Abstract
Mesenchymal stem cells (MSCs) critically contribute to bone formation, and proper induction of osteogenic differentiation can lead to an increase in bone mass. In the present study, we reported that an increased miR-194-5p level in plasma is inversely related to the degree of bone formation in osteoporosis patients. We also noted that increased miR-194-5p in the MSCs of ovariectomized (OVX) mice and agomiR-194-5p manipulation of miR-194-5p significantly suppressed bone formation, both in aged and OVX mice. Furthermore, our in vitro study showed that overexpression of miR-194-5p suppresses osteogenic differentiation, as evidenced by the decreased bone formation marker genes and matrix mineralization. The luciferase assay indicated that Wnt family member 5a (Wnt5a) is a target gene of miR-194-5p that positively regulates osteogenic differentiation. Collectively, these data indicated that miR-194-5p inhibition may be a potential strategy for osteoporosis prevention.
Keywords: miRNA, MSCs, osteoporosis, Wnt/β-catenin
Graphical Abstract
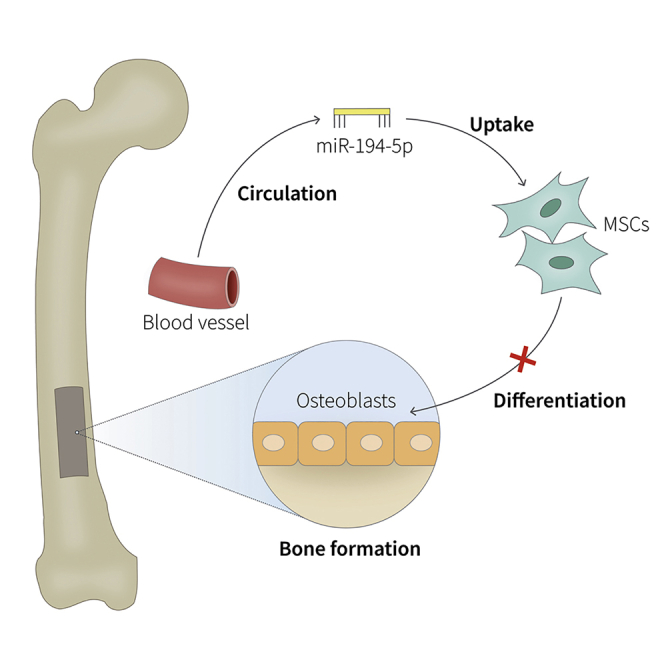
miRNAs have been reported to be closely related with osteoporosis progression. In this study, Mi et al. identified that the miR-194-5p level is highly increased in the plasma of osteoporosis patients, which suppresses bone formation both in vitro and in vivo. These findings suggested that miR-194-5p inhibition may be a potential strategy for osteoporosis prevention.
Introduction
Osteoporosis is a chronic systemic disease characterized by decreased mass and microarchitectural deterioration of bone. It is mainly seen in postmenopausal women and with aging, and, hence, with the aging population, the socioeconomic burden of osteoporosis is on the rise.1 Osteoporosis patients have increased bone fragility and fracture susceptibility, which are associated with significant mortality and poor quality of life.2 Although various drugs have been developed to inhibit bone resorption and increase bone formation, their side effects limit their long-term administration.3 Therefore, further research to elucidate the molecular and cellular factors in play is necessary.
Bone mesenchymal stem cell (BMSC) differentiation plays a major role in the maintenance of normal bone homeostasis.4 Accumulating evidence has suggested that induction of adipocyte differentiation impairs bone formation, while increased osteogenic differentiation is essential for bone mass increase.5, 6, 7 Therefore, a clear understanding of MSC fate regulation is required. Recent studies have focused on investigating the regulation of extrinsic and intrinsic regulators on MSC fate. For example, activation of the histone demethylation protein lysine demethylase 4B (KDM4B) induces osteogenic differentiation of MSCs.8 Although the molecular mechanism of MSC differentiation has been extensively studied, the role of microRNA (miRNA) control in MSC commitment, and consequently the connection between miRNAs and osteoporosis, is yet to be clarified. miR-194-5p, a vertebrate-specific miRNA, mainly regulates proliferation and energy production in tumor cells. For example, miR-194-5p promotes breast cancer cell proliferation, migration, and invasion.9 However, overexpression of miR-194-5p suppress the migration and invasion ability of colorectal cancer cells.10 Recent studies have indicated that miR-194-5p is involved in the differentiation of myoblast and hematopoietic progenitors,11,12 which highly suggests that miR-194-5p could modulate cell differentiation. Previous studies have reported that miR-194-5p is upregulated in women with osteoporosis, which may be a negative regulator for osteoporosis.13 It has been widely accepted that enhanced osteogenic differentiation of MSCs is essential to protect against osteoporosis. However, whether miR-194-5p affects osteoporosis progression via regulating MSCs differentiation and its underlying mechanism still needs further investigation.
Therefore, in the present study, we demonstrate that circulating miR-194-5p suppresses the osteogenic differentiation of MSCs. Injection of miR-194-5p directly into the bone marrow reduces osteoblast differentiation, thereby promoting osteoporosis. Furthermore, the underlying mechanism of miR-194-5p-mediated MSCs differentiation was investigated.
