Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus responsible for the coronavirus disease -19 (COVID-19). Since December 2019, SARS-CoV-2 has infected millions of people worldwide, leaving hundreds of thousands dead. Chloroquine (CQ) and Hydroxychloroquine (HCQ) are antimalarial medications that have been found to have in vitro efficacy against SARS-CoV-2. Several small prospective studies have shown positive outcomes. However, this result has not been universal, and concerns have been raised regarding the indiscriminate use and potential side effects. The clinicians are conflicted regarding the usage of these medications. Appropriate dose and duration of therapy are unknown. Here, we will discuss the pharmacokinetic and pharmacodynamic properties of CQ and HCQ, as well as review the antiviral properties. The manuscript will also examine the available data from recent clinical and preclinical trials in order to shed light on the apparent inconsistencies.
Key Indexing Terms: Chloroquine, Hydroxychloroquine, SARS-CoV-2, COVID-19
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus responsible for the coronavirus disease-19 (COVID-19). SARS-CoV-2 is a beta coronavirus. Coronaviruses (CoV) belong to the family Coronaviridae of the order Nidovirales. Infection by SARS-CoV-2 was first reported in Wuhan, China, in December of 2019 and has become a worldwide pandemic, infecting millions of people and causing hundreds of thousands of deaths. COVID-19 is a zoonotic disease, likely originating from bats and has infected humans from a yet unknown intermediate host.1 The virus spreads through contact with respiratory secretions, droplets, and aerosols.2 The United States has seen the highest number of cases and has had the largest COVID-19 fatality reported to date. Aggressive public health measures such as social distancing, contact tracing, and testing have seemingly slowed down the projected trajectory of the pandemic in the U.S., but concerns remain whether this will become the new norm. It is unknown whether SARS-CoV-2 will become a seasonal, cyclical disease like Influenza and other human coronavirus strains (HCoV): 229E, NL63, OC43, HKU1. The consequences of this pandemic could be devastating, especially for resource deprived countries where public health measures are inadequate and health care facilities are not as advanced as the western world. In general, HCoVs are associated with mild respiratory tract infections that represent 10-30% of seasonal cases.3 The disease can be severe in patients with preexisting lung diseases, such as chronic obstructive pulmonary disease.
In 2003, the world experienced devastation by the first novel coronavirus, severe acute respiratory syndrome associated coronavirus, SARS-CoV. Infection with SARS-CoV resulted in atypical pneumonia that affected more than eight thousand people and caused more than seven hundred deaths (9% mortality). The disease spread to 26 countries through international travel.4 Another coronavirus, the Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV), arose in 2012 in parts of the middle east and was associated with a thirty-four percent mortality affecting patients in twenty-seven countries.5 The latest threat, initially named 2019-novel coronavirus (2019-nCoV), and later, SARS-CoV-2, is closely related to the original SARS-CoV.6 Until recently, no treatment for COVID-19 showed definitive efficacy. The Food and Drug Administration (FDA) recently approved emergency use authorization for Remdesivir to be used in patients with severe illness due to SARS-CoV-2. This announcement followed a recent publication that showed a reduced duration of disease in patients treated with Remdesivir.7 Multiple other antivirals, anti-inflammatory, and immunomodulating medications are currently under investigation or being empirically used by physicians to treat COVID-19.8
An old antimalarial medication chloroquine (CQ), and its derivative Hydroxychloroquine (HCQ), have become increasingly popular in the attempt to find an effective treatment for COVID-19, especially after being endorsed by the U.S. president in March 2020. CQ has been used for more than seventy years as an antimalarial drug; however, the use of HCQ is rather recent. Although the efficacy of these compounds have declined as antimalarials, the identification of their role as immunomodulators has revolutionized modern medicine. HCQ is commonly used for many autoimmune diseases, including lupus, rheumatoid arthritis, Sjogren syndrome, inflammatory myopathies, and cutaneous sarcoidosis.9 In vitro studies have also demonstrated potent antiviral properties of CQ and HCQ. Based on this data, many clinicians and hospitals have incorporated CQ and HCQ into the treatment algorithm for COVID-19 patients, although, currently, there remains a significant paucity of high-quality data. The use of these medications, however, does not come without risks and could be associated with serious side effects. One such concern is the development of a potentially life-threatening malignant cardiac arrhythmia. Within the last few months, several prospective studies have revealed contradicting results regarding the utility of CQ and HCQ. In this manuscript, we will discuss the pharmacokinetic and pharmacodynamic properties of CQ and HCQ as well as review the antiviral properties; we will also examine the available data from recent clinical and preclinical studies involving COVID-19 patients to shed light on the apparent inconsistencies.
Pharmacokinetics and pharmacodynamic properties
CQ and HCQ are synthetic antimalarial drugs. CQ is a 4-aminoquinoline and was first synthesized in 1934.10 Large scale production of CQ was crucial during the second world war for the prevention and treatment of malaria among the coalition forces. The incidental improvement of rashes and inflammatory arthritis among soldiers provided a clue to the anti-inflammatory and immunomodulatory property of CQ.9 HCQ was later produced in 1955 by substitution of the N-diethyl group of CQ by N-dihydroxy-ethyl side chain. This side chain modification led to the preserved efficacy and reduced side effects. As a result, HCQ is currently the more commonly prescribed medication between the two.
HCQ and CQ have complex and somewhat different pharmacokinetic properties due to the large volume of distribution and long terminal half-life.11 , 12 Both are weakly basic compounds due to the presence of basic side chains and are absorbed quickly from the upper gastrointestinal tract.11 CQ and HCQ are administered as phosphate and sulfate salts, respectively. The bioavailability is relatively high, between 70-80%. The lag time between the ingestion of the medication and detectable blood level is about 0.43 hours, and peak serum concentration is reached within 1-2 hours.13 There is no significant first-pass metabolism, 40-50% of the drug binds with plasma protein and undergoes extensive hepatic metabolism.13 CQ and HCQ are converted totheir active metabolite desethylchloroquine and desethylhydroxychloroquine, respectively. The elimination half-life is between 3-5 days, although terminal half-lifes are 40-50 days due to an extensive volume of distribution (about 800 L/Kg). The plasma concentration of CQ and HCQ can be variable in an individual patient as well as among different individuals. Drug concentrations in the specific organs are difficult to assess as these compounds do not follow the pharmacological three compartment model of drug distribution.10 Physiologically based pharmacokinetic modeling is now available to accurately estimate drug concentration in the deeper tissue. One important feature is the ability of these molecules to bind with the pigment melanin. Prolonged exposure might cause irreversible retinopathy. Deposition in melanin rich tissue is responsible for long terminal half-life. Other than the eyes, CQ and HCQ are also found in the heart, liver, lungs, leukocytes, and liver for a prolonged period of time. At a cellular level, because of their basic nature, these drugs accumulate in the acidic organelles, such as the endosomes, lysosomes and Golgi apparatus, and increase the pH.14 This property is responsible for the very high volume of distribution and a rapid drop in serum concentration following absorption. The lysosomotropic effect is also thought to contribute to the antimicrobial effect. The renal excretion of unchanged medication is 21% for HCQ and 51% for CQ. Renal failure can increase the bioavailability of the drug, and there is no dosing recommendation for patients with severe kidney disease.12 There is some evidence that HCQ might be safer than CQ.15 , 16 However, it is important to emphasize that the pharmacokinetic and pharmacodynamic properties of these medications can vary significantly, especially in critically ill patients with hemodynamic instability, altered absorption, and rapid changes in volume status, among others. It is also uncertain whether COVID-19 itself can precipitate unpredictable and unanticipated drug effects. Observational and randomized control trials to ascertain the appropriate dosing and therapeutic window amid a pandemic would be challenging. A comparison of the pharmacokinetic and pharmacodynamic properties of CQ and HCQ is presented in Table 1 .
