Abstract
The most important aspect of controlling COVID-19 is its timely diagnosis. Molecular diagnostic tests target the detection of any of the following markers such as the specific region of the viral genome, certain enzyme, RNA-dependent RNA polymerase, the structural proteins such as surface spike glycoprotein, nucleocapsid protein, envelope protein, or membrane protein of SARS-CoV-2. This review highlights the underlying mechanisms, advancements, and clinical limitations for each of the diagnostic techniques authorized by the Food and Drug Administration (USA). Significance of diagnosis triaging, information on specimen collection, safety considerations while handling, transport, and storage of samples have been highlighted to make medical and research community more informed so that better clinical strategies are developed. We have discussed here the clinical manifestations and hospital outcomes along with the underlying mechanisms for several drugs administered to COVID-19 prophylaxis. In addition to favourable clinical outcomes, the challenges, and the future directions of management of COVOD-19 are highlighted. Having a comprehensive knowledge of the diagnostic approaches of SARS-CoV-2, and its pathogenesis will be of great value in designing a long-term strategy to tackle COVID-19.
Keywords: COVID-19, SARS-CoV-2, Antiviral drugs, Molecular diagnosis, RT-PCR, Immunochromatographic assay and Drug discovery
1. Introduction
The ongoing coronavirus pandemic (COVID-19) is the most catastrophic global crisis after the Second World War [1]. COVID-19 is a contagious disease, caused by a novel severe acute respiratory syndrome Coronavirus (SARS-CoV-2). The genome analysis of this new virus showed ~ 79.5% similarity with SARS-CoV and middle east respiratory syndrome Coronavirus (MERS-CoV) [2]. However, the highest sequence similarity (~96%) was observed for the bat Coronavirus. Therefore, it has been speculated that COVID-19 was transmitted from bats to humans. Recent studies suggest an intermediary animal host, which potentially could be pangolin [3] or dog [4].
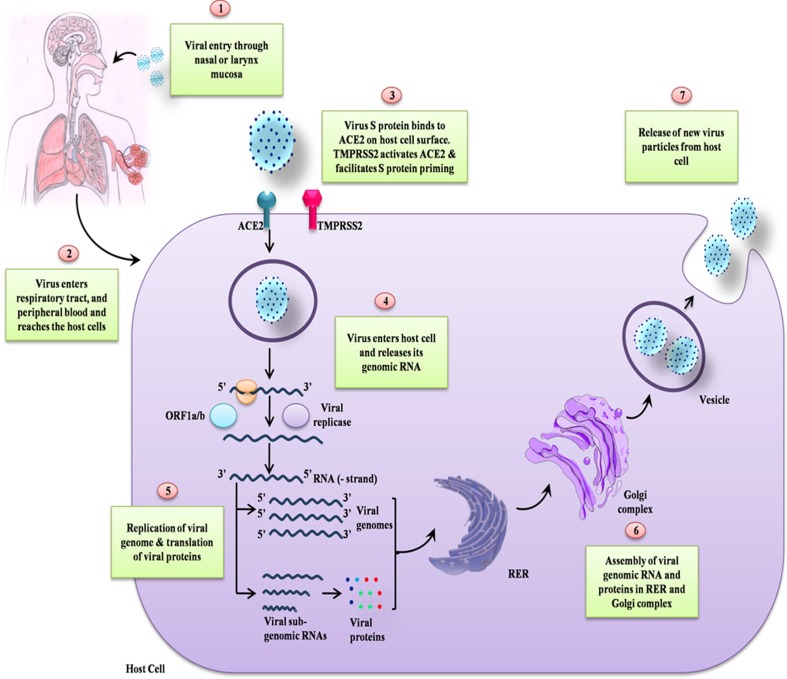
Earlier studies on COVID-19 suggest that this disease spreads through close contact through the infected person and with small respiratory droplets released while coughing, sneezing, or talking [5]. Tiny droplets of saliva or sputum released from the mouth may carry heavy loads of viruses that are known to stay in the air for a long time and hence potentially can act as carriers of infection [6]. The inhalation of these minute droplets leads to the spreading of viral infection from the sick to a healthy person even when a person is not in direct physical contact with the infected person. Even though COVID-19 is not an airborne disease, some medical practices such as cardiopulmonary resuscitation and intubation may trigger respiratory secretions that can get aerosolized and hence resulted in its spread [7]. The virus gets its way inside the human body through eyes, nose, and mouth and thus spread through contacting the virus from contaminated surfaces and then touching these body parts [8]. However, the extent of viral transmission through contaminated surfaces depends upon environmental conditions such as temperature and humidity [9]. The entry of SARS-CoV-2 into host cells is facilitated by the binding of homo-trimer spike protein (S) that is on the surface of the virus to ACE2 on the host’s cell membrane [10]. The recognition of the host cell receptor is a key determinant of the tissue tropism and pathogenicity of the virus [11]. A schematic representation of the life cycle of SARS-CoV-2 is illustrated in Fig. 1 . The life cycle of SARS-CoV-2 is similar to the SARS-CoV, MERS-CoV [12].
Fig. 1.
Schematic representation of the life cycle of SARS-CoV-2. The spike surface glycoprotein of SARS-CoV-2 attaches to the ACE2 receptor and subsequently enters to the host cell. After entering to the host cell, viral particles release their genome and subsequently translated into protein and new viral particles formed then released to infect next cells.
Sometimes, animal viruses get accidentally transferred to humans and can evolve itself causing sickness. Thus, it becomes a human host-virus, such as SARS-CoV, MERS-CoV, and SARS-CoV-2. The current COVID-19 pandemic has intensified the interest of scientists all over the world for developing treatment options to mitigate the impact of the disease on human life [12], [13]. More than 300 clinical trials are currently in progress for the treatment of COVID-19. Different strategies have been adopted to combat COVID-19. However, there is no approved treatment for the same till date. In this article, we evaluated literature for reports informing various diagnostic methods, potential antiviral chemical therapeutics, and effective treatment strategies towards clinical management of COVID-19 patients.
2. Diagnosis approached in SARS-CoV-2 pathogenesis
Diagnosis of COVID-19 is a critical step in tracing the virus and understanding its epidemiology. This helps to block the transmission and ensure proper patient care. The preliminary step of COVID-19 diagnosis is through observation of signs and symptoms such as initial loss of smell or taste or both [14], cough, mild to high fever, myalgia, or fatigue [15], etc. In addition, several gastrointestinal symptoms of vomiting, diarrhoea, and nausea in some individuals are observed [16]. However, inconsistencies in the manifestation of symptoms from asymptomatic to severe cases like, septic shock, imbalanced metabolic acidosis, coagulation dysfunction, and acute respiratory pneumonia-like syndrome are mostly reported [17]. The presentation of signs and symptoms should be considered as the basis for further tests and not for diagnostic purposes.
