Since the occurrence of novel coronavirus disease 2019 (COVID-19) pandemic, there have been many discussions on the repurposing of existing drugs for the treatment of COVID-19, one of which is the statins. However, there are two opposing views on the effects of statins on the clinical course of COVID-19. Dysregulation of the myeloid differentiation primary response protein (MYD) 88 pathway which results in overwhelming inflammation has been observed and associated with poor prognosis in other coronavirus infections; this could be the case for COVID-19 but has not been conclusively proven1 , 2. Statins are known inhibitors of MYD88 and could stabilize MYD88 levels in the presence of external stressors, which thus suggest their roles in protecting COVID-19 patients from the development of overwhelming inflammatory responses3. Besides, statins are known to experimentally up-regulate ACE2 expression, and therefore might be protective towards lung injury induced by coronavirus4. On the other hand, statins cause deficiency of endogenous cholesterol content in the cells, leading to upregulation of low-density lipoprotein receptors, which in turn results in constant incorporation of exogenous cholesterol onto the cell membrane and the subsequent formation of multiple lipid rafts, thus enhancing accessibility for coronaviruses5. Some researchers6 have also argued that statins might promote the development of a more severe course of COVID-19 due to activation of the inflammasome pathway in acute respiratory distress syndrome, leading to increased pro-inflammatory interleukin-18 (IL-18) levels and subsequent cytokine storm7 , 8. Individual observational studies9, 10, 11, 12 have since reported on this area and we carried out a meta-analysis to summarise the existing evidence on the effect of statins on the clinical outcomes of COVID-19 from adjusted analyses.
We searched PubMed, Google Scholar, and medRxiv (preprint repository) databases, up to 27 July 2020, for studies evaluating the risk of severe illness and/or mortality in COVID-19 among statin users compared to non-statin users, with the following keywords and their MeSH terms: “COVID-19”, “statin”, and “HMG-CoA reductase” without language restrictions. The reference lists of reviews and retrieved articles were also screened for additional pertinent papers. Studies were included if they are of cohort or case-control design, included patients with confirmed COVID-19, and with data available to compare the risk of severe illness and/or mortality among statin users compared to non-statin users in adjusted analyses. Each included article was independently evaluated by two authors (CSK and SSH) who extracted the study characteristics and measures of effect. In case of discrepancies in data extraction, the consensus was achieved through discussion. The quality of observational studies was evaluated using the Newcastle-Ottawa Scale13. Adjusted hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) from each study were pooled using a random-effects model using Meta XL, version 5.3 (EpiGear International, Queensland, Australia). The Cochran’s Q heterogeneity test and I2 statistic were performed to estimate the heterogeneity.
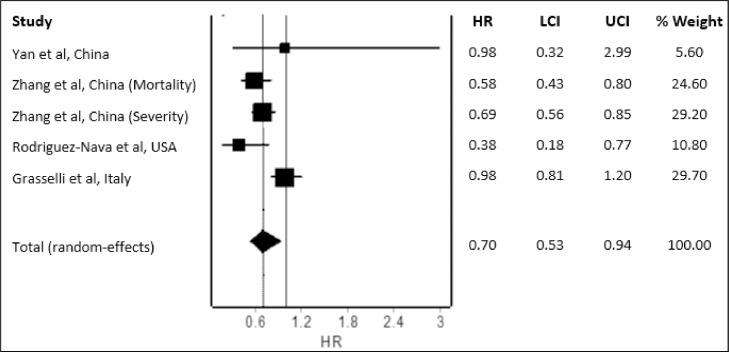
Our literature search yielded 274 potential studies. After deduplication and application of the eligibility criteria, four studies with a total of 8,990 COVID-19 patients were included for meta-analysis. Except for Yan et al. which is of moderate quality (5/9), other studies are of good quality (at least 7/9). Study characteristics are provided in Table 1 . The pooled analysis revealed a significantly reduced hazard for fatal or severe disease with the use of statins (Figure 1 ; pooled HR=0.70; 95% CI 0.53-0.94) compared to non-use of statins in COVID-19 patients. Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which 3 are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable. Current preliminary findings suggested a reduction in fatal or severe disease by 30% and discredited the suggestion of harms with the use of statins in COVID-19 patients. Much left to be determined on the regimen of statin for the treatment of COVID-19 though available evidence suggests that statin therapy of moderate-to-high intensity could be effective. Nevertheless, we await more data from prospective studies to substantiate our findings. Future well-designed randomized controlled trials are also needed to confirm the benefits of statins in COVID-19 patients.