Results
miR-194-5p Is Highly Increased in Osteoporotic Patients and Ovariectomized (OVX) Mice
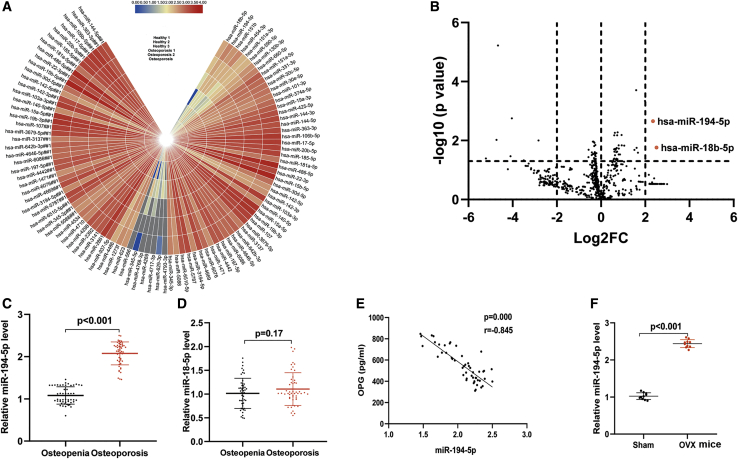
To investigate serum miRNA expression in osteoporosis, we obtained miRNA microarray expression profiling data from the Gene Expression Omnibus (GEO) databases (GEO: GSE64433). Subsequently, the miRNAs of healthy and osteoporotic patients were analyzed with a GEO2R tool, and the 50 most upregulated and 50 most downregulated miRNAs were constructed using a heatmap (Figure 1A). Total miRNA dysregulation was visualized using a volcano plot (Figure 1B). As shown in Figure 1B, both miR-194-5p and miR-18-5p levels were significantly increased in the osteoporosis serum samples in comparison to the healthy serum samples (p < 0.05, log2fold change [FC] > 3). To further confirm the level of miR-194-5p and miR-18-5p in serum, we collected blood samples from 50 healthy and 50 osteoporotic patients. As shown in Figure 1C, the miR-194-5p level was significantly higher in the plasma of osteoporotic patients in comparison to the healthy volunteers. However, no significant difference was observed in the miR-18-5p level between the two groups (Figure 1D). A strong negative association between serum miR-194-5p level and osteoprotegerin (OPG) concentration was also observed in the osteoporotic patients (Figure 1E). Subsequently, we assessed the miR-194-5p level in the bone MSCs of sham and OVX mice. Our data suggested that the miR-94-5p level was significantly increased in the MSCs of OVX mice in comparison to sham mice (Figure 1F). Collectively, these data suggest that an elevated miR-194-5p level is closely linked to osteoporosis.
Figure 1.
miR-194-5p Is Highly Increased in Osteoporosis Patients and OVX Mice
(A) A heatmap of the 50 most upregulated and most downregulated miRNAs was constructed. (B) The total dysregulated miRNAs were visualized using a volcano plot. (C and D) The level of miR-194-5p (C) and miR-18-5p (D) in the serum of healthy volunteers and osteoporosis volunteers was evaluated using quantitative real-time PCR. n = 50. (E) Association between serum miR-194-5p and bone formation marker (OPG) in osteoporosis patients. n = 50. (F) The miR-194-5p level in the bone MSCs of control (sham) and OVX mice was assessed using quantitative real-time PCR. n = 10.
miR-194-5p Suppresses Osteoblast Differentiation and Decreases Bone Mass In Vivo
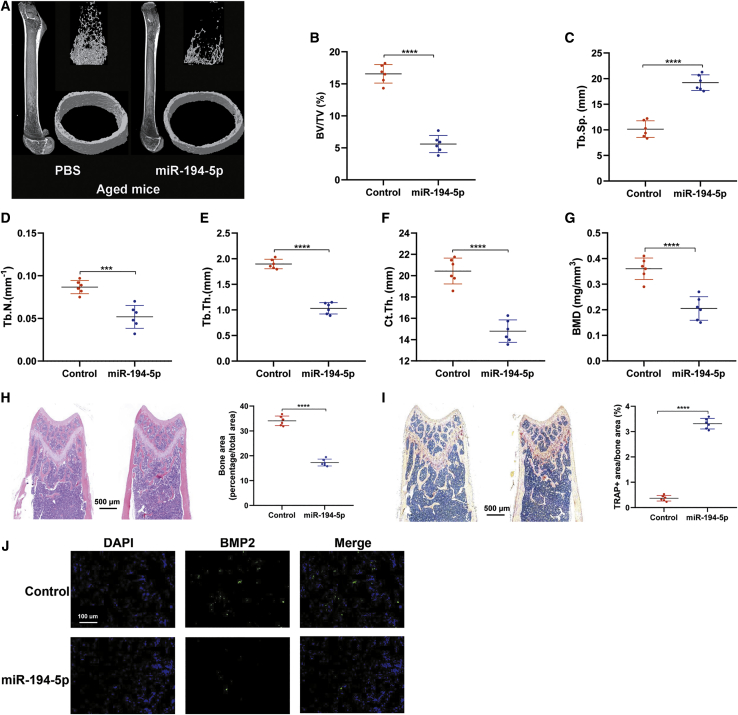
To determine whether increased miR-194-5p in vivo affects bone homeostasis in adult mice, 12-month-old male mice were treated with phosphate-buffered saline (PBS) or miR-194-5p injected into the femoral bone marrow. Three months later, micro-computed tomography (micro-CT) analysis of the femur revealed that miR-194-5p injection significantly decreased trabecular bone volume/tissue volume (BV/TV), trabecular numbers (Tb.N.), and trabecular thickness (Tb.Th.) and increased trabecular separation (Tb.Sp.) (Figures 2A–2E). The trabecular and cortical bone mass was clearly decreased in the miR-194-5p treatment group, as evidenced by lower cortical thickness (Ct.Th.) and bone mineral density (BMD) (Figures 2F and 2G). In agreement with the above results, decreased bone area with increased tartrate-resistant acid phosphatase (TRAP)-positive area was observed in the miR-194-5p treatment group in comparison to the control group (Figures 2H and 2I). Notably, miR-194-5p significantly decreased the bone morphogenetic protein 2 (BMP2)-positive osteoblast number when compared to control mice (Figure 2J). Collectively, these data suggest that miR-194-5p promotes osteoporosis progression in aged mice.
Figure 2.