The prime consideration in the diagnosis of COVID-19 is the detection of symptoms in clinical situations. Conventionally, samples for pathology are collected from upper and lower respiratory regions (throat, oropharyngeal, nasopharyngeal, bronchoalveolar fluid, and sputum) through swabs for RT-PCR test. In the blood and urine of the infected, there is still an absence of the virus; hence, these are not considered a useful clinical specimen. Reports of inconsistency in RT-PCR test results for CoV-SARS-2 in various tissues [18] and temporal variation of test results from the same tissues [19] suggests that the inter-link between the temporal surge of viral load and its bio-distribution in different tissues of the body would have a critical implication on the accuracy of various tests for diagnosis.
Many assays or tools have been commercialized for the diagnosis of COVID-19 and many more are currently in development. Various molecular assays and immunological assays are currently validated in different laboratories. We discuss here some techniques for the diagnosis of SARS-CoV-2 pathogenesis:
2.1. Molecular biology methods
The knowledge of the viral genome sequence has made it possible for us to use molecular techniques in the detection of SARS-CoV-2. Molecular diagnostic methods target to detect either specific regions of the viral genome or RNA-dependent RNA polymerase (RdRP) and/or structural proteins of SARS-CoV-2 (Table 1 ).
Table 1.
Tests that are approved for use in the diagnosis of COVID-19.
| Test | Description | Company | Stage of Use | Country |
|---|---|---|---|---|
| RDT | Lateral flow assay for IgG and IgM detection. (93.8% sensitivity and 95.6% specificity) | Cellex Inc. | FDA approved for EUA (the US only) | US/China |
| RDT | Detect IgM and IgG for Nucleocapsid (N) protein of SARS-CoV-2 | Chem Bio | FDA approved for EUA (the US only) | USA |
| Modified ELISA | Detects total IgM and IgG with a sensitivity of 83 – 100% depending onset of infection. | VITROS (Ortho Clinical Diagnostics) | FDA approved for EUA (the US only) | USA |
| ELISA | Qualitative detection for IgG for spike protein receptor domain | Mount Sinai Laboratory | FDA approved for EUA (the US only) | USA |
| RDT, SPICA | IgG and IgM for SARS-CoV-2 test in blood or serum | Atyu Bioscience/ Orient gene Biotech | CE approved for use in China. (in Use for other countries) | US/China |
| RDT | Lateral flow assay that assay IgM and IgG (no specification of sensitivity or specificity) | Guangzhou Wondfo Biotech Co Ltd | NMPA approved for use in China. (in Use for other countries) | China |
| RDT | Lateral flow assay with AuNP colloidal test for IgM against SARS-CoV-2 | Guangdong Hecin-Scientific | NMPA approved for use in China. (in Use for other countries) | China |
| RDT | Standard Q COVID 19 test is a rapid immunochromatographic test for IgM and IgG in blood samples | SD Biosensors | Approved for use outside the US.(in Us for Research use) | Republic of Korea |
| ELISA | ELISA test for SARS-CoV-2 (others not specified) | Mayo Clinic (Minnesota University) | Clinical Use | USA |
| RDT | RapCoV COVID 19 Test, is an in vitro test for IgM and IgG detection. (Sensitivity = 89%, specificity = 100%) | Advavite | Other countries (in USA for research use) | USA |
| RDT | Detection Kit for IgM and IgG for SARS-CoV-2 in blood samples within 15 min. (Specificity = 100%, Sensitivity = 87.3%) | ScanWell Health/ INNOVITA | CE approved in China FDA approval for the USA. (In use for other countries) | US/China |
| RDT-NA | Two tests- a rapid assay for detection of antibodies reactive to recombinant viral protein and neutralization assay | Wang lab | Currently in Use in Singapore | Singapore |
RDT = Rapid Diagnosis Test, SPICA = Solid Phase Immunochromatographic Assay, NA = Neutralization Assay). Johns Hopkins University, Retrieved fromhttps://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html.
Currently, the RT-PCR test is a single discrete standard method for the diagnosis of SARS-CoV-2. The RT-PCR protocol involves the extraction of RNA from the specimen and reverses the transcription to cDNA followed by PCR amplification of a specific region with SARS-CoV-2 specific primers and detection through specific probes. Evaluation of RdRp and Envelope (E) genes was originally devised [20] which was adopted by Charité Germany for the diagnosis of COVID-19. The assay screens the E gene which is a pan-SARS-beta-coronavirus gene. The confirmatory test is done by targeting the RdRp gene using specific primers and probes listed in Table 2 . The limit to detection is 3.6 copies (RdRp gene) and 3.9 copies (E gene) per reaction and cycle threshold value of less than 37.0 is treated as a positive test. Specific probes and primers target the 1ab gene (ORF1 ab gene or Transcriptase/Replicase gene) as a confirmatory assay. While the level of E gene confirms the presence of SARS related virus. The minimum limit of detection is taken as 1000 copies/ml [18]. The cycle threshold value of less than 40 is set as positive confirmation test criteria.
Table 2.
Primers and probes for targeting SARS-Cov-2 genes in an RT-PCR test for COVID-19 diagnosis.
| Target Gene | Primers | Probe | Host Country | ||
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| ORF | 1ab | CCCTGTGGGTTTTACACTTAA | ACGATTGTGCATCAGCTGA | FAM- CCGTCTGCGGTATGTGGAAAGGTTATGG- BHQ1 | CDC, China |
| 1b-nsp14 | TGGGGYTTTACRGGTAACCT | AACRCGCTTAACAAAGCACTC | FAM-TAGTTGTGATGCWATCATGACTAG-TAMRA | Hong Kong University | |
| RdRp | AGATTTGGACCTGCGAGCG | GAGCGGCTGTCTCACAAGT | FAM-TTCTGACCTGAAGGCTCTGCGCG- BHQ1 | IP, Paris, France | |
| ATGAGCTTAGTCCTGTTG | CTCCCTTTGTTGTGTTGT | HEX- AGATGTCTTGTGCTGCCGGTA- BHQ1 | |||
| GGTAACTGGTATGATTTCG | CTGGTCAAGGTTAATATAGG | FAM- TCATACAAACCACGCCAGG- BHQ1 | |||
| GTGAARATGGTCATGTGTGGCGG | CARATGTTAAASACACTATTAGCATA | FAM- CAGGTGGAACCTCATCAGGAGATGC- BBQ | Charité Germany | ||
| E | ACAGGTACGTTAATAGTTAATAGCGT | ATATTGCAGCAGTACGCACACA | FAM- ACACTAGCCATCCTTACTGCGCTTCG- BBQ | ||
| N | N1 | GACCCCAAAATCAGCGAAAT | TCTGGTTACTGCCAGTTGAATCTG | FAM- ACCCCGCATTACGTTTGGTGGACC- BHQ1 | CDC, USA |
| N2 | TTACAAACATTGGCCGCAAA | GCGCGACATTCCGAAGAA | FAM-ACAATTTVCCCCCAGCGCTTCAG-BHQ1 | ||
| N3 | GGGAGCTTCAATAGAGGAAAA | TGTAGCACGATTGCAGCATTG | FAM- AYCACAYYGGCACCCGCAATCCTG-BHQ1 | ||
| N | GGGGAACTTCTCCTGCTACAAT | CAGACATTTTGCTCTCAAGCTG | FAM-TTGCTGCTGCTTGACAGATT-TAMRA | CDC, China | |
| N | AAATTTTGGGGACCAGGAAC | TGGCAGCTGTGTAGGTCAAC | FAM-ATGTCGCGCATTGGCATGGA-BHQ | NIID, Japan | |
| N | CGTTTGGTGGACCCTCAGAT | CCCCACTGCGTTCTCCATT | FAM-CAACTGGCAGTAACCA-BHQ1 | NIH, Thailand | |
| N | TAATCAGACAAGGAACTGATTA | CGAAGGTGTGACTTCCATG | FAM-GCAAATTGTGCAATTTGCGG-TAMRA | Hong Kong University | |
The sequence of the primers and probes are written in 5′ to 3′- end pattern (Left to Right). Probes labelled at the 5′-end = Reporter (FAM = 6-carboxyfluorescein, HEX = Hexachloro-fluorescein) and at 3′-end = Quencher (BHQ1 = Black Hole Quencher1, BBQ = BlackBerry Quencher, TAMRA = Tetra-Methyl-Rhodamine).
