Highlights
-
•
Periodontal disease is associated with echolucent plaques.
-
•
Periodontal disease is associated with increased macrophages in plaques.
-
•
Periodontal disease is associated with decreased smooth muscle cells in plaques.
-
•
Periodontal disease is associated with plaque instability.
Keywords: Periodontal disease, Carotid endarterectomy, Inflammation, Vulnerable plaque
Abstract
Background
Periodontal disease (PD) is a chronic inflammatory oral condition with potentially important systemic sequelae. We sought to determine whether the presence of PD in patients with severe carotid disease was associated with morphological features consistent with carotid plaque instability.
Methods
A total of 52 dentate patients hospitalized for carotid endarterectomy (CEA) had standardized assessments of their periodontal status, including measurements of probing pocket depth (PPD), clinical attachment level (CAL) and bleeding on probing (BoP). Carotid plaque morphology was assessed by ultrasound using the gray scale median (GSM) score and by immunohistochemistry using anti-CD68 and anti-alpha-actin antibodies, markers for macrophages and smooth muscle cells (SMCs) respectively.
Results
In total 30/52 patients (58%) had PD. Significant associations were noted between low GSM on ultrasound and each mm in PPD (p = 0.001), each mm in CAL (p = 0.002) and with a 10% increase in BoP (p = 0.009). Using the standardized PERIO definition the association remained robust (aOR = 10.4 [95% CI:2.3–46.3], p = .002). Significant associations were also observed with high macrophage accumulation and each individual PD measure (p < 0.01 for PPD, CAL and BoP) and with the PERIO definition (aOR = 15 [95% CI:1.8–127.8], p = .01). Similarly, low SMC density was also significantly associated with individual measures of PD (p < 0.05 for PPD, CAL and BoP), but not with the PERIO definition (aOR 3.4 [95% CI:0.9–12.8], p = .07).
Conclusions
The presence of PD was significantly associated with both ultrasound and immunohistochemistry features of carotid plaque instability in patients undergoing CEA.
1. Introduction
Most cardiovascular (CV) events such as myocardial infarctions (MI) or strokes are the result of acute thrombus formation on ruptured atherosclerotic plaques. These plaques are frequently not critically stenosed prior to the acute event, but exhibit distinct morphological characteristics that render them vulnerable to rupture [1]. Local inflammation is considered the hallmark of vulnerable plaques. Accumulating macrophages scavenge lipids and expand the lipid core, secrete matrix metalloproteinases (MMPs) that degrade extracellular collagen, and also induce vascular smooth muscle cells (SMCs) apoptosis [2]. These alterations progressively lead to the reduction of the tensile strength of the plaque’s fibrous cap thereby making the plaques vulnerable and prone to rupture. Therefore, high macrophage accumulation and low SMCs density have been associated with more vulnerable plaques [1]. Advances in noninvasive imaging modalities make it now increasingly possible to detect vulnerable plaque features in vivo [3], [4]. The broad availability and ease of use make carotid ultrasound the cornerstone modality for carotid stenosis. Importantly, by determining the echogenic properties of a plaque, expressed as a Grey Scale Median (GSM) score, ultrasound can also provide insights into plaque content and morphology since vulnerable features like the presence of a necrotic core and high lipid content make plaques appear more echolucent.
The clinical implications of identifying modifiable risk factors linked to plaque vulnerability are obvious. One such novel factor may be periodontal disease (PD), a chronic oral condition with potentially important systemic sequelae. The spectrum of PD ranges from the mild form of gingivitis to the severe form of periodontitis that can be prevalent in 10% of the general population (Supplementary figure 1) [5]. The pathophysiology of PD centers on the presence of oral bacteria in the interface of gum and teeth that become organized in a complex biofilm and induce an inflammatory response that can lead to destruction of the supporting tissues. Importantly, this local inflammatory process is also associated with a chronic low grade systemic inflammatory response [6].
Over the past three decades a large body of literature has proposed an association between PD and atherosclerotic cardiovascular events [7], [8], [9], [10]. Mechanisms proposed as potentially underlying this association include elevated systemic levels of proinflammatory and prothrombotic mediators, cross-reactive systemic antibodies that can further promote inflammation, increases in pro-inflammatory lipid classes and subclasses, and finally a common genetic susceptibility for both PD and CV disease predisposing such individuals to an exaggerated inflammatory response [11], [12], [13], [14]. In addition to the systemic mechanisms, local mechanisms may also be at play. Specifically, live periodontal bacteria or their DNA, have been isolated in carotid plaques and linked to local inflammation and to intraplaque hemorrhages, both of which are features of plaque instability [15], [16], [17].
The aim of the current study was to determine whether an association exists between objective measures of PD and vulnerable plaque morphology in patients with severe carotid artery disease scheduled for endarterectomy.