Table 1.
| Study | Country | Design | Total number of patients | Age | Statin regimen | Mortality |
Severe diseasea |
Adjustment of Covariates | NOS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statin users (n/N; %) | Non-statin users (n/N; %) | Adjusted estimate | Statin users (n/N; %) | Non-statin users (n/N; %) | Adjsuted estimate | ||||||||
| Yan et al | China | Retrospective, multicenter | 610 | 48.8 (14.2) | N/A | - | - | - | 5/16; 31.3 | 123/594; 20.7 | HR=0.98 (0.32-2.99) |
Age, sex, body mass index | 5/9 |
| Zhang et al | China | Retrospective, multicenter | 4,305 | Statin=66.0 (59.0–72.0) Non-statin=57.0 (45.0–67.0) |
Atorvastatin, rosuvastatin, simvastatin, pravastatin, fluvastatin, pitavastatin Median duration: 22.0 days, Median dose (atorvastatin equivalent): 20.0 mg per day |
45/861; 5.2 | 325/3444; 9.4 | HR=0.58 (0.43-0.80) |
- | - | HR=0.69 (0.56-0.85) |
Mortality: Age, gender, and oxygen saturation at admission Severity: Age, gender, blood pressure, pre-existing comorbidities, indicators of disease severity and organ injuries, low-density-lipoprotein-cholesterol increase, total cholesterol increase |
8/9 |
| Rodriguez-Nava et al | USA | Retrospective, single center | 87 | 68 (58-75) | Atorvastatin 40 mg | N/A | N/A | HR=0.38 (0.18-0.77) |
- | - | - | Age, hypertension, cardiovascular disease, invasive mechanical ventilation, severity according to the National Institutes of Health criteria, number of comorbidities, and adjuvant therapies | 7/9 |
| Grasselli et al | Italy | Retrospective, multicenter | 3,988 | 63 (56-69) | N/A | N/A | N/A | HR=0.98 (0.81-1.20) |
- | - | - | Age, gender, type of respiratory support, comorbidities, angiotensin-converting enzyme inhibitor therapy, angiotensin receptor blocker, diuretic, positive end-expiratory pressure at admission, fraction of inspired oxygen at admission, arterial partial pressure of oxygen/fraction of inspired oxygen at admission | 7/9 |
HR=hazard ratio; NOS=Newcastle-Ottawa Scale.
The definition of severe disease in the study by Yan et al. is based on the definition given in Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia by Chinese National Health Commission while in the study by Zhang et al. is based on the admission into intensive care unit.
Figure 1.
Pooled mortality and/or disease severity risk in COVID-19 patients using or not using statins. (Heterogeneity: I2=71%; p = 0.01)
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Yuan S. Statins May Decrease the Fatality Rate of Middle East Respiratory Syndrome Infection. mBio. 2015;6:e01120. doi: 10.1128/mBio.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan X, Deng Y, Guo X, Shang J, Zhu D, Liu H. Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: partly involvement of TLR-4/MYD88 pathway. Biochem Biophys Res Commun. 2014;446(1):292–297. doi: 10.1016/j.bbrc.2014.02.091. [DOI] [PubMed] [Google Scholar]
- 3.Totura AL, Whitmore A, Agnihothram S. Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection. mBio. 2015;6(3) doi: 10.1128/mBio.00638-15. e00638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YH, Wang QX, Zhou JW. Effects of rosuvastatin on expression of angiotensin-converting enzyme 2 after vascular balloon injury in rats. J Geriatr Cardiol. 2013;10(2):151–158. doi: 10.3969/j.issn.1671-5411.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shresta S. Statin drug therapy may increase COVID-19 infection. Nepal Med J. 2020;5(3) [Google Scholar]
- 6.Thorp EB, Gallagher TM. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J Virol. 2004;78(6):2682–2692. doi: 10.1128/JVI.78.6.2682-2692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein MR, Poland GA, Graeber CW. Are certain drugs associated with enhanced mortality in COVID-19? QJM. 2020:hcaa103. doi: 10.1093/qjmed/hcaa103. [published online ahead of print, 2020 Mar 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers A, Guan J, Trtchounian A, Hunninghake G, Kaimal R, Desai M. Association of elevated plasma interleukin-18 level with increased mortality in a clinical trial of statin treatment for acute respiratory distress syndrome. Crit Care Med. 2019;47:1089–1096. doi: 10.1097/CCM.0000000000003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan H, Valdes AM, Vijay A. Role of Drugs Affecting the Renin-Angiotensin-Aldosterone System on Susceptibility and Severity of COVID-19: A Large Case-Control Study from Zheijang Province, China. Preprint. medRxiv. 2020 2020.04.24.20077875. [Google Scholar]
- 10.Zhang XJ, Qin JJ, Cheng X. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.06.015. [published online ahead of print, 2020 Jun 24] S1550-4131(20)30316-30318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Nava G, Trelles-Garcia DP, Yanez-Bello MA, Chung CW, Trelles-Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24(1):429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasselli G, Greco M, Zanella A. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3539. [published online ahead of print, 2020 Jul 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July 27, 2020)