ABSTRACT
Organic acids such as monocarboxylic acids, dicarboxylic acids or even more complex molecules such as sugar acids, have displayed great applicability in the industry as these compounds are used as platform chemicals for polymer, food, agricultural and pharmaceutical sectors. Chemical synthesis of these compounds from petroleum derivatives is currently their major source of production. However, increasing environmental concerns have prompted the production of organic acids by microorganisms. The current trend is the exploitation of industrial biowastes to sustain microbial cell growth and valorize biomass conversion into organic acids. One of the major bottlenecks for the efficient and cost-effective bioproduction is the export of organic acids through the microbial plasma membrane. Membrane transporter proteins are crucial elements for the optimization of substrate import and final product export. Several transporters have been expressed in organic acid-producing species, resulting in increased final product titers in the extracellular medium and higher productivity levels. In this review, the state of the art of plasma membrane transport of organic acids is presented, along with the implications for industrial biotechnology.
Keywords: industrial biotechnology, cell factories, carboxylic acids, transporter proteins, permease
Transporter protein expression enables the optimization of microbial cell factories for the bioproduction of organic acids.
INTRODUCTION
Organic acids are an essential group of platform chemicals produced by microbes. Most of the organic acids produced industrially are used in the food industry. Currently, the major source of production of these compounds is the chemical synthesis from petroleum derivatives. Nonetheless, several organic acids are already industrially generated via microbial cell-factories, including succinic, lactic, citric, gluconic and acetic acid (Alonso, Rendueles and Díaz 2015). Microbial production of organic acids comprises several membrane transport processes, mostly controlled by membrane proteins, namely substrate import, transport of metabolites between organelles and product export. These processes, critical for the bioproduction of organic acids, are the major topic of this review.
MICROBIAL CELL FACTORIES IN THE PRODUCTION OF BIO-BASED ORGANIC ACIDS
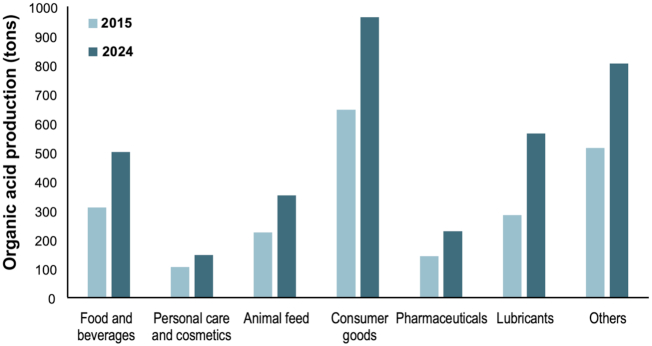
The global organic acids market was valued at 17 billion euros in 2016. The forecasts predict an annual growth of 8.3%, which should reach 30 billion euros by the year 2023 (Sahu 2017) with an impact in a broad range of industrial sectors (Fig. 1). The most significant contributions to this growth are the use of renewable resources, the rising market and the growing demand from developing countries for bio-based organic acids.
Figure 1.
Annual production of organic acids according to market sector/application in 2015 and estimated growth for 2024 (adapted from https://www.alliedmarketresearch.com/organic-acids-market).
The industry of microbial organic acid production is under continuous development to increase cell factory productivity, yields and range of products. Along with classical strain engineering approaches and adaptive laboratory evolution (ALE) strategies, the development of recombinant DNA technology together with synthetic biology has allowed the rational engineering of organic acid-producing microbes. Meanwhile, beyond the classical industrial microbes, such as Escherichia coli, Saccharomyces cerevisiae and Corynebacterium glutamicum, we have witnessed the appearance of other species isolated from natural sources, displaying a high capacity to generate organic acids (Buschke et al. 2013; Na et al. 2012; Becker and Wittmann 2015).
Membrane transporters as tools for the improvement of cell factories
Nowadays, most industrial microorganisms are metabolically engineered to produce specific products and/or to metabolize specific substrates. For decades, the transport mechanisms and energetics of these compounds were underestimated, and most attention was given to the engineering of metabolic pathways. Recently, the scientific community and biotech companies focused their efforts on transporter engineering, envisaging the development and improvement of microbial cell factories (Sauer et al. 2008; Boyarskiy and Tullman-Ercek 2015; Kell et al. 2015).
The microbial fermentation industry faces two major bottlenecks in the production line: the first relates to product accumulation and toxicity inside the cell and low product titers in the extracellular medium; the second is associated with cell factory capacity to assimilate carbon and energy sources for product biosynthesis. These two obstacles in microbial fermentation can be overcome by transport activity of endogenous or exogenous membrane transporters, importers and/or exporters, as well as their genetic manipulation regarding expression levels and generation of mutant alleles with increased transport capacity or altered specificity (Sauer et al. 2010; Boyarskiy and Tullman-Ercek 2015; Kell et al. 2015).
MEMBRANE TRANSPORTERS IN THE IMPORT OF RENEWABLE SUBSTRATES
The most used renewable feedstocks in the bio-based industrial production of carboxylic acids are cheese whey, lignocellulosic biomass, glycerol and pectin-rich wastes (Alonso, Rendueles and Díaz 2015). Lactose is the most abundant sugar in cheese whey; xylose along with arabinose are abundant sugars in lignocellulosic hydrolysates; pectin-rich wastes, such as citrus and beet pulp, are rich in galacturonic acid (Alonso, Rendueles and Díaz 2015; Deng, Wang and Yan 2016). Frequently, these sugars are hardly assimilated and metabolized by microbial cell factories. This bottleneck is associated with the lack of membrane proteins or extracellular enzymes capable of respectively taking up or converting these substrates into assimilated forms. Therefore, membrane transporters are engineered in microbial cell factories to increase the efficiency of substrate influx, by altering transporter specificity, affinity and/or capacity, ultimately leading to improved production yields (Van Dyk 2008; Kell et al. 2015; Deng, Wang and Yan 2016).
