI).
Inclusion criteria included maternal age ≥18 years, availability of maternal vaccination records, delivery between weeks 37 and 42 of gestation, and a non-twin newborn. Exclusion criteria included newborn weight <2500g, pregnant women with vaccination <15 days prior to delivery, multiple or recent (within three months) blood transfusions, immunosuppressive therapy (period ≥15 days), cardiac, renal, or neurologic disorders, cough illness lasting ≥2 weeks with one of the pertussis symptoms (paroxysms, “whoop,” or posttussive vomiting). Cord blood was collected at delivery and maternal blood was collected within 24 hours of delivery. Maternal and cord blood samples were centrifuged to collect plasma at the hospitals within 24 hours after withdrawal. Refrigerated plasma samples were transported to the Pertussis Serology Laboratory of the Instituto Adolfo Lutz, where they were aliquoted, coded, and frozen at −80°C for storage until testing.
2.2. Maternal Tdap
The vaccine for all pregnant women was administered intramuscularly into the deltoid muscle during a routine pregnancy check-up. The vaccine used was Boostrix® (GSK Biologicals, Rixensart, Belgium), licensed in Brazil as Refortrix®, which contains 20 IU of tetanus toxoid, 2 IU of diphtheria toxoid, 8 μg of inactivated pertussis toxoid, 8 μg of filamentous haemagglutinin, and 2.5 μg of pertactin.
2.3. Laboratory methods
Detection of IgG antibodies to PT in plasma was determined by ELISA using the Centers for Disease Control and Prevention (CDC) protocol as previously described [29–31]. Masked plasma samples were tested at a dilution of 1:100, with 5 μL of specimen, and plated in triplicate wells. Samples were tested twice by two different technicians. The final concentration was the average of the two results. Paired mother and cord blood samples were tested on the same plate.
Anti-PT IgG antibodies were quantified using standard curves constructed with six standards (15–480 International Units (IU)/mL) that are calibrated to the WHO International Standard 06/140 (NIBSC, England), provided by the CDC. Microplates were read at 450 nm with a Sunrise microplate reader (Tecan, Switzerland) and analyzed using the software Magellan 6.6 (Tecan, Switzerland) employing a 4–parameter logistic regression curve. The lower limit of quantitation (LLQ) was 15 IU/mL; for this study, all values <LLQ were assigned a concentration of 7.5 IU/mL.
2.4. Statistical Analysis
Sample sizes were estimated based on the following parameters: seropositivity of 50% of anti-PT IgG among newborns of vaccinated women, relative risk of 2.0, significance level of 5%, test power of 80% and an one-tailed test. The minimum number of sample units was 45 for each group (vaccinated and unvaccinated). Descriptive statistics consisted of absolute and relative frequencies or means and standard deviations of demographic and socioeconomic characteristics, obstetric history, prenatal and delivery data. These variables were compared between vaccinated and unvaccinated groups, using the chi-square test and Student t test, with a 5% significance level.
Geometric mean concentrations (GMC) with 95% confidence intervals (95% CI) of maternal and cord blood antibodies were calculated. Comparison of GMCs between groups (vaccinated and unvaccinated) was done using the Mann Whitney test. To assess the effect of maternal vaccination on anti-PT IgG levels, we performed a multiple linear regression model (with log10 transformation of antibody concentrations) adjusting for confounding variables. These control variables were selected in univariate analyses (p<0.20) and were significant in the final model (p<0.05). The Kruskal Wallis test was used to evaluate GMCs of maternal and cord blood antibodies in relation to vaccination week and time interval between vaccination and delivery. Pearson correlation coefficients were calculated for maternal and cord blood antibodies, cord blood antibodies with gestational weeks at Tdap vaccination, and cord blood antibodies with time interval between vaccination and delivery. All statistical analyses were performed using Stata 12 Software (StataCorp LLC, Texas, USA).
3. Results
3.1. General characteristics of the study population
A total of 362 pregnant women were recruited to take part in this study. There were 44 exclusions: birthweight <2500g (n=6); maternal age <18 years (n=2); preterm birth (n=4); no maternal/cord blood available (n=17); vaccination <15 days prior to delivery (n=2); quit participation (n=6); no vaccination information (n=7). Ultimately, 243 Tdap vaccinated women and 75 unvaccinated women with paired maternal and cord blood participated.
