Abstract
Association of titanium dioxide nanoparticles (TiO2 NPs) and biochar (BC) to assist phytoremediation of Sb contaminated soil was investigated in this study. Seedlings of Sorghum bicolor were exposed to different regimes of TiO2 NPs (0, 100, 250 and 500 mg kg−1) and BC (0, 2.5% and 5%), separately and in combination, to investigate the effects on plant growth, Sb absorption and accumulation and physiological response of the plant in Sb contaminated soil. Co-application of TiO2 NPs and BC had positive effects on plant establishment and growth in contaminated soil. Greater accumulation of Sb in the shoots compared to the roots of S. bicolor was observed in all treatments. Application of BC increased immobilization of Sb in the soil. Using TiO2 NPs significantly increased accumulation capacity of S. bicolor for Sb with the greatest accumulation capacity of 1624.1 μg per pot achieved in “250 mg kg−1 TiO2 NPs+2.5% BC” treatment (P < 0.05). Association of TiO2 NPs and BC significantly increased chlorophyll a (Chl a) and chlorophyll b (Chl b) contents of S. bicolor compared to the TiO2 NPs-amended treatments. Results of this study presented a promising novel technique by combined application of TiO2 NPs and BC in phytoremediation of Sb contaminated soils. Co-application of TiO2 NPs and BC could reduce the required amounts of TiO2 NPs for successful phytoremediation of heavy metal polluted soils. Intelligent uses of plants in accompany with biochar and nanomaterials have great application prospects in dealing with soil remediation.
Keywords: TiO2 NPs, Biochar, S. bicolor, Phytoremediation, Immobilization, Antimony, Soil science, Environmental engineering, Environmental hazard, Environmental pollution, Environmental toxicology, Toxicology, Environmental science
TiO2 NPs; Biochar; S. bicolor; Phytoremediation; Immobilization; Antimony, Soil science; Environmental engineering; Environmental hazard; Environmental pollution; Environmental toxicology, Toxicology, Environmental science
1. Introduction
Antimony (Sb) is a lustrous gray metalloid that belongs to the group 15 of the periodic table, which may occurs through naturally and anthropogenic sources. Sb is often referred to as a heavy metal in eco-toxicological assessments, but it has mixed metallic and nonmetallic characteristics, therefore it could be more properly described as a metalloid. The abundance of Sb in the earth's crust is around 0.2–0.3 and concentrations of Sb in non-contaminated soils usually range from 0.3 to 8.4 mg/kg (He, 2007), but human activities have led to elevated levels of Sb in soils at many locations in recent years. Antimony has a wide range of applications in industry such as manufacturing of semiconductors, medicine, fire retardants, lead hardeners, diodes, cable coverings, alloys, batteries, polyethylene terephthalate (PET), brake linings and pigments (Bagherifam et al., 2019; Pan et al., 2011). Leaching of Sb in contaminated lands such as mining areas and disposal sites may pose a serious human and environmental risk (Okkenhaug et al., 2016). Formation of antimoniosis, which is a particular form of pneumoconiosis, was reported in the literature as a result of chronic exposure to antimony. Long-term exposure to Sb may also trigger heart and gastrointestinal diseases (Cooper and Harrison, 2009). Antimony is listed as a priority pollutant by the US EPA due to its potential carcinogenicity (Pan et al., 2011). Environmental contamination of antimony is gaining growing attention worldwide because of its pretty high mobility and elevated man-made occurrence in the environment. The remediation of Sb-contaminated soils brings a techno-economical challenge for researchers and decision makers. For example, chemical stabilization of some heavy metals e.g. Pb with lime or phosphate has been applied successfully, but recent researches indicated that lime and phosphate could increase the mobility of Sb in soil (Okkenhaug et al., 2013), and therefore enhance the groundwater contamination risk. Among the various remediation technologies, phytoremediation is one of the most promising, ecologically friendly, and economical remediation approaches, which can uptake metal(loid)s and eliminate the contaminants from soil (Ehsan et al., 2014). Selected plants for phytoextraction should have rapid growth, extended root system, and high biomass production (Ashraf et al., 2019). Reduction in mobility of metal(loid)s in soil occurs through phytostabilization. Phytoremediation of Pb using L. perenne in soil contaminated with Pb (up to 21 g kg−1) showed accumulation of ca. 1.5 g kg−1 Pb in plant tissue after four months (Karami et al., 2011). However, Sb uptake and accumulation may vary widely among different plant species (Tschan et al., 2009). Antimony concentrations of up to 98.23 mg/kg were detected in plants growing adjacent to Sb deposit sites (Qi et al., 2011). Accumulation of Sb in roots of D. viscose with a limited translocation of Sb from the roots to the shoots was also reported in the literature (Pérez-Sirvent et al., 2012).
Although phytoremediation is a promising technology, its effectiveness could be affected by various factors such as plant species, rhizosphere microbes, climate situation, contaminant levels and bioavailability and soil condition (Abdelkrim et al., 2020). Biochar may be considered as a less expensive alternative to activated carbon in reducing mobilization and release of contaminants from contaminated soils. Biochar was found to be able to immobilize metal(loid)s in the environment and reduce their bioavailability and biotoxicity to the organisms (Gong et al., 2018). The alleviated toxicity effects of lead on plant growth and development in biochar treated soil was reported (Kiran and Prasad, 2019). In addition, positive effects of biochars on plant establishment and growth in heavy metals contaminated soils have been found (Kiran and Prasad, 2019; Rees et al., 2016). For instance, shoot biomass production of Z. mays and L. perenne significantly increased in heavy metal-contaminated soils treated with biochar (Rees et al., 2016); however, competition between heavy metals and nutrients to be adsorbed onto adsorption sites on biochar particles may lead to suppressed plant growth (Gong et al., 2019).
Using nanomaterials to facilitate phytoremediation of polluted soil is gently drawing global attention (Song et al., 2019). Many of the nanomaterials such as titanium dioxide nanoparticles (TiO2 NPs) were initially used in an effort to increase the productivity of edible plants (Gao et al., 2013). Most studies using TiO2 NPs have been focused in aqueous media with only few in soil. Sorption and immobilization of metal(loid)s in soil through applying nanoparticles has been found to be a significant mechanism by which remediation goals at metal(loid)-contaminated sites can be achieved. Cd uptake by soybean increased from 128.5 to 507.6 μg per plant as a result of addition of 100–300 mg/kg TiO2 NPs to soil (Singh and Lee, 2016). To the best of our knowledge, so far there has been no attempt to use the association of biochar and TiO2 NPs to promote phytoremediation of Sb in soil. The main aim of this study was to investigate the phytoremediation of antimony-polluted soil through co-application of biochar and TiO2 NPs. We specifically studied: (i) the effect of biochar and TiO2 NPs treatments, individually and in combination, on the biomass and length of plants in Sb-contaminated soil; (ii) the absorption and translocation of Sb in Sorghum bicolor under the effect of biochar, TiO2 NPs and association of biochar and TiO2 NPs; (iii) impacts of biochar, TiO2 NPs and biochar/TiO2 NPs treatments on accumulation capacity and phytoremediation potential of S. bicolor to eliminate Sb, and (iv) effects of biochar, TiO2 NPs and biochar/TiO2 NPs mixture on photosynthetic chlorophyll content of S. bicolor in Sb-contaminated soil.
2. Materials and methods
2.1. Soil preparation and analysis
The soil was collected from a depth of 5–25 cm from a non-contaminated area in Southern part of Tehran Province, Iran (35°32′N, 51°24′E). The collected soil samples were sieved through a 2-mm mesh to exclude the gravel and large debris, and then air-dried (22–25 °C) for one week. The sieved soil was thoroughly mixed by hand before adding Sb to soil. The obtained soil was placed in plastic pots. Each pot filled with 1 kg of soil mixed with 150 ml distilled water (DW) containing K2H2Sb2O7·4H2O, to provide desired level of Sb in soil. In the control pots 150 mL of clean DW was added (Papazoglou and Fernando, 2017). The pots were kept in a dark room (20–24 °C) and stabilized for eight weeks at 70% of field capacity using tap water before planting. Chemical analysis of the pots soil was carried out prior to sowing the seeds. Briefly, the soil pH was measured in suspension using a 1:2.5 (w/v) ratio of soil-water ratio. Phosphorus was determined by Olsen P extracting solution (0.5 M NaHCO3, pH 8.5), total nitrogen by the Kjeldahl measurement (VELP Scientifica, UDK 142, Italy), organic carbon (OC) content was measured according to the Walkley-Black method, in which organic carbon is oxidized using potassium dichromate (Walkley and Black, 1934). Electrical conductivity (EC) was measured using a conductivity meter in a soil-water extract (1:2.5 soil: water ratio (w/v)). The soil texture was determined using a Bouyoucos densitometer which is classified as Clay-Loam (CL). Selected characteristics of the used soil are given in Table 1. The obtained data represent the mean value of three replicates. Sb and Ti contents of soils were determined as described by the USEPA-3050B method (EPA, 1996); 0.5 g of soil sample was digested with a mixture of concentrated 14 mL of HNO3, HCLO4 and HF (5:1:1, v/v/v/) in a tightly closed Teflon vessel for 4h. Then the concentrations of elements in soil were determined by inductively coupled plasma optical emission spectrometry (ICP-OES) (Perkin Elmer Optima 5300 DV) (Singh and Lee, 2018). Sb and Ti could be detected at a limit of 0.002 mg/L. All the analytical determinations were carried out in triplicate and the mean values were reported.
Table 1.