Table 1.
Comparison of pharmacokinetic and pharmacodynamic properties of CQ and HCQ.
| Parameters | Chloroquine | Hydroxychloroquine |
|---|---|---|
| Formulation | Chloroquine phosphate | Hydroxychloroquine sulfate |
| Absorption | Upper gastrointestinal tract | Upper gastrointestinal tract |
| Bioavailability | 70-80% | 70-80% |
| Plasma protein binding | 40-50% | 40-50% |
| Volume of distribution in plasma | 65,000L | 42,257L |
| Volume of distribution in blood | 15,000L | 5,500L |
| Metabolism by liver | Desethylchloroquine | Desethylchloroquine Desethylhydroxychloroquine |
| Total blood clearance | 129 ± 35 mL/min | 96 ± 5 mL/min |
| Total plasma clearance | 1099 ± 155 mL/min | 667 ± 235 mL/min |
| Elimination half life | 288 hours | 1200 hours |
| Terminal half life | 45 ± 15 days | 41 ± 11 days |
| Renal clearance | 51% | 21% |
| Unmetabolized excretion | 58% | 62% |
Mechanism of action of CQ and HCQ as antivirals
CQ and HCQ are versatile agents. The antimicrobial properties stem from the modulation of different biologic processes. In the following section, we will review the antiviral properties of CQ and HCQ that are specific to the coronavirus.
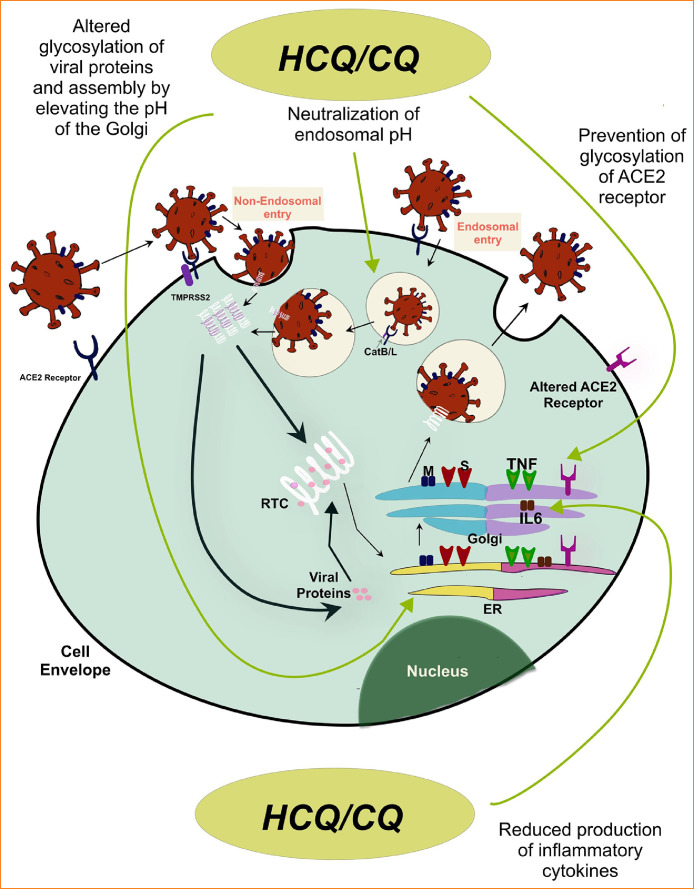
CoV belong to the family Coronaviridae of the order Nidovirales. CoV received their name due to their striking appearance, which resembles the solar corona. The virions are spherical, with an approximate diameter of 125 nm by electron microscopy.17 It is an enveloped, positive sense RNA virus composed of four main structural protein components, spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins. The trimeric surface glycoproteins (S proteins) projects as club-like spikes and is responsible for the unique morphology. The S protein also mediates attachment of the viral particle to the host receptor and is responsible for host specificity and tropism for a specific tissue.18 CoV that affect animals bind to receptors that are specific to the species. Human CoV, for example, the SARS-CoV uses angiotensin converting enzyme 2 (ACE 2) as the receptor to gain access to the cells, whereas, MERS-CoV uses dipeptidyl peptidase 4 (DPP4) as the receptor.19 , 20 Like the SARS-CoV, the SARS-CoV-2 also binds to ACE 2 as the receptor.21
Once SARS-CoV-2 gains access to a suitable host, it follows the following steps to infect host cells and establish infection18:
-
1.
Attachment of the ‘S protein’ to the ACE2 receptor
-
2.
Cleavage of S protein by acid dependent proteolytic enzyme transmembrane protease serine 2 (TMPRSS2) to expose the fusion protein 22
-
3.
Fusion of the viral fusion protein and cell membrane within the acidified endosome
-
4.
Release of viral genome in the cytoplasm
-
5.
Translation of replicase gene and formation of replicase-transcriptase complex (RTC)
-
6.
Replication, synthesis of subgenomic RNA and structural proteins
-
7.
Assembly and release of viral particles from the Golgi apparatus
-
8.
Release of viral particles by exocytosis
One viral replication cycle takes 5-6 hours, and the cytopathic effect in the cell culture is visible as early as 2 days after inoculation with SARS-CoV-2.23 Pathobiologically, the virus initially infects the epithelial cells in the nose. The patient remains asymptomatic at this stage. The infection can progress to affect the epithelial cells of the conducting airways. The body mounts an innate immune response, and the patients show clinical symptoms and signs of the disease. In most patients, the gas exchanging units remain unaffected, and the patients make a recovery with supportive therapy. However, in a minority of patients, the type 2 pneumocytes are affected, which can be manifested by ARDS and severe hypoxia.24 Although the rapidity of disease progression is variable among different hosts, there is usually an inevitable delay between the presentation with upper respiratory symptoms and progression to ARDS.
The exact mechanism of action by which the antimalarials exert antiviral properties is currently unknown. However, the following have been proposed and evaluated in research:
-
1.
The binding affinity of the S protein to ACE2 has been shown to be a major determinant of pathogenicity for SARS-CoV.21 Chloroquine has been shown to cause rapid transition of ACE2 through the Golgi apparatus and prevent the terminal glycosylation. The alteration of the receptor potentially results in reduced affinity of the S protein for surface ACE2 receptor.25
-
2.Formation of endosomes at the cell surface is one of the two mechanisms SARS-CoV-2 employs to gain access to the cytoplasm. The acidic endosomal environment is required for the acid dependent cysteine proteases cathepsin B and L (CatB/L) to prime the spike protein and expose the fusion protein, which mediates the attachment of the viral particle to the host cell membrane.22 The basic nature of CQ and HCQ results in accumulation in intracellular acidic organelles and render the environment more basic. This results in the inactivation of CatB/L, and reduces the ability of the virus to gain access inside the cell. The following provides direct evidence for this mechanism:Ammonium chloride increases the pH of the endosomes and deactivates CatB/L, and significantly reduces the entry of SARS-CoV-2 into the host cell.22 CQ and HCQ exert the same effect as ammonium chloride at a cellular level.