Extensive efforts were devoted to the use of genetically engineered E. coli as a sustainable platform for the production of industrially important compounds, including organic acids (for a review see Chen et al. 2013; Yang et al. 2020). One of the few examples reporting the engineering of membrane transporters for the uptake of substrates to improve organic acid production in E. coli is the study by Wu, Liu and Singh (2018). Here, the increased production of catechol (gluconic acid precursor) was achieved after the co-expression of the catechol biosynthetic pathway and the transporter CouP, which enabled the uptake of aromatic compounds present in lignin. In a more recent work, Khunnonkwao et al. (2018) described the improvement of succinic acid production upon re-engineering of xylose transporters in E. coli. The filamentous fungus Aspergillus niger is the oldest industrial workhorse due to its great robustness to extreme acid environments and better fitness for industrial fermentation (Tong et al. 2019). Genome design and metabolic engineering approaches to optimize the A. niger cell factory for industrial citric acid production can be found in Tong et al. (2019). However, few transporter engineering strategies for substrate influx were described in this species. The endogenous low-affinity glucose transporter Lgt1 was expressed in the citrate-producing A. niger H915–1 strain, under the control of the low-pH-inducible promoter Pgas, leading to enhanced glucose absorption during the acid producing period and enhanced citrate production (Liu et al. 2018).
Yeasts are considered one of the most promising groups of industrial microorganisms to produce organic acids and ethanol. Thus hereafter, emphasis will be given on the functional expression of xylose, arabinose, lactose, glycerol and galacturonic acid transporters in attempts to improve organic acid biorefinery applications in yeasts.
Xylose
The heterologous expression of xylose transporters in S. cerevisiae for the conversion of this lignocellulosic sugar into ethanol was extensively reported in the literature. More than 80 heterologous xylose transporters or putative xylose transporters have already been expressed in S. cerevisiae (for a review see Moysés et al. 2016). Significant examples include SUT1, SUT2, XUT1, XUT3 (Xyp33), XUT4, Xyp29 (STL12), SUT3 (Xyp37) from Scheffersomyces stipitis, GXF1 from Candida intermedia, At5g59250 from Arabidopsis thaliana, An29–2 and An25 from Neurospora crassa, xtrD from A. nidulans, MgT05196 from Meyerozyma guilliermondii and Xylh from Debaryomyces hansenii. More than 80% of these putative transporters or annotated sugar transporters were not functional in S. cerevisiae, probably due to misfolding or improper localization (Moysés et al. 2016). The ones that were properly expressed in the HXT-null S. cerevisiae strain displayed activity for xylose transport, but the majority showed a preference for glucose over xylose.
Arabinose
Along with xylose, arabinose is the second most abundant pentose sugar present in lignocellulosic hydrolysates. S. cerevisiae possesses endogenous arabinose transporters (Hxt9, Hxt10 and Gal2), Gal2 being the most prominent (Wang et al. 2013). However, the consumption of this sugar pentose is inefficient and inhibited by glucose, since Gal2 exhibits a much lower affinity for arabinose than for glucose or galactose (Becker and Boles 2003; Subtil and Boles 2011a). To improve the kinetics of arabinose uptake, transporter genes from other organisms have been functionally expressed in engineered arabinose-metabolizing S. cerevisiae strains. Two characterized L-arabinose transporters, LAT-1 from N. crassa and MtLAT-1 from Myceliophthora thermophila were expressed in this yeast (Li et al. 2015). The expression of both transporters in a S. cerevisiae strain containing a L-arabinose metabolic pathway resulted in a much faster L-arabinose utilization, greater biomass accumulation and higher ethanol production than the control strain. Expression of the PcAraT arabinose transporter from Penicillium chrysogenum enabled growth on arabinose in the presence of glucose in a S. cerevisiae strain deficient in hexose phosphorylation and able to metabolize arabinose (Bracher et al. 2018). This transporter showed significantly higher affinity for arabinose compared to the endogenous Gal2 and had far less pronounced inhibition of arabinose uptake in the presence of glucose or xylose.
Lactose
A recombinant S. cerevisiae flocculent strain heterologously expressing the β-galactosidase LAC4 and lactose permease LAC12 genes from Kluyveromyces lactis was used for ethanol production from lactose in a continuous culture operation (Domingues et al. 1999). This approach resulted in an ethanol production yield of 0.51 g/g of lactose. In continuous fermentation conditions, this engineered S. cerevisiae strain reached an ethanol productivity of 11 g/L/h, which represented a 7-fold rise compared with values of ethanol productivity from lactose previously mentioned in the literature (Domingues et al. 1999). The expression of the lactose transporter CDT-1, the intracellular β-galactosidase GH1–1 from N. crassa and the lactate dehydrogenase ldhA from Rhizopus oryzae in S. cerevisiae allowed the production of lactic acid from lactose, cow's milk, or whey (Turner et al. 2017). A lactic acid yield of 0.358 g/g lactose was achieved from a Yeast extract-Peptone medium containing about 80 g/L whey.
Glycerol
Heterologous expression of glycerol facilitators (Fps1 homologs) from non-Saccharomyces yeast species that show superior growth on glycerol, e.g. Cyberlindnera jadinii, Komagataella pastoris, Pachysolen tannophilus and Yarrowia lipolytica, improved the maximum specific growth rates of the S. cerevisiae CBS 6412–13A strain by 30–40% in synthetic glycerol medium (Klein et al. 2016). Conversely, no improvement was visible after the overexpression of the endogenous S. cerevisiae FPS1 gene. Deletion of the endogenous glycerol/H+ symporter STL1 did not impair the superior growth of these strains. A significant increase in ethanol production (from none to 8.5 g/L) was obtained upon the expression of the heterologous aquaglyceroporin CjFPS1 from C. jadinii in the strain CBS DHA, which catabolizes glycerol via the dihydroxyacetone (DHA) pathway(Asskamp, Klein and Nevoigt 2019). Further optimizations, including the reduction of oxygen availability in the shake flask cultures, increased the ethanol titer up to 15.7 g/L.