Selected properties of the soil used in experiments.
| Parameter | Value |
|---|---|
| Clay (%) | 28 |
| Silt (%) | 35 |
| Sand (%) | 37 |
| Texture | Clay Loam |
| Organic matter (%) | 1.22 |
| Organic C (%) | 0.62 |
| Soil pH | 7.7 |
| Electrical Conductivity (dS/m) | 2.24 |
| Total N (% wt) | 1.12 |
| Phosphorus (mg/kg) | 8.7 |
2.2. Preparation of biochar
Fresh urban yard trimmings with no pollution background was initially chopped into wood chips of 5–10 cm length and then oven-dried for 48 h. Fresh trimmings of pike trees were used as a feedstock for preparation of biochar. Leaves and fruits were excluded. Dried wood chips were placed in open crucibles, then weighted, and covered thoroughly with aluminum foil in order to provide an oxygen-limited environment. Biochar derived from the wood chips was produced under the pyrolytic temperature of up to 740 °C with a temperature gradient of ca. 10 °C/min until the desired pyrolysis temperature of 740 ± 5 °C was reached in the muffle furnace under the atmospheric pressure with residence time of 42 min. At the end, samples were kept in the furnace overnight to let them cool down to the room temperature. Obtained biochar chips were originally in granular form having a wide range of particle sizes. The produced biochar chips were air-dried over a week, ground using a ceramic mortar and pestle and sieved to gain homogenous crushed biochar, with the particle size of 1 mm–2 mm. Elemental composition of the produced biochar was as follow (dry basis): C (81.5%), O (11.2%), H (3.3%), N (0.5%), S (0.1%) and ash (3.4%). The produced biochar had particle density of 1.5 g/cm3. The biochar had bulk density of 0.68 g/cm3. The pH of the biochar was determined to be 9.1. BET surface area was measured using a Brunauer-Emmett-Teller Surface Area & Porosity Analyzer (NOVA 4200e) by nitrogen gas sorption analysis at 77K. Samples were vacuum degassed prior to analysis, at 300 °C for 5–15 h, based on the required time to reach a stable surface area measurement (Qiu et al., 2009). BET surface area of the biochar was determined to be 281.35 m2g-1. The biochar doses were chosen based on the literature where effective immobilization of contaminants achieved in soil by biochars (Kiran and Prasad, 2019; Kołtowski and Oleszczuk, 2016). The biochar levels of 2.5% and 5% were selected in this research.
2.3. Synthesis of nanoscale titanium dioxide particles
To prepare TiO2 NPs, 50 ml TiCl4 was slowly added to 200 mL of DW in an ice bath and stirred for 30 min using a magnetic stirrer to gain a homogeneous solution. Then, the bath temperature was increased to boiling point till the process of formation of nanoparticles completed. 150 mL of urea solution (104 mg/mL) was added under constant stirring rate till the solution turned into a white colloid without any precipitation. We used urea in preparation of TiO2 NPs because addition of urea as the nitrogen source can positively affect surface area and activity of the produced nanoparticles as suggested in the literature (Marques et al., 2019; Cheng et al., 2008). The obtained solution was then allowed to settle overnight and the precipitate was washed with DW for 5 times (Chavan et al., 2020). When TiCl4 hydrolyses, TiO2 particles in accompany with H+ and Cl− ions were generated, which can be described by the following reaction:
| (1) |
Then, the volume of the suspension was made up to 100 mL with deionized water, containing the desired concentration of the TiO2 NPs per 100 ml deionized water. The size of TiO2 NPs were determined using transmission electron microscopy (TEM), manufactured by PHILIPS (EM208 S), with an acceleration voltage of 100 kV. The size of the TiO2 NPs particles covers a range between 15 and 40 nm. Selection of TiO2 NPs concentration range was based on the preliminary experiments, which showed that TiO2 NPs concentration of lower than 100 mg kg−1 had negligible influence on plant growth and Sb uptake. Applying doses of 1000 and 1500 mg kg−1 TiO2 NPs to soil exhibited severe inhibitory effects on plant growth. Therefore, the TiO2 NPs concentration range was selected as 100–500 mg kg−1 to assess the impacts of TiO2 NPs on phytoremediation of Sb-contaminated soil. Synthesized TiO2 NPs were applied to the soil at desired levels of TiO2 NPs per kilogram of soil by suspending 100–500 mg of the TiO2 NPs in 300 mL of deionized water separately, sonicated in water bath for 30 min at 30 °C with occasional stirring.
2.4. Pot experiment
The S. bicolor seeds were obtained from the National Plant Gene Bank of Iran (NPGB), sterilized in 70% ethanol for 2 min and in 1% NAClO for 10 min, then rinsed three times with DW and sown in plastic pots treated with BC, TiO2 NPs and their combination. Air-dried Sb-contaminated soil was treated individually with 0, 2.5% and 5% (w/w) of the wood-derived biochar, 0, 100, 250 and 500 mg kg−1 TiO2 NPs, and biochar at doses of 2.5% and 5% (w/w) followed by TiO2 NPs water suspension at the concentrations of 100, 250 and 500 mg kg−1 as described in the literature (Gong et al., 2019). S. bicolor was cultivated over an 80-day period in a greenhouse. The seeds were planted in the 1.5–2.0 cm depth of the surface soil in each pot. Pots were kept in a greenhouse under natural sunlight (20–25 °C, 10–12 h light) to imitate real-world conditions, and irrigated two or three times per week to 70% of the field capacity of the soil. Pot experiments were carried out in three replicates. Pots were monitored to assess seedling emergence rate in different treatments and grown plants were harvested after 80 days, and the biomass and length of roots and shoots were measured. Plants were dried in an oven at 70 °C for 48 h to obtain dry weight of the biomass.
2.5. Determination of Sb and Ti in plant organs
After harvesting, plant roots and shoots were thoroughly rinsed with DW, dried at room temperature (22–25 °C), and then oven-dried at 60 °C for 12 h. The obtained samples were ground through a 200 mesh. The amount of 0.2 g ground sample was digested in a digestion tube. 15 mL of 70% HNO3 was added and heated at 120 °C for 90 min. After cooling down, 3 mL 30% H2O2 was added to the digestion block, then heated again to 120 °C for 90 min. The obtained solution then let to be cooled down and fillied up with Milli-Q water to 50 mL, and then analyzed for the Ti and Sb concentrations by inductively coupled plasma optical emission spectrometry (ICP-OES) (Perkin Elmer Optima 5300 DV) (Klingenfuss, 2014).
2.6. Bio concentration and translocation factors
The bio concentration factor (BCF) is used to calculate the metal uptake capacity from soil to plant tissues. It can be measured for each plant part, such as roots and shoots. Translocation factor (TF) is also an important tool to assess the potential of a given plant for phytoremediation purposes. It is determined from the ratio of the concentration of an element in plant's shoots compared to that in the plant's roots. BCF and TF were calculated using the following equations (Embrandiri et al., 2017):
| (2) |
| (3) |
2.7. Chlorophyll content
Fresh leaves materials were analyzed in dark condition to determine chlorophyll content in different treatments. Accurately weighted amount (0.5 g) of intact leaves were grinded thoroughly, 20 ml of 80% acetone was added. Milled mixture was then incubated at 4 °C for 3 h, and centrifuged for 5 min at 2500 rpm to remove particulate matter. The supernatant was filtered and transferred into a 50 ml volumetric flask and the volume was made up to 50ml by addition of 80% acetone. Chlorophyll content was measured by UV spectrophotometry at 645 nm and 663 nm (Wellburn, 1994). The chlorophyll a (Chl a), chlorophyll b (Chl b), and chlorophyll a+ b (total chlorophyll) contents were calculated using the following equations (Arnon, 1949):
| (4) |
| (5) |
| (6) |
2.8. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 24. All results in this paper were presented as the mean with standard errors (n = 3). Significance of differences was determined using one-way analysis of variance (ANOVA). Significance level was considered at P = 0.05.
3. Results and discussion
3.1. Germination of S. bicolor in Sb contaminated soil treated with TiO2 NPs, BC and their combination
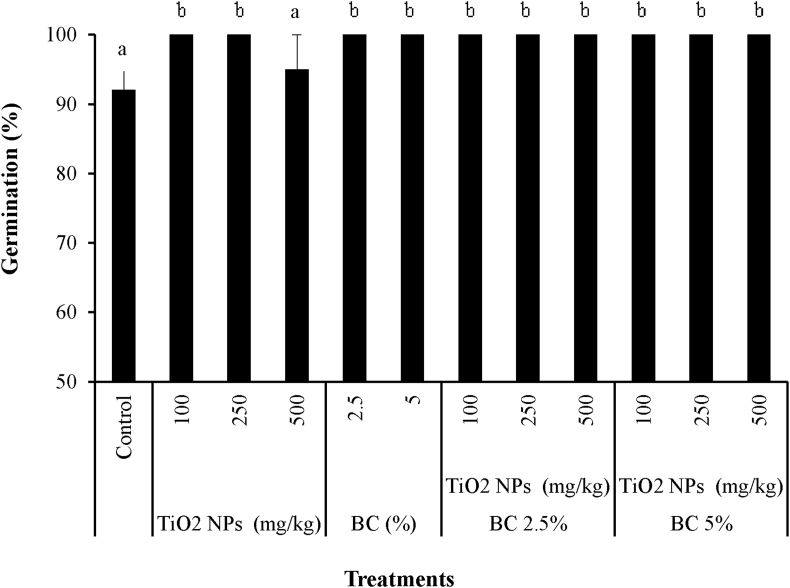
Seedling emergence of S. bicolor in different treatments was monitored during the experiment. The S. bicolor was found to be a tolerant plant with high germination rate in all treatments. Applying TiO2 NPs concentrations of 100–500 mg kg−1 to soil improved germination rate compared to the control (contaminated unamended soil) (Figure 1). In this study, delayed germination was not observed due to addition of 100–500 mg kg−1 TiO2 NPs; however, germination rate of S. bicolor in presence of 500 mg kg−1 TiO2 NPs in soil slightly declined compared with 100 and 250 mg kg−1 TiO2 NPs treatments. Enhanced germination of cabbage and corn in presence of low concentrations of titanium in soil has been reported (Lyu et al., 2017). Accelerated germination of S. bicolor seeds in Sb-contaminated soils received low concentrations of nano-TiO2 was observed in this study, which is consistent with the corresponding impact of applying 100–300 mg kg−1 nano-TiO2 on sprouting of soybean seeds in Cd-polluted soil (Singh and Lee, 2016). Positive effect of BC on germination of S. bicolor was also observed. Promoting effect of biochar on germination of various plant species such as oat (Chirakkara and Reddy, 2015) and sunflower (Kookana et al., 2011) has been reported. The highest possible germination rate (100%) was observed in all biochar-amended treatments, with or without TiO2 NPs. The ability to adapt harsh conditions under environmental stress is an important factor for a plant to be opted for remediation purposes.