Overexpression of miR-194-5p Decreases Bone Mass and Promotes Osteoporosis in Aged Mice
(A) Representative images of the femur of aged male mice. (B–E) Quantification of trabecular BV/TV (B), Tb.Sp. (C), Tb.N. (D), and Tb.Th. (E) of mice from the two groups. (F and G) Quantification of trabecular Ct.Th. (F) and BMD (G) of mice from the two groups. (H) H&E staining of the femoral metaphysis and quantification of the bone area. (I) TRAP staining of the femoral metaphysis and quantification of the TRAP-positive area. (J) Representative images of BMP2-positive osteoblasts on the surface of trabecular bone. n = 6. ∗∗∗p < 0.01, ∗∗∗∗p < 0.0001.
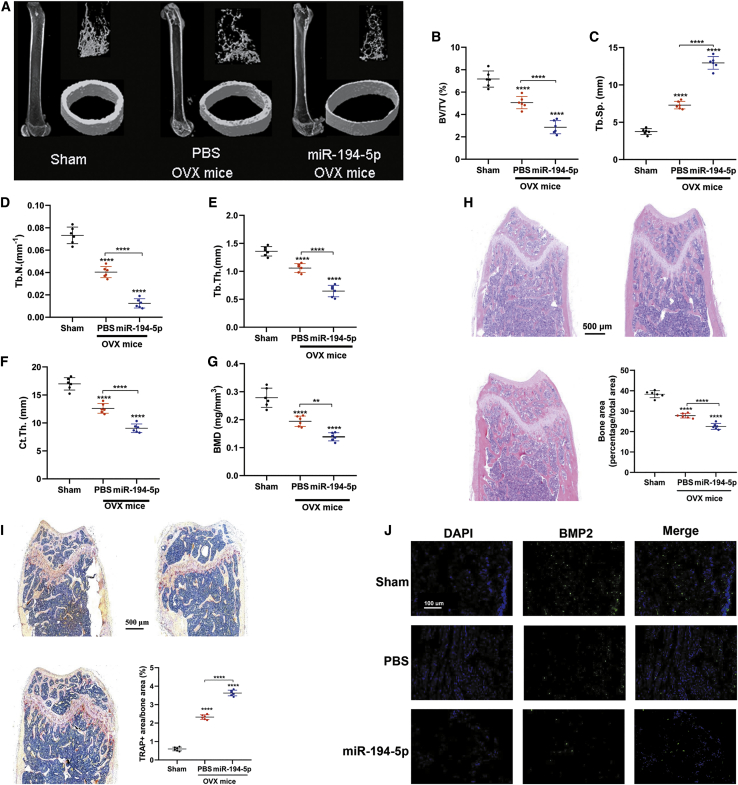
miR-194-5p Induces Bone Mass Loss in OVX Mice
To test the effect of miR-194-5p on osteoporosis, we treated OVX mice with miR-194-5p. After 3 months of administration, the miR-194-5p treatment group showed lower BV/TV, Tb.N., and Tb.Th. and higher Tb.Sp (Figures 3A–3E). In agreement with the above results, the Ct.Th. and BMD levels were significantly decreased in the miR-194-5p treatment group (Figures 3F and 3G). Importantly, the decreased bone area and TRAP-positive area also demonstrated the negative effect of miR-194-5p in osteoporosis (Figures 3H and 3I). This result was further supported by a decrease in BMP2-positive osteoblasts on the surface of trabecular bone (Figure 3J). Collectively, these data indicated that miR-194-5p contributes to the progression of osteoporosis in OVX mice.
Figure 3.
miR-194-5p Induce Bone Mass Loss in OVX Mice
(A) Representative images of the femur of OVX mice. (B–E) Quantification of trabecular BV/TV (B), Tb.Sp. (C), Tb.N. (D), and Tb.Th. (E) of mice from the three groups. (F and G) Quantification of trabecular Ct.Th. (F) and BMD (G) of mice from the three groups. (H) Representative images of H&E staining of the femoral metaphysis and quantification of the bone area. (I) Representative images of TRAP staining of the femoral metaphysis and quantification of the TRAP-positive area. (J) Representative images of BMP2-positive osteoblasts on the surface of trabecular bone. n = 6. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
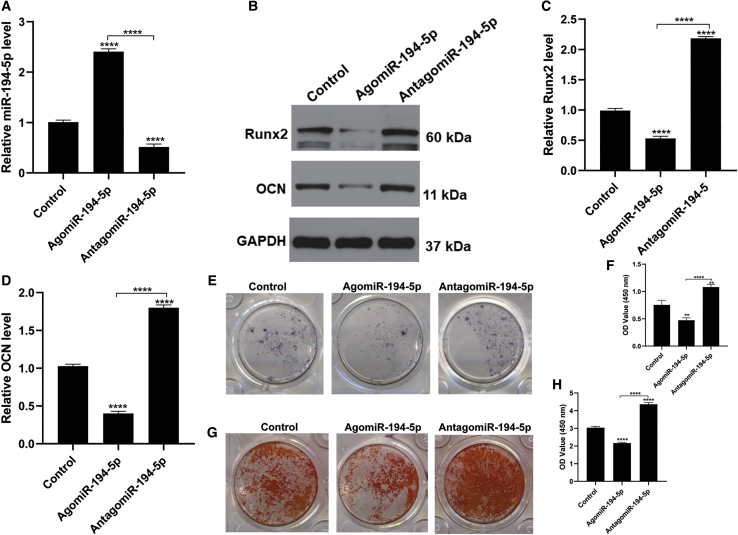
miR-194-5p Inhibits Osteogenic Differentiation In Vitro
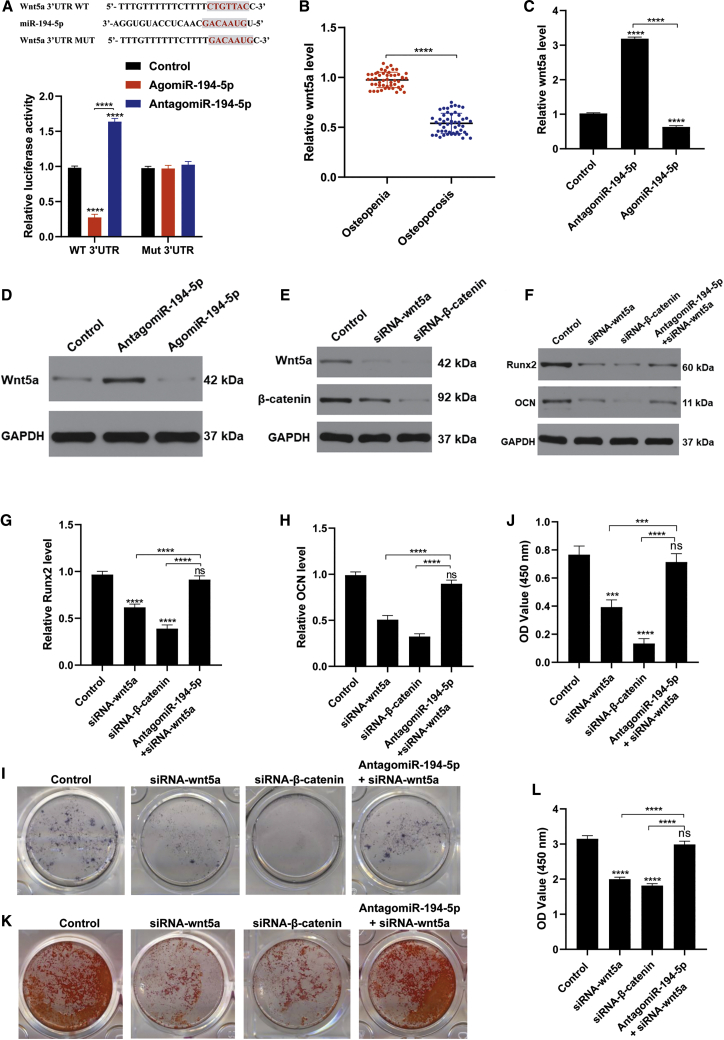
As miR-194-5p overexpression impairs bone mass and decreases osteoblasts in vivo, we hypothesized that miR-194-5p is involved in MSC differentiation. Therefore, to investigate whether miR-194-5p treatment can inhibit osteogenic differentiation we measured osteogenic-related genes, and performed alkaline phosphatase (ALP) and alizarin red staining. We found that agomiR-194-5p treatment significantly increased the level of miR-194-5p in MSCs, while antagomiR-194-5p treatment decreased the level of miR-194-5p (Figure 4A). Osteogenic-related gene expression was significantly suppressed with agomiR-194-5p treatment, evidenced through decreased Run family transcription factor 2 (Runx2) and osteocalcin (OCN) levels (Figures 4B-4D). In agreement with the above results, diminished ALP activity and mineralization capacity were observed in the agomiR-194-5p group (Figures 4E–4H). Collectively, these results demonstrate that miR-194-5p is a key regulator of osteogenic differentiation.