Three nucleocapsid protein gene (N gene) regions are targeted using primer and probe (Table 2). The assay uses individual probe and primers for three N genes (N1, N2, N3 gene) with an additional primer and probe for detecting human RNase P. N3 gene primer and probe sets are for detection of all SARS-like Coronaviruses. The sensitivity of this assay is lower than other assays as it has a limit of detection of 8.3 copies per reaction.
Reverse transcription-loop mediated isothermal amplification (RT-LAMP) has been developed to detect SARS-CoV-2 in patients by targeting spike gene and orf1ab gene of the virus with 4 primers (outer forward primer-F3, outer backward primer-B3, forward inner primer-FIP, and a backward inner primer-BIP). For accelerating the reaction, additional 1 or 2 primers are added (loop forward primer- LF) and/ or a loop backward primer- LB). The change in colour/ turbidity of the reaction mixture from fluorescent dye hydrolysis for every hit on target after 60 min of incubation at 65 °C is observed through a turbidimeter (O.D. at 650 nm) the value of 1.0 is considered as positive test [21].
For the qualitative detection of the specific gene sequence of SARS-CoV-2, the sample is generally collected as nasopharyngeal or oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, or nasopharyngeal wash/aspirate as recommended by the FDA. In addition, swabs of upper respiratory specimens including nasopharyngeal, nasal swabs, or mid-turbinate are collected from an individual, with or even without symptoms of COVID-19. Despite the great advantages of these methods, a well-trained technical person is required to carry out such diagnostic procedures. Potential of these molecular tools are restricted to the samples obtained from the respiratory tracts of the suspected individuals. Sputum, nasopharyngeal aspirates, BAL fluid, nasal aspirates, nasopharyngeal or oropharyngeal swabs can only be tested through this approach. Also, the chances of false-negative results become high when the lab reagents are contaminated, used past their expiry date, or samples are not timely collected from the right region. False-negative results are also obtained with improper storage and transport of specimen, the presence of amplification inhibitors in samples, and if the mutation rate of the virus is high during the PCR cycle [62].
DETECTOR assay is an RNA-sensing assay that uses synthetic SARS-CoV-2 RNA fragments to recognize the signature of E and N gene sequences of SARS-CoV-2. Viral RNA targets are reversed transcribed to cDNA and amplified which subsequently transcribed back to RNA isothermally. The RNA fragments in the reaction combine with Cas13a protein (ribonuclease) that binds with the amplified RNA product forming a SHERLOCK. Ribonuclease (Cas13a) then cleaves the surrounding fluorophore-quencher probes emitting signals. This CRISPR-Cas13 is a working protocol for SARS-CoV-2 detection that has been rolled out for clinical trials [22].
2.2. Immunological methods
For the diagnosis of COVID-19 by immunological technique, viral antigen (antigen test) present in the specimen or antibodies or immunoglobulins produced (antibody test) against specific components of SARS-CoV-2 are detected. These are blood-based tests commonly used to detect antibodies generated against SARS-CoV-2 after the infection. Antibody response to SARS-CoV-2 infection is similar to any typical viral infection [23]. Therefore, the chance of antibody cross-reactivity from infections of other pathogens can exist and sometimes be misinterpreted. Like most immunological diagnostic protocols, Enzyme-Linked Immunosorbent Assay (ELISA) for COVID-19 detection uses IgM and IgG antibody against nucleocapsid (N) and receptor binding domain spike proteins (S) of SARS-CoV-2.
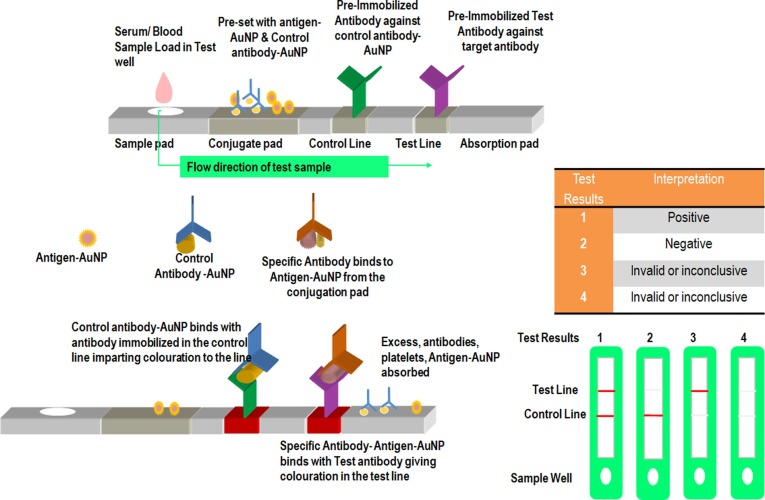
The immunochromatographic test is a rapid chromogenic double antigen/double antibody sandwich ELISA developed in the wake of the SARS-CoV outbreak during 2004 [24]. Recombinant N protein of SARS-CoV-2 was taken as an antigen which is immobilized on a nitrocellulose strip with IgG coupled to colloidal gold particles. Anti-SARS-CoV-2 N protein antibody IgG binds to the SARS-CoV-2 antigen to form red-coloured antibody-antigen-gold complex ( Fig. 2 ). The specificity of the immunochromatographic test is around 98.2% in various kits.
Fig. 2.
