Abstract
Objectives
Evidence of endovascular treatment (EVT) for acute large vessel occlusion (LVO) ischemic stroke in patients harboring substantial prestroke disability is lacking due to their exclusion from randomized trials. Here, we used routine care observational data to compare outcomes in patients with and without prestroke disability receiving EVT for LVO ischemic stroke.
Methods
Consecutive patients undergoing EVT for acute LVO ischemic stroke at the Sahlgrenska University Hospital from January 1st, 2015 to March 31st, 2018 were registered in the Sahlgrenska Stroke Recanalization Registry. Pre- and poststroke functional levels were assessed by the modified Rankin Scale (mRS). Outcomes were recanalization rate (mTICI = 2b/3), symptomatic intracranial hemorrhage [sICH], complications during hospital stay, and return to prestroke functional level and mortality at 3 months.
Results
Among 591 patients, 90 had prestroke disability (mRS ≥ 3). The latter group were older, more often female, had more comorbidities and higher NIHSS scores before intervention compared to patients without prestroke disability. Recanalization rates (80.0% vs 85.0%, p = 0.211), sICH (2.2% vs 6.3% p = 0.086) and the proportion of patients returning to prestroke functional level (22.7% vs 14.8% p = 0.062) did not significantly differ between those with and without prestroke disability. Patients with prestroke disability had higher complication rates during hospital stay (55.2% vs 40.1% p < 0.01) and mortality at 3 months (48.9% vs 24.3% p < 0.001).
Conclusion
One of five with prestroke disability treated with thrombectomy for a LVO ischemic stroke returned to their prestroke functional level. However, compared to patients without prestroke disability, mortality at 3 months was higher.
Keywords: Ischemic stroke, Large vessel occlusion, Thrombectomy, Acute care, Disability, Outcome, Mortality
Introduction
Endovascular treatment (EVT) has become the gold standard for treating large vessel occlusion (LVO) ischemic stroke in the anterior circulation following the publication of several randomized trials showing it to be safe and effective [1–8]. However, the participants in these trials were highly selected and almost exclusively functionally independent prior to stroke. Thus, despite the clear evidence of the benefit of EVT in anterior circulation LVO, in clinical daily practice, stroke physicians are often faced with acute stroke patients for whom evidence-based data do not explicitly contribute to making treatment decisions.
Prestroke disability with functional dependency is relatively common among patients presenting with acute ischemic stroke, with a reported frequency between 13 and 19.5% [9–11]. Currently, there is no consensus on the use of EVT in this patient group. The American Heart Association guidelines [12] do not include LVO ischemic patients harboring prestroke dependency among those with indication for EVT, although the guideline states that the treatment may be reasonable, while the European Stroke Organisation does not mention prestroke disability in their guidelines on thrombectomy in acute ischemic stroke [13]. So far, data from observational thrombectomy registers are scarce. Recently, Goldhoorn et al. reported prestroke dependency in 11% of patients who underwent EVT for an anterior circulation LVO in the observational MR CLEAN registry [14]. Despite lower absolute frequency of favorable outcomes at 90 days in prestroke-dependent patients compared to prestroke-independent patients, about one in four reached their prestroke functional level after EVT, indicating benefit of the treatment.
In the present study, we used data from a prospective high-volume single-center registry to investigate outcomes of EVT in LVO ischemic stroke patients harboring prestroke disability. According to local practice, based on the assumption that the benefit of EVT is reasonable also in prestroke-dependent patients, the treating physicians did not routinely exclude this patient group from EVT. Thus, a relatively large proportion of patients harboring prestroke dependency underwent EVT. Here, we hypothesized that LVO ischemic stroke patients harboring prestroke dependency treated in clinical routine benefit from EVT and return to their prestroke functional level to a similar extent as prestroke-independent patients.