Figure 4.
miR-194-5p Suppresses Osteogenic Differentiation of MSCs
(A) Quantitative real-time PCR showed that miR-194-5p expression was increased with agomiR-194-5p treatment. (B) Western blot showed that the osteogenic-related markers Runx2 and OCN were decreased with agomiR-194-5p treatment. (C and D) Quantitative real-time PCR showed that the osteogenic-related markers Runx2 (C) and OCN (D) were decreased with agomiR-194-5p treatment. (E) ALP staining of MSCs after treatment with the indicated agents for 3 days. (F) Quantification of ALP staining among the three groups. (G) Alizarin red staining of MSCs 21 days after the indicated treatment. (H) Quantification of alizarin red staining of MSCs on day 21. All data are expressed as mean ± SD. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Wnt5a/β-Catenin Signaling Is Essential for miR-194-5p-Mediated Inhibition of Osteogenic Differentiation
To investigate the molecular mechanisms of miR-194-5p-mediated osteogenic differentiation, we screened the miRDB database (http://mirdb.org/) to identify potential targets. Previous studies reported that the Wnt signaling pathway is closely associated to bone formation and osteoporosis;14 hence, we hypothesize that Wnt family member 5a (Wnt5a) may be a potential target of miR-194-5p. To identify the relationship between miR-194-5p and Wnt5a, we used a wild-type (WT) Wnt5a 3′ UTR or a mutant-type (Mut) Wnt5a 3′ UTR construct fused to a luciferase reporter. The results showed that agomiR-194-5p substantially suppressed WT Wnt5a 3′ UTR reporter activity, while antagomiR-194-5p increased WT Wnt5a 3′ UTR reporter activity. However, both agomiR-194-5p and antagomiR-194-5p had no impact on Mut Wnt5a 3′ UTR reporter activity (Figure 5A). The quantitative real-time PCR results showed that the serum level of Wnt5a in the osteopenia group was significantly higher than that in the osteoporosis group (Figure 5B). Next, we performed an in vitro study to test whether miR-194-5p affects the Wnt/β-catenin signaling pathway. When MSCs were transfected with the miRNA construct for 24 h, lower relative Wnt5a levels in the agomiR-194-5p group were noted, compared with the other groups (Figures 5C and 5D). MSCs were treated with Wnt5a and small interfering RNA (siRNA) β-catenin to verify the role of the Wnt/β-catenin signaling pathway during MSC differentiation. We demonstrated that Wnt5a and β-catenin levels were markedly decreased after siRNA-Wnt5a or siRNA-β-catenin treatment (Figure 5E). Inhibition of either Wnt5a or β-catenin significantly reduced osteogenic differentiation, evidenced through decreased osteogenic markers (Figures 5F–5H), diminished ALP activity, and decreased mineralization capacity (Figures 5I–5L). Not surprisingly, miR-194-5p inhibition (antagomiR-194-5p) partly rescued the negative effect of siRNA-Wnt5a on osteogenic differentiation. Taken together, these data suggest that the Wnt5a/β-catenin signaling pathway is involved in the osteogenic differentiation through interaction with miR-194-5p.