Characteristics of mothers included in this analysis are shown in Table 1. The mean maternal age was 28.5 years, slightly younger in the unvaccinated group, due to a higher proportion of <20 year old women (14.7%). There were no differences in the distribution of socioeconomic variables between groups. Overall, 63.5% women were non-white; 55.8% had studied ≥ 8 years; 62.7% had a monthly household income below R$ 1.576 (approximately US$ 440); 83.5% lived with <2 people aged 0-10 years. Regarding obstetric history, prenatal care, and delivery characteristics, a higher proportion of women that had <7 prenatal visits (33.8%) were in the unvaccinated group. Mean birthweight (3406.2 g) and gestation age at birth (39.3 weeks) were slightly higher in the vaccinated group. About one third of the women were nulliparous and almost all used public services for prenatal care (97.6%). A little over half of total births (55.3%) were through vaginal delivery. Newborn gender was equally distributed without difference between groups.
Table 1:
Maternal and infant characteristics, Sao Paulo, 2015-2016.
| Total | Vaccinated | Unvaccinated | p-value* | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
|
Demographic | |||||||
| Maternal age (yrs) † | 0.031 | ||||||
| <20 | 26 | 8.2 | 15 | 6.2 | 11 | 14.7 | |
| 20-30 | 175 | 55.0 | 132 | 54.3 | 43 | 57.3 | |
| 30-35 | 61 | 19.2 | 53 | 21.8 | 8 | 10.7 | |
| ≥35 | 56 | 17.6 | 43 | 17.7 | 13 | 17.3 | |
| Maternal race | 0.318 | ||||||
| White | 116 | 36.5 | 85 | 35.0 | 31 | 41.3 | |
| Non-white# | 202 | 63.5 | 158 | 65.0 | 44 | 58.7 | |
|
Socioeconomic | |||||||
| Mother educational level | 0.697 | ||||||
| <8 years | 133 | 44.2 | 102 | 43.6 | 31 | 46.3 | |
| ≥ 8 years | 168 | 55.8 | 132 | 56.4 | 36 | 53.7 | |
| Household income per month | 0.825 | ||||||
| < R$ 1.576 | 195 | 62.7 | 151 | 63.4 | 44 | 60.2 | |
| R$1.576 - 3.151 | 87 | 28.0 | 66 | 27.6 | 21 | 28.8 | |
| ≥ R$ 3.152 | 29 | 9.3 | 21 | 8.8 | 8 | 11.0 | |
| People living in household | 0.369 | ||||||
| < 2 people aged 0-10 years | 253 | 83.5 | 197 | 84.5 | 56 | 80.0 | |
| ≥ 2 people aged 0-10 years | 50 | 16.5 | 36 | 15.5 | 14 | 20.0 | |
|
Obstetrical history and antenatal care | |||||||
| Smoking during pregnancy | 0.051 | ||||||
| Yes | 35 | 11.1 | 22 | 9.2 | 13 | 17.3 | |
| No | 279 | 88.9 | 217 | 90.8 | 62 | 82.7 | |
| Nulliparous | 0.551 | ||||||
| Yes | 101 | 31.9 | 75 | 31.0 | 26 | 34.7 | |
| No | 216 | 68.1 | 167 | 69.0 | 49 | 65.3 | |
| Number of antenatal visits | 0.001 | ||||||
| <7 | 64 | 20.3 | 39 | 16.2 | 25 | 33.8 | |
| ≥7 | 251 | 79.7 | 202 | 83.8 | 49 | 66.2 | |
| Prenatal service | 0.058 | ||||||
| Public | 281 | 97.6 | 211 | 98.6 | 70 | 94.9 | |
| Private | 6 | 2.1 | 2 | 0.9 | 4 | 5.1 | |
| Mixed | 1 | 0.3 | 1 | 0.5 | 0 | 0.0 | |
|
Delivery | |||||||
| Gestation (wks)‡ | 0.074 | ||||||
| 37|−40 | 188 | 59.1 | 137 | 56.4 | 51 | 68.0 | |
| ≥40 | 130 | 40.9 | 106 | 43.6 | 24 | 32.0 | |
| Birthweight (g)• | 0.171 | ||||||
| <3000 | 54 | 17.0 | 37 | 15.2 | 17 | 22.7 | |
| 3000|−3500 | 157 | 49.4 | 117 | 48.1 | 40 | 53.3 | |
| ≥3500 | 107 | 33.6 | 89 | 36.6 | 18 | 24.0 | |
| Mode of delivery | 0.233 | ||||||
| Vaginal | 176 | 55.3 | 130 | 53.5 | 46 | 61.3 | |
| Cesarean | 142 | 44.7 | 113 | 46.5 | 29 | 38.7 | |
| 1-minute apgar <7 | 0.171 | ||||||
| Yes | 16 | 5.1 | 10 | 4.2 | 6 | 8.2 | |
| No | 296 | 94.9 | 229 | 95.8 | 67 | 91.8 | |