Methods
This registry-based study included all patients receiving an arterial puncture with the intention of EVT for treating an acute large vessel occlusion ischemic stroke at the Sahlgrenska University Hospital from January 1st 2015 to March 31st 2018. The data were obtained from the Sahlgrenska Stroke Recanalization Registry which combines data from the Swedish Stroke register, The EndoVascular treatment in Acute Stroke registry (EVAS-registry), the Regional population registry and the patients’ medical records. Data on causes of death were obtained from the National Board of Health and Welfare.
The modified Rankin Scale (mRS) was used to define pre- and poststroke functional level. The prestroke mRS values were obtained from the patients’ medical records and the poststroke mRS values were calculated from the answers of the Swedish Stroke Registers’ questionnaire at 3 months, a method previously used and validated [15], and from data obtained from patients’ medical records. Prestroke disability was defined as mRS score ≥ 3. A favorable outcome was defined as returning to the prestroke functional level (mRS score) 3 months after the intervention.
Recanalization was estimated using the modified Thrombolysis in Cerebral Infarction score (mTICI). Achieved recanalization was defined as an mTICI score of 2b, 2c or 3. All patients were evaluated with National Institutes of Health Stroke Scale (NIHSS) immediately upon arrival. We defined early neurological improvement as a decrease of at least 4 points or a score of 0 on NIHSS 24 h after the intervention. Early neurological deterioration was defined as an increase of 4 points or more on NIHSS 24 h after the intervention. Complications accounted for in this study were symptomatic intracranial hemorrhage (sICH) and complications during hospital stay including pulmonary embolism, pneumonia, pulmonary edema, significant arrhythmia, epilepsy, urinary infections, kidney failure, intracranial hypertension, delirium, problems in the groin after puncture requiring treatment and malignant cerebral media artery infarction. SICH was defined according to the European Cooperative Acute Stroke Study III (ECASS III) criteria as having an intracranial hemorrhage (ICH) on radiologic follow-up combined with a clinical deterioration equal of 4 points or more on NIHSS 24 h after intervention, or death within 7 days [16].
Prespecified primary outcomes were return to at least the prestroke functional level and mortality 3 months after intervention. Secondary outcomes were recanalization, complications and early neurological improvement or deterioration.
Statistical analyses
Differences in outcomes and baseline characteristics between patients with (mRS 3–5) and without (mRS 0–2) prestroke disability were investigated using the Mann–Whitney U test for continuous variables and Chi-squared or Fisher’s test for categorical variables. A binary logistic regression analysis was performed to investigate the associations between prestroke disability and return to at least the prestroke functional level as well as death at 3 months after intervention adjusted for the possible confounders’ age, sex, atrial fibrillation, stroke before index stroke, NIHSS score before intervention, recanalization, and complications during hospital stay. In this study, a two-tailed value of p < 0.05 was considered statistically significant. IBM SPSS version 25 was used for all statistical analyses.
Results
From January 1st 2015 to March 31st 2018, 593 patients with anterior or posterior LVO ischemic stroke underwent EVT at the Sahlgrenska University Hospital. Two patients were younger than 18 years and were excluded. Of the remaining 591 patients, 90 had a prestroke disability (15.2%). Figure 1 illustrates a flowchart of the included patients and proportions of patients with missing data at 3 months follow-up.
Fig. 1.
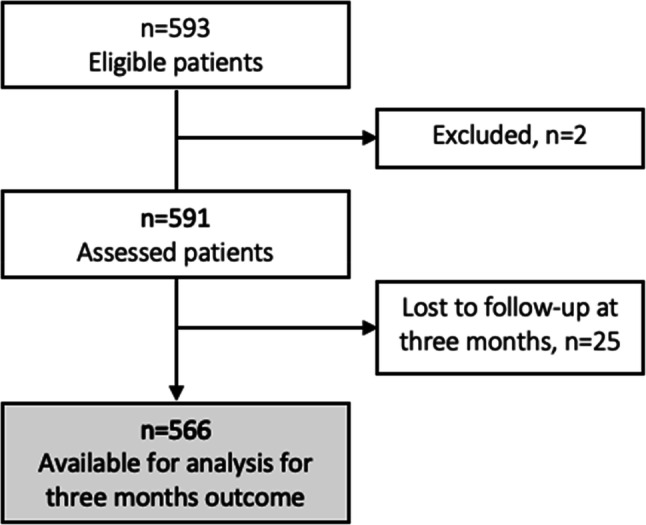
Flow chart of the study population. Two patients were underaged and were therefore excluded
Baseline characteristics for patients with and without prestroke disability are shown in Table 1. Patients with prestroke disability were older, more often women, and had more comorbidities such as atrial fibrillation and history of stroke compared to prestroke-independent patients. They also displayed higher NIHSS-scores before intervention. There were no significant differences in type of onset, the proportion of patients receiving IVT before intervention, nor vessel occlusion localization between the groups.