Figure 1.
Final seedling emergence of S. bicolor in Sb-contaminated soil treated with BC, TiO2 NPs and BC + TiO2 NPs. Error bars represent standard deviation of three replicates. Means that do not share a letter are significantly different at p = 0.05 (mean ± SD; n = 3).
3.2. Growth of S. bicolor in Sb contaminated soil treated with TiO2 NPs, BC and their combination
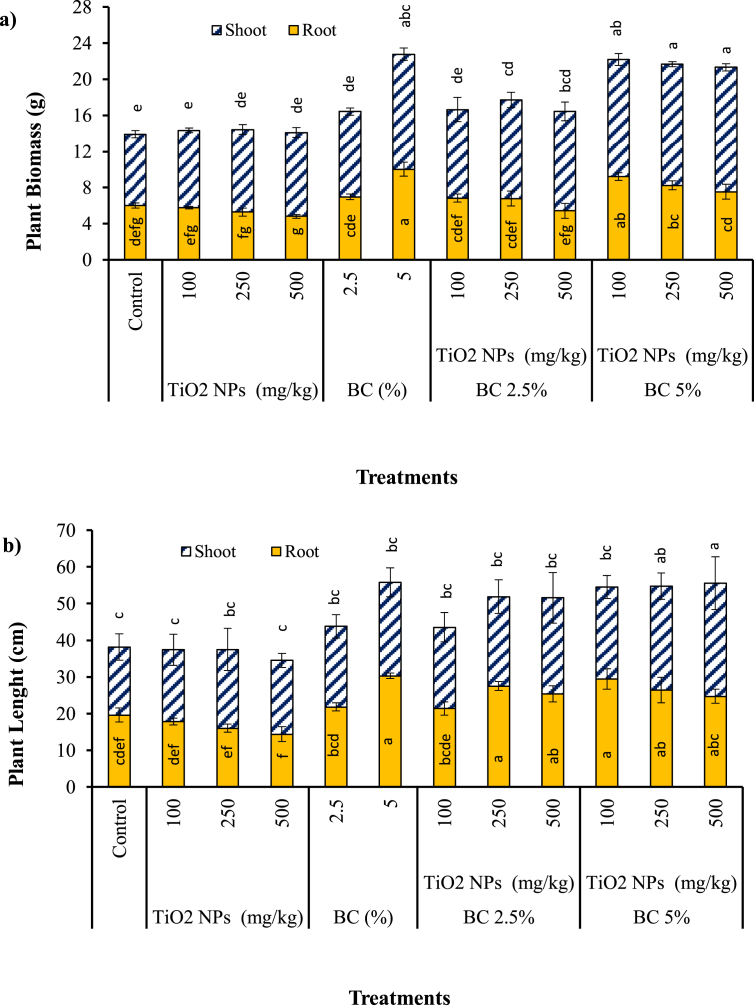
Biomass and length of roots and shoots were determined after harvesting to assess the effects of different TiO2 NPs and BC treatments as well as their combination impact on plant growth. Figure 2a shows that applying TiO2 NPs to Sb-contaminated soil reduced root biomass of S. bicolor particularly at higher TiO2 NPs dose of 500 mg kg−1, while enhanced shoot biomass of plants in all TiO2 NPs treated soils compared to the control. Contradictory results on the effects of TiO2 NPs on plant growth have been reported in the literature. For instance, a reduction in biomass of wheat was found at concentrations of 90 mg kg−1 TiO2 NPs (Du et al., 2011), while application of up to 300 mg kg−1 TiO2 NPs enhanced the biomass of soybean cultivated in Cd-contaminated soil (Singh and Lee, 2016). The most meaningful increase in shoots biomass among the TiO2 NPs treated soils (17.50%) was gained in 500 mg kg−1 TiO2 NPs treatment, compared to the control (Figure 2a). Total plant biomass increased slightly with the application of 100–500 mg kg−1 TiO2 NPs. The extent of plant growth promotion or inhibition in presence of nanoparticles in soil was suggested to be related to plant type as well as nanoparticles types and concentrations (Yoon et al., 2019).
Figure 2.
The biomass (a) and length (b) of S. bicolor grown in TiO2 NPs, BC and BC + TiO2 NPs treatments after 80 days. Error bars represent standard deviation of three replicates. Means that do not share a letter are significantly different at p = 0.05 (mean ± SD; n = 3).
Figure 2a indicates that addition of BC to soil increased root and shoot biomass of S. bicolor significantly, compared to the control and TiO2 NPs treatments (P < 0.05). Application of biochar coupled with TiO2 NPs also promoted S. bicolor growth and biomass. Increase in plant biomass with the rising of BC levels in Sb-contaminated soil was observed in this study. However, application of TiO2 NPs alone did not show a significant effect on S. bicolor biomass, compared to the control treatment (P > 0.05). Addition of 5% biochar increased plant biomass significantly by 63.76%, in comparison with the control (P < 0.05). It can also be inferred from Figure 2a that both 2.5% BC and 5% BC treatments significantly enhanced root biomass compared to the TiO2 NPs treatments (P < 0.05), with a greater impact by addition of 5% BC. “5% BC” treatment was found to yield the greatest plant biomass (22.78 g) followed by“100 mg kg−1 TiO2 NPs+5% BC” (22.21 g) and “250 mg kg−1 TiO2 NPs+5% BC” (21.68 g). Differences between the above-mentioned treatments were not statistically significant (P > 0.05). Furthermore, the positive effects of BC on the root and the shoot biomass of S. bicolor were found in presence of different concentrations of TiO2 NPs. In a previous study, cotton stick biochar amendment increased the biomass of S. oleracea by 29–36% in Cd polluted soil (Younis et al., 2015). Positive effect of maize biochar on growth of Chickpea (Cicer arietinum L.) has been reported (Egamberdieva et al., 2019); however pine-based biochar could not significantly promote growth of Lotus pedunculatus cv barsille (Shen et al., 2016). Biochar can immobilize metal(loid)s in soil through adsorption of metals, enhance biological activity in soil, release essential nutrients for plants, and improve water holding capacity and soil structure to promote plant growth (Chirakkara and Reddy, 2015). S. bicolor is a reasonably fast growing species that makes it suitable for phytoremediation purposes since establishment of considerable biomass is a crucial factor affecting phytoremediation potential of plants (Chen et al., 2016).
The length of roots followed the same trend as root biomass almost in all amended treatments (Figure 2b). Application of 100 mg/kg TiO2 NPs increased shoot length by 5.95% compared with the control, which was in agreement with the findings of Abdel Latef et al. (2018), where adding 0.01% TiO2 NPs to soil slightly increased shoot length of leguminous crops (Abdel Latef et al., 2018). Boosted ability of edible plants such as tomato to take up nutrients (N, P, Ca, Mg) from soil, and therefore enhanced growth of plants in soils treated with titanium was also reported (Kleiber and Markiewicz, 2013). However, the root extension of S. bicolor in the Sb-contaminated soil treated with 500 mg kg−1 TiO2 NPs (14.4 ± 2.0 cm) was suppressed by 26.53% in comparison with the extension yielded in the control treatment. Preliminary tests showed that growth of S. bicolor was only slightly altered in presence of applied concentration of Sb in soil compare to the clean soil. Moreover, pre-tests showed inhibitory effect of 1000 and 1500 mg kg−1 TiO2 NPs on germination and growth of S. bicolor, which markedly inhibit seedling emergence and plant growth. Negative effects of higher doses of TiO2 NPs on S. bicolor growth may be caused by the antibacterial effects of TiO2 NPs. Titanium dioxide has photocatalytic properties thereby generating reactive oxygen species (ROS) which has detrimental impacts on microbial cell walls (Bumbudsanpharoke et al., 2015). Higher doses of TiO2 NPs in soil may induce adverse effects on rhizosphere microbial community. In addition, higher uptake of nanoparticles of TiO2 at higher doses of applied TiO2 NPs in Sb-contaminated soil may cause toxicity to the plants. Therefore, concurrent impacts of Sb and excessive TiO2 NPs in soil may cause enhanced toxicity to the plants and adversely affect plant growth.
BC addition to the Sb-contaminated soil increased both the roots and the shoot lengths compared to the control. Treated soils with the combination of TiO2 NPs and BC yielded greater plant length compared to the treatments amended only with TiO2 NPs. Recently similar results on the positive effect of biochar on plant growth have been reported. For instance, Wang et al. (2018) observed stimulation of soybean growth by addition of bamboo biochar. Plant nutrient uptake and translocation could be enhanced by biochar application, which may result in promoted plant biomass (Rees et al., 2016). Application of 5% BC posed greater effect on plant growth promotion in both TiO2 NPs and non-TiO2 NPs amended soils, compared to 2.5% BC.