| Sex of newborn | 0.189 | ||||||
| Male | 161 | 50.6 | 128 | 52.7 | 33 | 44.0 | |
| Female | 157 | 49.4 | 115 | 47.3 | 42 | 56.0 | |
Chi-Squared test
Maternal age - mean (standard deviation): 29.0 (6.4) in vaccinated group and 27.0 (6.5) in unvaccinated group (p=0.020)
Non-white includes black, “pardo” (mixed race), Asian and Indigenous
Gestation (wks) - mean (standard deviation): 39.3(1.1) in vaccinated group and 39.0 (1.1) in unvaccinated group (p=0.051)
Birthweight (g) - mean (standard deviation): 3406.2(406.9) in vaccinated group and 3260.6(368.5) in unvaccinated group (p=0.006)
3.2. Anti-PT IgG levels
Maternal anti-PT (≥15 IU/mL) IgG levels were found in 30.7% of the unvaccinated women and 94.2% of the vaccinated ones. Cord anti-PT (≥15 IU/mL) IgG levels were found in 38.7% of the unvaccinated group and 96.3% of the vaccinated group. The GMC of anti-PT IgG in maternal plasma was 5.4 fold higher among the vaccinated mothers (66.5 IU/mL; 95%CI 60.1–73.6 IU/mL) compared to the unvaccinated ones (12.4 IU/mL; 95%CI 10.3–14.9 IU/mL) (p<0.001) (Table 2). The same occurred for anti-PT IgG levels in cord blood, which was 5.6 fold higher in the vaccinated group (89.8 IU/mL; 95%CI 80.9–99.6 IU/mL) compared to the unvaccinated group (16.1 IU/mL; 95%CI 12.7–20.4 IU/mL) (p<0.001).
Table 2:
Geometric mean concentration (GMC) of anti-pertussis toxin (PT) IgG in vaccinated and unvaccinated maternal and cord blood.
| Maternal anti-PT IgG |
p-value* | Cord blood anti-PT IgG |
p-value* | |||
|---|---|---|---|---|---|---|
| N | % | GMC (95% CI) | GMC (95% CI) | |||
| Unvaccinated | 75 | 23.6 | 12.4 (10.3-14.9) | <0.001 | 16.1 (12.7-20.4) | <0.001 |
| Vaccinated | 243 | 76.4 | 66.5 (60.1-73.6) | 89.8 (80.9-99.6) |
Mann-Whitney test
3.3. Regression analysis
The regression model showed that vaccination had an important effect on anti-PT IgG levels in maternal plasma (β=0.733; 95% CI 0.635 – 0.831) adjusted for pre-pregnancy body mass index BMI (β= −0.010; 95%CI −0.018 - −0.002), and cord plasma (β=0.739; 95%CI 0.641 – 0.838) adjusted for maternal race and gestational age at birth (β=0.091; 95% CI 0.003 – 0.177) (results not shown in tables). Pre-pregnancy BMI mean (standard deviation, SD): 26.5 (5.7) in vaccinated group and 25.7 (5.4) in unvaccinated group (p=0.302).
3.4. Time interval between vaccination and delivery
Table 3 shows maternal and cord blood anti-PT IgG GMCs by gestational week of vaccination and time interval between vaccination and delivery. Mothers received their Tdap vaccine at a mean (SD) of 30.1 (3.2) weeks of gestation and 9.3 (3.2) weeks prior to delivery. No significant differences were observed in maternal anti-PT IgG GMCs by gestational week of vaccination or time interval to delivery. Comparison of cord blood GMCs by gestational week at vaccination also did not show significant difference, but higher antibody levels were found in cord blood from mothers vaccinated at least 60 days (8 weeks) before delivery. The ratio between cord and maternal antibodies at delivery decreased relative to the progression of gestational week of vaccination, and increased with a more prolonged time interval between vaccination and delivery (p<0.001).
Table 3:
Geometric mean concentration (GMC) of anti-pertussis toxin (PT) IgG in maternal and cord blood stratified by gestational week at Tdap vaccination and time interval between vacination and delivery.