2. Methods
2.1. Study population
The recruitment of the study population took place at the Vascular Surgery Department of the “Red Cross” Hospital in Athens, Greece. Candidates included all patients that, on the predetermined days that the study periodontist visited the hospital, were hospitalized with ≥ 70% stenosis and were scheduled to undergo carotid artery endarterectomy (CEA). The periodontist informed the patients about the study and those who consented to participate received periodontal evaluation. Patients that did not meet the inclusion criteria were excluded from the study. From a total of 72 patients screened, 52 were finally included in this observational study. A flow chart of the study is presented in Supplementary figure 2. The inclusion criteria were: ≥70% stenosis on carotid ultrasound; not edentulous; no periodontal therapy or systemic antibiotics for the last 6 months; if diabetic under adequate control; no medications known to induce gingival overgrowth. Baseline demographic information, presence of CV risk factors and past medical history were collected from the patient’s medical record.
The study was conducted in full accordance with the World Medical Association’s Declaration of Helsinki, as revised in 2008. The study protocol was approved by the Committee of Research and Ethics of the Dental School of the National and Kapodistrian University of Athens, Greece. Written informed consent was provided by all study participants.
2.2. Carotid ultrasound
Pre-operative carotid ultrasound was performed with a linear array 12 MHz ultrasound transducer (General Electric LogiqE, Riverside, CA, USA). Peak systolic velocity (PSV) and internal carotid artery (ICA)/common carotid artery (CCA) PSV ratio were calculated and percent arterial stenosis was graded according to standard methodology [18]. Gray scale median (GSM) score was estimated from digital B-mode ultrasound image sequences as previously described [4]. A 15-second sequence from a longitudinal view was recorded, and image normalization and GSM calculation were performed by a blinded operator as previously described [4]. Plaques with GSM score ≤ 25 were considered unstable while plaques with GSM score > 25 were considered stable (Supplementary figure 3).
2.3. Tissue processing
During surgery, CEA specimens for immunohistochemistry were collected, washed gently with saline solution, examined, photographed and then fixed in 10% formaldehyde solution. Plaques were decalcified in 5% ethylenediaminetetraacetic acid solution for 24 h. Transverse paraffin blocks, 4-mm thick, were prepared from each atherosclerotic lesion. Sequential transverse sections were obtained and stained with hematoxylin and eosin (H&E). Transverse sections included the most stenotic site and sections distal and proximal to the maximal stenosis.
2.4. Immunohistochemistry
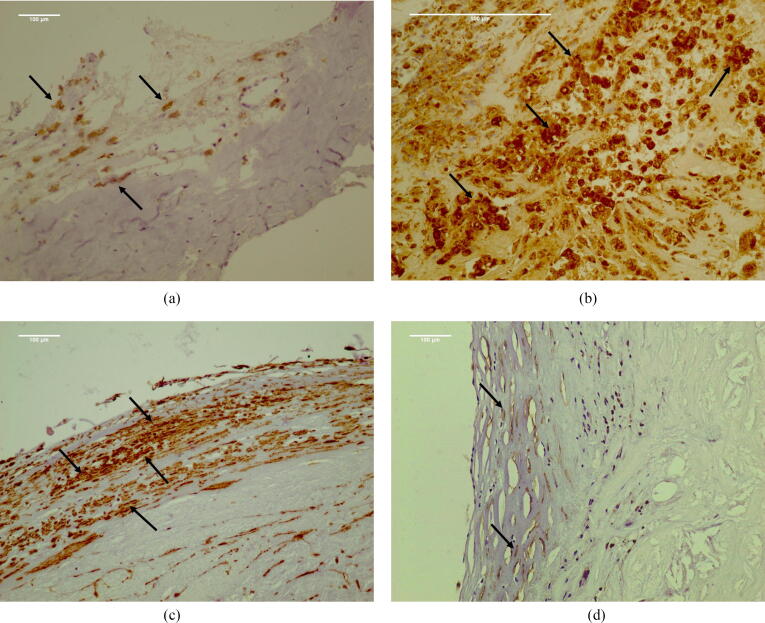
Adjacent serial sections were stained with an anti-CD68 antibody (clone KP1, DAKO, dilution 1:100) to identify macrophages. A semi-quantitative score was used to define macrophages along the cap and shoulders as either small scattered collections (1), masses of moderate cellular density (2), or confluent hypercellular areas (3). Sections from the same blocks were stained for SMCs with an anti-alpha-actin (anti-αSMA) antibody (Smooth Muscle Actin, clone 1A4, DAKO, dilution 1:200). Emphasis was placed on the density and thickness of SMCs along the cap and shoulders. Once again, scores ranging from 1 to 3 were applied for a thick-dense band of SMCs along the cap/shoulder considered (1), a thin-loose arrangement considered (3), and intermediate findings (2). Scores were assigned by two experienced calibrated pathologists blinded to both periodontal status and ultrasound characteristics. The inter-rater agreement was high (Kappa = 0.90, as evaluated by kappa statistics), however, where a difference was noted between scores, slides were placed on a large screen, and consensus was reached after discussion. In order to utilize a binary definition of plaque stability (stable vs. unstable) similar to that used in ultrasound evaluation, plaques with scores (1) and (2) for anti-CD68 and/or anti-αSMA were combined and considered as stable, while plaques with score (3) were considered as unstable (Fig. 1).
Fig. 1.
Demonstrative figure panel of the immunohistochemical evaluation of plaque stability. (a) Scanty macrophages assessed with low density of anti-CD68 staining (arrows). Plaque considered stable (Score 1). (b) Numerous macrophages around atheromatous core assessed with high density of anti-CD68 staining (arrows). Plaque considered unstable (Score 3). (c) Thick cellular cap assessed with high density of anti-alpha actin staining (arrows). Plaque considered stable (Score 1). (d) Collagenized cup with scanty SMCs, assessed with low density of anti-alpha actin staining (arrows). Plaque considered unstable (Score 3).