Galacturonic acid
The introduction of the galacturonic acid transporter GAT1 from N. crassa, along with a fungal reductive pathway for galacturonic acid catabolism (gaaA, gaaC and gaaD from A. niger and lgd1 from Trichoderma reesei), allowed the engineered S. cerevisiae strain to metabolize galacturonic acid (Biz et al. 2016). This strain was only able to catabolize galacturonic acid when a co-substrate was added (fructose). Tracing experiments with 13C-galacturonic acid revealed its conversion into glycerol (Biz et al. 2016). Recently, the expression of another galacturonic acid transporter, GatA from A. niger, allowed a more rapid consumption of this acid (Protzko et al. 2018). The involvement of endogenous yeast hexose transporters Hxt1–7 and Gal2 in the glucose-inhibited uptake of undissociated galacturonic acid in acidic conditions was also uncovered. Expression of glucose-insensitive GatA coupled with uronate dehydrogenase allowed the engineered S. cerevisiae strain to produce 8 g/L of meso-galactaric acid from citrus peel waste supplemented with additional glucose (Protzko et al. 2018).
TRANSPORTER EXPRESSION FOR THE OPTIMIZATION OF ORGANIC ACID EFFLUX
Regarding the microbial production of organic acids, several reports pointed to the effectiveness and contribution of membrane transporters for product efflux. The organic acid transporters that thus far have been functionally characterized in yeast, fungi and bacteria are listed in Table 1. Among these transporters, a great majority belong to the 2-hydroxycarboxylate transporter (2-HCT) (TC 2.A.24), Divalent Anion:Na+ Symporter (DASS) (TC 2.A.47) and Sialate:H+ symporter (SHS) (TC 2.A.1.12) families. Members of 2-HCT are involved in the transport of di- and tricarboxylate substrates (malate/citrate uptake) with either Na+ or H+ as the co-substrate and precursor/product exchangers (Sobczak and Lolkema 2005). Some members mediate the transport of monocarboxylate substrates, 2-hydroxyisobutyrate and D-lactate (Bandell et al. 1998; Pudlik and Lolkema 2012). The integral membrane proteins of the DASS family are conserved from bacteria to humans. DASS proteins typically mediate the coupled uptake of Na+ ions and dicarboxylate, tricarboxylate, or sulfate (for a review see Lu 2019). A total of six members of DASS present a broad range of substrates from mono-, di- to tricarboxylates. The SHS transporter family, despite only having two distinct family members, the sialic acid transporter NanT, and the lactate/pyruvate:H+ symporter orthologues, is the one with most members characterized in yeast, accepting mainly mono- and dicarboxylates as well as sugar acids (Casal et al. 2008; Ribas et al. 2017). Next, we will highlight the transporters that had an impact on the improvement of cell factories.
Table 1.
Microbial organic acid transporter proteins (experimentally verified). Table includes the transporter family, the species, the Transport Classification Database (TC number), the number of transmembrane segments (TMS), description of the transporter activity and references.
| Family | Transporter protein | Species | TC number | TMS* | Description | References |
|---|---|---|---|---|---|---|
| 2-HCT | CimH (YxkJ) | Bacillus subtilis | 2.A.24.2.4 | 10 | Electroneutral L-Malate/Citrate:H+ symporter; citrate (Km 10 μM), L-Malate (Km 1.5 mM) | Krom, Aardema and Lolkema (2001) |
| MaeN (YufR) | 2.A.24.2.3 | 11 | Malate:Na+ symporter | Tanaka, Kobayashi and Ogasawara (2003) | ||
| CitS | Klebsiella pneumoniae | 2.A.24.1.1 | 12 | Sodium:Citrate symporter | Kebbel et al. (2013) | |
| MleP | Lactococcus lactis | 2.A.24 | 13 | Sodium:Citrate symporter; malate (Km 0.46 mM), lactate (Km 4.6 mM) | Bandell et al. (1997); Poolman et al. (1991); Pudlik and Lolkema (2011) | |
| CitP (CitN) | 2.A.24.3.1 | 12 | Electrogenic citrate:L-Lactate exchanger; citrate (Km 56 µM), malate (Km 0.1 mM), lactate (Km 26 mM) | Pudlik and Lolkema (2012) | ||
| CitP | Leuconostoc mesenteroides | 2.A.24.3.2 | 13 | Citrate:Lactate antiporter; citrate, citramalate, malate, 2-Hydroxyisobutyrate and lactate | Bandell et al. (1998); Marty-Teysset et al. (1996) | |
| AAEx | SucE | Corynebacterium glutamicum | 2.A.81.1.3 | 9 | Succinate exporter | Fukui et al. (2011) |
| AceTr | SatP | Escherichia coli | 2.A.96.1.1 | 6 in hexameric channels | Acetate, lactate and succinate transporter; acetate (Km 1.24 mM) and succinate (Km 1.18 mM) | Sá-Pessoa et al. (2013) |
| AceP | Methanosarcina acetivorans | 2.A.96.1 | 6 | Acetate transporter; acetate (Km 0.49 mM) | Ribas et al. (2019) | |
| Ady2 (Ato1) | Saccharomyces cerevisiae | 2.A.96.1.4 | 6 | Acetate permease; acetate (Km 0.84 mM), lactate, propionate and formate | Pacheco et al. (2012); Paiva et al. (2004); Ribas et al. (2019) | |