Schematic representation of Rapid Immuno-Chromatographic Assay. Serum/ Blood Sample Load in Test well which was pre-set with antigen-AuNP & Control antibody-AuNP. Control antibody-AuNP binds with antibody immobilized in the control line imparting colouration to the line. Specific Antibody binds to Antigen-AuNP from the conjugation pad. Specific Antibody- Antigen-AuNP binds with test antibody giving colouration in the test line.
Most tests for rN are done through an indirect ELISA method using IgG where N protein is bounded with IgG/IgM antibody to a microplate or nitrocellulose membrane. A secondary antibody conjugated with horseshoe reddish peroxidase (HRP) binding to the complex is detected by the generation of colour on the addition of substrate TMB (3,3′5, 5′ tetramethylbenzidine). The sensitivity for the IgG tests is reported to be 99.0% [23].
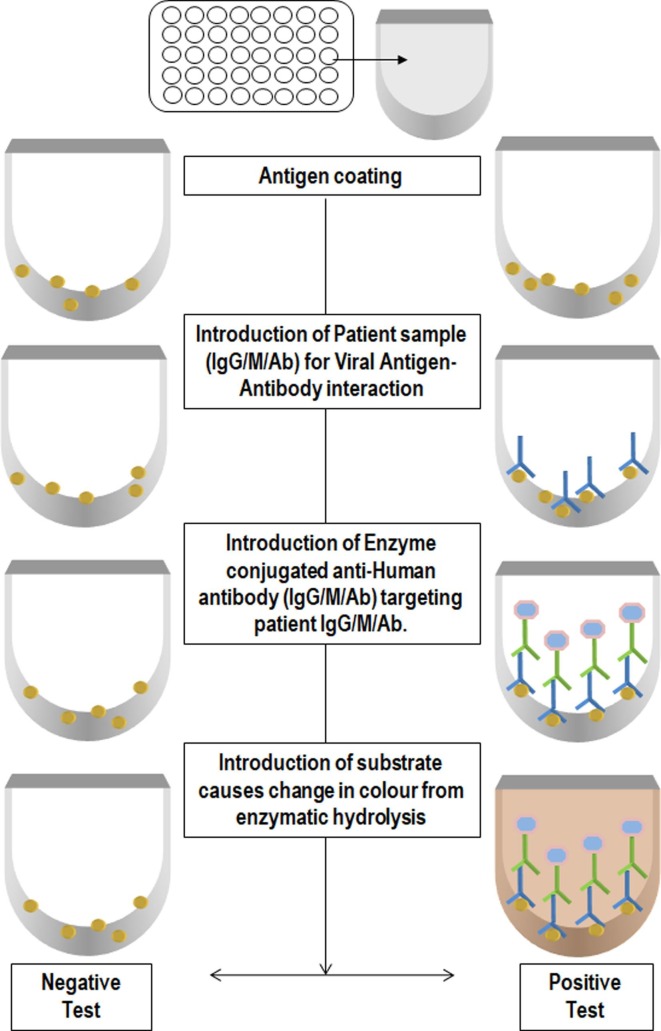
Ab-ELISA is a double-antigen sandwich immunoassay for the detection of total antibodies that binds to the SARS-CoV-2 spike protein receptor domain (RBD) in plasma or serum ( Fig. 3 ). In this method, immobilized mammalian cell-expressed recombinant antigen containing the receptor-binding domain of the virus spike proteins and HRP-conjugate antigen were used. The specificity of the Ab-ELISA test kit is 99.1% [23]. IgM specific for recombinant spike protein RBD detection is done using HRP-conjugate and TMB as a substrate for the colorimetric test following IgM µ-chain capture method. The specificity of IgM ELISA is 98.2% [23].
Fig. 3.
Schematic representation of the Enzyme-Linked Immunosorbent Assay Test. The 96 well plate was coated with the antigen. Patient sample (IgG/M/Ab) was loaded to the coated well for Viral Antigen-Antibody interaction. Introduction of Enzyme conjugated anti-Human antibody (IgG/M/Ab) targeting patient IgG/M/Ab. After washing of unbound antibody wells were subjected with substrate which causes change in colour from enzymatic hydrolysis. The intensity of color is proportional to the viral load.
SARS-CoV-2 IgG-IgM combined antibody test through a lateral flow immunoassay (LFIA) works on the principle that immunoglobulin class M (IgM) forms the first line of immune defense response against viral infection followed by IgG as a long-term immunity and memory response. Therefore, the detection of IgM would be an indication of recent exposure to SARS-CoV-2 while IgG would be an indication of exposure to the virus some time ago. In IgG-IgM assay, antigen from SARS-CoV-2 that can bind specifically to IgG and IgM are conjugated along with 40 nm AuNP and immobilized on a nitrocellulose pad. The presence of anti-SARS-CoV-2 antibodies (IgG and IgM) are then detected by binding of the anti-SARS-CoV-2 antibodies to their respective antibodies conjugated with AuNP colorimetric reagents giving a red/purple colour within 15 min. The sensitivity and specificity of the assay is 88.66% and 90.6%, respectively. IgG-IgM assay is recommended for Point of Care use due to its superiority in terms of sensitivity and utility over a single IgM or IgG test.
In an antigen test, the presence of viral antigens in swab samples is detected directly by binding of monoclonal antibodies specific for viral antigens. The positive binding of monoclonal antibodies to the viral antigen is observed through fluorescence/colour generation. Nucleocapsid protein test is based on the principle of fluorescence Immunochromatographic assay using goat Anti-rabbit IgG antibodies and mouse anti-nucleocapsid protein (SARS-CoV-2) monoclonal antibody immobilized on nitrocellulose membrane. Conjugation of anti-nucleocapsid monoclonal antibody or rabbit Ig with carboxylate-modified polystyrene Europium captures the nucleocapsid of SARS-CoV-2 from saline solution treated nasal swap of patients. The resulting fluorescence read from the immuno-fluorescence analyser was compared to the standard cut-off value from healthy individuals.
A sensitivity value of above 68% is taken as SARS-CoV-2 nucleocapsid protein test positive [25]. In principle, the detection of viral antigen would be the virus-specific marker for the diagnosis of COVID-19. These diagnostic methods are having a great advantage of convenience and rapidness but limited by inadequate sensitivity and specificity compared to standard RT-PCR. Due to these reasons, results from these methods need to be interpreted with caution.
2.3. Haematological
Change in physio-pathological parameters such as hematological and histopathological can act as a measure to detect SARS-CoV-2. A blood sample of suspected patient is taken for hematological diagnosis. Many studies have shown that individuals with SARS-CoV-2 exhibit clinical characteristics of systemic inflammation such as lymphopenia, neutrophilia, thrombocytopenia, lowered serum albumin and increased C-reactive proteins (CRPs), lactate dehydrogenase, aspartate aminotransferase, alanine transferase, cardiac troponin, erythrocyte sedimentation rate, D dimer, ferritin, creatine kinase, etc [26]. Until now, a well-established hematological parameter/ pathophysiological novel marker for COVID-19 is still lacking. However, monitoring of hematological parameters could serve as a clinical indicator of severity and prognostication of the disease for early intervention of treatment, but not as a diagnostic tool.