Figure 5.
Wnt5a/β-Catenin Signaling Is Involved in miR-194-5p-Mediated Osteogenic Differentiation
(A) Effect of miR-194-5p on the luciferase activity of WT Wnt5a 3′ UTR or Mut Wnt5a 3′UTR after treatment with agomiR-194-5p or antagomiR-194-5p in MSC cells. (B) The level of Wnt5a in the serum of healthy volunteers and osteoporosis volunteers was evaluated using quantitative real-time PCR. n = 50, (C and D) The Wnt5a levels were evaluated by quantitative real-time PCR (C) and western blot (B) after antagomiR-194-5p and agomiR-194-5p treatment. (E) The Wnt5a and β-catenin levels were assessed by western blot after indicated treatment. (F–H) Western blot (F) and quantitative real-time PCR were used to evaluate the osteogenic-related markers Runx2 (G) and OCN (H). (I) ALP staining of MSCs after indicated treatment for 3 days. (J) Quantification of ALP staining of MSCs on day 3. (K) Alizarin red staining of MSCs after 21 days following indicated treatment. (L) Quantification of alizarin red staining of MSCs on day 21. The data are expressed as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
A shift in BMSC lineage is a hallmark of osteoporosis, but the underlying mechanism of the MSC differentiation remains poorly understood. In the present study, we provide evidence for the role of circulating miR-194-5p in MSC differentiation and osteoporosis progression.
The role of miRNAs has been well established in the physiological and pathological processes of disease, and dysregulated miRNAs are thought to be main contributors in osteoporosis.15 Specifically, elevated miR-182 has been shown to increase bone resorption and induce osteoporosis.16 Conversely, miR-34a is protective in osteoporosis.17 In the present study, we identified that the miR-194-5p level is markedly increased in the serum of osteoporosis patients, a level negatively correlated with the bone formation marker OPG. Our result was consistent with previous studies that identified miR-194-5p as a potential biomarker for postmenopausal osteoporosis.18 Thus, elevated miR-194-5p levels are likely to contribute to the development of osteoporosis.
Prior studies have reported that miR-194-5p regulates the progression of various cancers.19,20 However, its mechanism in osteoporosis remains elusive. Osteoporosis is a complex pathophysiological process determined by the viability of different cells, including osteoblasts, osteoclasts, and MSCs.21 Based on the elevated miR-194-5p level in the MSCs of OVX mice, a new connection between miR-194-5p and MSCs has become clear, specifically that miR-194-5p suppresses the capacity of osteogenic differentiation. Furthermore, a decrease in BMP2-positive osteoblasts seen in the trabecular bone of aged and OVX mice after miR-194-5p treatment highlights the negative effect of miR-194-5p.
It is well established that the canonical Wnt pathway governs processes such as proliferation, migration, and differentiation. The significance of Wnt/β-catenin signaling in osteogenic differentiation has been well documented.22,23 In the present study, we identified that the Wnt signaling pathway is involved in miR-194-5p-mediated osteogenic differentiation. We then offer evidence that miR-194-5p inhibits Wnt5a expression, thereby suppressing Wnt/β-catenin pathway activation. Previous studies have suggested that Wnt activation is necessary for MSC differentiation.24 In line with prior studies, we found that inhibition of the Wnt pathway through treatment with siRNA-Wnt5a-conditioned medium markedly inhibits osteogenic differentiation. miR-194-5p may regulate multiple targets, with Wnt5a being just one of the main targets that affects MSC differentiation. Therefore, further studies are necessary to investigate the underlying mechanism of miR-194-5p on MSC differentiation.
Although multiple miRNAs have been reported to be involved in osteoporosis, the origin of such dysregulated miRNAs remains unclear. With aging, many molecules are released from senescent cells and transported in the blood or to other cells. Previous studies have suggested that dying cells can transfer miR-194-5p into residual repopulating cells.25 We infer that miR-194-5p may be the metabolites of senescent cells, which could further affect the metabolic processes of other normal cells. Better understanding of the mechanisms of MSC differentiation may shed new light on osteoporosis and other bone mass loss-related diseases. In the current study, we demonstrated that miR-194-5p regulates MSC differentiation through inhibition of the Wnt/β-catenin pathway. However, the pathogenesis of osteoporosis is complex, and the effect of miR-194-5p on other cell types, including osteoblasts and osteoclasts, requires further investigation.