| Gpr1 | Yarrowia lipolytica | 2.A.96.1.2 | 6 | Acetate transporter; acetate (Km 0.95 mM) | Augstein et al.(2003); Paiva et al. (2004); Ribas et al. (2019) | |
| Bestrophin | Best1 (AN2251) | Aspergillus nidulans | 1.A.46.2.1 | 4 | Ca2+-activated anion-selective channel; citrate, propionate, benzoate and sorbate | Galagan et al.(2005); Roberts, Milnes and Caddick (2011) |
| CitMHS | CitM | Bacillus subtilis | 2.A.11.1.1 | 9 | Citrate or D-Isocitrate divalent metal:H+ symporter; (Km 35–63 µM), metal (in order of preference): Mg2+, Mn2+, Ni2+, Zn2+ and Co2+ | Krom et al. (2000); Li and Pajor (2002) |
| CitH (CitN) | 2.A.11.1.2 | 11 | Citrate divalent metal:H+ symporter: (Km 35–63 µM) metal (in order of preference): Ca2+, Ba2+ and Sr2+ | Krom et al. (2000) | ||
| YRAO | 2.A.11.1.5 | 13 | Citrate:H+ symporter | Watanabe et al. (2012) | ||
| CitH | Corynebacterium glutamicum | 2.A.11.1.6 | 10 | Divalent cation:citrate; citrate transport in complex with Ca2+ or Sr2+ | Brocker et al. (2009) | |
| DAACS | Dct | Aspergillus carbonarius | 2.A.23.1.7 | 9 | Fumarate, L-Aspartate: symporter | Yang et al. (2017) |
| Actinobacillus succinogenes | 2.A.23 | 12 | Malate and citrate exporter | Darbani et al. (2019) | ||
| DctA | Bacillus subtilis | 2.A.23.1.6 | 8 | Dicarboxylate:H+ symporter; succinate (Km 2.6 μM), fumarate | Asai et al. (2000); Groeneveld et al. (2010) | |
| Corynebacterium glutamicum | 2.A.23 | 7 | Dicarboxylate:H+ symporter; L-Malate (Km 736 μM), fumarate (Km 232 μM), succinate (Km 218 μM), oxaloacetate and glyoxylate | Youn et al. (2008) | ||
| Escherichia coli | 2.A.23.1.7 | 8 | Aerobic dicarboxylate transporter; succinate (Km 25 µM), orotate, fumarate and L- and D-Malate | Baker et al. (1996); Karinou et al. (2013); Kay and Kornberg (1971) | ||
| DASS | DccT (DcsT) | Corynebacterium glutamicum | 2.A.47.1.12 | 14 | Aerobic sodium dicarboxylate transporter; succinate (Km 30 μM), fumarate (Km 79 μM), malate (Km 360 μM) and oxaloacetate | Ebbighausen, Weil and Krämer (1991); Teramoto et al. (2008); Youn et al. (2008) |
| TtdT (YgjE) | Escherichia coli | 2.A.47.3.3 | 12 | L-Tartrate:Succinate antiporter; L-Tartrate (Km 700 µM) uptake, succinate (Km 400 µM) efflux | Kim and Unden (2007) | |
| CitT | 2.A.47.3.2 | 13 | Citrate:Succinate antiporter; citrate uptake and efflux of succinate, fumarate and tartrate. | Pos, Dimroth and Bott (1998) | ||
| SLC13 | Actinobacillus succinogenes | 2.A.47 | 14 | Citrate exporter | Darbani et al. (2019) | |
| SdcA | 2.A.47.5.3 | 13 | Dicarboxylate: Na+ transporter; fumarate (Km 536 µM) and succinate (Km 389 µM) uptake | Rhie et al. (2014) | ||
| SdcS | Staphylococcus aureus | 2.A.47.1.11 | 14 | Dicarboxylate: Na+ symporter; succinate (Km 7 mM), malate (Km 8 mM), fumarate (Km 15 mM), aspartate and α-Ketoglutarate transporter | Hall and Pajor (2005); Hall and Pajor (2007) | |
| Dcu | DcuA | Escherichia coli | 2.A.13.1.1 | 11 | Anaerobic antiporter of aspartate, malate, fumarate and succinate; uptake and efflux of fumarate | Six et al. (1994); Zientz et al. (1996) |
| DcuB | 2.A.13.1.2 | 11 | Anaerobic antiporter of aspartate, malate, fumarate and succinate; uptake and efflux of fumarate and citrate exporter | Darbani et al. (2019); Kim and Unden (2007); Six et al. (1994); Zientz et al. (1996) | ||
| DcuC | DcuC | Escherichia coli | 2.A.61.1.1 | 12 | Anaerobic electroneutral C4-dicarboxylate exchanger; dicarboxylate-proton symporter; citrate exporter | Chen et al. (2014); Darbani et al. (2019); Zientz et al. (1999,1996) |
| DHA1 | CexA | Aspergillus niger | 2.A.1.2 | 12 | Citrate exporter | Steiger et al. (2019) |
| FNT | FocA | Escherichia coli | 1.A.16.1.1 | 6 in pentameric channels (PDB 3KCU) | Exporter of acetate; (Km 23.9 mM), lactate (Km 96 mM) and pyruvate (Km 11.6 mM); uptake/efflux of formate (Km 11.7 mM) | Lü et al. (2012); Wang et al. (2009) |
| PfFNT | Plasmodium falciparum | 1.A.16.2.7 | 6 | Lactate:H+ symporter; D-Lactate, pyruvate, acetate and formate | Marchetti et al. (2015); Wu et al.(2015) | |
| LctP | LldP | Escherichia coli | 2.A.14.1.1 | 12 | Lactate permease; L-Lactate, D-Lactate and glycolate | Núñez et al. (2001) |
| GlcA (YghK) | 2.A.14.1.2 | 13 | Glycolate permease; L-Lactate, D-Lactate and glycolate | Núñez et al. (2002); Núñez et al. (2001) | ||
| LutP | Bacillus subtilis | 2.A.14.1.3 | 14 | Lactate permease | Chai, Kolter and Losick (2009) | |
| Bacillus coagulans | 2.A.14.1 | 14 | Lactate permease | Wang et al. (2019) | ||
| MFS | MfsA | Aspergillus terreus | 2.A.1 | 12 | Dicarboxylate transporter; Itaconate exporter | Hossain et al. (2016); Huang et al. (2014) |
| Itp1 | Ustilago maydis | 2.A.1 | 12 | Itaconate exporter | Geiser et al. (2016) | |