Table 1.
Baseline characteristics
| Prestroke mRS ≥ 3 (n = 90) | Prestroke mRS 0–2 (n = 501) | P value | |
|---|---|---|---|
| Age, median (range) | 86 (57) | 74 (82) | < 0.001 |
| Women [n/N (%)] | 54/90 (60.0) | 218/501 (43.5) | < 0.01 |
| Medical history | |||
| Hypertension [n/N (%)] | 65/88 (73.9) | 322/496 (64.9) | 0.102 |
| Diabetes [n/N (%)] | 20/90 (22.2) | 84/499 (16.8) | 0.217 |
| Atrial fibrillation [n/N (%)] | 56/90 (62.2) | 204/499 (40.9) | < 0.001 |
| Dyslipidemia [n/N (%)] | 30/88 (34.1) | 197/487 (40.5) | 0.261 |
| Stroke before index stroke [n/N (%)] | 25/90 (27.8) | 66/501 (13.2) | < 0.001 |
| Onset characteristics | |||
| Onset time | 0.699 | ||
| Confirmed [n/N (%)] | 54/90 (60.0) | 306/501 (61.1) | |
| Estimated [n/N (%)] | 18/90 (20.0) | 116/501 (23.2) | |
| Wake-up [n/N (%)] | 17/90 (18.9) | 72 /501(14.4) | |
| Unknown [n/N (%)] | 1/90 (1.1) | 7/501 (1.4) | |
| NIHSS before intervention—median (IQR) | 18 (9) | 16 (8) | < 0.01 |
| IVT before intervention [n (%)] | 38/90 (42.2) | 252/500 (50.4) | 0.153 |
| Occluded artery | |||
| Internal carotid artery [n/N (%)] | 30/90 (33.3) | 152/501 (30.3) | 0.563 |
| Middle cerebral artery [n/N (%)] | 43/90 (47.8) | 275/501 (54.8) | 0.220 |
| Anterior cerebral artery [n/N (%)] | 1/90 (1.1) | 17/501 (3.4) | 0.247 |
| Vertebral arteries [n/N (%)] | 1/90 (1.1) | 12/501 (2.4) | 0.446 |
| Basilar artery [n/N (%)] | 8/90 (8.9) | 50/501 (10.0) | 0.753 |
| Posterior cerebral artery [n/N (%)] | 2/90 (2.2) | 18/501 (3.6) | 0.510 |
| Spontaneously recanalized [n/N (%)] | 9/90 (10.0) | 51/501 (10.2) | 0.959 |
P values below the threshold for statistical significance (p < 0.05) are given in bold
mRS modified Rankin Scale, IQR interquartile range, sICH symptomatic intracranial hemorrhage, IVT intravenous thrombolysis, NIHSS National Institutes of Health Stroke Scale
Differences between groups were investigated with the Mann–Whitney U test for continuous and the Chi-squared tests for categorical variables. Fisher’s test was used when any category comprised five patients or less. NIHSS before treatment was missing for 1 patient with prestroke mRS 3–5 and for 5 patients with prestroke mRS 0–2
Early outcome
Prestroke-dependent and independent patients did not differ with respect to recanalization rate or time from groin puncture to recanalization (Table 2). NIHSS score at 24 h after the intervention was significantly higher among prestroke-dependent patients, and the proportion of patients with postintervention improvement of ≥ 4 points on the NIHSS was somewhat higher among prestroke-independent than prestroke-dependent patients (62.0 versus 54.4%, respectively), although this difference was not statistically significant (p = 0.179, Table 2). There were also no significant differences between the groups for early neurological deterioration, sICH, or in-hospital death, but prestroke-dependent patients were more often affected by complications during hospital stay compared to prestroke-independent patients (55.2% vs. 40.1%, p < 0.01).