However, SARS-CoV-2 also enters the cell cytoplasm through a non-endosomal pathway. Transmembrane serine protease 2 (TMPRSS2) can enzymatically cleave the spike protein at the cell surface resulting in the fusion of the viral envelope to the plasma membrane and internalization of the viral nuclear material. A similar mode of entry was also seen with SARS-CoV.26 Moreover, most data regarding the viral entry has been obtained from either in vitro studies or animal models. Whether neutralization of endosomal pH by CQ and HCQ contributes to significant antiviral effect is debatable. Additionally, the inhibitor of TMPRSS2, camostat mesylate partially prevents the entry of SARS-CoV into the host cell.27
-
3.
Altered terminal glycosylation of the structural viral proteins, viral assembly, transport, and release of viral particles by altering the pH of the Golgi apparatus.28 Although this was not the case in all experiments.25
-
4.
CQ and HCQ have been shown to have reduced generation of pro-inflammatory cytokines such as tumor necrosis factor alpha and interleukin 6 (IL-6).29 However, an interim analysis of a phase 3 trial of sarilumab (a monoclonal IL-6 receptor blocker) revealed no significant benefit in ‘severely ill’ patients with COVID-19 and the protocol was modified to include only ‘critically ill’ patients.30
-
5.
Inhibition of lysosomal enzymes and impaired antigen presentation by antigen presenting cells.10
Fig. 1 summarizes the proposed site of action for CQ and HCQ.
FIGURE 1.
SARS-CoV-2 replication by endosomal and non-endosomal pathways and proposed site of action for CQ and HCQ.
In Vitro studies of CQ and HCQ
Savarino et al. first proposed the theoretical efficacy of CQ and HCQ against SARS-CoV.29 Following this hypothesis, the anti-malarial drugs were tested by in-vitro studies during and after the SARS-CoV epidemic in 2003.
Keyaerts et al. reported the efficacy of CQ against SARS-CoV in 2004.31 The authors used the Vero E6 cell lines to culture the virus. Different concentrations of CQ were employed to pretreat the cells. When cells were pretreated with a four micro mol concentration of CQ, no detectable viral replication was identified after 24 hours. A concentration of 16 mico mol was needed to abolish viral replication by 99% after 72 hours. The half-maximal effective concentration, EC50, was 8.8 mmol. The efficacy was also seen when the drug was used post-infection. In a study by Vincent et al., the cultured cell lines pretreated with CQ for 18-24 hours were refractory to infection by SARS-CoV. CQ was also effective in the reduction of the viral load when used within 3-5 hours after established infection. The drug concentration necessary to attain similar efficacy of pretreated cells was higher. The authors concluded that not only was CQ effective in preventing infection, it was also an effective therapy.25 The EC50 was 4.4 mmol, comparable with the trial by Keyaerts. No randomized control clinical control trials were performed with these compounds during the SARS outbreak.
Due to the prior observed efficacy of CQ in cell cultures against SARS-CoV, no known treatment for SARS-CoV-2, and out of desperation, clinicians and researchers in China utilized CQ and HCQ early in the treatment of COVID-19 pandemic. The use of medications with unproven benefits has led to a significant controversy regarding the safety and efficacy of these drugs. The earliest data of the in vitro efficacy of HCQ against SARS-CoV-2 was reported by Wang et al., where the authors tested multiple medications, including ribavirin, an anti-protozoal, remdesivir, and HCQ for in-vitro efficacy. CQ was found to be extremely effective against the SARS-CoC-2 with an EC50 1.13 and EC90 of 6.9 micromol.32 Yao et al. performed an elegant study to compare the effectiveness of CQ and HCQ and used physiologically based pharmacokinetic (PBPK) modeling to find the optimal dose of these medications to achieve therapeutic drug concentration in the lungs. The simulation model was validated, and the projected data was within 90% confidence interval of the observed values. In this study, the HCQ was found to be more effective than CQ (EC50 0.72 vs. 5.47 mmol in 48 hours), and interestingly, the concentration required to achieve EC50 on day 2 was lower than day 1. This was thought to be due to the accumulation of the drug intracellularly and enhancing the antiviral effect. In the study, the concentration of HCQ in the lungs was found to be 400 times higher than that of plasma. This higher lung concentration was expected, given the drug's known large volume of distribution and accumulation in intracellular acidic organelles. The tissue concentration of HCQ was also higher than CQ. The authors simulated different dosing for HCQ and based on the result, recommended an initial loading dose of 400mg twice a day, followed by 200mg twice a day for a total of 5 days.33
It is important to note that the authors only used the Vero cell lines for all of the studies discussed above. Vero cells are derived from simian kidney epithelial cells and may not be a true representation of in vivo infection. Airway epithelial cell cultures with air liquid interface is likely a better physiologic model to study respiratory viral pathogens.34 In addition to SARS-CoV and SARS-COV-2, CQ and HCQ have demonstrated in vitro efficacy against many viruses, including Influenza, HIV, Herpes simplex virus, Epstein-Barr virus (EBV), and hepatitis viruses, among others.35, 36, 37, 38, 39 Unfortunately, the in vivo data was less encouraging. A randomized trial failed to show any efficacy of CQ as a prophylactic agent against Influenza.40 In vivo data against herpes and EBV were also not promising; in fact, CQ enhanced EBV replication in Burkitt lymphoma cells.41 , 42 On the other hand, the use of CQ in patients with HIV resulted in a significant reduction of viral load, delay in the development of resistance against anti-retroviral medications, and an attenuated rate of vertical transmission.43, 44, 45
Efficacy of CQ and HCQ in clinical studies
Several clinical trials have assessed the efficacy and safety of CQ or HCQ alone or in combination with Azithromycin (AZ) in patients with COVID-19. It is noteworthy that not all trials had a control arm, and the patient population represented in these studies might have been considered ‘too stable’ or ‘too sick’. Additionally, the dosing regimen used varied among trials. Nonetheless, these studies provide valuable insight into the potency and safety of these medications.
The early reports from China
Chinese scientists and physicians started to use CQ early in the epidemic. At the peak of the COVID-19 outbreak in China, in mid-February 2020, the Chinese authority reported significant efficacy of CQ in the prevention of pneumonia, reduced duration of the illness, improved radiologic appearance, and enhanced viral clearance in 100 patients without significant side-effects.46 This perceived benefit prompted rapid endorsement by the Chinese health authority for CQ to be used in patients with mild, moderate, and severe infection, in whom there was no contraindication.47 The better potency of HCQ over CQ in vitro was later reported by Yao et al. leading to the increased interest of the medical community in the use of HCQ instead of CQ.33
The immensely positive French study
In a non-randomized French trial that included 36 patients (20 on HCQ and 16 control), Gautret et al. reported rapid virologic clearance in patients treated with HCQ.48 The patients received HCQ at a dose of 200 mg three times daily for a total of 10 days. The authors included any patient that was admitted to the hospital for this study, which included asymptomatic patients, as well as those with mild upper respiratory tract infection or lower respiratory tract infection (LRTI). The patients who were diagnosed with LRTI had computed tomographic (CT) evidence of pneumonia. The patients were enrolled within a mean delay of 4 days following symptom onset. The percentage of patients with different severity of disease was not statistically different between the treatment and control arm. The patients in the treatment group were older (51.2 versus 37.3 years). A subgroup of patients in the treatment arm also received AZ (500 mg on day one followed by 250 mg for four more days). The primary outcome of the study was virologic clearance on post inclusion day 6. Patients treated with HCQ had a viral clearance rate of 70% compared to 12.5% for the control group. Patients who received HCQ and AZ (six patients) had a response rate of 100% by day 6. Although a total of 36 patients were used for statistical calculations, six patients that were initially enrolled in the control group were excluded as they were lost to follow up. Three of these patients required ICU support, one died, one left the hospital, and one stopped taking the medication due to nausea. There was a significant difference in the nasopharyngeal viral carriage even by day three between the treated and untreated patients. This study generated tremendous enthusiasm among clinicians worldwide as rapid viral clearance was attained in days compared to a Chinese study where the median duration of viral shedding was 20 days.49 The authors did not report the details about disease progression and clinical outcome in the cohort.