2.4. Radiology
Computed Tomography (CT) is serving as a valuable technique for the diagnosis and progression of the disease. CT scan results of COVID-19 infected individual shows a variety of abnormalities that are unique from other viral pneumonia infections of SARS, MERS, and Adenoviruses [27]. CT scan was found to be more appropriate in providing insights about pneumonia but the results were variable in different patients highlighting the different stages the virus might have been at that time [28]. The scan manifestation unique to COVID-19 patients includes patchy lesion- that are nodular to irregularly shaped have ground-glass opacity (GGO) and are consolidated in the subpleural region of the central lung lobes [29]. They have distinct reversed halo sign and pulmonary nodules with halo sign [27]. A Follow-up CT scan revealed that the lesions were migratory with the phenomenon of absorption of the primary lesion to the emergence of new lesions in the pleural and sub-pleural regions [30]. Since the varied representation of pneumonia is obtained by CXR and CT scan in different patients, therefore it was difficult to conclude the actual pathogenic mechanism [31]. Also, certain asymptomatic patients showed early changes under CT scan which is an excellent approach to identify the patients without symptoms however; this was not always the case [32].
2.5. Nanomaterial-based diagnostic methods
The crucial factors of diagnosis and treatment of virus-related diseases can be adequately addressed through nanotechnological platforms. The nanomaterials and microfabrication technologies offer valuable diagnostic tools for viral detection in ultralow concentration in blood, serum, and plasma ( Figure S1 ). Nanomaterials respond to a minor stimulus and provide a signal even to ultra-low concentrations. Plasmonic metal nanoparticles, such as gold nanoparticles (AuNPs), offer high surface area, which immobilizes various analytes and induces a significant change in plasmonic signal. A colorimetric detection methodology has been reported for the detection of influenza virus A (IVA) using gold nanoparticles (NPs) modified with monoclonal anti-hemagglutinin antibodies (mAb). The AuNPs-mAb probes are sensitive for surface recognition sites of the virus and induce the plasmon effect in the attached AuNPs, leading to visual detection of viral strains [33]. The enveloped virus proteins like hemagglutinin primarily may be utilized for detection parameters for the rapid and easy detection of viral infections.
Zhao et al. [34] reported the fluorescent quantum dots (QDs) and superparamagnetic nanoparticle beads functionalized with mAb for diagnosis of avian influenza virus (AIV). The detection of infectious viruses using various other nanomaterials such as SiNPs [35], AgNPs [36], CuNPs [37], and CNTs [38] have been explored in recent years, which opens the possibilities for detection of Coronaviruses shortly. More recently, Seo et al. [39] demonstrated the graphene-based biosensor for SARS-CoV-2 detection in clinical samples. Authors exploited the immobilization of the SARS-CoV-2 spike antibody onto the fabricated device with 1-pyrene butyric acid N-hydroxysuccinimide ester (PBASE) acting as probe linker. Despite the great advantage of nanomaterial, still, several clinical validations are required to be established between a definite correlation of disease status and diagnostic outcomes.
3. Therapeutic approaches in COVID-19
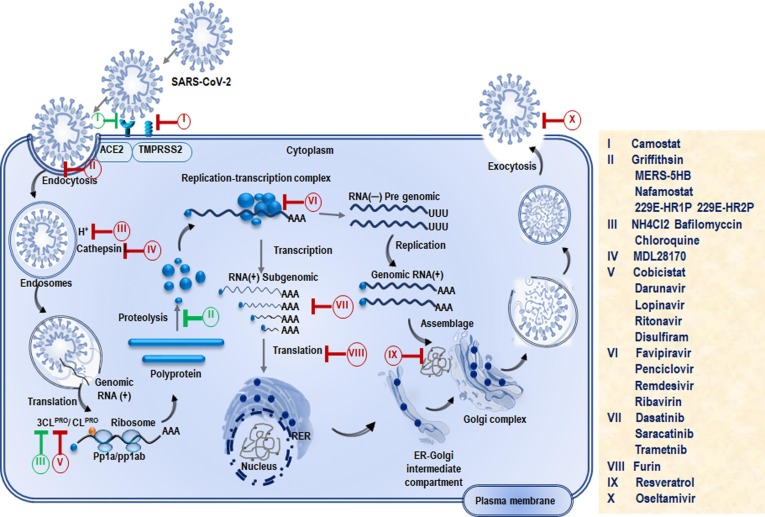
Despite the challenges associated with COVID-19 therapy, there are still several approaches currently being undertaken which show significant outcomes [40]. In this section, we shall discuss the positive impacts of some of the clinically used medications for the treatment of severe COVID-19 patients ( Figure S2 , Table S1). Several drugs are in clinical trials and some of them have shown significant promise in addressing COVID-19 patients [13], [41], [42]. The site of action of these drugs is illustrated in Fig. 4 . In addition, to drugs under clinical trials, several vaccines are in the pipeline which is expected to play a significant role in controlling the COVID-19 pandemic (Table S2). This section provides a brief discussion of some of the promising drugs which are used to fight SARS-CoV-2 infection.
Fig. 4.
Showing the phases of the life cycle of SARS-CoV-2 and the sites of action of potential drugs. The life cycle of SARS-CoV-2 begins by binding of S protein to the ACE2 receptor on the host cells which is the potential target for antibody. RNA-dependent RNA polymerase (RdRp) and main protease play pivotal role in mediating viral replication and transcription, making them attractive targets for majority of drugs.
3.1. Remdesivir
Remdesivir (GS-5734™) is a novel small molecule, a broad-spectrum antiviral nucleoside analog that is now being investigated as a potential treatment option for COVID-19 [43]. It belongs to the class of polymerase inhibitors. It is reported to show activity against different RNA viruses, including SARS-CoV, MERS-CoV, Nipah virus, Hendra viruses, and Ebola viruses. Remdesivir is a prodrug of its parent adenosine analog, GS-441524. Both of these medicines metabolized into the active component as nucleoside triphosphate (GS-443902) after ingestion and they show antiviral activity against SARS-CoV [44]. Being a nucleoside analog, Remdesivir targets the viral genome replication process by acting as an RdRp inhibitor. Yin et al. [45] established the structural basis leading to inhibition of the RNA-dependent RNA polymerase of SARS-CoV-2 using Remdesivir. On metabolization of remdesivir into active nucleoside triphosphate (NTP) which competes with ATP for incorporation into nascent RNA strand resulting in premature RNA synthesis leading to termination and ceasing of growth of RNA strands [44]. It has been reported that Remdesivir and chloroquine are highly effective in the control of SARS-CoV-2 infection in-vitro. Recently, WHO has claimed that antiviral drug Remdesivir failed in the first trials for the treatment of COVID-19 [46]. However, the drug now has become a matter of debate between the clinical experts, scientists, and US-based pharmaceutical companies. There are still controversies regarding the results obtained from this trial, as clinicians suggest no benefit in COVID-19 treatment using Remdesivir; whereas, the company claims it as a promising drug for the same [47].