Amplified phytotoxicity of heavy metals by addition of TiO2 NPs to soil has been reported, especially at higher nZVI doses (Huang et al., 2020). However, co-application of BC with TiO2 NPs may reduce toxicity effects of TiO2 NPs even at higher TiO2 NPs levels, which is in agreement with the findings of this study. Greater plant biomass and length were obtained in 500 mg kg−1 TiO2 NPs treatments in presence of 2.5% and 5% BC, compared to the corresponding non-BC treatment. Sensibility of root and shoot growth of S. bicolor to the higher applied concentration of TiO2 NPs (500 mg kg−1) was found to be decreased by addition of BC to soil. The applied concentrations of TiO2 NPs and BC to the Sb-contaminated soil, individually and in combination, favored plant growth, with greater positive effect obtained in BC treatments. Slight inhibitory effect of TiO2 NPs on root growth of S. bicolor was alleviated by using biochar. To be brief, plant growth in Sb-contaminated soil was positively affected by TiO2 NPs and BC in the following order: 5% BC treatment > combined BC and TiO2 NPs treatments > TiO2 NPs treatments.
3.3. Phytoremediation of Sb from soil under the effect of TiO2 NPs, BC and their combination
In this study, TiO2 NPs and biochar were used individually and in combination to promote Sb uptake and translocation in S. bicolor or enhance immobilization of Sb in the rhizosphere of S. bicolor. Phytoextraction is known to be the main mechanism by which metal(loid)s are remediated in soil during the phytoremediation process (Bhargava et al., 2012). Table 2 presents the distribution of Sb in plant roots and shoots in different treatments. Concentrations of Sb in roots of S. bicolor were 45.75, 51.89 and 56.80 mg kg−1 in treated soils with 100, 250 and 500 mg kg−1 TiO2 NPs, respectively, compared to 38.03 mg kg−1 of the control. Promoting effect of low doses of TiO2 NPs on Sb uptake by S. bicolor is consistent with the literature, where application of 100–300 mg kg−1 TiO2 NPs to soil enhanced Cd uptake by G. max (Singh and Lee, 2016). Addition of 5% biochar to soil reduced Sb uptake by S. bicolor significantly, compared to the control treatment (P < 0.05). Sb concentration in roots of S. bicolor declined to 27.01 and 16.56 mg kg−1 in soils treated only with, respectively, 2.5% and 5% biochar. Co-application of BC and TiO2 NPs increased Sb immobilization in soil and reduced Sb uptake by the plant compared with the corresponding treatments amended only with TiO2 NPs. However, Sb uptake by S. bicolor increased in TiO2 NPs + BC treatments compared to the contaminated soils received only BC. In other words, immobilization of Sb in soil was more promoted in BC-amended treatments, while the applied range of TiO2 NPs enhanced phytoextraction of Sb. The influence of BC on stabilization of Sb in soil was more highlighted with increase in BC content of soil. For instance, Sb concentration in the roots of S. bicolor declined significantly from 49.78 mg kg−1 in “500 mg kg−1 TiO2 NPs+ 2.5% BC” treatment to 35.98 mg kg−1 in “500 mg kg−1 TiO2 NPs+ 5% BC” treatment (27.72% reduction; P < 0.05). Sorption of metals onto biochar particles is believed to occur mainly on surface functional groups, particularly oxygen containing functional groups (Silvani et al., 2019). However, higher metal absorption by plants due to addition of BC to soil was also reported in the literature (Simiele et al., 2020) that could be attributed to an increase of root surface, thus a higher exchange surface between soil and plant, and hence promoted metal uptake. Indeed, biochar can have contradictory impacts on plant metal uptake by reducing metal availability, on one hand, and through increasing root biomass and proliferation, on the other hand as also suggested by Rees et al. (2016) (Rees et al., 2016).
Table 2.
Distribution of Sb in roots and shoots of S. bicolor cultivated in Sb-contaminated soil, bioconcentration factors (BCF) and translocation factors (TF) for TiO2 NPs, BC and TiO2 NPs + BC treatments after 80 days. Standard deviations for three replicates are presented. Means that do not share a letter are significantly different at p = 0.05 (mean ± SD; n = 3).
| BC (%) | TiO2 NPs (mg/kg) | Sb concentration (mg/kg) |
Absorption and translocation factors |
||
|---|---|---|---|---|---|
| Root | Shoot | BCF | TF | ||
| 0 | 0 | 38.03 ± 8.51cdef | 75.12 ± 10.85cd | 1.33 ± 0.10cd | 2.05 ± 0.52a |
| 100 | 45.75 ± 9.33abcd | 95.63 ± 14.15bc | 1.41 ± 0.07c | 2.11 ± 0.15a | |
| 250 | 51.89 ± 8.58ab | 128.64 ± 9.61a | 1.78 ± 0.07ab | 2.54 ± 0.53a | |
| 500 | 56.8 ± 6.14a | 138.21 ± 29.41a | 1.91 ± 0.11a | 2.42 ± 0.29a | |
| 2.5 | 0 | 27.01 ± 7.31fgh | 53.87 ± 13.61de | 1.01 ± 0.09e | 2.02 ± 0.27a |
| 100 | 41.08 ± 7.68bcde | 84.02 ± 23.62c | 1.31 ± 0.17cd | 2.16 ± 0.97a | |
| 250 | 47.56 ± 11.22abcd | 119.26 ± 21.02ab | 1.67 ± 0.16b | 2.54 ± 0.23a | |
| 500 | 49.78 ± 5.18abc | 122.83 ± 19.41ab | 1.8 ± 0.08ab | 2.50 ± 0.61a | |
| 5 | 0 | 16.56 ± 6.78h | 29.45 ± 8.43e | 0.59 ± 0.06f | 1.83 ± 0.21a |
| 100 | 20.92 ± 4.58gh | 44.26 ± 5.33e | 0.74 ± 0.09f | 2.15 ± 0.22a | |
| 250 | 31.19 ± 5.06efg | 75.83 ± 16.43cd | 1.07 ± 0.08e | 2.51 ± 0.78a | |
| 500 | 35.98 ± 7.86def | 88.07 ± 11.24c | 1.16 ± 0.07de | 2.53 ± 0.60a | |
Concentrations of Sb in roots and shoots of S. bicolor grown in presence of 500 mg kg−1 TiO2 NPs were 56.80 mg kg−1 and 138.21 mg kg−1 with a significant increase of 49.36% and 83.98%, respectively, compared to the control. The concentrations of Sb in roots and shoot of S. bicolor cultivated in treated soil with 5% BC were 16.56 mg kg−1 and 29.45 mg kg−1, showing a significant decrease of 56.45% and 60.79%, respectively, compared with the control treatment. Application of 2.5% biochar together with 250 mg kg−1 TiO2 NPs significantly increased Sb concentration in the roots and shoots by 25.06% and 58.76%, respectively, compared with the control (P < 0.05). Reduction in concentrations of Sb in roots of S. bicolor under the effect of BC could be related to the large specific surface area of BC and their high adsorption capacity, which might reduce availability of Sb for being uptaken by the plant roots (Gong et al., 2019). In this research, the applied range of TiO2 NPs did not exhibit adverse effects on plant growth and phytoextraction potential; however, inhibitory effects of high doses of TiO2 NPs due to their toxicity on contaminant uptake by plants has been reported in the literature (Cao et al., 2017).
Accumulation of metal(loid)s such as Sb in plants may induce oxidative stress due to the production and accumulation of reactive oxygen species (ROS). Low concentrations of TiO2 NPs were found to be able to slightly relieve the oxidative stress in plants cultivated on heavy metal-contaminated soils, while elevated oxidative stress due to addition of high concentrations of nanomaterials has been reported in the literature (Huang et al., 2018). Therefore, the high accumulation of both TiO2 NPs and Sb is likely to induce oxidative stress and damage to antioxidant enzymes of plants. For this reason low to medium concentrations of TiO2 NPs was applied in this research to promote phytoremediation potential of S. bicolor in Sb-contaminated soil, while avoiding extra toxicity which might be caused by high TiO2 NPs levels. Higher application rates of TiO2 NPs may occupy adsorption sites on biochar surface and compete with Sb for adsorption onto biochar, which could limit biochar effectiveness in immobilization of Sb in the rhizosphere zone. High retention capacity of biochar for titanium particles was reported in the literature (Zhuravlev, 2019). Moreover, co-application of biochar with TiO2 NPs could reduce availability of metal(loid)s in soil followed by alleviated metal uptake by plant, which may result in reduced toxicity of metal(loid)s to plants and their promoted growth as observed in this study. Our results indicated that the impacts of TiO2 NPs, combined with BC, on growth and metal uptake of S. bicolor could vary with the concentration at which amendments are incorporated to soil.