Table 2.
Outcomes
| Prestroke mRS ≥ 3 (n = 90) | Prestroke mRS 0–2 (n = 501) | p value | |
|---|---|---|---|
| Early outcomes | |||
| Recanalization rate [n (%)] | 72/90 (80.0) | 426/501 (85.0) | 0.211 |
| Groin puncture to recanalization, hh:mm, median (IQR) | 00:45 (00:48) | 00:52 (01:04) | 0.174 |
| Improvement on NIHSS ≥ 4 points or back to 0 [n (%)] | 49/86 (57.0) | 310/486 (63.7) | 0.184 |
| Deterioration on NIHSS ≥ 4 points [n (%)] | 10/86 (11.6) | 54/486 (11.1) | 0.918 |
| NIHSS 24 h after intervention, median (IQR) | 13 (12) | 8 (13) | < 0.01 |
| In-hospital deaths [n (%)] | 10/90 (11.2) | 51/501 (10.2) | 0.758 |
| sICH [n (%)] | 2/90 (2.2) | 32/500 (6.4) | 0.086 |
| Complications during hospital stay [n (%)] | 48/90 (55.2) | 192/501 (40.1) | < 0.01 |
| Outcomes 3 months after intervention | |||
| Return to at least prestroke mRS [n (%)] | 20/88 (22.7) | 71/478(14.8) | 0.062 |
| Death [n (%)] | 43/88 (48.9) | 116/478 (24.3) | < 0.001 |
P values below the threshold for statistical significance (p < 0.05) are given in bold
NIHSS National Institutes of Health Stroke Scale, sICH symptomatic Intracranial Hemorrhage
Recanalization defined as mTICI score of 2b-3. Differences between groups were investigated with the Mann–Whitney U- test for continuous and the Chi-squared tests for categorical variables. Fisher’s test was used when any category comprised five patients or less. Time from groin puncture to recanalization was missing in 2 and 36 among patients with and without prestroke disability, respectively
Functional outcome at 3 months
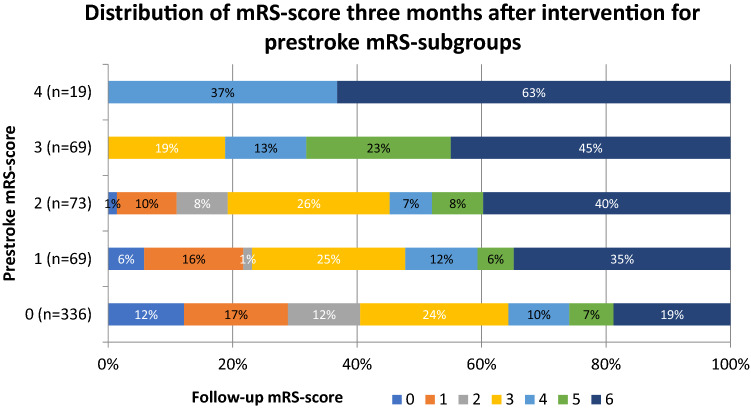
Figure 2 shows the distribution of mRS scores 3 months after intervention for each prestroke mRS score. The proportion of patients returning to at least prestroke functional level did not differ significantly between prestroke-dependent and independent patients (22.7% vs. 14.8%, p = 0.062, Table 2). Reperfusion status was strongly associated with the chance for returning to prestroke functional level in both groups. None of the 17 patients with unsuccessful recanalization returned to prestroke functional level compared to 20 of 71 (28%) with successful recanalization among the prestroke-dependent (p < 0.01), and only 2/73 (2.7%) compared with 68/405 (17%) among prestroke-independent patients (p < 0.001). The strong association between successful recanalization and favorable outcome at 3 months remained in the multivariable regression model (Table 3). In this model, prestroke disability showed independent positive association, and NIHSS score before the intervention independent negative association with favorable outcome.