2.5. Periodontal examination
Prior to surgery, patients underwent an oral examination by a periodontist who was blinded to the ultrasound findings. Measures of PD included clinical attachment loss (CAL), probing pocket depths (PPD) and bleeding on probing (BoP) at six sites per tooth. Specifically, CAL is the distance from the bottom of the gingival pocket to the cemento-enamel junction and represents the amount of periodontal destruction. PPD is the distance from the bottom of the gingival pocket to the crest of the gingival margin representing the area where subgingival bacteria may accumulate. BoP is the percent of sites that bleed upon probing and is considered a marker of active inflammation. Accordingly, higher values of CAL, PPD and BoP are consistent with more severe PD and inflammation. The combination of CAL, PPD, and BoP constitute the most thorough and complete assessment of PD as outlined in the consensus report of the 5th European workshop in Periodontology [19]. For the binary classification of the presence of PD the definition PERIO: ≥4 teeth with ≥ 1 pockets with PPD ≥ 4 mm and CAL ≥ 3 mm was used. In addition, the number of missing teeth were evaluated, since this could also, in part, be considered a surrogate indication of more severe PD.
2.6. Statistics
Continuous variables were reported as mean ± SD and categorical variables were reported as relative frequencies. The Shapiro-Wilk test was used to assess normality of continuous variables. Associations between categorical variables were tested using the Chi-Square test and between continuous variables and binary variables through Student’s t-test or Mann-Whitney when scores were normally or skewed distributed, respectively.
Logistic regression was performed to determine the association of independent variables with the dependent variable. Multiple logistic regression models were controlled for potential confounders, such as gender, age, smoking habits, hypertension, hypercholesterolemia and diabetes mellitus. The results were presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). SPSS 20.0 was used for statistical analyses (SPSS Inc, Chicago, Il, USA) and a p value < 0.05 was considered significant. Finally, a linear regression with GSM and number of teeth present as covariates was performed.
3. Results
3.1. Study participants
The baseline characteristics of the 52 study participants are shown in Table 1. The mean age was 68.1 ± 8.9 years, 73% were males, and 57.7% had PD. Forty eight percent of patients were symptomatic having experienced a transient ischemic attack (TIA) and 48% had ≥ 90% carotid stenosis.
Table 1.
Demographic and baseline characteristics of study participants (n = 52). Abbreviations: Transient ischemic attack (TIA); probing pocket depth (PPD); clinical attachment level (CAL); bleeding on probing (BoP).
| Variables | Values |
|---|---|
| Age (years ± SD) | 68.1 ± 8.9 |
| Men, n (%) | 38 (73.1) |
| Caucasian, n (%) | 52 (100) |
| Hypertension, n (%) | 44 (84.6) |
| Hypercholesterolemia, n (%) | 33 (63.5) |
| Diabetes mellitus, n (%) | 15 (28.9) |
| Alcohol consumption a, n (%) | 8 (15.4) |
| Smokingb, n (%) | 23 (44.2) |
| Symptomatology (TIA), n (%) | 25 (48.1) |
| Degree of stenosis, n (%) | |
| 70–90% | 27 (51.9) |
| ≥ 90% | 25 (48.1) |
| Degree of stenosis at contralateral, n (%) | |
| ≥ 50% | 14 (26.9) |
| Anti-platelet therapy, n (%) | 23 (44.2) |
| Periodontitis (PERIO) c, n (%) | 30 (57.7) |
| PPD, mean (SD) | 3.28 (0.9) |
| CAL, mean (SD) | 4.20 (1.6) |
| BoP, mean % (SD) | 50.34 (35.5) |
| Remaining teeth, mean (SD) | 14 (7.7) |
>2 glasses of wine per day or >1 glass of liquor per day
Current smokers and ex-smokers that have quit smoking less than 6 months.
PERIO: ≥4 teeth with more than one sites with PPD ≥ 4 mm and CAL ≥ 3 mm.
3.2. Association between PD parameters and plaque morphology
The main results of our analysis are shown in Table 2 where the association between PD parameters and plaque stability are displayed according to both ultrasound and immunohistochemistry criteria.
Table 2.
Association of periodontal disease parameters with GSM, CD68, αSMA, and combinations of CD68 & αSMA. Abbreviations: bleeding on probing (BoP), clinical attachment level (CAL), gray scale median (GSM), probing pocket depth (PPD), periodontitis (PERIO), alpha-smooth muscle actin (αSMA).