The negative French study
Due to the unexpected and overwhelming success of the trial by Gautret et al., Molina et al. performed a prospective trial of 11 COVID-19 patients to replicate the efficacy of HCQ and AZ combination on viral clearance. These authors found that following the combination therapy, 80% of patients tested positive by PCR on day 5 and 6 of therapy, contradicting the result of the prior study.50 One patient died during the trial. However, in contrast to the previous study, the patients enrolled were older (58.7 Vs. 51.2 years), all but one patient on this trial was on supplemental oxygen and eight had significant comorbidities, including cancer in 5 and HIV in 1. The authors did not report the duration between the onset of symptoms and recruitment. There was no control group for this study. In one patient, the combination of HCQ and AZ led to QTc prolongation and discontinuation of the drugs. The pretreatment QTC was 405 msec, which increased to 470 msec after 4 days of therapy. The interruption was likely premature, as many patients in the intensive care unit receive potential QTc prolonging medications safely as long as the QTc remains below 500 msec.51
The contradictory results in China
Two subsequent Chinese studies revealed conflicting results. In the first study reported by Chen et al. from the Shanghai Public Health Clinical Center, no difference was found in viral clearance and normalization of temperature between the HCQ treated and the control group.52 The study included 15 patients in the intervention and 15 in the control arm. All patients were treatment naïve. The second study by Chen et al. from the Renmin Hospital of Wuhan University revealed intriguing results.53 The researchers randomly assigned 62 patients to intervention (200mg twice a day of HCQ for five days) and control group. All patients had pneumonia by CT scan and PaO2/FiO2 >300. The primary outcome was time to clinical recovery (TTCR) characterized by resolution of fever and cough for 72 hours, and CT imaging improvement after 5 days of therapy. The mean age for all patients was 44.7 years, there was no age or sex difference between the groups. The authors did not report the length of time between symptom onset and inclusion in the study. Patients who received HCQ, demonstrated statistically significant reduction in TTCR compared to the control group. The radiologic improvement was also more commonly seen in the HCQ group (80.6 vs 54.8%). In the HCQ group, 61.3% of patients had a greater than 50% resolution of pneumonia. Four patients in the control arm of this study developed severe illness.
The first multicenter randomized control trial
To date, the largest multicenter, open-label, randomized control trial was published by Tang et al.54 According to the preliminary report, the trial was stopped after a preplanned interim analysis, due to the observed accelerated clinical improvement with HCQ. An independent research organization was employed to conduct the study. One hundred fifty patients were recruited from 16 centers in China between February 11th to 29th after meeting the eligibility criteria, they were randomized in a 1:1 ratio assignment to receive standard of care (SOC) or HCQ in addition to the SOC. The patients in the SOC group received therapy based on the Chinese national clinical practice guideline, which included antiviral medications. Based on the disease severity, the patients received massive doses of HCQ (1200 mg daily for three days, then 800 mg daily for two weeks for mild to moderate disease or for three weeks for severe disease). The mean age was 46 and the mean duration from symptom onset to randomization was 16.6 days. Mild to moderate infection was present in 99% of patients without any significant hypoxia or tachypnea. The primary outcome was the negative viral conversion rate within 28 days after inclusion, as determined by PCR. Although the plan was to enroll 360 patients (for an estimated power of 80%) for the study, an interim analysis was conducted on March 14th after the enrollment of the initial 150 patients. There was no difference in the rate of viral clearance between the two groups (85.4% vs. 81.3%) at day 28. No difference was observed in prespecified secondary outcomes of viral clearance after 4,7,10, 14, and 21-days. Post hoc analysis did not reveal any differences among different subgroups.
The researchers however observed more rapid improvement of clinical symptoms with HCQ compared to SOC when the confounding factors, such as the use of antiviral medications, were removed. The inflammatory marker CRP was lower, and there was a faster recovery of lymphopenia in the HCQ treated group. No life-threatening cardiac arrhythmia or visual difficulty was reported even with such massive doses of HCQ, suggesting a significant safety margin with more conventional dosing (described above). The predominant side effect was gastrointestinal. The authors suggested the anti-inflammatory property of HCQ as the potential cause for the observed benefit and proposed that no significant viral clearance was obtained from the addition of HCQ to the standard of care as no difference was notable between patients receiving HCQ within seven days of symptoms onset versus after seven days. However, it is essential to emphasize that there were only ten patients that received HCQ who presented before one week of symptoms, and the mean delay between symptom onset and therapy was 16.6 days. Additionally, quantitative viral load was also not measured in this study; instead, the negativity of two viral PCRs 24 hours apart was used as the measure of viral clearance.
The retrospective VA study
Magagnoli et al. published a retrospective chart review study from patients admitted to the VA hospitals across the United States.55 The cohort comprised of proven cases of 368 hospitalized male COVID-19 patients from March 9 to April 11. Patients were divided into three groups based on the medications they received, HCQ alone, HCQ and AZ, and no HCQ. The primary outcome that the authors looked into was the overall rate of hospital death among the different cohorts as well as the number of patients that required mechanical ventilation in each group and the rate of death among ventilated patients. The authors claimed that there was worse mortality among patients who received HCQ versus no HCQ (27.8% for HC, 22.1% for HCQ+AZ, and 11.4% for no HCQ). There was no difference among cohorts regarding the number of patients that required mechanical ventilation and death rate of mechanically ventilated patients. The authors concluded that since no difference was seen among groups regarding the pulmonary complications, non-pulmonary organ complications could have been responsible for this observed worse outcome. However, there are several flaws to this conclusion. The patients that received HCQ, with or without AZ, were significantly sicker than the no HCQ group. For example, 73.4% of patients in the no HCQ group had an oxygen saturation of 95% or higher compared to 57.5% in the HCQ+AZ and 62.9% in the HCQ group. Similarly, more patients in the HCQ groups had evidence of end-organ dysfunction (liver function tests), anemia, higher mean systolic blood pressure, lymphopenia, and higher inflammatory markers. The fact that these patients received HCQ was an indication that the clinicians perceived them to be sicker at the very beginning of the hospitalization. Moreover, no report regarding the symptom onset was provided. In an earlier study from China, the presence of a higher mean systolic blood pressure, lymphopenia, elevated transaminase levels, and an oxygen saturation less than 93% on admission was associated with significantly worse mortality.56 Overall, the data provided in this study is far from being conclusive and has all the limitations of a retrospective study.