Fig. 2.
Distribution of mRS score 3 months after intervention for prestroke mRS subgroups. mRS modified Rankin Scale
Table 3.
Multivariable odds ratios and 95% confidence intervals of return to at least prestroke functional level at 3 months after intervention
| Odds ratio | CI lower | CI higher | p value | |
|---|---|---|---|---|
| Agea | 0.98 | 0.96 | 1.00 | 0.052 |
| Female sex | 1.12 | 0.69 | 1.83 | 0.643 |
| Prestroke disability | 2.64 | 1.34 | 5.19 | < 0.01 |
| Atrial fibrillation | 1.18 | 0.70 | 1.98 | 0.540 |
| Stroke before current stroke | 1.17 | 0.60 | 2.30 | 0.649 |
| NIHSS before interventionb | 0.93 | 0.90 | 0.97 | 0.001 |
| Successful recanalization | 9.90 | 2.36 | 41.6 | < 0.01 |
| Complications during hospital stay | 1.60 | 0.96 | 2.66 | 0.074 |
P values below the threshold for statistical significance (p < 0.05) are given in bold
NIHSS National Institutes of Health Stroke Scale, CI confidence interval
aPer 1 year increase
bPer one point increase in NIHSS score
Mortality at 3 months
Three months after the intervention, patients with prestroke disability displayed significantly higher mortality compared to prestroke-independent patients (48.9% vs. 24.3%, p < 0.001, Table 2). Among patients with prestroke disability, mortality at 3 months did not differ between those with and without successful recanalization (46.2% vs 56.3%, p = 0.16).
The association between prestroke disability and mortality 3 months after intervention was further analyzed using multivariable logistic regression adjusting for the potential confounders’ age, sex, atrial fibrillation, history of stroke, NIHSS score before intervention, recanalization and complications during hospital admission. In this model, increasing age, prestroke disability, higher score of NIHSS before intervention, history of stroke, and unsuccessful recanalization were independently associated with a higher risk of death (Table 4), whereas no association between death and complications during hospital stay, or sex were observed.
Table 4.
Multivariable odds ratios and 95% confidence intervals of death at 3 months after intervention
| Odds ratio | CI lower | CI higher | p value | |
|---|---|---|---|---|
| Agea | 1.03 | 1.01 | 1.05 | < 0.01 |
| Female sex | 1.21 | 0.79 | 1.86 | 0.380 |
| Prestroke disability | 1.93 | 1.12 | 3.33 | < 0.05 |
| Atrial fibrillation | 1.51 | 0.96 | 2.32 | 0.074 |
| Stroke before current stroke | 1.84 | 1.08 | 3.16 | < 0.05 |
| NIHSS before interventionb | 1.10 | 1.06 | 1.14 | < 0.001 |
| No recanalization | 3.29 | 1.94 | 5.56 | < 0.001 |
| Complications during hospital stay | 1.48 | 0.97 | 2.24 | 0.069 |
P values below the threshold for statistical significance (p < 0.05) are given in bold
NIHSS National Institutes of Health Stroke Scale, CI confidence interval
aPer one year increase
bPer one point increase in NIHSS score
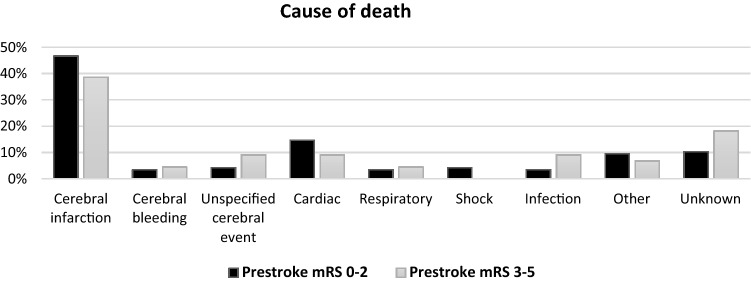
Figure 3 shows causes of death for patients with and without prestroke disability. Cerebrovascular disease was the most frequent cause of death followed by cardiac disease and infections, with no significant difference between prestroke-dependent and independent patients.