| MHS | Dehp2 | Burkholderia caribensis | 2.A.1.6.11 | 12 | Acetate/haloacid transporter; acetate, chloroacetate, bromoacetate, 2-chloropropionate; low-affinity to glycolate, lactate and pyruvate | Su et al. (2016); Su and Tsang (2013) |
| Deh4p | 2.A.1.6.13 | 11 | Acetate/Monochloroacetate (haloacid) permease; acetate (Km 5.5 μM) and monochloroacetate (Km 9 μM) | Su et al. (2016); Su and Tsang (2013) | ||
| NhaC | MleN (YqkI) | Bacillus subtilis | 2.A.35.1.2 | 10 | Malate:Lactate antiporter coupled with proton uptake and sodium efflux; Malic2−-2H+: Na+-Lactate1− | Wei et al. (2000) |
| SHS | Jen1 | Saccharomyces cerevisiae | 2.A.1.12.2 | 12 | Lactate/Pyruvate:H+ symporter; acetate (Km 4.8 mM), lactate (Km 0.2 mM), propionate, pyruvate (Km 0.7 mM), selenite | Casal et al. (1999); McDermott, Rosen and Liu (2010); Soares-Silva et al. (2007, 2003) |
| CaJen1 | Candida albicans | 2.A.1.12 | 10 | Monocarboxylate permease; lactate (Km 0.33 mM), pyruvate and propionate | Soares-Silva et al. (2004) | |
| CaJen2 | 2.A.1.12 | 10 | Dicarboxylate permease; succinate (Km 0.49 mM), malate (Km 0.12 mM); affinity for the sugar acids gluconate, xylarate and mucate | Ribas et al. (2017); Vieira et al. (2010) | ||
| DH17 | Debaryomyces hansenii | 2.A.1.12 | 12 | Malate permease (Km 0.27 mM) | Soares-Silva et al. (2015) | |
| SHS | DH18 | Debaryomyces hansenii | 2.A.1.12 | 12 | Succinate permease (Km 0.31 mM) | Soares-Silva et al. (2015) |
| DH24 | 2.A.1.12 | 12 | Succinate permease (Km 0.16 mM) | Soares-Silva et al. (2015) | ||
| DH27 | 2.A.1.12 | 12 | Acetate permease (Km 0.94 mM) | Soares-Silva et al. (2015) | ||
| KlJen1 | Kluyveromyces lactis | 2.A.1.12 | 12 | Monocarboxylate permease; lactate (Km 2.08 mM), pyruvate | Lodi et al. (2004); Queirós et al. (2007) | |
| KlJen2 | 2.A.1.12 | 11 | Dicarboxylate permease; malate (Km 0.15 mM), succinate (Km 0.11 mM), fumarate; affinity for the sugar acids gluconate and saccharate | Lodi et al. (2004); Queirós et al. (2007); Ribas et al. (2019) | ||
| SSS | MctC | Corynebacterium glutamicum | 2.A.21.7.3 | 13 | Acetate/Propionate:H+ symporter; pyruvate (Km 250 µM), acetate (Km 31 µM), propionate (Km 9 µM) | Jolkver et al. (2009) |
| ActP (YjcG) | Escherichia coli | 2.A.21.7.2 | 13 | Acetate (Km 5.4 μM) and glyoxylate coupling ion: proton transporter, with affinity for tellurite | Elías et al. (2015); Gimenez et al. (2003) | |
| ActP1 | Rhodobacter capsulatus | 2.A.21.7.4 | 14 | Acetate permease; acetate (Km 1.89 mM), pyruvate, lactate, tellurite (Km 163 μM) | Borghese et al. (2016); Borghese Cicerano and Zannoni (2011); Borghese and Zannoni (2010) | |
| ST | KgtP (WitA) | Escherichia coli | 2.A.1.6.2 | 12 | α -Ketoglutarate (Oxoglutarate) :symporter; arabinose exporter | Koita and Rao (2012) |
| SulP | DauA (YchM) | Escherichia coli | 2.A.53.3.11 | 11 | Aerobic succinate transporter; succinate (Km 0.56 mM), aspartate and fumarate | Karinou et al. (2013) |
| TDT | Mae1 | Schizosaccharomyces pombe | 2.A.16.2.1 | 10 | Malate:H+ symporter; oxaloacetate, malonate, succinate, fumarate and thio-malate; exporter of fumarate, succinate and malate | Camarasa et al. (2001); Darbani et al. (2019); Grobler et al.(1995); Osawa and Matsumoto (2006) |
| Ssu1 | Ustilago trichophora | 2.A.16 | 9 | Malate transporter | Zambanini et al. (2017) | |
| Ssu2 | 2.A.16 | 9 | Malate transporter | Zambanini et al. (2017) | ||
| TRAP-T | DctPQM | Rhodobacter capsulatus | 2.A.56.1.1 | 12 (DctM) + 4 (DctQ) + receptor | Tripartite dicarboxylate: H+ symporter; malate (Kd 8.4 μM) competitively inhibited by fumarate (Ki 2 μM) and succinate (Ki 8 μM) | Forward et al. (1997) |
| TTT | TctABC | Corynebacterium glutamicum | 2.A.80.1.4 | 12(TctA) + 4(TctB) + 1(TctC) | Citrate transport in complex with Ca2+ or Mg2+ | Brocker et al. (2009) |
Number of TMS predicted with the TMHMM software (http://www.cbs.dtu.dk/services/TMHMM/) or verified.
TCDB Families: 2-HCT–2-Hydroxycarboxylate Transporter; AAEx—Aspartate:Alanine Exchanger; AceTr—Acetate Uptake Transporter; Bestrophin—Anion Channel-forming Bestrophin; CitMHS—Citrate-Mg2+:H+ (CitM) Citrate-Ca2+:H+ (CitH) Symporter; DAACS—Dicarboxylate/Amino Acid:Cation (Na+ or H+) Symporter; DASS—Divalent Anion:Na+ Symporter; Dcu—C4-Dicarboxylate Uptake; DcuC—C4-dicarboxylate Uptake C; DHA1–Drug:H+ Antiporter-1; FNT—Formate-Nitrite Transporter; LctP—Lactate Permease; MFS—Major Facilitator Superfamily; MHS—Metabolite:H+ Symporter; NhaC—Na+:H+ Antiporter; SHS—Sialate:H+ Symporter; SSS—Solute:Sodium Symporter; ST—Sugar transporter; SulP—Sulfate Permease; TDT—Telurite-resistance/Dicarboxylate Transporter; TRAP-T—Tripartite ATP-independent Periplasmic Transporter; TTT—Tripartite Tricarboxylate Transporter. na—not annotated at TC Database.