The trial from Brazil
Borba et al. reported a single-center double-blind, randomized controlled study that evaluated the efficacy and safety of high dose versus low dose CQ in patients hospitalized with confirmed or suspected COVID-19. The patients enrolled in this trial were generally sick with hypoxia, respiratory failure, shock requiring vasopressors, and renal failure. The authors had a predefined target to enroll 441 patients; however, an unplanned interim analysis following the enrollment of 81 patients revealed a higher risk of mortality (39% versus 15%) in the high dose group (600 mg twice daily for ten days versus 450 mg twice a day for the first day followed by 450 mg daily for four days), and the study was prematurely terminated.57 There is no difference in mortality among critically ill patients. Following the publication of the study, the use of CQ by clinicians for COVID-19 has fallen out of favor.
The New York City experience
An observational study by Geleris et al. looked into the primary outcome of worsening respiratory status requiring intubation or death following admission in patients who had received treatment with HCQ compared to patients who had not.58 The single-center study reviewed the data from 1446 patients who were admitted to the hospital from March 7th to April 8th, during the peak of the epidemic in New York City. The authors excluded 70 patients as they were intubated or died within 24 hours after admission. Out of 1376 patients, 811 patients received HCQ (45.8% and 85.9% received the first dose within 24 and 48 hours respectively) and 565 did not. Patients who received HCQ were sicker at baseline with a lower PaO2/FiO2 ratio (233 versus 360). The hospital adopted a policy of treating patients with HCQ if they had an oxygen saturation below 94% on room air, which likely explains the baseline difference in the patient characteristics. There was a significant difference between the unmatched cohorts regarding age, sex, ethnicity, concurrent medications, BMI, and smoking status. Twenty-seven patients received remdesivir (22 in the HCQ group), 70 patients were on anti-IL-6 therapy (58 in HCQ group), and 613 patients received concomitant AZ (486 in HCQ and 127 in no HCQ group). A total of 346 patients developed the primary endpoint of respiratory failure; 166 patients died without intubation and 180 were intubated. In the nonadjusted analysis, patients who received HCQ were more likely to have reached the primary endpoint compared to non HCQ receivers. However, on primary multivariate analysis and analysis of propensity score-matched data, there was no difference in the primary outcome.
Another retrospective study by Rosenberg et al. that included 1438 patients did not show any reduction in mortality among patients treated with HCQ, HCQ and AZ, or AZ. The study also did not reveal any increased likelihood of abnormal EKG with these medications.59
The multicontinental registry analysis
An analysis of patient registry that included 671 hospitals and over ninety-six thousand patients spanning six continents, was reported by Mehra et al.60 In this study, the authors compared the outcome among patients who received CQ or HCQ with or without a Macrolide to patients who received no such therapy. Patients who received the treatment were divided in four distinct groups (CQ alone, CQ+ Macrolide, HCQ alone, and HCQ+ Macrolide). The primary endpoint was in-hospital mortality. Only patients who received the study drug within 48 hours of hospital admission were included. The cohort consisted of patients from North America (65.9%), Europe (17.3%), Asia (8.4%), Africa (4.6%), South America (3.7%), and Australia (0.1%). In contrary to the previous studies, no baseline difference was seen among different groups, including in disease severity and degree of hypoxia. Compared to the control group (9.3%), the mortality was increased in CQ (16.4%), CQ+Macrolide (22.2%), HCQ (18%), and HCQ+Macrolide (23.8%). Compared to the control group (0.3%), CQ (4.3%), CQ+Macrolide (6.5%), HCQ (6.1%), and HCQ+Macrolide (8.1%) were independently associated with the development of de-novo ventricular arrhythmia. Surprisingly, the disease severity among groups was not different in this observational study. However, the integrity of this database has been questioned and an independent review is being conducted by the journal editors due to an expression of concern regarding the data reported in this paper, the article has since been retracted.** Table 2 summarizes the studies discussed above.
Table 2.
Recently published clinical studies on CQ and HCQ.
| Study | Type | Peer review | Number of patients | Study drug | Dose | Primary outcome | Results | Safety concerns | Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Guatret et al. 48 | Prospective double arm study | No | 36 Control: 16 Intervention: 20 |
HCQ With or without AZ |
HCQ: 200 mg three times daily for 10 days AZ: 500 mg on D1 250 mg D2-5 |
Virologic clearance post inclusion day 6 | 70% vs 12.5% clearance with HCQ 100% clearance for HCQ+AZ |
None | Small study Patients with mild infection was included 6 patients in the intervention arm were excluded during analysis Clinical outcome was not reported |
| Molina et al. 50 | Prospective single arm trial | Yes | 11 | HCQ with AZ | HCQ: 200 mg three times daily for 10 days AZ: 500 mg on D1 250 mg D2-5 |
Virologic clearance and clinical outcome on day 5 and 6 | 80% of patients tested positive at the end of day 5 or 6 | One patient developed QTc prolongation more than 50 milliseconds | Patients were older compared to the study by Guatret Most patients were on oxygen and had significant comorbidity |
| Chen et al. 52 | Prospective double arm study | Yes | 30 Control: 15 Intervention: 15 |
HCQ | HCQ: 400md daily for 5 days | Virologic clearance and normalization of temperature | No difference between the control and HCQ group | One patient in HCQ group developed serious illness | Small study to identify any meaningful difference |
| Chen et al. 53 | Randomized parallel group trial | No | 62 Control: 31 Intervention: 31 |
HCQ | HCQ: 400md daily for 5 days | Time to clinical recovery, clinical and radiologic changes after 5 days | Shortened time to clinical recovery and improved chest radiology in the HCQ group | Mild adverse effect in two patients, rash and headache Four patients in the control arm developed severe illness |
Small study Young patient population, mean age 44.7 years |
| Tang et al. 54 | Multicenter open labelled randomized control trial | No | 150 Control: 75 Intervention: 75 |
HCQ | HCQ: 1200 mg daily for 3 days followed by 800 mg daily for a total of 2-3 weeks | Virologic clearance at 28 days | No difference in primary outcome HCQ group had more symptomatic improvement Rapid normalization of CRP and lymphopenia in the HCQ cohort |
Adverse effect more common (30% vs 8.8%) in the HCQ group, diarrhea the commonest One patient developed blurred vision |
Open labelled trail Very high and prolonged HCQ dosing |
| Magagnoli et al. 55 | Retrospective chart review | No | 368 No HCQ: 158 HCQ+AZ: 113 HCQ: 97 |
HCQ HCQ+AZ No HCQ |
Risk of death or mechanical ventilation | Increased risk of mortality among patients who received HCQ likely secondary to non-pulmonary organ dysfunction. No difference in the rate of intubation |
Observational study Sicker patients in the HCQ cohort All patients are men over the age of 65 years |
||
| Borba et al. 57 | Single center double blind randomized control trial | Yes | 81 High dose CQ: 41 Low dose CQ: 40 |
High dose CQ Low dose CQ |