Fig. 3.
Cause of death for those who died within 3 months after intervention divided by prestroke mRS score. Total number of deaths 159 (n = 116 for prestroke mRS 0–2, n = 43 for prestroke mRS 3–5). mRS modified Rankin Scale
Patients with non-stroke-related prestroke disability
Of 90 patients with prestroke disability, 25 had a prior clinical stroke and 65 had non-stroke related disability. The two groups did not differ with respect to NIHSS score before intervention (median score 18 and 19 in those with and without prior stroke, respectively, p = 0.725). Neither did they differ significantly with respect to recanalization rates (84% vs 78%), early neurological improvement (56% vs 54%), sICH (4% vs 1.5%), complications during hospital stay (44% vs 60%), return to at least prestroke functional level (28% vs 20%), nor mortality at 3 months (44% vs 49%), p > 0.183 all through.
Discussion
Prestroke-dependent patients undergoing EVT for LVO ischemic stroke in clinical routine were older, had more comorbidities (atrial fibrillation and stroke before index stroke) and had more severe strokes (higher NIHSS before intervention) compared to prestroke-independent patients. Even so, recanalization rates, sICH, and the proportion of patients returning to their prestroke functional level were not significantly worse when compared to prestroke-independent patients. The findings that more than half of the patients with prestroke disability showed early neurological improvement and that one of five improved back to prestroke functional level 3 months after the intervention indicate a benefit of the treatment also in this patient group. However, complications during hospital stay were more common in the prestroke-dependent group, and mortality 3 months after the intervention was higher.
Outcomes of EVT in LVO ischemic stroke patients harboring prestroke disability were previously reported from two observational registries [14, 17]. The proportion of patients with prestroke disability in these registries were comparable to ours, with 157 of 1441 (11%) in the MR CLEAN registry [14] and 23 of 131 (17%) in a single-center study from Israel [17]. The present study adds another 90 patients to the previously systematically reported 180 patients with prestroke disability undergoing EVT for LVO ischemic stroke.
Similar to findings in our study, the previous reports from the two observational registries [14, 17] indicated beneficial effects of EVT in prestroke-dependent patients. In both studies, a substantial proportion (27% and 35%) of prestroke-dependent patients maintained their previous functional level at follow-up after 3 months. Moreover, early neurological improvement did not differ between prestroke-dependent and independent patients, and successful recanalization predicted a favorable outcome in both groups. Thus, results from our study are in concordance with the two previous real-life reports and further strengthens the evidence suggesting benefit of EVT in LVO ischemic stroke patients harboring prestroke disability. Taken together, the combined result from the three existing observational studies suggests that clinicians can be recommended to not routinely exclude patients harboring prestroke disability from EVT.
However, despite a substantial proportion of patients maintaining their prestroke functional level after EVT, our study and the two previous real-life reports show high mortality, up to 50%, 3 months after the intervention among patients harboring prestroke disability. We found that mortality at 3 months was independently associated with higher age, unsuccessful recanalization, comorbidities, stroke severity, and prestroke disability, whereas there was no increase in in-hospital death nor sICH among prestroke-dependent patients. Thus, the higher mortality in prestroke-dependent patients seems partly associated with the increased fragility related to older age and comorbidity. An important key question is how the positive effects of EVT with prevention of accumulated disability in prestroke-dependent patients stand against a relatively high short-term mortality. To better understand the balance between the beneficial effects and mortality after EVT in this particular patient group, both with respect to the individual and the society at large, further studies, including cost-effectivity analyses as well as more detailed measurements of disability and causes of death are required.