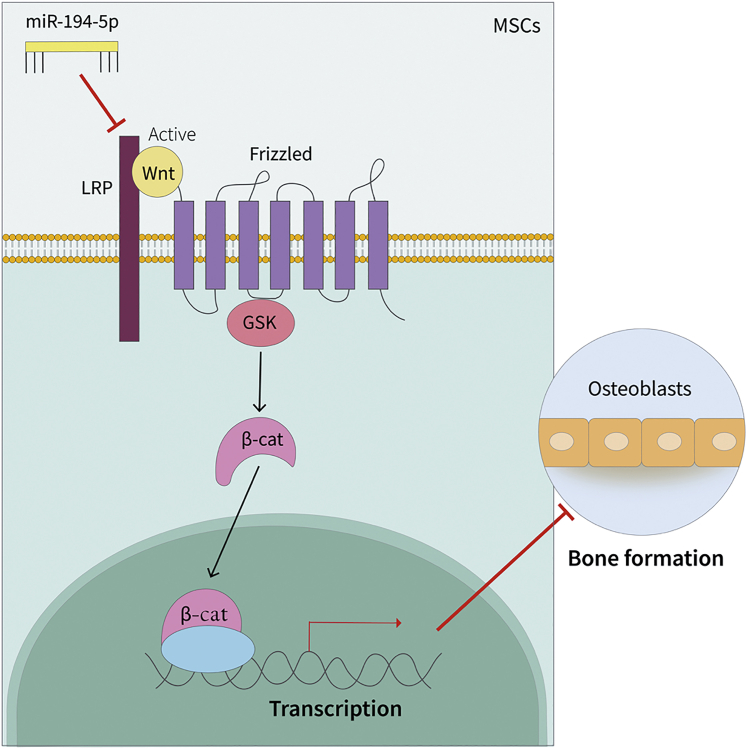
Our findings establish a new mechanism of miR-194-5p in MSC differentiation. Circulating miR-194-5p suppresses Wnt5a expression, thereby inhibiting Wnt/β-catenin activation and in turn suppressing osteogenic differentiation and inducing osteoporosis in vivo (Figure 6). Thus, therapeutic interventions targeting miR-194-5p offer great promise in the treatment of osteoporosis.
Figure 6.
Diagrammatic Sketch of miR-194-5p-Medicated Osteoblast Differentiation
Circulating miR-194-5p is transported to the bone marrow, where it is taken up by MSCs. Subsequently, miR-194-5p suppresses Wnt/β-catenin pathways, resulting in osteogenic differentiation inhibition.
Materials and Methods
Statement of Human and Animal Rights
Blood was collected from 50 healthy and 50 osteoporosis volunteers. All clinical procedures were approved by the Committee on Clinical Ethics in the Union Hospital (Wuhan, China). All samples were obtained with signed informed consent. Detailed information on the volunteers is listed in Table S1. C57BL/6 mice were purchased from the Center of Experimental Animal, Tongji Medical College, Huazhong University of Science and Technology. All animal protocols were in compliance with the Ethics Committee of the Institutional Animal Care and Use Committee at Tongji Medical College, Huazhong University of Science and Technology.
Cell Culture and Transfection
6- to 8-week-old male C57BL/6 mice were sacrificed and the femurs and tibias were dissected. The bone marrow was flushed with PBS, followed by centrifugation at 300 × g and suspension in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) with 1% penicillin/streptomycin (P/S). Cells were incubated in a humidified incubator at 37°C with 5% CO2. Before transfection, cells were seeded at 50% confluency in DMEM containing 10% FBS for 24 h.
The control siRNA (#37007), mouse Wnt5a siRNA (#sc-41113), and mouse β-catenin siRNA (#29210) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). AgomiR-194-5p and antagomiR-194-5p were purchased from GenePharma (Shanghai, China). Cells were transfected with miR-194-5p (200 μm) or siRNA (50 nM) using Lipofectamine 3000 (Invitrogen, USA). All experiments were repeated at least three times independently.
Osteogenic Differentiation and Staining
For osteogenic differentiation of MSCs, cells were incubated in an osteogenic induction medium (Cyagen Biosciences, USA). After induction for 3 days, quantitative ALP staining was performed according to the manufacturer’s instructions. After induction for 21 days, BMSCs were stained using alizarin red at room temperature for 10 min, followed by rinsing with PBS.
Western Blot
Total protein was collected from cells using a lysis buffer. Protein concentration was evaluated using a bicinchoninic acid (BCA) kit. Ten micrograms of protein was separated using a 10% SDS-PAGE gel, followed by transfer to a polyvinylidene fluoride (PVDF) membrane. The membranes were incubated with primary antibodies at 4°C overnight, followed by a 1-h incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature. The primary antibodies used were as follows: anti-Wnt5a (1:500, Abcam, MA, USA, #ab229200), anti-β-catenin (1:500, Abcam, MA, USA, #ab16051), anti-Runx2 (1:500, Abcam, MA, USA, #ab23981), anti-OCN (1:500, Abcam, USA, #ab93876), and anti-GAPDH (1:10,000, Abcam, USA, #ab37168). All experiments were performed in triplicate.
Quantitative Real-Time PCR Analysis
Total RNA was isolated from cells using TRIzol (Invitrogen, USA). For mRNA, the equivalent of 1 μg of total RNA was reverse transcribed in a 12-μL reaction containing 1× mRNA-specific reverse transcriptase (RT) primers (1 μL), total RNA (1 μg), 10 mM deoxyribonucleotide triphosphate (dNTP) mix (1 μL), and distilled water. The above solution was mixed at 65°C for 5 min and quick-chilled on ice. Subsequently, 5× first-strand buffer (4 μL), 0.1 M DTT (2 μL), and RNaseOUT recombinant ribonuclease inhibitor (1 μL) were added to the above solution. The solution was incubated at 37°C for 2 min, followed by adding 1 μL of Moloney murine leukemia virus (M-MLV) RT and incubating the solution at 25°C for 10 min and 37°C for 50 min. This was followed by heat inactivation for 15 min at 70°C. Amplification reactions were performed using FastStart Universal SYBR Green Master (Rox) (Roche) with 1 μL of RT under optimized conditions at 95°C for 5 s, 58°C for 30 s, and 72°C for 30 s for 40 cycles, followed by a melting curve analysis. For miRNA extraction, the miRNeasy serum kit (QIAGEN, CA, USA) was used according to the manufacturer’s instructions. The miR-194-5p level was measured using an all-in-one miRNA quantitative real-time PCR detection kit (GeneCopoeia, MD, USA) according to the manufacturer’s instruction. PCRs were performed and GAPDH and U6 were selected as the reference gene for mRNA and miRNA, respectively. The comparative Ct method (2−ΔΔCt) was used to calculate the results. The sequences of the primers are listed in Table S2. Each sample was analyzed five times during each experiment. The experiments were carried out at least three times.