High dose CQ: 600 mg twice daily for 10 days Low dose CQ: 450 mg twice daily on day one followed by 450 mg daily for four more days |
Reduction in mortality by 50% in the high CQ group compared to low CQ | Increased mortality in high dose CQ group (39% vs 15%) | Higher instances of QTc prolongation with the higher dose | Very high dosing regimen in the high dos group Other antiviral medications that can increase QTc were allowed |
| Geleris et al. 58 | Observational single center study | Yes | 1376 HCQ: 811 No HCQ: 565 |
HCQ vs no HCQ | Risk of intubation or death | No difference in the risk of intubation or death in multivariate analysis or analysis of propensity score matched data | Observational study A significant number of patients received azithromycin, remdesivir and anti-IL-6 therapy |
||
| Rosenberg et al. 59 | Retrospective multi-center cohort study | Yes | 1438 No drug: 221 HCQ: 271 HCQ+AZ: 735 AZ alone: 211 |
No drug vs HCQ vs HCQ+AZ vs AZ | Mortality difference | No difference in mortality among groups when adjusted for confounders | Increased risk of cardiac arrest in patients receiving HCQ+AZ but not HCQ | Retrospective study Inflammatory markers were not reported |
|
| Mehra et al. 60 | Retrospective registry analysis | Yes | 96,032 No drug: 81,144 CQ alone: 1868 CQ+ Macrolide: 3783 HCQ alone: 3016 HCQ+ Macrolide: 6221 |
CQ: 765 mg for 6.6 days HCQ: 596 mg for 4.2 days CQ+ Macrolide: 790 mg for 6.8 days HCQ+ Macrolide: 597mg for 4.3 days |
In-hospital mortality De-nono ventricular arrhythmia |
The mortality was higher in CQ, CQ+ Macrolide, HCQ and HCQ+ Macrolide groups compared to no drugs No baseline difference in disease severity among groups were identified |
All study groups were associated with higher risk of de-novo ventricular arrhythmia | Retrospective study No difference in baseline disease severity is surprising **This study has since been retracted due to inconsistencies with the data** |
|
| Boulware et al. 61 | Randomized, double-blind, pragmatic, placebo-controlled trial | Yes | 821 HCQ: 414 Placebo: 407 |
HCQ vs Placebo | HCQ: 800mg loading dose, 600mg 6-8 hours later, then 600mg daily for 4 days | Symptomatic illness confirmed by a positive molecular assay or, if testing was unavailable, COVID-19 related symptoms | Onset of new symptoms associated with Covid-19 did not differ significantly between the hydroxychloroquine and placebo groups | Mild side effects including nausea, diarrhea, and abdominal discomfort in 40.1% HCQ patients compared to 16.8% in placebo group | Based largely on subjective data Delayed start of prophylactic treatment Limited testing ability for all subjects |
| Arshad et al. 63 | Multi-center retrospective observational study | Yes | 2541 HCQ+AZ: 783 HCQ: 1202 AZ: 147 No intervention: 409 |
HCQ with or without AZ | HCQ 400mg twice daily for 2 doses followed by 200mg daily for 4 days AZ 500mg daily for one day followed by 250mg daily for 4 days |
In-hospital mortality | Increased survival probability in the HCQ and HCQ+AZ groups. Patients with AZ alone or no HCQ/AZ intervention had the highest cumulative mortality hazard |
No significant cardiac arrhythmia including torsades de pointes | Retrospective Study Single-system data |
Abbreviations: AZ, Azithromycin; CQ, Chloroquine; HCQ, Hydroxychloroquine; IL-6, Interleukin 6.
The post-exposure prophylaxis study
In a recently published double-blind pragmatic study, Boulware et al. examined the prophylactic efficacy of HCQ in asymptomatic individuals with known exposure to patients with COVID-19.61 The study included 821 asymptomatic adult participants (recruited via internet-based survey and social media) who reported moderate or high-risk exposure. Moderate risk exposure consisted of individuals wearing a mask but no eye protection whereas, high-risk exposure denoted the absence of both mask and eye protection. These self-reported subjects had been within 6 feet of an individual diagnosed with COVID-19 for greater than 10 minutes. Of these patients, 414 were assigned to the HCQ group, and 407 were assigned to the placebo group. The HCQ dosing regimen included 800mg orally as the first dose, followed by 600mg 6-8 hours later, and 600mg daily for the next four days for a total of 5 days of therapy. Patients began their treatment within four days of exposure, and they were then evaluated for the next 14 days for the development of signs and symptoms suggestive of COVID-19. The primary endpoint was the development of COVID-19, confirmed by a positive molecular assay or symptomatic illness indicative of the disease when testing was not available. A total of 107 of 821 participants were deemed to have reached the primary outcome. The study found a 2.4% absolute reduction (95% confidence interval -7 to 2.2%) in the incidence of COVID-19, which did not reach the prespecified relative risk reduction of 50% with HCQ prophylaxis. However, there were several significant limitations of the study. The recruitment of study subjects via internet-based survey and social media platforms could have introduced a selection bias. Similarly, self-reported data as outcome measures might also be questionable, especially in the absence of objective testing. As discussed by Avidan et al., initiation of HCQ up to 4 days post-exposure might be considered to be early treatment rather than post-exposure prophylaxis. In addition, the authors pointed out that at the upper range of the 95% confidence interval (-7%), there would have been a relative reduction of about 50%.62 Future studies on preexposure prophylaxis would be necessary to further investigate the efficacy of HCQ in the prevention of SARS-CoV-2 infection.
A positive, system-wide study in Detroit
A multi-center, single system, retrospective observational study conducted by Arshad et al. showed promising results with the use of HCQ alone, and in combination with AZ in hospitalized patients with COVID-19.63 The primary outcome of this study focused on in-hospital mortality. The authors retrieved relevant data by retrospective review of the electronic medical record of 2,541 patients with an average hospital stay of 6 days. Patients who expired within 24 hours of admission were excluded from the study, as well as patients who were readmitted. Treatments were protocol-based, patients who received HCQ received 400mg twice daily on day 1, followed by 200mg daily for four days. Patients who received AZ received 500mg on day 1, followed by 250mg for four days. Only patients with severe COVID-19 and minimum cardiac risk (QTc< 500ms) received both HCQ and AZ. The total in hospital mortality of all patients involved was 18.1%, the mortality rate of patients who received HCQ alone was 13.5%, 20.1% in patients who received HCQ and AZ, 22.4% in patients who received only AZ, and 26.4% in patients who did not receive AZ or HCQ. The estimated survival among treatment groups exhibited increased survival probability in both the HCQ and HCQ+AZ groups. It is important to note that among all patients within the study, no patients suffered from torsades de points, 4% of total mortality was due to cardiac arrest, 88% from respiratory failure, and 8% from cardiopulmonary arrest or other causes. Key factors that may have contributed to the success/results of this study include early administration of HCQ (started within one day of admission), standardized and safe dosing, specific inclusion criteria, and a larger cohort. Important limitations include the retrospective study design as well as data retrieval from a single system. Overall, the results of this study are promising and can be used as the basis of a larger, controlled trial.
Why are the results incongruent?