| Maternal anti-PT IgG |
p-value* | Cord blood anti-PT IgG |
p-value* | Ratio ** (95% CI) | |||
|---|---|---|---|---|---|---|---|
| N | % | GMC (95% CI) | GMC (95% CI) | ||||
| Gestational Week | |||||||
| Mean (SD) | 30.1 (3.2) | ||||||
| 17|-|26 | 21 | 8.6 | 72.7 (58.3-90.6) | 0.764 | 114.3 (95.4-136.9) | 0.060 | 1.6 (1.4-1.8) |
| 27|-|29 | 87 | 35.8 | 64.5 (54.1-76.8) | 100.1 (83.6-119.8) | 1.5 (1.4-1.7) | ||
| 30|-|33 | 97 | 40.0 | 64.6 (54.4-76.6) | 84.9 (72.2-99.9) | 1.3 (1.2-1.4) | ||
| 34|-|40 | 38 | 15.6 | 73.7 (56.9-95.4) | 70.4 (51.7-96.1) | 1.0 (0.8-1.1) | ||
| Time interval between vaccination and delivery (days) | |||||||
| Mean (SD) | 65.0 (22.1) | ||||||
| 15|-|30 | 17 | 7.0 | 74.7 (50.2-111.2) | 0.411 | 54.0 (34.4-84.8) | 0.006*** | 0.8 (0.6-0.9) |
| 31|-|60 | 77 | 31.7 | 66.4 (54.8-80.6) | 82.8 (68.2-100.5) | 1.2 (1.1-1.3) | ||
| 61|-|90 | 128 | 52.7 | 62.9 (54.7-72.3) | 93.6 (81.7-107.2) | 1.5 (1.4-1.6) | ||
| ≥ 91 | 21 | 8.6 | 86.1 (64.8-114.7) | 141.2 (101.1-197.1) | 1.6 (1.4-1.9) | ||
Kruskal Wallis test
p<0.001
15|-|30 vs 61|-|90 p= 0.014; 15|-|30 vs ≥ 91p= 0.001; 31|-|60 vs ≥ 91 p= 0.016
Figure 1 shows a strong positive correlation (r=0.917) between the anti-PT IgG levels in maternal and cord blood at delivery (p<0.001). Figure 2A shows a negative correlation (r=−0.150) between the anti-PT IgG levels in cord blood and gestational week at Tdap vaccination (p=0.020) and Figure 2B shows a positive correlation (r=0.196) with time interval between vaccination and delivery (days) (p=0.002).
Figure 1:
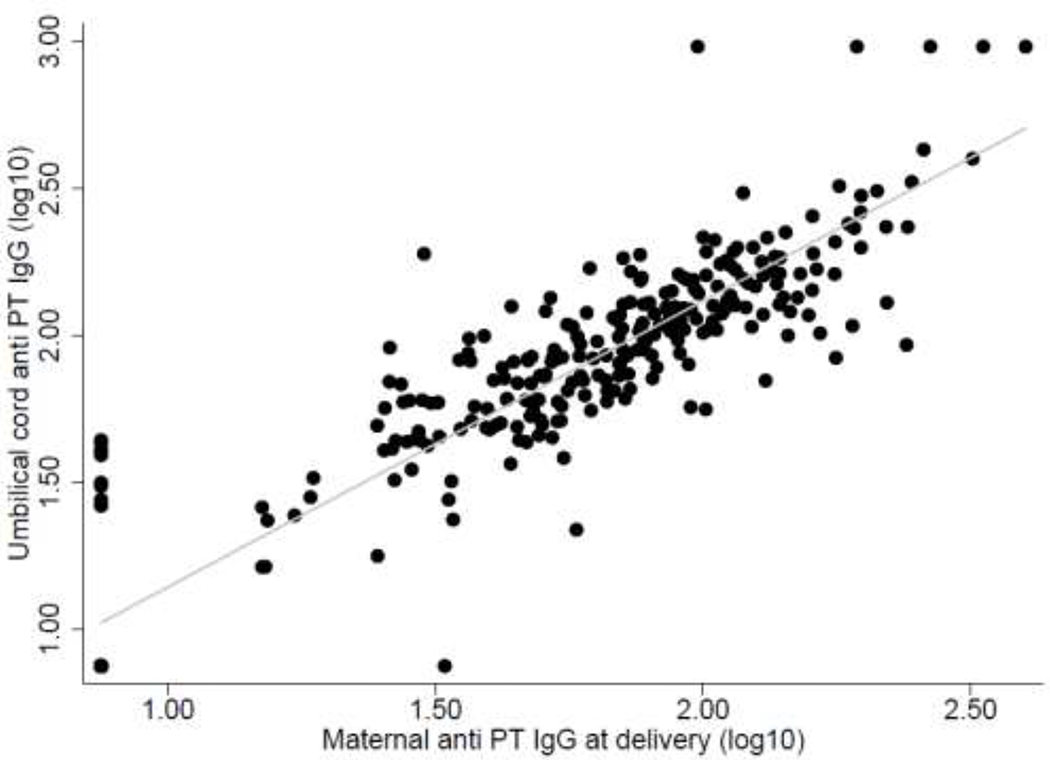
Correlation between the anti-PT IgG levels in maternal and cord blood (log 10) (r=0.9171, p value<0.001).