| Plaque characterization | PPD (mm) | CAL (mm) | BoP (%) | Non PERIO n (%) |
PERIO n (%) |
|
|---|---|---|---|---|---|---|
| (mean ± SD) | (mean ± SD) | (mean ± SD) | ||||
| Periodontal disease parameters and GSM | ||||||
| GSM > 25 | Stable | 2.49 ± 0.7 | 3.13 ± 1.1 | 29.87 ± 30.8 | 13 (72.2%) | 5 (27.8%) |
| GSM ≤ 25 | Unstable | 3.70 ± 0.9 | 4.77 ± 1.5 | 61.18 ± 33.4 | 9 (26.5%) | 25 (73.5%) |
| p-value | <0.001 a | <0.001a | .001a | .001b | ||
| Periodontal disease parameters and CD68 | ||||||
| anti-CD68 score (1) or (2) | Stable | 3.01 ± 0.9 | 3.71 ± 1.2 | 40.14 ± 30.9 | 20 (52.6%) | 18 (47.4%) |
| anti-CD68 score (3) | Unstable | 3.95 ± 0.9 | 5.42 ± 1.8 | 75.48 ± 34.6 | 2 (14.3%) | 12 (85.7%) |
| p-value | <0.001 a | 0.001 a | <0.001 a | .001b | ||
| Periodontal disease parameters and αSMA | ||||||
| anti-αSMA score (1) or (2) | Stable | 2.72 ± 0.6 | 3.52 ± 1.0 | 36.98 ± 33.5 | 15 (55.6%) | 12 (44.4%) |
| anti-αSMA score (3) | Unstable | 3.83 ± 1.0 | 4.89 ± 1.8 | 63.69 ± 32.9 | 7 (28.0%) | 18 (72.0%) |
| p-value | <0.001 a | 0.001 a | 0.004 a | .004b | ||
| Periodontal disease parameters and combinations of CD68 & αSMA | ||||||
| anti-CD68/anti-αSMA score (1) or (2) anti-CD68/anti-αSMA score (3) |
Stable | 2.99 ± 0.9 | 3.77 ± 1.2 | 40.06 ± 32.0 | 21 (51.2%) | 20 (48.8%) |
| Unstable | 4.21 ± 0.9 | 5.64 ± 1.9 | 84.60 ± 23.5 | 1 (9.1%) | 10 (90.9%) | |
| p-value | <0.001a | 0.001 a | <0.001 a | .001b | ||
PERIO: ≥4 teeth with more than one sites with PPD ≥ 4 mm and CAL ≥ 3 mm.
Mann-Whitney test was used.
Chi-Square test was used.
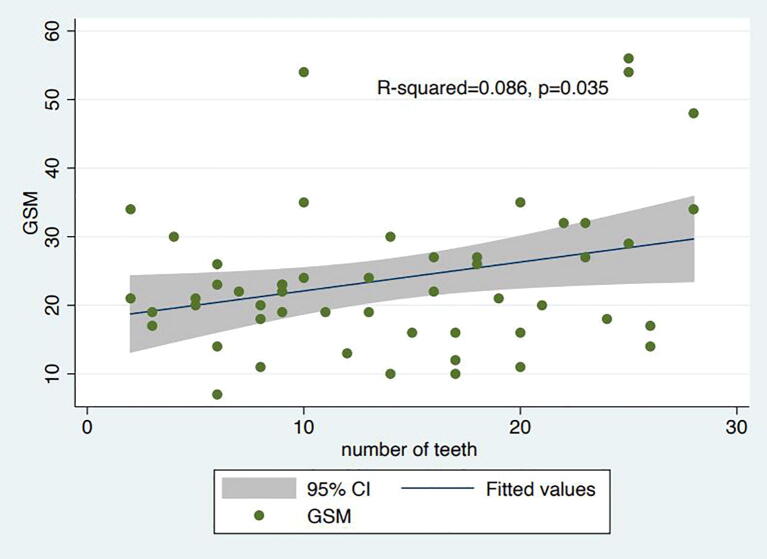
In the case of ultrasound-based classification of plaque stability (dichotomous at GSM score 25), all PD parameters were significantly worse in patients with unstable plaques compared to patients with stable plaques (preliminary data previously published [20]). Specifically, CAL was 4.8 mm vs 3.1 mm (p < .001), PPD was 3.7 mm vs 2.5 mm (p < .001) and BoP was 61.2% vs 29.9% (p = .001). Importantly, significant associations with GSM defined unstable plaques were also noted when the binary PERIO definition was used. The linear regression analysis revealed, also, a positive association between GSM and the number of teeth present with R2 = 0.086, p = .038 (Supplementary figure 4).
Consistent observations were also seen when plaque stability was based on histopathological observations (Table 2). High macrophage accumulation (anti-CD68) and low SMC density at the fibrous cap (anti-αSMA) were significantly associated with all individual PD parameters and with the PERIO definition. Finally, when plaque stability was determined by the combination of both anti-CD68 and anti-αSMA, then once again all clinical PD parameters (CAL, PPD and BoP) and the PD definition (PERIO) were significantly worse in patients with the unstable plaques compared to patients with stable plaques.
In order to account for potential confounding, we assessed the association between PD and plaque stability by multiple logistic regression adjusting for common confounders such as sex, age, smoking, hypertension, hypercholesterolemia and diabetes mellitus (Table 3). Consistent with the raw associations, regression results also demonstrated significant associations between PD and ultrasound-detected plaque instability with adjusted odds ratio (aOR) of 15.5 (95% CI:3.1–77.4, p = .001) for each mm increase in PPD, aOR = 3.9 (95% CI:1.6–9.1, p = .002) for each mm increase in CAL and aOR = 1.4 (95% CI:1.1–1.8, p = .009) for every 10% increase in BoP.