The bio concentration factors (BCF) and translocation factors (TF) were calculated and presented in Table 2, to gain better understanding on the effects of different amendments on absorption and translocation of Sb in S. bicolor. Increase in BCF values was observed in S. bicolor grown in 100–500 mg kg−1 TiO2 NPs treated soil, compared with the control soil. BCF values were also enhanced compared to the control by adding 250–500 mg kg−1 TiO2 NPs in combination with 2.5% BC, though application of 5% BC reduced BCF values considerably, regardless of the TiO2 NPs presence in soil. In the present study, BCF values were enhanced by addition of 100–500 mg kg−1 TiO2 NPs to the soil, compared to the control indicating the enhanced capability of S. bicolor to extract Sb from the soil in presence of TiO2 NPs. The greatest value of BCF (1.91) was achieved by addition of 500 mg kg−1 TiO2 NPs. BCF values increased in all treatments receiving TiO2 NPs, with or without BC, with the rising TiO2 NPs concentrations. However, application of BC, with or without TiO2 NPs, reduced BCF values significantly, such that the BCF value in soil treated only with 5% BC declined by 55.63% compared to the control (P < 0.05). Reduction in BCF values in BC amended soils compared to other treatments indicates enhanced immobilization of Sb in soil through adsorption onto biochar particles, which is consistent with the findings of Gong et al. (2019), where availability of Cd in sediments amended with tea waste derived biochar decreased during a phytoremediation experiment (Gong et al., 2019). In the present study, BCF values in most treatments were greater than one, indicating the high capability of S. bicolor to extract Sb from the soil. Sb uptake from soil was promoted by using TiO2 NPs, while the uptake of Sb from soil was alleviated by application of biochar.
Translocation of metal(loid)s from the roots to shoots and their accumulation in shoots is another important index to evaluate the phytoextraction potential in plants. High TF values of Sb for S. bicolor indicate great movement and translocation ability of Sb in the plant. However, translocation of Sb from the roots to the shoots was somewhat limited by application of biochar. On the other hand, TF values were considerably increased in presence of TiO2 NPs. TF values in TiO2 NPs treatments ranged from 2.11 to 2.54 for Sb, with the highest TF values obtained for 250 mg kg−1 TiO2 NPs. Promoted uptake of Sb in S. bicolor due to the addition of low levels of TiO2 NPs might be due to the fact that applying TiO2 NPs to soil may reduce the pH of root exudates (Ghoto et al., 2020), which in turn alter availability of Sb in the rhizosphere to be uptaken by plans. In another study, TF values of Cd for soybean were found to be less than one for all treatments including contaminated soils received TiO2 NPs (Singh and Lee, 2016), which indicates higher translocation potential for Sb than Cd. The limited ability of plants to transfer heavy metals to the aerial parts of plants has also been reported in the literature as a result of blocking the root apex in plants due to the exposure to heavy metals (Wang et al., 2014). Apoplastic barriers might develop near the root apex in plants grown under the stress of heavy metals, which reduce the translocation of sorbed metals from root to aerial parts (Vatehová et al., 2012).
Applying 500 mg kg−1 TiO2 NPs slightly reduced obtained TF value. No adverse effect of TiO2 NPs on translocation of Sb in plant, compared to the control, was observed in this study; however, application of higher doses of TiO2 NPs may cause inhibitory effects on translocation of metal(loid)s in plants. Oxygen deficiency in soil is a possible phenomenon due to the oxidation of TiO2 NPs, particularly in presence of high concentrations of TiO2 NPs (El-Temsah et al., 2016). In addition, high levels of nanomaterials could also adversely affect transfer of nutrients from roots to aerial parts and disturb plant growth (Huang et al., 2020), which was not observed in this study mainly because of using low to moderate concentrations of TiO2 NPs. However, co-application of TiO2 NPs and BC might alleviate inhibitory effects of high doses of TiO2 NPs on heavy metal translocation in plants due to the promoted adsorption of TiO2 NPs on biochar surfaces. Application of high doses of TiO2 NPs in presence of BC to look at the effect on translocation of metal(loid)s in plants has not been reported in the literature and could be an interesting subject to further study in the future. Overally, results showed that TiO2 NPs addition to soil increased the uptake and translocation of Sb by S. bicolor in comparison with non-TiO2 NPs treatments. Applying TiO2 NPs to soil may increase soil organic matter through root exudates of plants, which in turn promotes absorption and translocation of S. bicolor. On the other hand, TiO2 NPs may also move along the plant pipeline upon internalization of TiO2 NPs by plants and the plant metabolism may be disturbed by TiO2 NPs (Huang et al., 2020). However, stomatal opening of plant leaves could be enhanced by nanomaterials, leading to increased CO2 uptake and photosynthesis (Kim et al., 2015). Our results also indicated that the impacts of TiO2 NPs, combined with BC, on growth and metal uptake of S. bicolor depend on the concentration at which amendments are incorporated to soil. Studying the Sb speciations in different treatments could better justify the effect of biochar, TiO2 NPs, and their coupled use on phytoremediation performance of plants. More complicated reactions are anticipated in phytoremediation involving the co-application of biochar and TiO2 NPs, compared to their individual application.
3.4. Accumulation capacity of Sb
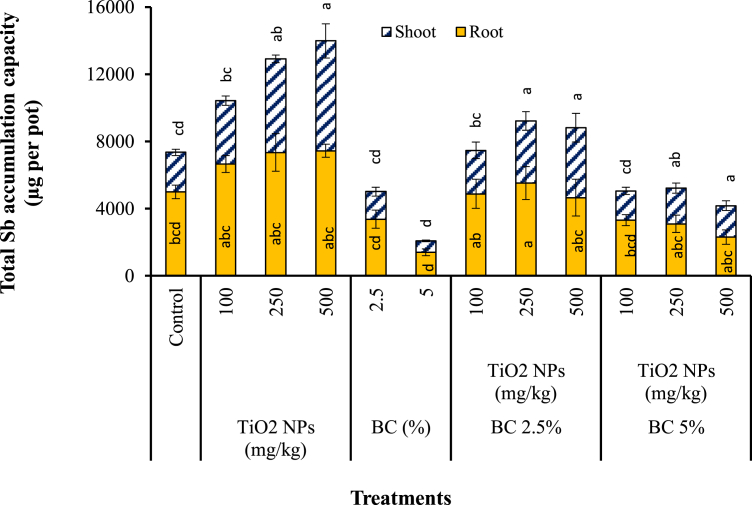
Sb accumulation capacity of S. bicolor does not only depend on Sb concentration in plant organs, but also depends on plant dry biomass, which was calculated and illustrated in Figure 3. The greatest total Sb accumulation capacity in S. bicolor was reached 1624.1 μg per pot, which was achieved in “2.5% BC+250 mg kg−1 TiO2 NPs” treatment followed by the “2.5% BC+500 mg kg−1 TiO2 NPs” treatment (1622.7 μg per pot). This might be posed by the slight reduction in root biomass in presence of 500 mg kg−1 TiO2 NPs compared to 250 mg kg−1 TiO2 NPs and positive effect of BC on gained plant biomass. One the other hand, the least Sb accumulation capacity was obtained in the soil amended only with 5% biochar (541.6 μg per pot), followed by 2.5% BC treatment (697.4 μg per pot), indicating enhanced immobilization of Sb in BC amended soil and non-positive effect of biochar addition to soil on Sb accumulation capacity of S. bicolor. In fact, the increase of root biomass and surface induced by biochar could enhance Sb transfer to the plant and therefore counter-balance the reduction of Sb availability in biochar-treated soil. The BC in such combined systems can provide adsorption sites for metal(loid)s, therefore more effective contact between TiO2 NPs with sorbed metal(loid)s on BC is likely to take place. Sb accumulation capacity in control treatment was found to be 821.6 μg per pot. Overally, results indicated that TiO2 NPs addition to soil increased Sb accumulation capacity of S. bicolor, whereas biochar application reduced Sb accumulation capacity of the plant in this study. Combined application of TiO2 NPs and BC had positive effect on Sb accumulation capacity of S. bicolor only when 2.5% biochar was used. Increased As accumulation capacity of wheat in sediments amended with graphene oxide has also been reported (Hu et al., 2014).
Figure 3.
The Sb accumulation capacity in roots and shoots of S. bicolor grown under the effect of TiO2 NPs, BC and BC + TiO2 NPs treatments after 80 days. Error bars represent standard deviation of three replicates. Means that do not share a letter are significantly different at p = 0.05 (mean ± SD; n = 3).
It can be inferred from the results that co-application of TiO2 NPs and BC could reduce the required amounts of TiO2 NPs to promote plant growth and metal accumulation capacity of plants. Apart from economical benefits, this could reduce risks associated with elevated levels of nanomaterials used for remediation purposes. Based on the plant growth and accumulation capacity of Sb in S. bicolor, it is suggested that using of low levels of BC in accompany with low concentrations of TiO2 NPs to assist phytoremediation of Sb in soil could be promising.
3.5. Ti accumulation and translocation in presence of TiO2 NPs, BC and their combination
Concentrations of Ti in all harvested plants were determined in this research to assess the effect of different treatments on absorption of Ti. Titanium is the second most abundant transition metal, after iron, and its elemental abundance is about 100 times more than copper. Based on the literature, Ti contents of plants typically range from 1 to 578 mg kg−1, in non-hyper accumulator plant species (Lyu et al., 2017). Titanium is a beneficial nutrient for plants and its application via roots or leaves could improve nutritional status of plant species, promote enzymatic activity and photosynthesis, and facilitate uptake of other nutrients; however, low mobility of titanium in soil may limit its uptake by plants (Bacilieri et al., 2017). Like most transition elements, root-absorbed titanium is mainly accumulated in plant roots with a small fraction transferred to the aerial parts via xylem stream (Kelemen et al., 1993). Table 3 indicates that the total Ti levels in roots were significantly higher than those in shoots in all treatments (P < 0.05), indicating that S. bicolor roots were the preferential Ti storage organ. Application of TiO2 NPs increased Ti uptake by roots significantly (P < 0.05), which may be attributed to the direct exposure of the S. bicolor roots to the TiO2 NPs in soil. Concentrations of Ti in roots increased with TiO2 NPs concentration to hit a plateau of 1539.78 mg kg−1 in treated soil with 500 mg kg−1 TiO2 NPs, which was 1.9 times greater than the corresponding value in control treatment. In 100–500 mg kg−1 TiO2 NPs treatments, the concentrations of Ti in shoots as well as TF values increased with the rising of TiO2 NPs; which suggested that low to moderate contents of TiO2 NPs could promote translocation of Ti in S. bicolor.