Figure 2:
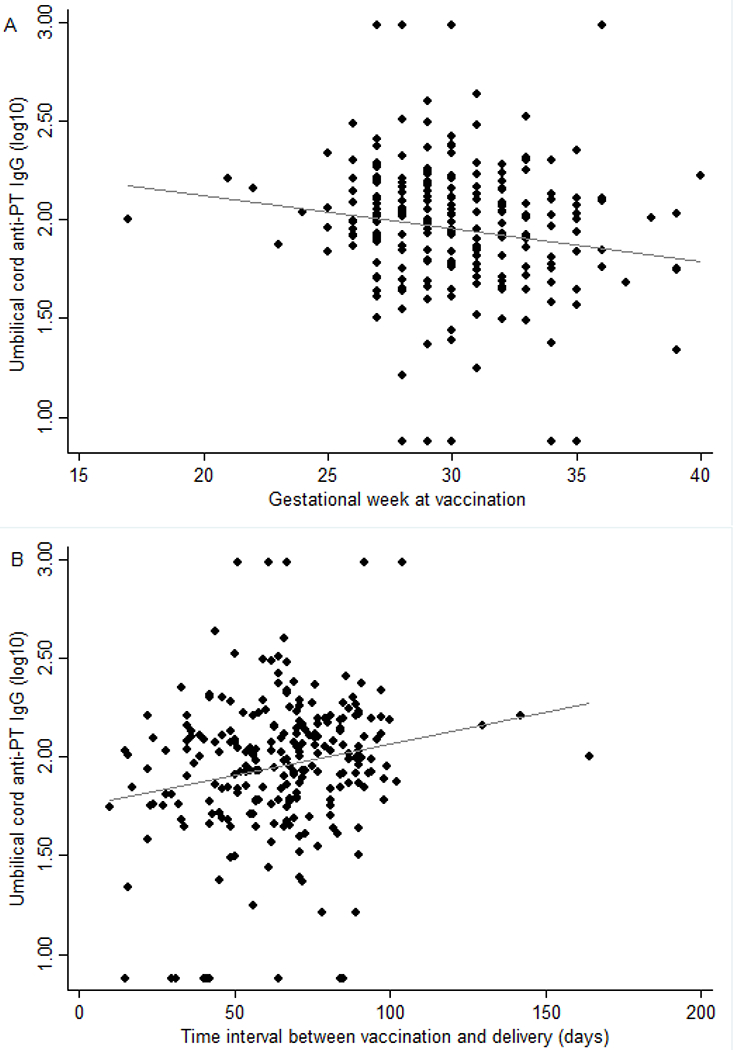
Correlation between the cord blood anti-PT IgG levels (log10) and gestational weeks at Tdap vaccination (A: r= −0.150, p value =0.020) and time interval between vaccination and delivery (days) (B: r= 0.196, p value=0.002).
4. Discussion
This is the first study to evaluate the maternal vaccination strategy in Brazil assessing the effects of Tdap vaccine during pregnancy on the anti-PT IgG response in mothers and their newborns. In Latin America, Fallo et al. [24] and Hincapié-Palacio et al. [22] conducted similar studies on expectant mothers and their newborns; however, in Argentina the scenario is different from Brazil and Colombia as an additional Tdap booster vaccination for adolescents is routinely used. To date and to the best of our knowledge, only Colombia and Brazil have introduced the Tdap booster during pregnancy in combination with a wP for infants without a Tdap adolescent booster [22]; our results corroborate Hincapié-Palacio’s findings. Regression analysis showed the GMCs of anti-PT IgG in maternal and cord blood were higher among vaccinated mothers compared to the unvaccinated ones (p<0.001), and these results are consistent with those from studies conducted in other countries [25–28].
The optimal timing of pertussis vaccination in pregnancy remains uncertain [32]. It is known that transplacental transfer of IgG pertussis antibodies increases starting from week 17 of gestation [33] and the predominance of this transfer across the placenta occurs during the third trimester; however, the efficiency of transplacental transport is affected by several factors, including the mother’s immune response, her age at delivery, and placental dysfunction or diseases [34]. In our study, higher cord blood antibody levels were observed when the time interval between vaccination and delivery was at least 60 days (8 weeks), suggesting that vaccination early in the third trimester or late in the second trimester may be more effective than later in pregnancy. Though not statistically significant, the results in the cord blood by gestational week of vaccination support this finding. Other studies with the same vaccine also found that vaccination early in the third trimester may be more effective than later in pregnancy [35, 36] and when time intervals between vaccination and delivery are at least 4 weeks long [28, 32]. Finally, although transplacental transfer of antibody is more efficient near term delivery, consideration should also be made that vaccination early in the third trimester or late in the second trimester may be more beneficial to preterm infants [37].