Table 3.
Association of GSM, CD68 and αSMA with periodontal disease/parameters (PPD, CAL, BoP, PERIO) after multiple logistic regression. Abbreviations: bleeding on probing (BoP), Confidence Interval (CI), clinical attachment level (CAL), gray scale median (GSM), Odds Ratio (OR), probing pocket depth (PPD), periodontitis (PERIO), alpha-smooth muscle actin (αSMA).
| Comparison | Variables | Category or increment | adjusted OR (95% CIs)a | p value |
|---|---|---|---|---|
| GSM ≤ 25 (Unstable plaque) vs. GSM > 25 (Stable plaque) | PPD | one mm increase in PPD | 15.5 (3.1–77.4) | 0.001 |
| CAL | one mm increase in CAL | 3.9 (1.6–9.1) | 0.002 | |
| ΒοP | 10% increase in ΒοP | 1.4 (1.1–1.8) | 0.009 | |
| PERIO | No PERIO | Reference | ||
| PERIO | 10.4 (2.3–46.3) | 0.002 | ||
| CD68 score(3) Unstable plaque vs. CD68 score (1) or (2) (Stable plaque) | PPD | one mm increase in PPD | 4.0 (1.5–10.9) | 0.007 |
| CAL | one mm increase in CAL | 3.1 (1.3–7.4) | 0.009 | |
| ΒοP | 10% increase in ΒοP | 1.5 (1.1–1.9) | 0.004 | |
| PERIO | No PERIO | Reference | ||
| PERIO | 15.0 (1.8–127.8) | 0.013 | ||
| αSMA score (3) (Unstable plaque) vs. αSMA score (1) or (2) (Stable plaque) | PPD | one mm increase in PPD | 7.2 (2.1–24.9) | 0.002 |
| CAL | one mm increase in CAL | 2.4 (1.2–4.8) | 0.012 | |
| ΒοP | 10% increase in ΒοP | 1.3 (1.0–1.6) | 0.030 | |
| PERIO | No PERIO | Reference | ||
| PERIO | 3.4 (0.9–12.8) | 0.07 | ||
PERIO: ≥4 teeth with more than one sites with PPD ≥ 4 mm and CAL ≥ 3 mm.
Adjustment for sex, age, smoking, hypertension, hypercholesterolemia, diabetes mellitus.
The PERIO definition association with low GSM also remained robust aOR = 10.4 (95% CI:2.3–46.3, p = .002). Significant associations were also observed with immunohistochemistry based vulnerable plaques classification. Specifically, for elevated macrophage staining the aORs were 4.0 (95% CI:1.5–10.9, p = .007) for each mm increase in PPD; 3.1 (95% CI:1.3–7.4, p = .009) for each mm increase in CAL; 1.5 (95% CI:1.1–1.9, p = .004) for every 10% increase in BoP and 15 (95% CI:1.8–127.8, p = .013) for PERIO. For low SMCs density the aORs were 7.2 (95% CI:2.1–24.9, p = .002) for each mm increase in PPD; 2.4 (95% CI:1.2–4.8, p = .012 for each mm increase in CAL and 1.3 (95% CI:1.0–1.6, p = .03) for every 10% increase in BoP. For PERIO the association was also present but did not reach significance with aOR 3.4 (95% CI:0.9–12.8, p = .07). Univariate logistic regression analysis showed no association between symptοmatic status and PPD [OR = 1.28 (95% CI: 0.73–2.58, p = .39)], CAL [OR = 0.98 (95% CI: 0.70–1.38, p = .92)], BoP [OR 1.01 (95% CI: 0.99–1.02, p = .49)] and PERIO [OR 0.83 (95% CI: 0.28–2.51, p = .75)].
4. Discussion
The major observation of the current study is that among patients with severe carotid artery disease undergoing endarterectomy the presence of PD is associated with vulnerable features in the culprit carotid plaque. This was consistent across multiple objective and clinically accepted measures of PD as well as when laque vulnerability was determined using either ultrasonographic or histopathologic criteria. Finally, these positive associations remained significant following adjustment for potential confounders.
We selected patients with severe carotid disease requiring endarterectomy and utilized both imaging and tissue criteria for the characterization of vulnerable plaques. The choice of ultrasound as the noninvasive imaging modality was primarily related to its ease of use, but also because evidence exists that plaque echolucency on ultrasound is strongly correlated with vulnerable features by histology such as a large necrotic lipid-rich core and a thin fibrous cap [21], [22]. In addition, echolucent plaques may carry prognostic significance with studies reporting higher risk for ischemic cerebrovascular events with such plaques independent of the degree of underlying stenosis or coexisting clinical risk factors [23], [24]. However, it is important to note that magnetic resonance imaging (MRI) or positron emission tomography (PET) are also excellent options for evaluation of plaque vulnerability and may actually provide even more detailed assessment of plaque morphology due to either higher tissue spatial resolution or ability to assess cellular activity [25]. Contrast enhanced ultrasound has also proven to be safe and effective in the evaluation of plaque vulnerability and could serve as a valuable alternative, especially since it is more easily accessible than PET scans and MRI [26], [27]. Optimally, our observations can be duplicated in future studies using these most advanced imaging modalities.