There are many limitations to the studies discussed above; namely, small sample size, the lack of uniformity in disease severity, the inclusion of antivirals, steroid, interferon or immunoglobulins in the standard treatment group, loss of patients to follow up which could have changed the outcome, nonreporting of disease onset, delayed recruitment to intervention, and of being retrospective. The Food and Drug Administration had issued an emergency use authorization in March of 2020 for CQ and HCQ for patients with COVID-19. However, this was not based on high-quality evidence. Additionally, a common dilemma in clinical practice is the decision of when to use these medications and which patients are likely to receive the maximal benefit while minimizing the potential side effects. Based on the in vitro studies, the potency of CQ and HCQ as an antiviral is unquestionable. Studies also suggest that the use of these medications as a prophylactic agent or early in the infectious process is likely to yield the most benefit. However, a preclinical success does not always translate into clinical wonder, and there are two major limitations of an empiric approach. Firstly, there are concerns for the safety of these medications, namely malignant arrhythmia due to prolongation of the QTc, and retinopathy. Secondly, the perceived positive outcome from HCQ can be misleading if used for patients with mild disease who would have recovered from the infection anyway. This is supported by the fact that a large percentage of patients infected with SARS-CoV-19 do not experience severe illness and many are entirely asymptomatic. Thus, the actual mortality might be significantly lower than reported.64 Conversely, some of the patients enrolled in the above-mentioned studies were late in the disease process showing signs of advanced infection, cytokine storm, and multiple organ failure. A perceived lack of benefit in this patient population could also be a false negative outcome. For example, a delay in appropriate antibiotic administration in hypotensive septic patients is associated with an increased mortality of 7.6 % per hour delay, and patients receiving delayed therapy might have a worse outcome despite receiving appropriate antibiotics.65 The contradictory results in the reported clinical trials therefore, might be a result of the heterogeneous patient population rather than the inefficacy of HC and HCQ. It is also noteworthy that the anti-inflammatory properties of the drugs also provide a beneficial effect for patients with COVID-19.54 Based on the data discussed in this manuscript, CQ/HCQ might have a potential role in the prevention and treatment of early infection by SARS-CoV-2. Multiple clinical trials, both by federal institutions and investigator-initiated, are currently underway to evaluate the role of HCQ as a prophylactic agent as well as a therapeutic option for patients with mild to moderate COVID-19.
Side effects and safety of CQ and HCQ
The antimalarial medications CQ and HCQ have a long list of safety data associated with their use. Long term use in rheumatologic diseases has proved to be safe. HCQ is safer than CQ and currently the drug of choice for rheumatologic diseases. The primary concerns for short term use of these mediations include QTc prolongation and cardiac arrhythmias, specifically, torsade de pointes. This risk could increase when a second medication such as a macrolide is added to the regimen, as well as if there are significant electrolyte abnormalities; which are commonly observed in critically ill patients. In recent clinical trials of antimalarials, only one patient developed prolongation of the QTc, ultimately leading to the discontinuation of the medications (405 to 470 msec after 4 days).50 However, for most patients admitted to the hospital, an EKG can be obtained daily without significant difficulty. QTc prolongation up to 500 msec can be safely tolerated and QTc can be closely monitored, especially within the ICU setting.51 In the largest prospective trial of 150 patients with COVID-19, administration of 12,400 mg of HCQ over two weeks was not associated with any cardiac events. This was a massive dose compared to the dose that has been previously recommended and validated (2,400 mg in 5 days).33 , 54 The reports of apparent safety are contradicted by several recently published reports that pointed towards potential safety concerns.60 , 66 Based on the recently published multi-national registry analysis that showed worse mortality and a higher risk of ventricular arrhythmia in patients treated with CQ and HCQ, with or without macrolides, the World Health Organization has stopped enrolling new patients into the HCQ arm of the Solidarity trial.60 Retinopathy secondary to HCQ occurs after long term use and is usually absent when the daily dose is less than 7.8 mg/kg/day.67 When used for a period of five to ten days, the risks are nearly nonexistent. Since more than 50% of unchanged HCQ is renally excreted, toxicity with HCQ is more likely in patients with significant renal impairment. No definitive data exists regarding the dosing adjustments in patients with severe kidney disease, and this patient population was largely excluded from trials.68
Conclusions
Based on current literature, the role of chloroquine (CQ) or hydroxychloroquine (HCQ) in the treatment or prevention of COVID-19 infection remains unclear. The National Institute of Health COVID-19 guideline has not made any official recommendation for or against the use of CQ or HCQ due to insufficient data. The Infectious Disease Society of America only recommends the use of these drugs in the setting of a clinical trial. Large prospective randomized control trials would be necessary to definitively answer the question of the efficacy of these agents both as a prophylactic and a therapeutic choice. Although many trials are currently underway, this might prove to be complicated during an ongoing health crisis. The published clinical trial results are contradictory, however the in vitro data suggests significant antiviral activity. It would be essential for clinicians to remember that many previous preclinical successes have not provided positive results in clinical trials. CQ and HCQ are still likely to be considered a potential therapy by many in the fight against COVID-19, especially in resource-poor countries. Active and close monitoring of patients receiving HCQ will be crucial, and early identification of a possible side effect would be of monumental importance.
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020 Feb 22;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolifarhood G, Aghaali M, Mozafar Saadati H, Taherpour N, Rahimi S, Izadi N, et al. Epidemiological and Clinical Aspects of COVID-19; a Narrative Review. Arch Acad Emerg Med. 2020 Apr 1;8(1) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7117787/ [cited 2020 Apr 15]; Available at: Accessed August 13, 2020. [PMC free article] [PubMed] [Google Scholar]
- 3.Paules CI, Marston HD, Fauci AS. Coronavirus Infections—More Than Just the Common Cold. JAMA. 2020 Feb 25;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 4.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO | Middle East respiratory syndrome coronavirus (MERS-CoV). WHO. World Health Organization; [cited 2020 Apr 15]. Available at: http://www.who.int/emergencies/mers-cov/en/. Accessed August 13, 2020.