Glutamic acid
The bacterium Corynebacterium glutamicum is used in microbial biotechnology for the production of glutamic acid. Glutamate efflux, triggered by increased mechanic tension, was associated with the activation of the channel NCgl1221 (MscCG; Nakamura et al. 2007; Becker et al. 2013), belonging to the MscS Family (TCDB 1.A.23 The Small Conductance Mechanosensitive Ion Channel). However, the activation mechanism of C. glutamicum mechanosensitive channels is not fully understood (for a review see Nakayama et al. 2019). Several channels of this family are described to play a critical role in product efflux of other amino acids, namely lysine, isoleucine, threonine, methionine and others (Van Dyk 2008; Kell et al. 2015).
Malic acid
In the natural malic acid producer Ustilago trichophora RK089, the overexpression of two endogenous malate transporter genes improved the production yields by 54% (Zambanini et al. 2017). The overexpression of pyruvate carboxylase (pyc) together with two malate dehydrogenases (mdh1, mdh2), and two malate transporters (ssu1, ssu2) was carried out in a laboratory-evolved U. trichophora strain that reached an extracellular malate titer of 120 g/L. Wild-type S. cerevisiae strains produce low levels of malate. High yield production of malic acid required the elimination of alcoholic fermentation, which in this yeast occurs under fully aerobic conditions when high concentrations of sugar are present (Zelle et al. 2008). The metabolic engineering of a S. cerevisiae strain allowed an increase up to 10-fold of malic acid titer relative to the control strain (Zelle et al. 2008). This was achieved through the engineering of a glucose-tolerant, C2-independent pyruvate decarboxylase-negative strain, together with: (i) the overexpression of the endogenous pyruvate carboxylase encoded by PYC2, (ii) the overexpression of an allele of the peroxisomal malate dehydrogenase MDH3 gene targeted to the cytoplasm and (iii) the functional expression of the S. pombe malate transporter SpMae1. These modifications per se improved malate production, and the combination of all genetic modifications reached a malate titer of approximately 59 g/L(Zelle et al. 2008). Recently, seven dicarboxylic acid transporters were expressed in a S. cerevisiae strain engineered for dicarboxylic acid production (Darbani et al. 2019). In this work, the expression of the SpMae1 homologous gene from Aspergillus carbonarius, AcDct, increased malate titer up to 12-fold. Upon SpMae1 expression, the following titers were obtained for malate (8 fold-4.3 g/L), succinate (3 fold-2.6 g/L) and fumarate (5 fold-0.33 g/L).
Fumaric acid
The overexpression of the S. cerevisiae mitochondrial succinate-fumarate carrier SFC1 gene enhanced fumarate export and production by 47.6% in this yeast (Xu et al. 2012). A S. cerevisiae strain engineered for the production of fumarate, deleted in the fumarase FUM1 gene and expressing the RoPYC pyruvate carboxylase gene of R. oryzae and the endogenous SFC1 gene, resulted in a titer of 1.7 g/L of fumarate in batch culture.
Using a different approach, fumarate production in Candida glabrata was improved by overexpressing the Sfc1 mitochondrial carrier in combination with the heterologous expression of SpMae1 (Chen et al. 2015). This work established the metabolic engineering of the tricarboxylic acid cycle in C. glabrata to construct the oxidative pathway for fumarate production. Thus, a set of genetic modifications to manipulate the oxidative pathway was applied in the α-ketoglutarate dehydrogenase complex, succinyl-CoA synthetase and succinate dehydrogenase. As a result, the C. glabrata producer strain reached a fumarate titer of 8.24 g/L. Overexpression of the argininosuccinate lyase gene led to a fumarate increase up to 9.96 g/L. The additional expression of two dicarboxylic acid transporters, Sfc1 and SpMae1, allowed an improvement of fumarate production (15.76 g/L; Chen et al. 2015).
In E. coli a set of C4-dicarboxylate transporters from different organisms were cloned in a fumaric acid-producing strain deleted in the genes fumABC, frdABCD, iclR and arcA, to evaluate their impact on the production of this acid (Zhang et al. 2017b). It was the overexpression of the endogenous transporters DcuB, an anaerobic fumarate–succinate antiporter (Zientz, Six and Unden 1996), and DcuC, a C4-dicarboxylate carrier that promotes succinate efflux during glucose fermentation (Zientz et al. 1999), that displayed the highest impact on the production of fumaric acid. These lead to an increase of fumaric acid yield by 48.5% and 53.1%, respectively. In fed-batch fermentation culture, the fumaric acid producer strain overexpressing the dcuB gene reached 9.42 g/L of fumaric acid after 50 h (Zhang et al. 2017b).
Succinic acid
Modulation of the simultaneous expression of E. coli transporter genes dcuB and dcuC led to a 34% increase of succinic acid titer in an engineered E. coli strain (Chen et al. 2014). In this work, four E. coli Dcu C4-dicarboxylate transporters were exploited for succinate export. Single deletion of dcuA or dcuD did not affect the export of this organic acid, while dcuB and dcuC deletion led to 15% and 11% decrease of succinate extracellular titer, respectively. The combined deletion of dcuB and dcuC genes resulted in a 90% decrease of succinate titer. As a result, a ribosome binding site library was investigated to modulate and increase the co-expression of dcuB and dcuC, which led to a 34% increase of succinate titer produced by E. coli (Chen et al. 2014).
In C. glutamicum, the overexpression of the endogenous succinate exporter, SucE, increased succinate yield in an engineered strain (Zhu et al. 2014). A dual-route for anaerobic succinate production was devised, involving the reconstruction of the glyoxylate pathway by overexpressing isocitrate lyase, malate synthase and citrate synthase. This succinate producer strain reached a succinate yield of 1.34 mol/mol of glucose. The additional overexpression of the endogenous succinate exporter, SucE, increased succinate yield to 1.43 mol/mol of glucose. In anaerobic fed-batch fermentation, the C. glutamicum succinate producer strain overexpressing SucE led to a titer of 109 g/L succinate.