The histological criteria used to identify vulnerable plaques in our analysis, namely elevated macrophage accumulation and attenuation in SMC concentrations on endarterectomy samples, are considered some of the hallmark features of rupture-prone vulnerable plaques and several studies have reported their presence in unstable atherosclerotic lesions [28], [29], [30]. Accordingly, both the ultrasound and histology definitions used in our study are well validated in their correlation with vulnerable plaque features. As anticipated to a certain degree, the number of patients with vulnerable plaques did not precisely match when plaques were evaluated by ultrasound or histological criteria. Therefore, to further explore the diagnostic accuracy of ultrasound testing for the detection of unstable plaques, we evaluated the sensitivity of the GSM use during duplex ultrasound. The sensitivity of GSM was estimated at 100.00% (95% CI: 78.20–100.00) and 80.77% (95% CI: 60.65–93.45%) with anti-CD68 and anti-αSMA as the reference method respectively. The high sensitivity of GSM implies a zero or very low rate of false negatives, indicating that the ultrasound method is unlikely to miss existing unstable plaques and underlines the accuracy of the ultrasound method.
We also strived for the most objective and accurate detection of PD by using multiple, objective, and to a degree complimentary parameters of disease activity in addition with a standardized and validated summary PD definition. Importantly, all periodontal evaluations were performed by a specialized periodontist further ensuring the validity and accuracy of the measurements. The associations we observed between PD and carotid plaque vulnerability were robust, present with both noninvasive and histological plaque classification and across the spectrum of the PD parameters used. Importantly, these associations persisted following adjustment for potential confounders. Although causation cannot be established by our findings, the consistency of our observations requires consideration of the plausible mechanisms that may be underlying these associations.
One potential mechanism revolves around the systemic low-grade inflammatory state that is almost always present in patients with PD and that may contribute to atherosclerotic plaque progression or destabilization [6]. A second potential mechanism is the seeding of periodontal bacteria into the systemic circulation and subsequently into atherosclerotic plaques. At least one study reported live periodontal bacteria in atherosclerotic plaques of coronary and carotid arteries, while other studies have demonstrated the frequent presence of their DNA in CEA specimens [15], [31]. Previous studies have proposed mechanisms on how these oral pathogens may contribute to accelerated plaque progression, destabilization, plaque hemorrhage and even a propensity for in situ thrombosis that could lead to cardiovascular events [8], [9], [32], [33].
Our study further contributes to the existing evidence by demonstrating that the presence of a chronic, festering oral infection is associated with vulnerable carotid plaque features thereby possibly providing clues for the reported increased risk for ischemic stroke in PD patients [34]. It is also very tempting to propose that similar biologic mechanisms may also apply for the coronary circulation contributing to the increased incidence of myocardial infarctions also observed in patients with PD [7], [35].
The results from this study may have significant implications for clinicians caring for patients with atherosclerotic vascular disease, including cardiologists, neurologists, vascular and cardiac surgeons. Over the last decades, the successful campaign to reduce CV risk has centered around the identification and treatment of modifiable risk factors. Smoking cessation, lipid lowering, blood pressure and glycemic control have all significantly reduced the risk for CV events by controlling plaque progression and destabilization. Our observations suggest that PD may be also contributing to plaque instability in patients with severe carotid artery stenosis and may provide a mechanistic insight to the reported link between PD and CV events [7]. Since PD is modifiable with appropriate treatment could this represent a novel opportunity to reduce CV risk? Existing evidence suggests that PD therapy reduces markers of systemic inflammation (C-reactive protein) and improves endothelial function, both of which are associated with CV events [36], [37]. In addition, PD therapy significantly decreased carotid intima-media thickness at 6 months [38], [39]. Therefore, the stage may be set now for interventional PD studies that prospectively investigate the impact on plaque stabilization and potentially reduction in CV events [40].
The primary limitations of our study are its observational design and the limited sample size. Other potential limitations may include the use of duplex ultrasound as the only imaging modality. As discussed, other imaging techniques such as MRI and PET-scan may have more accurately evaluated plaque morphology. Finally, although the vast majority of the participants (77%) had 8 or more teeth, some patients had a small number of remaining teeth. However, tooth loss could be, largely, attributed to more severe PD and, hence, be considered a surrogate of PD severity. Indeed, the linear regression analysis revealed a positive association between GSM score and the number of teeth present which further supports our principle findings of the association between PD and plaque vulnerability.
In conclusion, our data suggest that PD is associated with vulnerable plaque features in patients with severe carotid disease, an observation that provides a clue for the excess CV risk associated with PD.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100601.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Clinical presentation of periodontal disease. (a) Gingivitis and (b) Periodontitis.
Flow chart of the study.
Supplementary figure 3.