Several studies have been carried out with a similar objective, but there is some variability due to different acellular vaccine formulations that differ both in antigen composition and the method of detoxification. In this study, pregnant women were vaccinated with Boostrix, which is prepared with double detoxification (formaldehyde and glutaraldehyde) that might cause differences in immunogenicity [38]. Different settings, vaccine programs, and populations targeted should also be considered.
We performed the ELISA assay using only one dilution to measure anti-PT IgG antibodies. Although this approach is not perhaps the best way to get the most accurate imunogenicity results, single dilution assays have been used in many other studies [22, 27, 28, 35, [39].
There are some limitations to our study: pre-vaccination maternal plasma was not obtained, and the number of pregnant women who received the vaccine in second trimester (20-26 weeks gestation) was small, because most mothers were vaccinated during the third trimester as recommended by the Brazilian Immunization Program during the study period. Furthermore, clinical and background data was obtained from two public maternity hospitals which attend a low income population. Their clients are mostly non-white and have less than eight years of education. These hospitals are located in two different regions of the city of Sao Paulo (South and East), and may not be representative of the other regions of the city.
We had 52 samples with values <15 IU/mL in the unvaccinated mothers (69.3%) and 14 samples in the vaccinated mothers (5.8%). The former result could reflect a natural exposure to B. pertussis, whereas the latter could point to a possible pertussis vaccine failure, which constitutes an important factor that still remains to be elucidated. In fact, it is very complex to discuss this failure here since only anti-PT antibodies were measured and other aspects of the immune response were not investigated. Furthermore, serological correlates of protection are still unknown in pertussis.
Studies of immunogenicity against other pertussis antigens, such as PRN, that are correlated with protection should be carried out to investigate the effect of timing of maternal acellular vaccination in pregnancy. Likewise, analyzing the immune response differences to antigens that are not included in this Tdap formulation (that contains 3 components: PT, FHA, and PRN) but are included in the whole cell vaccine, like fimbrial antigens or adenylate cyclase toxin [40], deserves additional investigation. Also, other studies about the functionality of the antibodies or their neutralizing capacity, as well as the investigation of the clinical protection of young infants, should be performed [36].
A cohort study of mothers and their infants is currently ongoing, using antibody detection against other pertussis antigens by a microsphere-based, multiplex antibody capture assay (MMACA) to reaffirm this finding and determine the influence of maternal Tdap on a child’s immunological response after their own primary vaccination series is underway.
5. Conclusion
Tdap maternal immunization yielded significantly higher anti-PT IgG levels in vaccinated mothers and their infants compared to their unvaccinated counterparts. We observed higher GMCs of anti-PT IgG when the maternal Tdap occurred at least 60 days before delivery, suggesting that vaccination early in the third trimester may be more effective than later in pregnancy. This finding supports the current recommendation of the Brazilian Immunization Program.
Acknowledgements
The Maternal Pertussis Vaccine Working Group consists of: Edna M. de Souza, Jane H. Atobe, Carmem A F Oliveira for the Immunological Center, Instituto Adolfo Lutz and Daniela Leite for the National Reference Center for Pertussis, Bacteriology Center, Instituto Adolfo Lutz; Marcela R. Silva for the Respiratory Division, Centro de Vigilância Epidemiológica, Secretaria de Estado de Saúde SP; Corintio Mariani-Neto and the Scientific Researcher Ana Bersusa for the Hospital e Maternidade Leonor Mendes de Barros; Rita de Cássia S. Calabresi and the nurses Rosemary A. dos Santos and Diva T Tesser for the Hospital Maternidade Interlagos and Maria Ligia Bacciotte Ramos Nerger for the Centro de Controle de Doenças, Coordenação de Vigilância em Saúde, Secretaria Municipal da Saúde SP .The authors would like to thank all participating pregnant women and their newborn infants. We also thank all collaborators at the Hospital Maternidade Interlagos, especially, the pediatrician Orlando Pauletti Júnior for patient attendance and the nurses for their help with recruitment and blood specimen collection, and all collaborators at the Hospital Leonor Mendes de Barros, for their help with blood specimen collection.