Table 3.
Distribution of Ti in roots and shoots of S. bicolor grown in Sb-contaminated soil, bioconcentration factors (BCF) and translocation factors (TF) in TiO2 NPs, BC and TiO2 NPs + BC treatments after 80 days. Standard deviations for three replicates are presented. Means that do not share a letter are significantly different at p = 0.05 (mean ± SD; n = 3).
| BC (%) | TiO2 NPs (mg/kg) | Ti concentration (mg/kg) |
Absorption and translocation factors |
||
|---|---|---|---|---|---|
| Root | Shoot | BCF | TF | ||
| 0 | 0 | 830.93 ± 27.61d | 297.34 ± 40.61ef | 2.41 ± 0.28cd | 0.36 ± 0.04c |
| 100 | 1154.45 ± 59.80c | 440.18 ± 35.32c | 2.62 ± 0.13abcd | 0.38 ± 0.04abc | |
| 250 | 1387.89 ± 87.19b | 608.42 ± 29.05b | 3.24 ± 0.30ab | 0.44 ± 0.02abc | |
| 500 | 1539.78 ± 83.07a | 706.40 ± 98.71a | 3.42 ± 1.32a | 0.46 ± 0.04a | |
| 2.5 | 0 | 482.03 ± 53.38f | 173.96 ± 30.06g | 1.83 ± 0.41def | 0.36 ± 0.08bc |
| 100 | 713.11 ± 83.46e | 263.91 ± 36.93f | 2.44 ± 0.49bcd | 0.37 ± 0.06bc | |
| 250 | 814.64 ± 40.21d | 338.01 ± 30.61de | 3.03 ± 0.31abc | 0.41 ± 0.02abc | |
| 500 | 856.90 ± 71.618 | 377.42 ± 39.90cd | 3.14 ± 0.65abc | 0.44 ± 0.04ab | |
| 5 | 0 | 140.71 ± 12.13h | 52.07 ± 6.32h | 1.08 ± 0.09f | 0.37 ± 0.02bc |
| 100 | 358.46 ± 24.05g | 134.48 ± 20.98g | 1.37 ± 0.07ef | 0.37 ± 0.03bc | |
| 250 | 375.59 ± 47.33g | 158.52 ± 23.21g | 1.92 ± 0.17de | 0.43 ± 0.09abc | |
| 500 | 307.27 ± 26.92g | 134.38 ± 18.67g | 2.09 ± 0.21de | 0.44 ± 0.04abc | |
Despite the increase in concentrations of Ti in S. bicolor organs in TiO2 NPs treatments, Ti concentration in the plant decreased significantly in BC amended soils (P < 0.05). Co-application of 5% BC with TiO2 NPs also reduced Ti concentration in S. bicolor organs compared to the control treatment. Application of BC could reduce availability of Ti in soil through adsorption onto biochar particles as observed in this study, but biochar addition to soil did not exhibit inhibitory effects on translocation of Ti within the plant. Inhibitory effects of high TiO2 NPs content in soil on Ti translocation in plant was reported in the literature, which might be due to the plugging of the pathway of Ti from the root to the shoot by TiO2 NPs as also suggested by Wang et al. (2018)). In addition, high concentrations of Ti in plant tissues pose competition between Ti and Fe for ligands and proteins, which may result in Ti phytotoxicity (Lyu et al., 2017). Co-application of BC and TiO2 NPs could help reducing the required amounts of TiO2 NPs for successful phytoremediation of heavy metal polluted soils. In other words, this signifies beneficial outcome of co-application of appropriate levels of BC and low concentrations of TiO2 NPs to successfully assist phytoremediation of heavy metals in soil because when the pathway of metals from the roots to the shoots are blocked by excessive amounts of TiO2 NPs, target metal(loid)s could not transfer effectively from the roots to the shoots, therefore accumulation of metal(loid)s in shoots could be decreased. Table 3 shows that BCF and TF values of Ti in TiO2 NPs treatments ranged from 2.62 to 3.42 and 0.38 to 0.46, respectively, with the highest values obtained in 500 mg kg−1 TiO2 NPs treatment. Based on Table 4, the highest total Ti accumulation capacity in S. bicolor was found to be 13990.3 μg per pot, which was achieved in 500 mg kg−1 TiO2 NPs treatment. Table 4 illustrates that accumulation capacity of Ti in S. bicolor enhanced in TiO2 NPs treatments compared to the control. In contrast, application of BC significantly reduced total Ti accumulation capacity in S. bicolor (P < 0.05), such that Ti accumulation capacity in the roots and shoots of S. bicolor dropped to 1411.3 and 663.9 μg per pot, respectively, in presence of 5% biochar in soil.
Table 4.
The Ti accumulation capacity in roots and shoots of S. bicolor grown under the effect of TiO2 NPs, BC and TiO2 NPs + BC treatments after 80 days. Means that do not share a letter are significantly different at p = 0.05 (mean ± SD; n = 3).
| BC (%) | TiO2 NPs (mg/kg) | Ti accumulation capacity (μg per pot) |
|
|---|---|---|---|
| Root | Shoot | ||
| 0 | 0 | 5002.1 ± 403.2c | 2345.8 ± 182.8de |
| 100 | 6657.7 ± 507.1ab | 3765.1 ± 278.7c | |
| 250 | 7346.1 ± 1115.8a | 5559.3 ± 228.1b | |
| 500 | 7444.5 ± 386.0a | 6545.7 ± 1015.6a | |
| 2.5 | 0 | 3366.3 ± 542.8d | 1643.1 ± 262.3e |
| 100 | 4877.7 ± 866.1c | 2586.5 ± 489.7d | |
| 250 | 5531.4 ± 978.5bc | 3687.7 ± 554.9c | |
| 500 | 4653.2 ± 1092.4c | 4155.1 ± 852.9c | |
| 5 | 0 | 1411.3 ± 203.4e | 663.9 ± 52.6f |
| 100 | 3307.6 ± 327.1d | 1745.3 ± 215.5e | |
| 250 | 3098.6 ± 525.9d | 2128.9 ± 295.5de | |
| 500 | 2316.8 ± 425.3de | 1853.1 ± 287.1de | |
3.6. Chlorophyll content
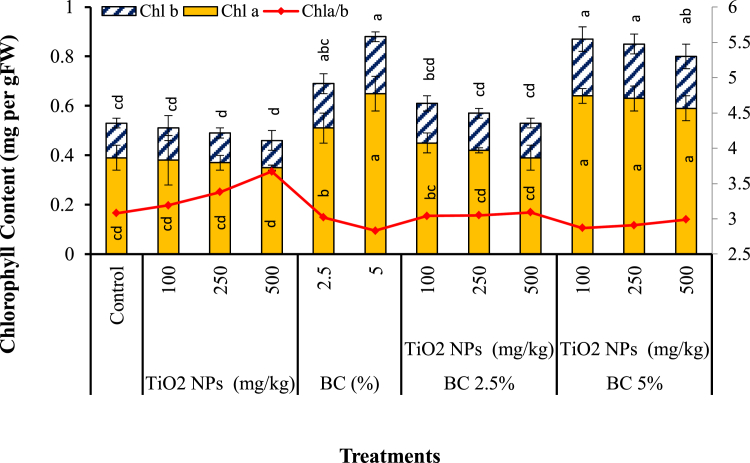
Figure 4 shows that the accumulation of TiO2 NPs and Sb in S. bicolor caused physiological changes. Chl a and Chl b were used as the biomarkers of photosynthesis ability of S. bicolor in different treatments. Addition of TiO2 NPs (100–500 mg kg−1) to the Sb-contaminated soil slightly altered Chl a and Chl b contents in this study. Application of TiO2 NPs may increase the level and activity of RuBisCO activase, which is an enzyme involved in the Calvin cycle, by which plants fix carbon dioxide. Increased level and activity of RuBisCO activase could enhance the photosynthesis rate (Gao et al., 2013). On the other hand, rreduction in the chlorophyll content at higher levels of TiO2 NPs due to their adverse impacts on the biochemical factors i.e. lipid peroxidation in photosynthesis membranes has been reported (Singh and Lee, 2016; Zhao et al., 2015).
Figure 4.
Changes of the chlorophyll content of S. bicolor grown in TiO2 NPs, BC and BC + TiO2 NPs treatments after 80 days. Error bars represent standard deviation of three replicates. Means that do not share a letter are significantly different at p = 0.05 (mean ± SD; n = 3).
Application of biochar increased Chl a and Chl b significantly with the greater positive effect in 5% BC treatment (P < 0.05). All the Sb-contaminated soils received 2.5% or 5% BC exhibited enhanced chlorophyll content compared to the control, regardless of the presence of TiO2 NPs in soil which is in agreement with the reported literature where addition of 4% biochar increased the chlorophyll content of M. arvensis by 15% in a Pb-spiked soil (Nigam et al., 2019). Photosynthesis of B. chinensis in cadmium contaminated soil treated with biochar was also found to be enhanced (Kamran et al., 2019). Application of BC along with TiO2 NPs may reduce the required amounts of TiO2 NPs for remediation works, which could in turn avoid the probable adverse effects of high TiO2 NPs concentrations on plant photosynthesis and chlorophyll content. Reduction in the chlorophyll content at higher levels of TiO2 NPs due to the adverse impact of high doses of TiO2 NPs on the biochemical factors i.e. lipid peroxidation in photosynthesis membranes has been reported (Zhao et al., 2015). The ratio of Chl a/Chl b was also used as a useful indicator for environmental stress. Chl a/Chl b ratio was somewhat increased by addition of TiO2 NPs to soil, compared to the control; however, application of biochar reduced the ratio of Chl a/Chl b in this study (Figure 4). Applying 5% BC posed the greatest increase for the total chlorophyll content, compared to the control (66.04%). It was concluded that co-application of BC and TiO2 NPs could induce positive effect on plant photosynthesis, compared with individual use of TiO2 NPs.