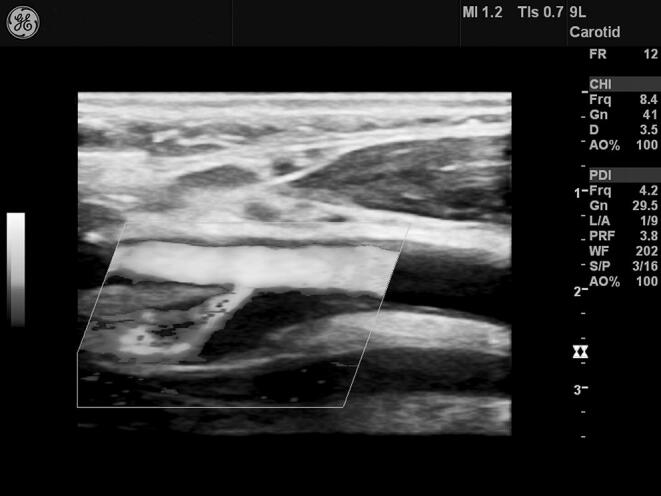
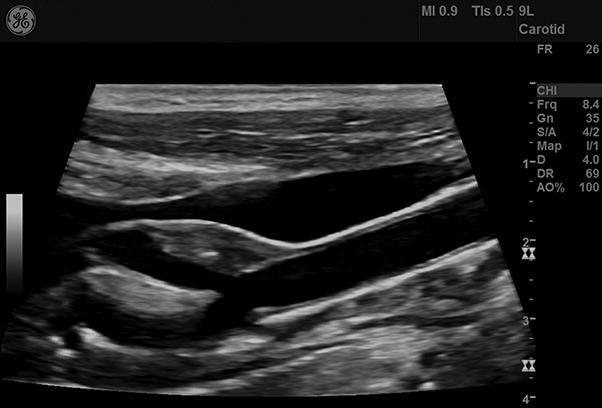
Demonstrative figure panel of the ultrasound evaluation of plaque stability. (a) Atherosclerotic lesion with gray scale median <25, predominantly echolucent. Plaque considered unstable. The boundaries of the plaque are depicted only on power Doppler image (marked area). (b) Atherosclerotic lesion with gray scale median >25, homogenous echogenic. Plaque considered stable.
Supplementary figure 4.
Linear regression analysis with grey scale median (GSM) score and number of remaining teeth as covariates.
References
- 1.Falk E., Shah P.K., Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 2.Davies M.J., Richardson P.D., Woolf N., Katz D.R., Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan C., Kerwin W.S., Yarnykh V.L. MRI of atherosclerosis in clinical trials. NMR Biomed. 2006;19:636–654. doi: 10.1002/nbm.1065. [DOI] [PubMed] [Google Scholar]
- 4.Biasi G.M., Froio A., Diethrich E.B. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756–762. doi: 10.1161/01.CIR.0000138103.91187.E3. [DOI] [PubMed] [Google Scholar]
- 5.Papapanou P.N. Epidemiology of Periodontal diseases: an update. J. Int. Acad. Periodontol. 1999;1:110–116. [PubMed] [Google Scholar]
- 6.Moutsopoulos N.M., Madianos P.N. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann. N. Y. Acad. Sci. 2006;1088:251–264. doi: 10.1196/annals.1366.032. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich T., Sharma P., Walter C., Weston P., Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Clin. Periodontol. 2013;40:S70–84. doi: 10.1111/jcpe.12062. [DOI] [PubMed] [Google Scholar]
- 8.Brodala N., Merricks E.P., Bellinger D.A. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler. Thromb. Vasc. Biol. 2005;25:1446–1451. doi: 10.1161/01.ATV.0000167525.69400.9c. [DOI] [PubMed] [Google Scholar]
- 9.Jain A., Batista E.L., Jr., Serhan C., Stahl G.L., Van Dyke T.E. Role for periodontitis in the progression of lipid deposition in an animal model. Infect. Immun. 2003;71:6012–6018. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonetti M.S., Van Dyke T.E. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013;40:S24–29. doi: 10.1111/jcpe.12089. [DOI] [PubMed] [Google Scholar]
- 11.Schenkein H.A., Loos B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Clin. Periodontol. 2013;40:S51–69. doi: 10.1111/jcpe.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Aiuto F., Orlandi M., Gunsolley J.C. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Periodontol. 2013;84:S85–S105. doi: 10.1902/jop.2013.134007. [DOI] [PubMed] [Google Scholar]
- 13.Paquette D.W., Brodala N., Nichols T.C. Cardiovascular disease, inflammation, and periodontal infection. Periodontol. 2007;2000(44):113–126. doi: 10.1111/j.1600-0757.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 14.Nichols T.C., Fischer T.H., Deliargyris E.N., Baldwin A.S., Jr. Role of NF-kB in Inflammation, Periodontitis and Atherogenesis. Ann. Periodontol. 2001;6:20–29. doi: 10.1902/annals.2001.6.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Kozarov E.V., Dorn B.R., Shelburne C.E., Dunn W.A., Jr., Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 2005;25:e17–18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 16.Brun A., Range H., Prouvost B. Intraplaque hemorrhage, a potential consequence of periodontal bacteria gathering in human carotid atherothrombosis. Bull. Group. Int. Rech. Sci. Stomatol. Odontol. 2016;53 [PubMed] [Google Scholar]
- 17.Range H., Labreuche J., Louedec L. Periodontal bacteria in human carotid atherothrombosis as a potential trigger for neutrophil activation. Atherosclerosis. 2014;236:448–455. doi: 10.1016/j.atherosclerosis.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Grant E.G., Benson C.B., Moneta G.L. Carotid artery stenosis: gray-scale and Doppler US diagnosis–Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003;229:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 19.Tonetti M.S., Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J. Clin. Periodontol. 2005;32:S210–213. doi: 10.1111/j.1600-051X.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 20.Kotsikoris I., Tsiantula P.V., Andrikopoulos V. Association of periodontal disease with the instability carotid plaque. Hellenic J. Surg. 2014;86:212–215. [Google Scholar]