- 6.Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 — Preliminary Report. N Engl J Med. 2020 May 22 doi: 10.1056/NEJMc2022236. NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 8.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. 2020 Apr 13 doi: 10.1001/jama.2020.6019. https://jamanetwork.com/journals/jama/fullarticle/2764727 [cited 2020 Apr 15]; Available at: Accessed August 13, 2020. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: From malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020 Mar;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 11.Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989 Jun;27(6):771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015 Oct;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 13.Furst DE. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996 Jun;5(Suppl 1):S11–S15. [PubMed] [Google Scholar]
- 14.O'Neill PM, Bray PG, Hawley SR, Ward SA, Park BK. 4-Aminoquinolines–past, present, and future: a chemical perspective. Pharmacol Ther. 1998 Jan;77(1):29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 15.McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983 Jul 18;75(1A):11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- 16.Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110(6):481–489. doi: 10.1034/j.1600-0463.2002.100606.x. [DOI] [PubMed] [Google Scholar]
- 17.Neuman BW, Adair BD, Yoshioka C, Quispe JD, Orca G, Kuhn P, et al. Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J Virol. 2006 Aug;80(16):7918–7928. doi: 10.1128/JVI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fehr AR, Perlman S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses. 2015 Feb 12;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raj VS, Mou H, Smits SL, Dekkers DHW, Müller MA, Dijkman R, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020 Mar 17;94(7) doi: 10.1128/JVI.00127-20. https://jvi.asm.org/content/94/7/e00127-20 [cited 2020 Apr 16]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harcourt J, Tamin A, Lu X, Kamili S, Sakthivel SK, Murray J, et al. Early release - severe acute respiratory syndrome coronavirus 2 from patient with 2019 novel coronavirus disease, United States. Emerging Infectious Diseases journal - CDC. June 2020;26 doi: 10.3201/eid2606.200516. https://wwwnc.cdc.gov/eid/article/26/6/20-0516_article [cited 2020 Apr 22]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020 Apr 1;55(4) doi: 10.1183/13993003.00607-2020. https://erj.ersjournals.com/content/55/4/2000607 [cited 2020 Apr 22]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005 Aug 22;2 doi: 10.1186/1743-422X-2-69. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1232869/ [cited 2020 Apr 11]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol. 2019 Mar 5;93(6) doi: 10.1128/JVI.01815-18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6401451/ [cited 2020 May 27]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012 Jun;86(12):6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st Century - PubMed. [cited 2020 Apr 16]. Available at: https://pubmed.ncbi.nlm.nih.gov/17629679/. Accessed August 13, 2020 [DOI] [PMC free article] [PubMed]
- 29.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003 Nov;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regeneron and Sanofi Provide Update on U.S. Phase 2/3 Adaptive-Designed Trial of Kevzara® (sarilumab) in Hospitalized COVID-19 Patients. Regeneron Pharmaceuticals Inc.[cited 2020 May 27]. Available at: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-us-phase-23-adaptive. Accessed August 13, 2020
- 31.Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004 Oct 8;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Mar;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. [cited 2020 Apr 18]; Available at:https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa237/5801998. Accessed August 13, 2020. [DOI] [PMC free article] [PubMed]
- 34.Dijkman R, Jebbink MF, Koekkoek SM, Deijs M, Jónsdóttir HR, Molenkamp R, et al. Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. J Virol. 2013 Jun 1;87(11):6081–6090. doi: 10.1128/JVI.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ooi EE, Chew JSW, Loh JP, Chua RCS. In vitro inhibition of human Influenza A virus replication by chloroquine. Virol J. 2006 May 29;3:39. doi: 10.1186/1743-422X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishop NE. Examination of potential inhibitors of hepatitis A virus uncoating. Intervirology. 1998;41(6):261–271. doi: 10.1159/000024948. [DOI] [PubMed] [Google Scholar]
- 37.Singh AK, Sidhu GS, Friedman RM, Maheshwari RK. Mechanism of enhancement of the antiviral action of interferon against herpes simplex virus-1 by chloroquine. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 1996 Sep;16(9):725–731. doi: 10.1089/jir.1996.16.725. [DOI] [PubMed] [Google Scholar]
- 38.Chiang G, Sassaroli M, Louie M, Chen H, Stecher VJ, Sperber K. Inhibition of HIV-1 replication by hydroxychloroquine: mechanism of action and comparison with zidovudine. Clin Ther. 1996 Dec;18(6):1080–1092. doi: 10.1016/s0149-2918(96)80063-4. [DOI] [PubMed] [Google Scholar]
- 39.Miller N, Hutt-Fletcher LM. Epstein-Barr virus enters B cells and epithelial cells by different routes. J Virol. 1992 Jun;66(6):3409–3414. doi: 10.1128/jvi.66.6.3409-3414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paton NI, Lee L, Xu Y, Ooi EE, Cheung YB, Archuleta S, et al. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. 2011 Sep 1;11(9):677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- 41.Banfield WJ, Kisch AL. The effect of chloroquine on herpesvirus infection in vitro and in vivo. Proc Soc Exp Biol Med. 1973 Mar 1;142(3):1018–1022. doi: 10.3181/00379727-142-37166. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Burton EM, Bhaduri-McIntosh S. Chloroquine triggers Epstein-Barr virus replication through phosphorylation of KAP1/TRIM28 in Burkitt lymphoma cells. PLoS Pathog. 2017;13(3) doi: 10.1371/journal.ppat.1006249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paton NI, Aboulhab J, Karim F. Hydroxychloroquine, hydroxycarbamide, and didanosine as economic treatment for HIV-1. Lancet Lond Engl. 2002 May 11;359(9318):1667–1668. doi: 10.1016/S0140-6736(02)08557-4. [DOI] [PubMed] [Google Scholar]
- 44.Paton NI, Aboulhab J, Hydroxychloroquine hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Med. 2005 Jan;6(1):13–20. doi: 10.1111/j.1468-1293.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 45.Neely M, Kalyesubula I, Bagenda D, Myers C, Olness K. Effect of chloroquine on human immunodeficiency virus (HIV) vertical transmission. Afr Health Sci. 2003 Aug;3(2):61–67. [PMC free article] [PubMed] [Google Scholar]
- 46.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 47.multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin J Tuberc Respir Dis. 2020 Mar 12;43(3):185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Méd Mal Infect. 2020 Mar 30 doi: 10.1016/j.medmal.2020.03.006. http://www.sciencedirect.com/science/article/pii/S0399077X20300858 [cited 2020 Apr 18]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Khatib SM, LaPointe NMA, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003 Apr 23;289(16):2120–2127. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 52.CHEN Jun LD, CHEN Jun LD. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ Med Sci. 2020 Mar 6;49(1) doi: 10.3785/j.issn.1008-9292.2020.03.03. 0–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. Epidemiology. 2020 Mar http://medrxiv.org/lookup/doi/10.1101/2020.03.22.20040758 [cited 2020 Apr 18]. Available at: Accessed August 13, 2020. [Google Scholar]
- 54.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. medRxiv. 2020 Apr 14 2020.04.10.20060558. [Google Scholar]
- 55.Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020 Apr 23 doi: 10.1016/j.medj.2020.06.001. 2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 Mar 26;368 doi: 10.1136/bmj.m1091. https://www.bmj.com/content/368/bmj.m1091 [cited 2020 Apr 23]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A randomized clinical trial. JAMA Netw Open. 2020 Apr 1;3(4) doi: 10.1001/jamanetworkopen.2020.8857. e208857–e208857. [DOI] [PubMed] [Google Scholar]
- 58.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 May 7;0(0) doi: 10.1056/NEJMoa2012410. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020 May 11 doi: 10.1001/jama.2020.8630. http://jamanetwork.com/journals/jama/fullarticle/2766117 [cited 2020 May 30]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet. 2020 May 22;0(0) doi: 10.1016/S0140-6736(20)31180-6. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/abstract [cited 2020 May 30]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020 Aug 6;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020 Aug;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avidan MS, Dehbi H-M, Delany-Moretlwe S. Hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020 Jul 15;383 doi: 10.1056/NEJMc2023617. [DOI] [PubMed] [Google Scholar]
- 64.Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020 Mar 27;0(0) doi: 10.1016/S1473-3099(20)30244-9. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30244-9/abstract [cited 2020 Apr 18]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 66.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 May 1 doi: 10.1001/jamacardio.2020.1834. https://jamanetwork.com/journals/jamacardiology/fullarticle/2765631 [cited 2020 May 11]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med. 1983 Jul 18;75(1):40–45. doi: 10.1016/0002-9343(83)91269-x. [DOI] [PubMed] [Google Scholar]
- 68.Tailor R, Elaraoud I, Good P, Hope-Ross M, Scott RAH. A case of severe hydroxychloroquine-induced retinal toxicity in a patient with recent onset of renal impairment: A review of the literature on the use of hydroxychloroquine in renal impairment. Case Rep Ophthalmol Med. 2012;2012 doi: 10.1155/2012/182747. https://www.hindawi.com/journals/criopm/2012/182747/ Hindawi [cited 2020 Apr 23]; Available at: Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]