This work was supported by the Fundo Especial de Saúde para Imunização em Massa e Controle de Doenças (FESIMA), Instituto Adolfo Lutz, Coordenadoria de Controle de Doenças, Secretaria de Estado da Saúde de São Paulo SP, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2015/16157–1), and the Latin American Pertussis Project, a collaboration between the US Centers for Disease Control and Prevention, the Pan American Health Organization, the Sabin Vaccine Institute, and selected ministries of health to strengthen pertussis surveillance in Latin America.
Footnotes
Conflict of Interest
All authors report no Conflicts of Interest
References
- [1].Guimarães LM, Carneiro EL, Carvalho-Costa FA. Increasing incidence of pertussis in Brazil: a retrospective study using surveillance data. BMC Infect Dis. 2015;15:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brazil. Coqueluche no Brasil: análise da situação epidemiológica de 2010 a 2014. Brazil: Ministry of Health, Boletim Epidemiológico; 2015. [Google Scholar]
- [3].Fernandes EG, Sartori AMC, de Soárez PC, Carvalhanas TRMP, Rodrigues M, Novaes HMD. Challenges of interpreting epidemiologic surveillance pertussis data with changing diagnostic and immunization practices: the case of the state of São Paulo, Brazil. BMC Infect Dis. 2018;18:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sistema Nacional de Agravos de Notificação. 2012. [Google Scholar]
- [5].Falleiros Arlant LH, de Colsa A, Flores D, Brea J, Avila Aguero ML, Hozbor DF. Pertussis in Latin America: epidemiology and control strategies. Expert Rev Anti Infect Ther. 2014;12:1265–75. [DOI] [PubMed] [Google Scholar]
- [6].Wiley KE, Zuo Y, Macartney KK, McIntyre PB. Sources of pertussis infection in young infants: a review of key evidence informing targeting of the cocoon strategy. Vaccine. 2013;31:618–25. [DOI] [PubMed] [Google Scholar]
- [7].Skoff TH, Kenyon C, Cocoros N, Liko J, Miller L, Kudish K, et al. Sources of Infant Pertussis Infection in the United States. Pediatrics. 2015;136:635–41. [DOI] [PubMed] [Google Scholar]
- [8].Heininger U, Riffelmann M, Bär G, Rudin C, von König CH. The protective role of maternally derived antibodies against Bordetella pertussis in young infants. Pediatr Infect Dis J. 2013;32:695–8. [DOI] [PubMed] [Google Scholar]
- [9].Gkentzi D, Katsakiori P, Marangos M, Hsia Y, Amirthalingam G, Heath PT, et al. Maternal vaccination against pertussis: a systematic review of the recent literature. Arch Dis Child Fetal Neonatal Ed. 2017;102:F456–F63. [DOI] [PubMed] [Google Scholar]
- [10].Pertussis vaccines: WHO position paper - September 2015. Wkly Epidemiol Rec. 2015;90:433–58. [PubMed] [Google Scholar]
- [11].Skoff TH, Blain AE, Watt J, Scherzinger K, McMahon M, Zansky SM, et al. Impact of the US Maternal Tetanus, Diphtheria, and Acellular Pertussis Vaccination Program on Preventing Pertussis in Infants <2 Months of Age: A Case-Control Evaluation. Clin Infect Dis. 2017;65:1977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brazil. Programa Nacional de Imunizações. 2015. [Google Scholar]
- [13].Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. 1998;16:1907–16. [DOI] [PubMed] [Google Scholar]
- [14].Taranger J, Trollfors B, Lagergård T, Sundh V, Bryla DA, Schneerson R, et al. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis. 2000;181:1010–3. [DOI] [PubMed] [Google Scholar]
- [15].Brazil. Informe Técnico para Implantação da Vacina Adsorvida Difteria, Tétano e Coqueluche (Pertussis Acelular) Tipo adulto - dTp. Brazil: Ministry of Health; 2014. [Google Scholar]
- [16].Brazil. Novo calendário vacinal de 2017. 2017. [Google Scholar]
- [17].(CDC) CfDCaP. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62:131–5. [PMC free article] [PubMed] [Google Scholar]
- [18].UK. Pregnant women to be offered whooping cough vaccination. UK Department of Health; 2012. [Google Scholar]
- [19].Leuridan E Pertussis vaccination in pregnancy: State of the art. Vaccine. 2017;35:4453–6. [DOI] [PubMed] [Google Scholar]
- [20].Becker-Dreps S, Butler AM, McGrath LJ, Boggess KA, Weber DJ, Li D, et al. Effectiveness of Prenatal Tetanus, Diphtheria, Acellular Pertussis Vaccination in the Prevention of Infant Pertussis in the U.S. Am J Prev Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Calvert A, Jones CE. Placental transfer of antibody and its relationship to vaccination in pregnancy. Curr Opin Infect Dis. 2017;30:268–73. [DOI] [PubMed] [Google Scholar]