Itaconic acid
Expression of two Aspergillus terreus genes encoding organic acid transporters, mttA and mfsA, increased itaconic acid production in an A. niger strain expressing the cis-aconitate decarboxylase (Li et al. 2011, 2012). MttA is a mitochondrial tricarboxylic acid transporter that preferentially transports cis-aconitate instead of citrate (Steiger et al. 2016). MfsA is an itaconate plasma membrane exporter (Huang et al. 2014; Hossain et al. 2016). The strains expressing mttA or mfsA displayed an increased itaconic acid (1.5 g/L) production when compared with an A. niger strain expressing only cis-aconitate decarboxylase (0.8 g/L; Li et al. 2013). Interestingly, the production did not increase further when both transporters were co-expressed (0.9 g/L). In a previous study in A. terreus, the overexpression of a bacterial hemoglobin (vgb) led to an increased dissolved oxygen level, having a strong effect on itaconic acid production (Lin et al. 2004). Additional optimization was achieved by overexpression of the fungal hemoglobin domain hbd1 and deletion of the oxaloacetate acetylhydrolase oahA gene, in combination with controlled batch fermentation conditions, resulting in the increase of the production level from 0.8 to 2.5 g/L of itaconic acid (Li et al. 2013). In a subsequent study, a titer of 26.2 g/L and a maximum production rate of 0.35 g/L/h were reached by overexpressing the cytosolic citrate synthase citB (Hossain et al. 2016).
Lactic acid
The S. cerevisiae genome encodes at least two plasma membrane monocarboxylate transporters, Jen1 (Casal et al. 1999; Soares-Silva et al. 2003) and Ady2 (Paiva et al. 2004; Ribas et al. 2019) with distinct specificities, mode of action and regulation mechanisms (Casal et al. 2008, 2016). In a S. cerevisiae strain engineered for lactate production, the constitutive expression of these two transporters resulted in a higher accumulation of lactic acid in the extracellular medium (Pacheco et al. 2012). In this study, the authors expressed the lactate-dehydrogenase LDH gene from L. casei in the S. cerevisiae W303–1A parental strain and in the three isogenic strains jen1∆, ady2∆ and jen1∆ ady2∆ to allow lactate production. All the deleted strains expressing LDH were able to produce higher titers of lactic acid compared with the parental isogenic strain. Moreover, the constitutive expression of JEN1 or ADY2 genes, along with LDH, resulted in the higher external accumulation of lactic acid in the presence of glucose. Upon glucose depletion, lactate consumption was also more pronounced in cells expressing Jen1 and/or Ady2, suggesting the involvement of these transporters in both the import and export of lactic acid (Pacheco et al. 2012).
Citric acid
In a recent work, Steiger et al. (2019) identified CexA, the longtime sought citrate exporter from A. niger. The constitutive and inducible overexpression of CexA in the native citric acid-producing species A. niger, resulted in significant increases in secreted citric acid (Steiger et al. 2019). The inducible system reached 109 g/L citric acid, five times higher than the parental wild-type strain and three times higher than the constitutive expression system.
ENGINEERING MEMBRANE TRANSPORTERS
Membrane transporters, like any protein, can display substrate promiscuity, altered conformation, distinct affinity and capacity depending on physiological conditions, as well as alterations in folding and stability. Finding a membrane transporter, either for import or export, might not be enough to achieve the levels of cell factory productivity needed to obtain a cost-effective and sustainable bioproduction process. The engineering of membrane proteins can diminish these constraints by tuning the activity towards specific conditions and substrates. This approach is frequently achieved by ALE experiments, mutagenesis or recombination involving methods of synthetic biology (Van Dyk 2008; Kell et al. 2015; Moysés et al. 2016). ALE of host organisms combined with the identification of responsible genetic changes and subsequent reverse engineering, is a powerful approach to obtain novel or improved substrate specificity of membrane transporters.
Engineering sugar transporters
Whereas it is common to look for exogenous transporters to be cloned into producer strains, the endogenous transportome can be used as a pool of transporters for cell factory optimization. One such example is the complex landscape of the S. cerevisiae genome that includes 20 transporter proteins belonging to the Hexose Transporter (HXT) Family, with great potential to be exploited in cell factories for the uptake of renewable sugars from lignocellulosic wastes (Kruckeberg 1996; Moysés et al. 2016). By using molecular modeling and docking studies, the endogenous S. cerevisiae Gal2 transporter was engineered to improve L-arabinose transport capacity (Subtil and Boles 2011b). In this study, nine residues were found to interact with L-arabinose. Rational protein design by directed mutagenesis allowed an increase of transporter capacity for L-arabinose. Besides the gain of function associated with arabinose transport capacity, the F85S mutation specifically improved xylose transport (Wang et al. 2017). In another study, the combination of computer-assisted modeling, site-directed mutagenesis, error-prone PCR approaches and selective growth conditions, resulted in the identification of residues in both Hxt7 and Gal2 that yielded glucose-insensitive xylose transporters (Farwick et al. 2014). The mutant Gal2-N376F had the highest affinity for D-xylose, along with a moderate transport rate for this pentose sugar, and completely lost the ability to transport hexoses (Farwick et al. 2014).
To obtain a transporter able to sufficiently import arabinose in the presence of glucose and xylose, a strain deficient in glucose phosphorylation and able to metabolize arabinose was created (Verhoeven et al. 2018). Subsequently, the engineered strain was grown in medium with these three sugars. This way, conditions were met where arabinose became the only metabolizable sugar within the media, while glucose and xylose were exerting selective pressure towards the evolution of an arabinose transporter uninhibited by glucose and xylose. Consequently, mutations within hexose transporter Gal2 in residues T89 and N376 were found to significantly increase the Km value of Gal2 for glucose, and decrease the Km value for arabinose, enabling superior growth of the engineered strain in a medium containing the three sugars (Verhoeven et al. 2018).
Engineering organic acid transporters
In an attempt to evolve an efficient fumarate exporter in S. cerevisiae, a knock-out strategy was implemented in which fumaric acid was turned into the energetically more favorable catabolic product, by deletion of the fumarase (FUM1) and glucose 6-phosphate dehydrogenase (ZWF1) genes (Shah 2016). The malate and succinate transporter DCT_02 from A. niger was used as a template expected to evolve into an efficient fumarate exporter. However, the evolution experiment did not yield the desired results, since only malate and succinate were secreted to the extracellular medium, and further strategy refinement is required (Shah 2016).
Through a rationally designed site-directed mutagenesis strategy, the substrate specificity of the yeast Jen1 monocarboxylate transporter was altered to acquire the ability to transport the dicarboxylic acids succinate (F270A and F270G; Soares-Silva et al. 2011) and saccharate (S271Q; Ribas et al. 2017).