3.2. Favipiravir
Favipiravir a new type of RdRp-inhibitor which acts as an antiviral drug, has been approved by the National Medical Products Administration of China, for the treatment of CoVs. The use of Favipiravir has been explored in the treatment of influenza as it selectively inhibits the RdRp of influenza virus. Favipiravir acts against RNA viruses by working on viral genetic copying to prevent its reproduction. A phase 3 clinical trial involving a double-blind, placebo-controlled, multi-centric study for the treatment of a moderate type of COVID-19 disease using Favipiravir drug is in process. Favipiravir combined with supportive care is being compared to placebo. The drug has been administered in the dosage of 1800 mg twice a day for the first day and 600 mg thrice a day from 2nd day onwards for a maximum of 14 days. The primary outcomes include the normalization of pyrexia, respiratory rate, and relief from cough maintained for at least 72 h [48].
3.3. Lopinavir/ritonavir
Lopinavir/ritonavir has been used in combination with other anti-retroviral medicinal products for the treatment of HIV-1 infected people. During the present pandemic of COVID-19, this combination of drugs has shown potential for the treatment, and many trials are under progress. Lopinavir/ritonavir act as a protease inhibitor drug and inhibit the action of 3CLpro, chymotrypsin-like protease enzyme, that plays a vital role in the processing of virus and thus interfere with the process of viral replication and its release from host cells. In an adaptive, randomized controlled clinical trial, Lopinavir/Ritonavir has been administered twice orally as tablets (200 mg and 50 mg) for 14 days to patients. While the others who couldn't swallow, 5 mL oral suspension was provided twice daily, followed by a daily assessment. The study is in phase 2 clinical trial [49]. In another phase-2/phase-3 clinical trial, a random, double-blind, multi-centric investigation is being carried out on COVID-19 patients. Four different groups of patients are receiving lopinavir/ritonavir and hydroxychloroquine. The study started on the 6th of April 2020 and due completion in April 2021 [50].
3.4. Novaferon
Novaferon has shown great potential as an antiviral drug against COVID-19 [51]. It is a synthesized protein consisting of 167 amino acids, designed in the laboratory on the technical basis of DNA shuffling technology (US patent US 7, 625,555 B2). Researchers have conducted a randomized, open-label, parallel-group study to explore the antiviral effects of novaferon for COVID-19 alone and in combination with lopinavir/ritonavir. Novaferon inhibited the viral replication in infected cells (EC50 = 1.02 ng/ml) and protected healthy cells from SARS-CoV-2 infection (EC50 = 0.1 ng/ml). Results from the 89 enrolled COVID-19 patients showed that both novaferon and novaferon plus lopinavir/ritonavir groups had significantly higher SARS-CoV-2 clearance rates on day six than the lopinavir/ritonavir group [51].
3.5. Chloroquine and hydroxychloroquine
Chloroquine is a widely used antimalarial drug that was observed to have a broad-spectrum antiviral activity [52]. It has been reported to block SARS-CoV-2 infection at low micromolar concentrations [53]. Chloroquine has the inhibition capability of replication of several intracellular micro-organisms, including SARS-CoV-2. The use of chloroquine increases endosomal pH and thereby interferes with the glycosylation of the cellular receptor of SARS-CoV. Therefore, it has the potential to block viral infection. Hydroxychloroquine (HCQ) is a 4-aminoquinoline with immunosuppressive, anti-autophagy with antimalarial effects [54]. It has been purposed that HCQ interferes with the processing and presentation of antigens and production of cytokines and hence suppresses immune functions. During the pandemic of COVID-19, HCQ has been studied and under numerous trials for the treatment of coronavirus disease [55]. In two small, uncontrolled studies, HCQ and its congener, chloroquine, were reported to be effective against COVID-19, however, the publishing journal later declared that the trial did not meet the Society's expected standard [56]. Due to severe complications observed in the COVID-19 patients, WHO recently discouraged the use of both CQ and HCQ [57].
3.6. Auranofin
Auranofin is an FDA approved lipophilic organogold compound which has been used to treat rheumatoid arthritis. It exhibits anti-inflammatory and potential antineoplastic activities. These gold-containing drugs have known anti-inflammatory properties, and this specific triethyl phosphine can reduce cytokine production and stimulate cell-mediated immunity [58]. The effect of auranofin was investigated on human cell lines (Huh7 derived from liver cells) infected with the COVID-19 virus. After 24 h of treatment, viral concentration was found to decrease by 85%, going up to 95% within 48 h. At the same time, auranofin was non-toxic, with reduced inflammation of the cells used in this trial. The drug has reported a reduction in the expression of cytokines, the signaling proteins that attract immune cells to the infection site. These are responsible for causing cytokine storms accountable for organ failure and death. Overall results suggest that auranofin inhibits replication of SARS-CoV-2 in human cells at a low micromolar concentration. However, further animal studies are needed to confirm this proof of concept before it can be tried in the clinical setting [59].
3.7. Famotidine
Famotidine is a competitive histamine H2-receptor antagonist that inhibits gastric secretion. Famotidine is the active compound in the heartburn drug, Pepcid. According to a report, on the 27th of April 2020, Northwell Health in the New York City area began a clinical trial to test famotidine against SARS-CoV-2. The trials have been underway since 7th of April with 1,174 patients, including 187 who were critically ill. Famotidine is structured in such a way that it could prevent the replication of SARS-CoV-2 protease [60]. Huaier Granule is under phase 2 and phase 3 clinical trial for the treatment of COVID-19, in a study started by Chen Xiaoping at Tongji Hospital on 1st of April 2020, Over 550 patients are under investigation primarily for their mortality rate, and ICU stays [61]. This study is likely to be completed by September 2020.
3.8. Ruxolitinib and Baricitinib
Ruxolitinib [62] and Baricitinib [63] are the FDA approved drugs for the treatment of myelofibrosis, polycythemia vera, and graft-versus-host disease and rheumatoid arthritis. These drugs are under phase 2 and phase 3 trials for the treatment of COVID-19, respectively. Under the trial studies, the effect of Ruxolitinib on the severity of COVID-19 has been investigated. It has been proposed that these two drugs have the potential to minimize the hyper inflammation caused by the SARS-CoV-2, and hence damage to the lungs and possibly other organs. Total 80 patients are under recruitment for the assessment of Ruxolitinib and Baricitinib (20 patients in trial 2 and 60 patients in phase 3 trial) at the University of Colorado Hospital, are receiving 10 mg Ruxolitinib twice daily for 14 days extended up to 29 days and 2 mg Barticinib orally regularly and similar manner. The study has been started by Joaquin Espinosa in April 2020 and is proposed to be completed in October 2020.