- [22].Hincapié-Palacio D, Hoyos MC, Ochoa J, Montoya N, García D, Osorio E, et al. Effect of maternal immunization against pertussis in Medellin and the metropolitan area, Colombia, 2016–2017. Vaccine. 2018;36:3984–91. [DOI] [PubMed] [Google Scholar]
- [23].Warfel JM, Papin JF, Wolf RF, Zimmerman LI, Merkel TJ. Maternal and neonatal vaccination protects newborn baboons from pertussis infection. J Infect Dis. 2014;210:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fallo AA, Neyro SE, Manonelles GV, Lara C, Hozbor D, Zintgraff J, et al. Prevalence of Pertussis Antibodies in Maternal Blood, Cord Serum, and Infants From Mothers With and Those Without Tdap Booster Vaccination During Pregnancy in Argentina. J Pediatric Infect Dis Soc. 2018;7:11–7. [DOI] [PubMed] [Google Scholar]
- [25].Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;204:334.e1-5. [DOI] [PubMed] [Google Scholar]
- [26].Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311:1760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vilajeliu A, Ferrer L, Munrós J, Goncé A, López M, Costa J, et al. Pertussis vaccination during pregnancy: Antibody persistence in infants. Vaccine. 2016;34:3719–22. [DOI] [PubMed] [Google Scholar]
- [28].Abu Raya B, Srugo I, Kessel A, Peterman M, Bader D, Gonen R, et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels - a prospective study. Vaccine. 2014;32:5787–93. [DOI] [PubMed] [Google Scholar]
- [29].Baughman AL, Bisgard KM, Edwards KM, Guris D, Decker MD, Holland K, et al. Establishment of diagnostic cutoff points for levels of serum antibodies to pertussis toxin, filamentous hemagglutinin, and fimbriae in adolescents and adults in the United States. Clin Diagn Lab Immunol. 2004;11:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Menzies SL, Kadwad V, Pawloski LC, Lin TL, Baughman AL, Martin M, et al. Development and analytical validation of an immunoassay for quantifying serum anti-pertussis toxin antibodies resulting from Bordetella pertussis infection. Clin Vaccine Immunol. 2009;16:1781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kapasi A, Meade BD, Plikaytis B, Pawloski L, Martin MD, Yoder S, et al. Comparative study of different sources of pertussis toxin (PT) as coating antigens in IgG anti-PT enzyme-linked immunosorbent assays. Clin Vaccine Immunol. 2012;19:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eberhardt CS, Blanchard-Rohner G, Lemaître B, Boukrid M, Combescure C, Othenin-Girard V, et al. Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis. Clin Infect Dis. 2016;62:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Englund JA. The influence of maternal immunization on infant immune responses. J Comp Pathol. 2007;137 Suppl 1:S16–9. [DOI] [PubMed] [Google Scholar]
- [34].Gall SA. Vaccines for pertussis and influenza: recommendations for use in pregnancy. Clin Obstet Gynecol. 2008;51:486–97. [DOI] [PubMed] [Google Scholar]
- [35].Naidu MA, Muljadi R, Davies-Tuck ML, Wallace EM, Giles ML. The optimal gestation for pertussis vaccination during pregnancy: a prospective cohort study. Am J Obstet Gynecol. 2016;215:237.e1-6. [DOI] [PubMed] [Google Scholar]
- [36].Abu Raya B, Srugo I, Bamberger E. Optimal Timing of Immunization Against Pertussis During Pregnancy. Clin Infect Dis. 2016;63:143–4. [DOI] [PubMed] [Google Scholar]
- [37].Ercan TE, Sonmez C, Vural M, Erginoz E, Torunoğlu MA, Perk Y. Seroprevalance of pertussis antibodies in maternal and cord blood of preterm and term infants. Vaccine. 2013;31:4172–6. [DOI] [PubMed] [Google Scholar]
- [38].Abraham C, Pichichero M, Eisenberg J, Singh S. Third-Trimester Maternal Vaccination Against Pertussis and Pertussis Antibody Concentrations. Obstet Gynecol. 2018;131:364–9. [DOI] [PubMed] [Google Scholar]
- [39].Healy CM, Rench MA, Swaim LS, Smith EO, Sangi-Haghpeykar H, Mathis MH, et al. Association Between Third-Trimester Tdap Immunization and Neonatal Pertussis Antibody Concentration. JAMA : the journal of the American Medical Association. 2018;320:1464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guiso N Bordetella Adenylate Cyclase-Hemolysin Toxins. Toxins (Basel). 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]