4. Conclusions
The main aim of this research was to assess the feasibility of phytoremediation of Sb-contaminated soil assisted by co-application of TiO2 NPs and BC. Results indicated that TiO2 NPs at low to moderate concentrations can be successfully coupled with BC to promote remediation of Sb in soil. Application of 100–500 mg kg−1 TiO2 NPs barely exhibited adverse effects on S. bicolor establishment and growth; however, co-application of BC and TiO2 NPs enhanced plant biomass and length in Sb-contaminated soil, which may be attributed to the alleviated toxicity of Sb in presence of biochar. Using TiO2 NPs significantly increased the accumulation capacity of S. bicolor for Sb, while individual application of BC reduced the accumulation capacity of S. bicolor in this study, which may be caused by reduced availability of Sb in soil due to adsorption on biochar particles. However, using low to moderate concentrations of TiO2 NPs in combination with lower doses of BC is suggested to promote remediation of Sb by S. bicolor as a practicable approach. Inhibitory effects of TiO2 NPs, BC and TiO2 NPs + BC at applied levels were not observed on plant growth and performance in this study. Accumulation and translocation of Ti by S. bicolor in the TiO2 NPs, BC and TiO2 NPs + BC treated soils showed similar behavior as Sb; though higher transfer ability of Sb than Ti in plants was observed. Leaf physiological structure was slightly affected by addition of TiO2 NPs to soil, but addition of BC to soil improved physiological structure of the plant significantly. The Chl a and Chl b contents of S. bicolor increased significantly by using BC, regardless of the presence of TiO2 NPs in soil. Significantly higher accumulation of Sb in shoots of S. bicolor compared to the roots in all amended and unamended treatments suggested S. bicolor shoots were the preferential Sb storage organ. Based on the obtained results, the best phytoremediation performance of S. bicolor was achieved by co-application of the 250 mg kg−1 TiO2 NPs and 2.5% biochar. Further studies are needed to assess the mechanisms of interactions involved in plant-TiO2 NPs-biochar association in soils contaminated by Sb. In addition, biochar particle size may affect its performance in immobilization of metal(loid)s in soil during a phytoremediation process, which is suggested to be further investigated. In summary, co-application of TiO2 NPs and BC, which was studied for the first time in this study to support phytoremediation of Sb contaminated soil, could promisingly support S. bicolor growth and performance. Engineering use of nanomaterials coupled with biological methods require significant cautions to be taken into account to avoid spreading excess amounts of nanomaterials in the environment. Co-application of TiO2 NPs and BC could help reducing the required amounts of TiO2 NPs for successful phytoremediation of heavy metal polluted soils, and could be a promising approach to remediate Sb-contaminated soils.
Declarations
Author contribution statement
Ali Daryabeigi Zand: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alireza Mikaeili Tabrizi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Azar Vaezi Heir: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by University of Tehran.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdel Latef A.A.H., Srivastava A.K., El-sadek M.S.A., Kordrostami M., Tran L.S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018;29:1065–1073. [Google Scholar]
- Abdelkrim S., Jebara S.H., Saadani O., Abid G., Taamalli W., Zemni H., Mannai K., Louati F., Jebara M. In situ effects of Lathyrus sativus-PGPR to remediate and restore quality and fertility of Pb and Cd polluted soils. Ecotoxicol. Environ. Saf. 2020;192:110260. doi: 10.1016/j.ecoenv.2020.110260. [DOI] [PubMed] [Google Scholar]
- Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S., Ali Q., Zahir Z.A., Ashraf S., Asghar H.N. Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019;174:714–727. doi: 10.1016/j.ecoenv.2019.02.068. [DOI] [PubMed] [Google Scholar]
- Bacilieri F.S., Pereira de Vasconcelos A.C., Quintao Lana R.M., Mageste J.G., Torres J.L.R. Titanium (Ti) in plant nutrition-A review. Aust. J. Crop. Sci. 2017;11:382. [Google Scholar]
- Bagherifam S., Brown T.C., Fellows C.M., Naidu R. Bioavailability of arsenic and antimony in terrestrial ecosystems: a review. Pedosphere. 2019;29:681–720. [Google Scholar]
- Bhargava A., Carmona F.F., Bhargava M., Srivastava S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012;105:103–120. doi: 10.1016/j.jenvman.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Bumbudsanpharoke N., Choi J., Ko S. Applications of nanomaterials in food packaging. J. Nanosci. Nanotechnol. 2015;15:6357–6372. doi: 10.1166/jnn.2015.10847. [DOI] [PubMed] [Google Scholar]
- Cao J., Feng Y., Lin X., Wang J., Xie X. Iron oxide magnetic nanoparticles deteriorate the mutual interaction between arbuscular mycorrhizal fungi and plant. J. Soils Sediments. 2017;17:841–851. [Google Scholar]
- Chavan S., Sarangdhar V., Nadanathangam V. Toxicological effects of TiO2 nanoparticles on plant growth promoting soil bacteria. Emerg. Contam. 2020;6:87–92. [Google Scholar]
- Chen Z.-J., Tian Y.-H., Zhang Y., Song B.-R., Li H.-C., Chen Z.-H. Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol. Eng. 2016;92:243–250. [Google Scholar]
- Cheng P., Deng C., Gu M., Dai X. Effect of urea on the photoactivity of titania powder prepared by sol–gel method. Mater. Chem. Phys. 2008;107(1):77–81. [Google Scholar]
- Chirakkara R.A., Reddy K.R. Biomass and chemical amendments for enhanced phytoremediation of mixed contaminated soils. Ecol. Eng. 2015;85:265–274. [Google Scholar]
- Cooper R.G., Harrison A.P. The exposure to and health effects of antimony. Indian J. Occup. Environ. Med. 2009;13:3. doi: 10.4103/0019-5278.50716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Sun Y., Ji R., Zhu J., Wu J., Guo H. TiO 2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011;13:822–828. doi: 10.1039/c0em00611d. [DOI] [PubMed] [Google Scholar]
- Egamberdieva D., Li L., Ma H., Wirth S., Bellingrath-Kimura S.D. Soil amendment with different maize biochars improves chickpea growth under different moisture levels by improving symbiotic performance with Mesorhizobium ciceri and soil biochemical properties to varying degrees. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsan S., Ali S., Noureen S., Mahmood K., Farid M., Ishaque W., Shakoor M.B., Rizwan M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014;106:164–172. doi: 10.1016/j.ecoenv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- El-Temsah Y.S., Sevcu A., Bobcikova K., Cernik M., Joner E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere. 2016;144:2221–2228. doi: 10.1016/j.chemosphere.2015.10.122. [DOI] [PubMed] [Google Scholar]
- Embrandiri A., Rupani P., Shahadat M., Singh R., Ismail S., Ibrahim M., Kadir M.A. The phytoextraction potential of selected vegetable plants from soil amended with oil palm decanter cake. Int. J. Recycl. Org. Waste Agric. 2017;6:37–45. [Google Scholar]
- EPA U. Environmental Protection Agency; Washington, DC, USA: 1996. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. [Google Scholar]
- Gao J., Xu G., Qian H., Liu P., Zhao P., Hu Y. Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings. Environ. Pollut. 2013;176:63–70. doi: 10.1016/j.envpol.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Ghoto K., Simon M., Shen Z.-J., Gao G.-F., Li P.-F., Li H., Zheng H.-L. Physiological and root exudation response of maize seedlings to TiO 2 and SiO 2 nanoparticles exposure. BioNanoScience. 2020:1–13. [Google Scholar]
- Gong X., Huang D., Liu Y., Zeng G., Chen S., Wang R., Xu P., Cheng M., Zhang C., Xue W. Biochar facilitated the phytoremediation of cadmium contaminated sediments: metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 2019;666:1126–1133. doi: 10.1016/j.scitotenv.2019.02.215. [DOI] [PubMed] [Google Scholar]
- Gong X., Huang D., Liu Y., Zeng G., Wang R., Wei J., Huang C., Xu P., Wan J., Zhang C. Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: for heavy metals stabilization and dye adsorption. Bioresour. Technol. 2018;253:64–71. doi: 10.1016/j.biortech.2018.01.018. [DOI] [PubMed] [Google Scholar]
- He M. Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ. Geochem. Health. 2007;29:209–219. doi: 10.1007/s10653-006-9066-9. [DOI] [PubMed] [Google Scholar]
- Hu B., Liang D., Liu J., Lei L., Yu D. Transformation of heavy metal fractions on soil urease and nitrate reductase activities in copper and selenium co-contaminated soil. Ecotoxicol. Environ. Saf. 2014;110:41–48. doi: 10.1016/j.ecoenv.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Huang D., Qin X., Peng Z., Liu Y., Gong X., Zeng G., Huang C., Cheng M., Xue W., Wang X. Nanoscale zero-valent iron assisted phytoremediation of Pb in sediment: impacts on metal accumulation and antioxidative system of Lolium perenne. Ecotoxicol. Environ. Saf. 2018;153:229–237. doi: 10.1016/j.ecoenv.2018.01.060. [DOI] [PubMed] [Google Scholar]