Enzyme-Linked Immunosorbent Assay (ELISA)
The plasma of patients was collected and the OPG level was assessed using a human OPG kit purchased from MultiSciences (Hangzhou, China, #70-EK11342) according to the manufacturer’s instructions.
Animal Experiments
Ten-month-old C57BL/6 mice were randomly divided into two groups. The control group received 30 μL of PBS twice monthly for 3 months through periosteal injection into the bone marrow cavity of the femur. The experimental group received agomiR-194-5p (20 μmol/L in 100 μL of PBS) twice monthly for 3 months through periosteal injection into the bone marrow cavity of the femur. Eight-week-old C57BL/6 mice were randomly divided into a sham or an OVX group. The sham group underwent fat tissue excision near the ovaries, and the OVX group underwent bilateral ovariectomy surgery. Four weeks later, when the osteoporotic mouse model was established, the sham group received 30 μL of PBS, and the OVX group received 30 μL of agomiR-194-5p (20 μmol/L in 100 μL of PBS) twice monthly for 3 months. All mice were sacrificed after 3 months, and the femurs were collected for further analysis (n = 6 per group).
Micro-CT Analysis
The femurs of mice were collected for micro-CT scanning (Bruker SkyScan 1176 scanner mCT system). Bones were scanned at a high resolution with an energy of 37 kV and 121 mA. The Bruker mCT evaluation software was used to construct and analyze the 3D images of the bone. To evaluate the bone structure, we collected the following parameters: BV/TV (%), Tb.Th., Tb.N., Tb.Sp., Ct.Th., and BMD.
Hematoxylin and Eosin (H&E) Staining and TRAP Staining
The femurs were harvested from mice after the experiments and fixed in 10% neutral buffered formalin for 24 h. The samples were then decalcified and sectioned into 4-μm-thick slices for further analysis. For H&E staining, the sections were deparaffinized in xylene and rehydrated through a graded series of ethanol. The sections were stained with hematoxylin for 5 min and eosin for 1 min. TRAP staining was performed according to the instructions of the TRAP kit (Sigma-Aldrich, St. Louis, MO, USA). Histological analyses of H&E-stained and TRAP-stained samples were performed with ImageJ software.
Immunofluorescence Staining
For bone sample immunofluorescence staining, the slides were deparaffinized for antigen retrieval. Next, the slides were permeabilized with 0.1% Triton X-100 and blocked with 5% normal goat serum. The slides were incubated with primary antibodies against BMP2 (1:1,000, Abcam) at 4°C overnight, followed by incubation with the specified secondary antibodies. The nuclei were counterstained with DAPI. Images were obtained with a fluorescence microscope in five random fields of each group.
Microarray Procedure
The miRNA-sequencing data (GEO: GSE64433) were obtained from the GEO database, which included whole-blood samples from three healthy volunteers and three osteoporosis volunteers. The differentially expressed miRNAs were analyzed using the online tool GEO2R. A volcano plot was constructed to illustrate all differential miRNAs, and a heatmap was used to exhibit the 50 most upregulated miRNAs and 50 most downregulated miRNAs using TBtools (http://www.tbtools.com/).
Statistical Analysis
All data were expressed as mean ± SD. The Student’s unpaired t test was performed to compare the difference between two groups. Comparison between three or more groups was performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test. A p value <0.05 was considered statistically significant.
Author Contributions
B.M. and G.L. conceived and designed the experiments. Y.X., C.Y., L.C., H.X., L.H., and Y.H. performed the experiments. F.C., Y.S. and W.Z. performed statistical analyses. B.M., A.C.P. and G.L. wrote and edited the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (grant nos. 2018YFC2001502 and 2018YFB1105705); the National Science Foundation of China (grant no. 81772345); the Wuhan Science and Technology Bureau (grant no. 2017060201010192); the National Health Commission of the People’s Republic of China (grants nos. ZX-01-018 and ZX-01-C2016153); and by the Health Commission of Hubei Province (grant no. WJ2019Z009).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.07.023.
Contributor Information
Yuan Xiong, Email: xiongyuanmed@163.com.
Guohui Liu, Email: liuguohui@hust.edu.cn.