3.9. Ribavirin
Another example of nucleoside analog used to stop viral RNA synthesis and viral mRNA capping with broad-spectrum antiviral activity is ribavirin, which has been analysed for its action against SARS-CoV-2 [64]. It is a prodrug, which when metabolized resembles purine RNA nucleotides that interferes with RNA metabolism required for viral replication. It has been observed during a comparative study on SARS-CoV-2 patients treated with combined therapy of lopinavir/ritonavir and ribavirin; that they are at lower risk of death than when receiving ribavirin monotherapy [65].
3.10. Ivermectin
Ivermectin is an FDA-approved anti-parasitic drug having broad-spectrum antiviral activity in vitro. A laboratory level study demonstrated that ivermectin has antiviral action against the SARS-CoV-2 clinical isolate in vitro in the Vero-hSLAM cells. Researchers have claimed that a single dose of ivermectin was able to cause ~ 5000-fold reduction in viral RNA at 48 h. It has led to the hypothesis that this is likely through inhibition of IMPα/β1-mediated nuclear import of viral proteins [66]. However, on the 10th of April 2020, the FDA issued guidance not to use ivermectin intended for animals as a treatment for COVID-19 in humans [67].
3.11. Arbidol
It is an antiviral widely used to treat the influenza virus. Arbidol can effectually prevent SARS-CoV-2 infection in vitro [41] . Lopinavir/ritonavir and Arbidol have been recently recommended by the National Health Commission and National Administration of Traditional Chinese Medicine for dealing with COVID-19 [68]. A recent study, suggests that arbidol monotherapy is more effective than lopinavir/ritonavir in treating COVID-19. No viral load was observed in the arbidol group as compared to 44.1% viral load in patients having lopinavir/ritonavir on the 14th day of treatment. Additionally, no apparent side effects were found in both groups [69].
3.12. Darunavir
Darunavir is a second generation of HIV-1 protease inhibitors used to prevent SARS-CoV-2 infection in-vitro [70] by inhibiting viral replication at 300 μM and this inhibition efficiency was 280-fold compared to the untreated group.
3.13. Carmofur
Carmofur is a pyrimidine analog used as an antineoplastic agent. It is an orally delivered lipophilic masked analog of 5-fluorouracil, a highly potent acid ceramidase mainly used for the treatment of breast and colorectal cancers. Carmofur is considered to inhibit the SARS-CoV-2 main protease (Mpro) [71]. The crystal structure of Mpro in complex with carmofur indicates that the carbonyl reactive group of carmofur is covalently bound to catalytic Cys145, whereas its fatty acid tail occupies the hydrophobic S2 subsite. Carmofur inhibits viral replication in cells (EC50 = 24.30 μM) and is a promising lead compound that can be developed as a new antiviral treatment for COVID-19.
4. Conclusions
SARS-CoV-2 is more contagious, sporadic, and deadly as compared to previously known CoVs, and therefore it has become a global health challenge. The highly infectious nature of the virus makes medical research imperative to develop active therapeutic agents and a preventive vaccine against the SARS-CoV-2 swiftly. Despite several new methods that are rapidly emerging for the diagnosis of COVID-19 patients, the RT-PCR-based method is still most promising. Various chemical therapeutics and antibody-based agents are being screened for their anti-SARS-CoV-2 activity, and some of them are showing promising results in the treatment of this disease. Drugs such as chloroquine is a broad-spectrum inhibitor of viral cell entry, and Remdesivir is a broad-spectrum RNA polymerase inhibitor. SARS-CoV-2 infection simultaneously triggers the host immune system and an inflammatory cascade response (cytokine storm). These are currently being targeted in the treatment of COVID-19 patients. In the evaluation of emerging vaccine candidates for SARS-CoV-2, the prime consideration should be given to the safety of the patients. The nanotechnological platform provides a new direction to diagnostics and treatment approaches in an efficient way. The more unique nanomaterials act as outstanding antiviral drug delivery systems and improve the efficacy of the procedure. Immediate efforts are needed to find effective diagnostic and therapeutic methods for the rapid and efficient management of severe COVID-19 patients.
Declaration of Competing Interest
The authors declared to have no conflict of interest.
Acknowledgments
SSS thanks to the Clifford Craig Foundation Launceston General Hospital, Tasmania, Australia. IKS is thankful to Department of Health Reseach for financial support (Grant no. No.12013/11/2019-HR/E). MIH thanks to the Indian Council of Medical Research for financial support (Grant No. BIC/12(01)/2015). Authors are also thankful to Dr. Amit Kumar (Hansraj College) for the help in drawing of figures.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2020.08.013.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Boccaletti S., Ditto W., Mindlin G., et al. Modeling and forecasting of epidemic spreading: the case of covid-19 and beyond. Chaos, Solitons Fractals. 2020;135 doi: 10.1016/j.chaos.2020.109794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lillie P.J., Samson A., Li A., et al. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J. Infect. 2020;80:578–606. doi: 10.1016/j.jinf.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia X. Extreme Genomic CpG Deficiency in SARS-CoV-2 and Evasion of Host Antiviral Defense. Mol. Biol. Evol. 2020 doi: 10.1093/molbev/msaa094. msaa:094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 6.To K.K., Tsang O.T., Yip C.C., et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin. Infect. Dis. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization WH. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (29 March 2020).
- 8.Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell 2020, Shang J, Wan Y, Luo C et al. Cell entry mechanisms of SARS-CoV-2, Proceedings of the National Academy of Sciences 2020;117:11727-11734.
- 9.Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020;7:1. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- 10.Shereen MA, Khan S, Kazmi A et al. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses, Journal of Advanced Research 2020, He F, Deng Y, Li W. Coronavirus disease 2019: What we know?, Journal of medical virology 2020;92:719-725. [DOI] [PMC free article] [PubMed]
- 11.Walls A.C., Park Y.-J., Tortorici M.A., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naqvi AAT, Fatima K, Mohammad T et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach, Biochim Biophys Acta Mol Basis Dis 2020:165878. [DOI] [PMC free article] [PubMed]
- 13.Shamsi A., Mohammad T., Anwar S., et al. Glecaprevir and Maraviroc are high-affinity inhibitors of SARS-CoV-2 main protease: possible implication in COVID-19 therapy. Biosci. Rep. 2020;40 doi: 10.1042/BSR20201256. BSR20201256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y., Min P., Lee S., et al. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Korean Med. Sci. 2020;35:e174. doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wölfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 20.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan C., Cui J., Huang L., et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020;26:773–779. doi: 10.1016/j.cmi.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26(7):771–783. doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J., Yuan Q., Wang H., et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa344. ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H.-S., Chiu S.-C., Tseng T.-C., et al. Serologic and molecular biologic methods for SARS-associated coronavirus infection Taiwan. Emerging Infectious Diseases. 2004;10:305. doi: 10.3201/eid1002.030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolaev E.N., Indeykina M.I., Brzhozovskiy A.G., et al. Mass Spectrometric detection of SARS-CoV-2 virus in scrapings of the epithelium of the nasopharynx of infected patients via Nucleocapsid N protein. J. Proteome Res. 2020 doi: 10.1021/acs.jproteome.0c00412. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. “WHO Director-General's opening remarks at the media briefing on COVID-19 – 11 March 2020”. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (11 March 2020.