This study has some obvious limitations. First, it is not a randomized study, and all comparisons between prestroke-dependent and independent patients must be interpreted with caution as the two groups differ with respect to a number of essential characteristics. Most importantly, the difference in baseline mRS between the groups results in a non-equal definition of favorable outcome (return to prestroke functional level), as a shift between different steps of the scale do not correspond to the same size of change. Moreover, different methods were used to assess the pre- and poststroke functional levels, the use of mRS for assessing prestroke disability is less standardized compared to poststroke mRS and is probably more vulnerable to interrater variability. Lastly, we did not have information on the cause of prestroke disability and could therefore not identify patients with transient conditions contributing to the restricted functional level, such as surgery, bone fractures, infections or heart conditions. The strengths of the study include a relatively large series of consecutive patients undergoing EVT for LVO ischemic stroke in clinical routine which is reflected by the higher mean age and higher proportions of patients with previous strokes and comorbidities compared to the selected patients included in randomized studies [18]. We also included patients with posterior LVO ischemic stroke. Moreover, a relatively large proportion of patients had prestroke disability, and there were few missing data and low rates of loss to follow-up.
Conclusion
About one-fifth of patients with prestroke disability and LVO treated with EVT returned to their prestroke functional level which is comparable to levels of patients without prestroke disability, suggesting that prestroke-dependent patients should not routinely be excluded from EVT. However, mortality at 3 months was higher, partly related to older age and increased fragility, which should be kept in mind when selecting patients for EVT in clinical practice. To better understand the balance between the beneficial effects and short-term mortality after EVT, further studies are required.
Acknowledgements
Open access funding provided by University of Gothenburg. The authors thank Judith Klecki for English language editing.
Funding
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-720211 and ALFGBG-726821).
Compliance with ethical standards
Conflicts of interest
None. Turgut Tatlisumak’s disclosures are reported at ORCID ID 0000-0002-2430-8988.
Ethical approval
The Regional Ethical Review Board in Gothenburg, Sweden, approved the study and it was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was completely observational, data for the study were collected from registries and medical records, and informed consent was not required according to national law.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Mocco J, Zaidat OO, von Kummer R, Yoo AJ, Gupta R, et al. Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47:2331–2338. doi: 10.1161/STROKEAHA.116.013372. [DOI] [PubMed] [Google Scholar]
- 7.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurology. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 8.Muir KW, Ford GA, Messow CM, Ford I, Murray A, et al. Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88:38–44. doi: 10.1136/jnnp-2016-314117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Swedish Stroke Register (2018) Riksstrokes årsrapport 2017 riksttroke.org: Riksstroke; 2018 [updated 2018–10. https://www.riksstroke.org/wp-content/uploads/2018/10/Riksstroke_Årsrapport-2017-WEBB.pdf. Accessed Jan 2019
- 10.Ganesh A, Luengo-Fernandez R, Pendlebury ST, Rothwell PM. Long-term consequences of worsened poststroke status in patients with premorbid disability. Stroke. 2018;49:2430–2436. doi: 10.1161/STROKEAHA.118.022416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn TJ, Taylor-Rowan M, Coyte A, Clark AB, Musgrave SD, et al. Pre-stroke modified rankin scale: evaluation of validity, prognostic accuracy, and association with treatment. Front Neurol. 2017;8:275. doi: 10.3389/fneur.2017.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 13.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic Stroke Endorsed by Stroke Alliance for Europe (SAFE) Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldhoorn RB, Verhagen M, Dippel DWJ, van der Lugt A, Lingsma HF, et al. Safety and outcome of endovascular treatment in prestroke-dependent patients. Stroke. 2018;49:2406–2414. doi: 10.1161/STROKEAHA.118.022352. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson M, Appelros P, Norrving B, Terent A, Stegmayr B. Assessment of functional outcome in a national quality register for acute stroke: can simple self-reported items be transformed into the modified Rankin Scale? Stroke. 2007;3:1384–1386. doi: 10.1161/01.STR.0000260102.97954.9c. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 17.Leker RR, Gavriliuc P, Yaghmour NE, Gomori JM, Cohen JE. Increased risk for unfavorable outcome in patients with pre-existing disability undergoing endovascular therapy. J Stroke Cerebrovasc Dis. 2018;27:92–96. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]