Supplemental Information
References
- 1.Ensrud K.E., Kats A.M., Boyd C.M., Diem S.J., Schousboe J.T., Taylor B.C., Bauer D.C., Stone K.L., Langsetmo L., Study of Osteoporotic Fractures (SOF) Research Group Association of disease definition, comorbidity burden, and prognosis with hip fracture probability among late-life women. JAMA Intern. Med. 2019;179:1095–1103. doi: 10.1001/jamainternmed.2019.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuggle N.R., Curtis E.M., Ward K.A., Harvey N.C., Dennison E.M., Cooper C. Fracture prediction, imaging and screening in osteoporosis. Nat. Rev. Endocrinol. 2019;15:535–547. doi: 10.1038/s41574-019-0220-8. [DOI] [PubMed] [Google Scholar]
- 3.Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G., Zhang Y., Yu S., Sun W., Miao D. Bmi1 overexpression in mesenchymal stem cells exerts antiaging and antiosteoporosis effects by inactivating p16/p19 signaling and inhibiting oxidative stress. Stem Cells. 2019;37:1200–1211. doi: 10.1002/stem.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Peppel J., Strini T., Tilburg J., Westerhoff H., van Wijnen A.J., van Leeuwen J.P. Identification of three early phases of cell-fate determination during osteogenic and adipogenic differentiation by transcription factor dynamics. Stem Cell Reports. 2017;8:947–960. doi: 10.1016/j.stemcr.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang M., Zheng C., Shou P., Li N., Cao G., Chen Q., Xu C., Du L., Yang Q., Cao J. SHP1 regulates bone mass by directing mesenchymal stem cell differentiation. Cell Rep. 2016;16:769–780. doi: 10.1016/j.celrep.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Wang P., Xie Z., Wang S., Cen S., Li M., Liu W., Tang S., Ye G., Zheng G. TRAF4 positively regulates the osteogenic differentiation of mesenchymal stem cells by acting as an E3 ubiquitin ligase to degrade Smurf2. Cell Death Differ. 2019;26:2652–2666. doi: 10.1038/s41418-019-0328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye L., Fan Z., Yu B., Chang J., Al Hezaimi K., Zhou X., Park N.H., Wang C.Y. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11:50–61. doi: 10.1016/j.stem.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao Q., Lv M., Li L., Sun Y., Liu S., Shen Y., Liu Z., Luo S. Long intergenic noncoding RNA 00641 inhibits breast cancer cell proliferation, migration, and invasion by sponging miR-194-5p. J. Cell. Physiol. 2020;235:2668–2675. doi: 10.1002/jcp.29170. [DOI] [PubMed] [Google Scholar]
- 10.Wu S., Sun H., Wang Y., Yang X., Meng Q., Yang H., Zhu H., Tang W., Li X., Aschner M., Chen R. MALAT1 rs664589 polymorphism inhibits binding to miR-194-5p, contributing to colorectal cancer risk, growth, and metastasis. Cancer Res. 2019;79:5432–5441. doi: 10.1158/0008-5472.CAN-19-0773. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Li M., Wang Y., Liu J., Zhang M., Fang X., Chen H., Zhang C. A Zfp609 circular RNA regulates myoblast differentiation by sponging miR-194-5p. Int. J. Biol. Macromol. 2019;121:1308–1313. doi: 10.1016/j.ijbiomac.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Dell’Aversana C., Giorgio C., D’Amato L., Lania G., Matarese F., Saeed S., Di Costanzo A., Belsito Petrizzi V., Ingenito C., Martens J.H.A. miR-194-5p/BCLAF1 deregulation in AML tumorigenesis. Leukemia. 2017;31:2315–2325. doi: 10.1038/leu.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng J., Zhang D., Pan N., Sun N., Wang Q., Fan J., Zhou P., Zhu W., Jiang L. Identification of miR-194-5p as a potential biomarker for postmenopausal osteoporosis. PeerJ. 2015;3:e971. doi: 10.7717/peerj.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat. Rev. Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- 15.Dou C., Ding N., Zhao C., Hou T., Kang F., Cao Z., Liu C., Bai Y., Dai Q., Ma Q. Estrogen deficiency-mediated M2 macrophage osteoclastogenesis contributes to M1/M2 ratio alteration in ovariectomized osteoporotic mice. J. Bone Miner. Res. 2018;33:899–908. doi: 10.1002/jbmr.3364. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K., Deng Z., Chen Y., Giannopoulou E., Xu R., Gong S., Greenblatt M.B., Mangala L.S., Lopez-Berestein G., Kirsch D.G. Bone protection by inhibition of microRNA-182. Nat. Commun. 2018;9:4108. doi: 10.1038/s41467-018-06446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krzeszinski J.Y., Wei W., Huynh H., Jin Z., Wang X., Chang T.C., Xie X.J., He L., Mangala L.S., Lopez-Berestein G. Retraction. Nature. 2020;582:134. doi: 10.1038/s41586-020-2273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding H., Meng J., Zhang W., Li Z., Li W., Zhang M., Fan Y., Wang Q., Zhang Y., Jiang L., Zhu W. Medical examination powers miR-194-5p as a biomarker for postmenopausal osteoporosis. Sci. Rep. 2017;7:16726. doi: 10.1038/s41598-017-17075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G., Zhou H., Yao W., Meng L., Lang B. lncRNA TUG1 promotes cisplatin resistance by regulating CCND2 via epigenetically silencing miR-194-5p in bladder cancer. Mol. Ther. Nucleic Acids. 2019;16:257–271. doi: 10.1016/j.omtn.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M., Han D., Yuan Z., Hu H., Zhao Z., Yang R., Jin Y., Zou C., Chen Y., Wang G. Long non-coding RNA H19 confers 5-Fu resistance in colorectal cancer by promoting SIRT1-mediated autophagy. Cell Death Dis. 2018;9:1149. doi: 10.1038/s41419-018-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing H., Liao L., An Y., Su X., Liu S., Shuai Y., Zhang X., Jin Y. Suppression of EZH2 prevents the shift of osteoporotic MSC fate to adipocyte and enhances bone formation during osteoporosis. Mol. Ther. 2016;24:217–229. doi: 10.1038/mt.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui Y., Liu Z., Park S.H., Thatcher S.E., Zhu B., Fernandez J.P., Molina H., Kern P.A., Zhou C. IKKβ is a β-catenin kinase that regulates mesenchymal stem cell differentiation. JCI Insight. 2018;3:e96660. doi: 10.1172/jci.insight.96660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muruganandan S., Govindarajan R., McMullen N.M., Sinal C.J. Chemokine-like receptor 1 is a novel Wnt target gene that regulates mesenchymal stem cell differentiation. Stem Cells. 2017;35:711–724. doi: 10.1002/stem.2520. [DOI] [PubMed] [Google Scholar]
- 24.Brun J., Fromigué O., Dieudonné F.X., Marty C., Chen J., Dahan J., Wei Y., Marie P.J. The LIM-only protein FHL2 controls mesenchymal cell osteogenic differentiation and bone formation through Wnt5a and Wnt10b. Bone. 2013;53:6–12. doi: 10.1016/j.bone.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Jiang M.J., Chen Y.Y., Dai J.J., Gu D.N., Mei Z., Liu F.R., Huang Q., Tian L. Dying tumor cell-derived exosomal miR-194-5p potentiates survival and repopulation of tumor repopulating cells upon radiotherapy in pancreatic cancer. Mol. Cancer. 2020;19:68. doi: 10.1186/s12943-020-01178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.