- 27.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. Am. J. Roentgenol. 2020;214:1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Xia L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. AJR Am. J. Roentgenol. 2020;14(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y., Guan H., Zhou S., et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan. Eur. Radiol. 2020;30:3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y., Liu Y.-L., Li Z.-P., et al. Clinical and CT imaging features of 2019 novel coronavirus disease (COVID-19) The Journal of infection. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K.S. Pneumonia associated with 2019 novel coronavirus: can computed tomographic findings help predict the prognosis of the disease? Korean J. Radiol. 2020;21:257–258. doi: 10.3348/kjr.2020.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet. Infect. Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Zhang L., Wei W., et al. Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst. 2015;140:3989–3995. doi: 10.1039/c5an00407a. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W., Zhang W.-P., Zhang Z.-L., et al. Robust and Highly Sensitive Fluorescence Approach for Point-of-Care Virus Detection Based on Immunomagnetic Separation. Anal. Chem. 2012;84:2358–2365. doi: 10.1021/ac203102u. [DOI] [PubMed] [Google Scholar]
- 35.Singhal C., Ingle A., Chakraborty D., et al. Impedimetric genosensor for detection of hepatitis C virus (HCV1) DNA using viral probe on methylene blue doped silica nanoparticles. Int. J. Biol. Macromol. 2017;98:84–93. doi: 10.1016/j.ijbiomac.2017.01.093. [DOI] [PubMed] [Google Scholar]
- 36.Sepunaru L., Plowman B.J., Sokolov S.V., et al. Rapid electrochemical detection of single influenza viruses tagged with silver nanoparticles. Chem. Sci. 2016;7:3892–3899. doi: 10.1039/c6sc00412a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Chen L., Su X., et al. Electrochemical immunosensor with graphene quantum dots and apoferritin-encapsulated Cu nanoparticles double-assisted signal amplification for detection of avian leukosis virus subgroup J. Biosens. Bioelectron. 2013;47:171–177. doi: 10.1016/j.bios.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed S.R., Kim J., Suzuki T., et al. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosens. Bioelectron. 2016;85:503–508. doi: 10.1016/j.bios.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 39.Seo G., Lee G., Kim M.J., et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS. Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 40.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proceedings of the National Academy of Sciences. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discoveries & Therapeutics. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 42.Mohammad T., Shamsi A., Anwar S., et al. Identification of high-affinity inhibitors of SARS-CoV-2 main protease: towards the development of effective COVID-19 therapy. Virus Res. 2020:198102. doi: 10.1016/j.virusres.2020.198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao Y-c, Deng Q-x, Dai S-x. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence, Travel medicine and infectious disease 2020:101647, Agostini ML, Andres EL, Sims AC et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, MBio 2018;9:e00221-00218. [DOI] [PMC free article] [PubMed]
- 44.Amirian ES, Levy JK. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses, One Health 2020:100128. [DOI] [PMC free article] [PubMed]
- 45.Yin W., Mao C., Luan X., et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roussel Y., Raoult D. Influence of conflicts of interest on public positions in the COVID-19 era, the case of Gilead Sciences. New Microbes and New Infections. 2020:100710. doi: 10.1016/j.nmni.2020.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arab-Zozani M., Hassanipour S., Ghoddoosi-Nejad D. Favipiravir for treating patients with novel coronavirus (COVID-19): protocol for a systematic review and meta-analysis of randomised clinical trials. BMJ open. 2020;10(7):e039730. doi: 10.1136/bmjopen-2020-039730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ClinicalTrials.gov. Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial (CATCO). https://clinicaltrials.gov/ct2/show/NCT04330690 (5 May 2020).
- 50.ClinicalTrials.gov. COVID MED Trial - Comparison Of Therapeutics for Hospitalized Patients Infected With SARS-CoV-2 (COVIDMED). https://clinicaltrials.gov/ct2/show/NCT04328012 (8 April 2020).
- 51.Zheng F., Zhou Y., Zhou Z., Ye F., Huang B., Huang Y., Ma J., Zuo Q., Tan X., Xie J., Niu P. SARS-CoV-2 Clearance in COVID-19 Patients with Novaferon Treatment: A Randomized, Open-label, Parallel Group Trial. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savarino A., Di Trani L., Donatelli I., et al. New insights into the antiviral effects of chloroquine. Lancet. Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geleris J., Sun Y., Platt J., et al. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortegiani A., Ingoglia G., Ippolito M., et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavalcanti A.B., Zampieri F.G., Rosa R.G., et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2019014. NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatre C., Roubille F., Vernhet H., et al. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug safety. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 58.Drug Bank. Auranofin. https://www.drugbank.ca/drugs/DB00995.
- 59.Rothan H.A., Stone S., Natekar J., Kumari P., Arora K., Kumar M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology. 2020;547:7–11. doi: 10.1016/j.virol.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borrell B. New York clinical trial quietly tests heartburn remedy against coronavirus. Science. 2020 [Google Scholar]
- 61.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Revista Panamericana de Salud Pública. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.La Rosée F., Bremer H.C., Gehrke I., Kehr A., Hochhaus A., Birndt S., Fellhauer M., et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020:1–11. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. The Journal of Infection. 2020 doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: Groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92(7):740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu C., Cheng V., Hung I., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caly L., Druce J.D., Catton M.G., et al. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FDA. FDA Letter to Stakeholders: Do Not Use Ivermectin Intended for Animals as Treatment for COVID-19 in Humans. https://www.fda.gov/animal-veterinary/product-safety-information/fda-letter-stakeholders-do-not-use-ivermectin-intended-animals-treatment-covid-19-humans (10 April 2020).
- 68.Xu K., Cai H., Shen Y., et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J. Zhejiang Univ. (Med. Sci.) 2020;49 [Google Scholar]
- 69.Zhu Z., Lu Z., Xu T., et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81:e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J., Xia L., Liu L., et al. Antiviral Activity and Safety of Darunavir/Cobicistat for the Treatment of COVID-19. Open Forum Infect. Dis. 2020;7(7):241. doi: 10.1093/ofid/ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin Z., Zhao Y., Sun Y., et al. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat. Struct. Mol. Biol. 2020;27:529–532. doi: 10.1038/s41594-020-0440-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.