- 21.Czernuszewicz T.J., Homeister J.W., Caughey M.C. Non-invasive in vivo characterization of human carotid plaques with acoustic radiation force impulse ultrasound: comparison with histology after endarterectomy. Ultrasound Med. Biol. 2015;41:685–697. doi: 10.1016/j.ultrasmedbio.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem M.K., Bown M.J., Sayers R.D. Identification of patients with a histologically unstable carotid plaque using ultrasonic plaque image analysis. Eur. J. Vasc. Endovasc. Surg. 2014;48:118–125. doi: 10.1016/j.ejvs.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Mathiesen E.B., Bonaa K.H., Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromso study. Circulation. 2001;103:2171–2175. doi: 10.1161/01.cir.103.17.2171. [DOI] [PubMed] [Google Scholar]
- 24.Reiter M., Effenberger I., Sabeti S. Increasing carotid plaque echolucency is predictive of cardiovascular events in high-risk patients. Radiology. 2008;248:1050–1055. doi: 10.1148/radiol.2483071817. [DOI] [PubMed] [Google Scholar]
- 25.Owen D.R., Lindsay A.C., Choudhury R.P., Fayad Z.A. Imaging of atherosclerosis. Annu. Rev. Med. 2011;62:25–40. doi: 10.1146/annurev-med-041709-133809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motoyama R., Saito K., Tonomura S. Utility of complementary magnetic resonance plaque imaging and contrast-enhanced ultrasound to detect carotid vulnerable plaques. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada H., Ogata T., Takano K. Evaluation of the time-dependent changes and the vulnerability of the carotid plaques using contrast-enhanced carotid ultrasonography. J. Stroke Cerebrovasc. Dis. 2018;27:321–325. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Silvestre-Roig C., de Winther M.P., Weber C., Daemen M.J., Lutgens E., Soehnlein O. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ. Res. 2014;114:214–226. doi: 10.1161/CIRCRESAHA.114.302355. [DOI] [PubMed] [Google Scholar]
- 29.Meuwissen M., van der Wal A.C., Niessen H.W. Colocalisation of intraplaque C reactive protein, complement, oxidised low density lipoprotein, and macrophages in stable and unstable angina and acute myocardial infarction. J. Clin. Pathol. 2006;59:196–201. doi: 10.1136/jcp.2005.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cojocaru E., Trandafirescu M., Leon M., Cotutiu C., Foia L. Immunohistochemical expression of anti-CD68 antibody in atherosclerotic plaque. Rom. J. Morphol. Embryol. 2012;53:61–66. [PubMed] [Google Scholar]
- 31.Szulc M., Kustrzycki W., Janczak D., Michalowska D., Baczynska D., Radwan-Oczko M. Presence of Periodontopathic Bacteria DNA in Atheromatous Plaques from Coronary and Carotid Arteries. Biomed. Res. Int. 2015 doi: 10.1155/2015/825397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fak F., Tremaroli V., Bergstrom G., Backhed F. Oral microbiota in patients with atherosclerosis. Atherosclerosis. 2015;243:573–578. doi: 10.1016/j.atherosclerosis.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 33.Chukkapalli S.S., Velsko I.M., Rivera-Kweh M.F., Zheng D., Lucas A.R., Kesavalu L. Polymicrobial Oral Infection with Four Periodontal Bacteria Orchestrates a Distinct Inflammatory Response and Atherosclerosis in ApoE null Mice. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen S., Sumner R., Hardin J. Periodontal disease and recurrent vascular events in stroke/transient ischemic attack patients. J. Stroke Cerebrovasc. Dis. 2013;22:1420–1427. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deliargyris E.N., Madianos P.N., Kadoma W. Periodontal disease in patients with acute myocardial infarction: prevalence and contribution to elevated C-reactive protein levels. Am. Heart J. 2004;147:1005–1009. doi: 10.1016/j.ahj.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Tonetti M.S., D'Aiuto F., Nibali L. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 37.Paraskevas S., Huizinga J.D., Loos B.G. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 38.Toregeani J.F., Nassar C.A., Nassar P.O. Evaluation of periodontitis treatment effects on carotid intima-media thickness and expression of laboratory markers related to atherosclerosis. Gen Dent. 2016;64:55–62. [PubMed] [Google Scholar]
- 39.Kudo C., Shin W.S., Sasaki N. Effects of periodontal treatment on carotid intima-media thickness in patients with lifestyle-related diseases: Japanese prospective multicentre observational study. Odontology. 2018;106:316–327. doi: 10.1007/s10266-017-0331-4. [DOI] [PubMed] [Google Scholar]
- 40.Bouchard P., Boutouyrie P., D’Aiuto F. European Workshop in Periodontal Health and Cardiovascular Disease Consensus Document. Eur. Heart J. 2010 Suppl B13–B22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of the study.