- Huang R., Dong M., Mao P., Zhuang P., Paz-Ferreiro J., Li Y., Li Y., Hu X., Netherway P., Li Z. Evaluation of phytoremediation potential of five Cd (hyper) accumulators in two Cd contaminated soils. Sci. Total Environ. 2020:137581. doi: 10.1016/j.scitotenv.2020.137581. [DOI] [PubMed] [Google Scholar]
- Kamran M., Malik Z., Parveen A., Zong Y., Abbasi G.H., Rafiq M.T., Shaaban M., Mustafa A., Bashir S., Rafay M. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019;250:109500. doi: 10.1016/j.jenvman.2019.109500. [DOI] [PubMed] [Google Scholar]
- Karami N., Clemente R., Moreno-Jiménez E., Lepp N.W., Beesley L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard Mater. 2011;191:41–48. doi: 10.1016/j.jhazmat.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Kelemen G., Keresztes A., Bacsy E., Feher M., Fodor P., Pais I. Distribution and intracellular localization of titanium in plants after titanium treatment. Food Struct. 1993;12:8. [Google Scholar]
- Kim J.-H., Oh Y., Yoon H., Hwang I., Chang Y.-S. Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana. Environ. Sci. Technol. 2015;49:1113–1119. doi: 10.1021/es504375t. [DOI] [PubMed] [Google Scholar]
- Kiran B.R., Prasad M. Biochar and rice husk ash assisted phytoremediation potentials of Ricinus communis L. for lead-spiked soils. Ecotoxicol. Environ. Saf. 2019;183:109574. doi: 10.1016/j.ecoenv.2019.109574. [DOI] [PubMed] [Google Scholar]
- Kleiber T., Markiewicz B. Application of “Tytanit” in greenhouse tomato growing. Acta Sci. Polonorum. Hortorum Cultus. 2013;12:117–126. [Google Scholar]
- Klingenfuss F. University of Gothenburg; Gothenburg: 2014. Testing of TiO2 Nanoparticles on Wheat and Microorganisms in a Soil Microcosm. [Google Scholar]
- Kołtowski M., Oleszczuk P. Effect of activated carbon or biochars on toxicity of different soils contaminated by mixture of native polycyclic aromatic hydrocarbons and heavy metals. Environ. Toxicol. Chem. 2016;35:1321–1328. doi: 10.1002/etc.3246. [DOI] [PubMed] [Google Scholar]
- Kookana R.S., Sarmah A.K., Van Zwieten L., Krull E., Singh B. Advances in Agronomy. Elsevier; 2011. Biochar application to soil: agronomic and environmental benefits and unintended consequences; pp. 103–143. [Google Scholar]
- Lyu S., Wei X., Chen J., Wang C., Wang X., Pan D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017;8:597. doi: 10.3389/fpls.2017.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques J., Gomes T.D., Forte M.A., Silva R.F., Tavares C.J. A new route for the synthesis of highly-active N-doped TiO2 nanoparticles for visible light photocatalysis using urea as nitrogen precursor. Catal. Today. 2019;326:36–45. [Google Scholar]
- Nigam N., Khare P., Yadav V., Mishra D., Jain S., Karak T., Panja S., Tandon S. Biochar-mediated sequestration of Pb and Cd leads to enhanced productivity in Mentha arvensis. Ecotoxicol. Environ. Saf. 2019;172:411–422. doi: 10.1016/j.ecoenv.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Okkenhaug G., Amstätter K., Lassen Bue H., Cornelissen G., Breedveld G.D., Henriksen T., Mulder J. Antimony (Sb) contaminated shooting range soil: Sb mobility and immobilization by soil amendments. Environ. Sci. Technol. 2013;47:6431–6439. doi: 10.1021/es302448k. [DOI] [PubMed] [Google Scholar]
- Okkenhaug G., Gebhardt K.-A.G., Amstaetter K., Bue H.L., Herzel H., Mariussen E., Almås Å.R., Cornelissen G., Breedveld G.D., Rasmussen G. Antimony (Sb) and lead (Pb) in contaminated shooting range soils: Sb and Pb mobility and immobilization by iron based sorbents, a field study. J. Hazard Mater. 2016;307:336–343. doi: 10.1016/j.jhazmat.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Pan X., Zhang D., Chen X., Bao A., Li L. Antimony accumulation, growth performance, antioxidant defense system and photosynthesis of Zea mays in response to antimony pollution in soil. Water, Air, Soil Pollut. 2011;21:517–523. [Google Scholar]
- Papazoglou E.G., Fernando A.L. Preliminary studies on the growth, tolerance and phytoremediation ability of sugarbeet (Beta vulgaris L.) grown on heavy metal contaminated soil. Ind. Crop. Prod. 2017;107:463–471. [Google Scholar]
- Pérez-Sirvent C., Martínez-Sánchez M.J., Martínez-López S., Bech J., Bolan N. Distribution and bioaccumulation of arsenic and antimony in Dittrichia viscosa growing in mining-affected semiarid soils in southeast Spain. J. Geochem. Explor. 2012;123:128–135. [Google Scholar]
- Qi C., Wu F., Deng Q., Liu G., Mo C., Liu B., Zhu J. Distribution and accumulation of antimony in plants in the super-large Sb deposit areas, China. Microchem. J. 2011;97:44–51. [Google Scholar]
- Qiu Y., Zheng Z., Zhou Z., Sheng G.D. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour. Technol. 2009;100(21):5348–5351. doi: 10.1016/j.biortech.2009.05.054. [DOI] [PubMed] [Google Scholar]
- Rees F., Sterckeman T., Morel J.L. Root development of non-accumulating and hyperaccumulating plants in metal-contaminated soils amended with biochar. Chemosphere. 2016;142:48–55. doi: 10.1016/j.chemosphere.2015.03.068. [DOI] [PubMed] [Google Scholar]
- Silvani L., Cornelissen G., Smebye A.B., Zhang Y., Okkenhaug G., Zimmerman A.R., Thune G., Sævarsson H., Hale S.E. Can biochar and designer biochar be used to remediate per-and polyfluorinated alkyl substances (PFAS) and lead and antimony contaminated soils? Sci. Total Environ. 2019;694:133693. doi: 10.1016/j.scitotenv.2019.133693. [DOI] [PubMed] [Google Scholar]
- Simiele M., Lebrun M., Miard F., Trupiano D., Poupart P., Forestier O., Scippa G.S., Bourgerie S., Morabito D. Assisted phytoremediation of a former mine soil using biochar and iron sulphate: effects on as soil immobilization and accumulation in three Salicaceae species. Sci. Total Environ. 2020;710:136203. doi: 10.1016/j.scitotenv.2019.136203. [DOI] [PubMed] [Google Scholar]
- Shen Q., Hedley M., Camps Arbestain M., Kirschbaum M.U.F. Can biochar increase the bioavailability of phosphorus? J. Soil Sci. Plant Nutr. 2016;16(2):268–286. [Google Scholar]
- Singh J., Lee B.K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): a possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016;170:88–96. doi: 10.1016/j.jenvman.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Singh J., Lee B.K. Effects of Nano-TiO2 particles on bioaccumulation of 133Cs from the contaminated soil by Soybean (Glycine max) Process Saf. Environ. Protect. 2018;116:301–311. [Google Scholar]
- Song B., Xu P., Chen M., Tang W., Zeng G., Gong J., Zhang P., Ye S. Using nanomaterials to facilitate the phytoremediation of contaminated soil. Crit. Rev. Environ. Sci. Technol. 2019;49:791–824. [Google Scholar]
- Tschan M., Robinson B.H., Schulin R. Antimony in the soil–plant system–a review. Environ. Chem. 2009;6:106–115. [Google Scholar]
- Vatehová Z., Kollárová K., Zelko I., Richterová-Kučerová D., Bujdoš M., Lišková D. Interaction of silicon and cadmium in Brassica juncea and Brassica napus. Biologia. 2012;67:498–504. [Google Scholar]
- Walkley A., Black I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38. [Google Scholar]
- Wang C., Alidoust D., Yang X., Isoda A. Effects of bamboo biochar on soybean root nodulation in multi-elements contaminated soils. Ecotoxicol. Environ. Saf. 2018;150:62–69. doi: 10.1016/j.ecoenv.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Wang S., Shi X., Sun H., Chen Y., Pan H., Yang X., Rafiq T. Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PloS One. 2014;9 doi: 10.1371/journal.pone.0108568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994;144:307–313. [Google Scholar]
- Yoon H., Kang Y.-G., Chang Y.-S., Kim J.-H. Effects of zerovalent iron nanoparticles on photosynthesis and biochemical adaptation of soil-grown Arabidopsis thaliana. Nanomaterials. 2019;9:1543. doi: 10.3390/nano9111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis U., Qayyum M.F., Shah M.H.R., Danish S., Shahzad A.N., Malik S.A., Mahmood S. Growth, survival, and heavy metal (Cd and Ni) uptake of spinach (Spinacia oleracea) and fenugreek (Trigonella corniculata) in a biochar-amended sewage-irrigated contaminated soil. J. Plant Nutr. Soil Sci. 2015;178:209–217. [Google Scholar]
- Zhao L., Sun Y., Hernandez-Viezcas J.A., Hong J., Majumdar S., Niu G., Duarte-Gardea M., Peralta-Videa J.R., Gardea-Torresdey J.L. Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ μ-XRF mapping of nutrients in kernels. Environ. Sci. Technol. 2015;49:2921–2928. doi: 10.1021/es5060226. [DOI] [PubMed] [Google Scholar]
- Zhuravlev I. Titanium silicates precipitated on the rice husk biochar as adsorbents for the extraction of cesium and strontium radioisotope ions. Colloid. Interface. 2019;3:36. [Google Scholar]