In two independent evolution experiments, S. cerevisiae strains deficient in Jen1 were evolved for growth on lactate as sole carbon and energy source (de Kok et al. 2012). Whole-genome resequencing of evolved strains uncovered the presence of single nucleotide changes in the acetate transporter gene ADY2 (C755G/L219V and C655G/A252G). These Ady2 mutated alleles encode efficient lactate transporters.
Presently, new relevant roles of protein transporters are being uncovered, namely at the level of improving industrial strain's tolerance to by-products. An example of the complexity of the roles of transporters in regulatory networks is reported by Zang et al. (2017a) who, in the presence of 3.6 g/L acetic acid pH 3.7, observed an increment of 14.7% in the final ethanol concentration for the S. cerevisiae strain lacking the ADY2 gene. By impairing acetate uptake from the extracellular space, the accumulation of intracellular acetate was reduced, and as consequence cells acquired increased tolerance towards this organic acid.
FUTURE PERSPECTIVES FOR TRANSPORT ENGINEERING
It is expected that in the near future, biorefineries increase the production of platform chemicals from renewable resources (Takkellapati, Li and Gonzalez 2018). The exploitation of industrial biowastes to sustain microbial cell growth and valorize biomass conversion into organic acids is one of these current trends. Achieving optimal processes requires industrially robust strains. One of the major bottlenecks for the efficient and cost-effective bioproduction of organic acids is their export through the microbial plasma membrane. Membrane transporter proteins are thus crucial elements for the optimization of this process. In this review, we presented examples of the most relevant and emerging cell factories for the production of organic acids, as well as the engineering strategies used to turn them into efficient producers of this family of compounds.
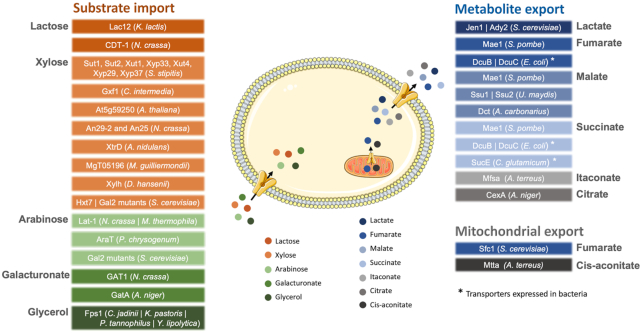
In recent years, a great effort was dedicated to transporter engineering, envisaging the development and improvement of microbial cell factories. Examples of transporters engineered in producer strains, especially in the yeast S. cerevisiae, are summarized in Fig. 2. Despite these advances, as the functional and structural characterization of membrane proteins is still a cumbersome process, the redesigning and engineering of optimized cell membrane transporters for industrial organic acid production is still at an early stage (Boyarskiy and Tullman-Ercek 2015; Kell et al. 2015). Different strategies can be followed to obtain improved transporters, namely with higher activity, altered substrate specificity and product selectivity. ALE can be a suitable approach when the desired transport process is directly linked to a selective advantage, such as the import of a sole carbon source necessary for growth. Nevertheless, its employment to generate improved transporters for the efflux of solutes can represent a demanding challenge. The complexity of biorefineries relies on many factors, including the optimization of several transporters, with complementary kinetic and regulatory properties (Verhoeven et al. 2018). Structure-based or computer simulation-based protein engineering is a powerful approach. However, these methods are hampered by the low number of robust three-dimensional structural models of transporter proteins. According to the Protein Data Bank (https://www.rcsb.org), transporter 3D structures account for less than 10% of the total database entries, showing that membrane proteins remain until now mostly uncharacterized, which evidences the need to increase the existing knowledge on this field. The recent identification of the gene encoding the long-sought citrate exporter from A. niger (Odoni et al. 2019; Steiger et al. 2019), is an example of the effort that must be carried out towards the identification of new transporters.
Figure 2.
The expression of endogenous or exogenous membrane transporter genes in engineered bacteria, yeast and filamentous fungi, allows the uptake of renewable substrates, as well as the export and extracellular accumulation of specialty organic acids. Transporters on the left were expressed in the plasma membrane, to promote the import of substrates. The transporters on the right were expressed either in the inner mitochondrial membrane or in the plasma membrane, to promote the export of organic acids. The black arrows indicate the direction of the transport, either to the cytoplasm, out of the mitochondria or to the extracellular medium. Transporters expressed in bacteria are marked with *. The figure was produced using the vector image bank of Servier Medical Art (http://smart.servier.com/).
The biodiversity of the microbial world is an excellent pool to uncover relevant transporters for organic acid production. Achieving the efficient heterologous expression of transporters is crucial to improve the robustness of microbial cell factories. For instance, the proper expression of bacterial transporters in fungi is constrained due to membrane incompatibility, low expression levels and folding difficulties (Young et al. 2011), limiting the options for prokaryotic transporter expression in eukaryotic cells. Still, the versatility and plasticity of membrane transporters suggest a promising future towards the optimization and implementation of platform chemical bioproduction at the industrial scale.
ACKNOWLEDGEMENTS
This work was supported by the strategic programme UID/BIA/04050/2019 funded by Portuguese funds through the FCT I.P., and the projects: PTDC/BIAMIC/5184/2014, funded by national funds through the Fundação para a Ciência e Tecnologia (FCT) I.P. and by the European Regional Development Fund (ERDF) through the COMPETE 2020–Programa Operacional Competitividade e Internacionalização (POCI), and EcoAgriFood: Innovative green products and processes to promote AgriFood BioEconomy (operação NORTE-01–0145-FEDER-000009), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). DR acknowledges FCT for the SFRH/BD/96166/2013 PhD grant. MSS acknowledges the Norte2020 for the UMINHO/BD/25/2016 PhD grant with the reference NORTE-08–5369-FSE-000060. TR acknowledges Yeastdoc European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 764927.
Contributor Information
I Soares-Silva, Centre of Molecular and Environmental Biology (CBMA), Department of Biology, University of Minho, Campus de Gualtar, Braga 4710-057, Portugal; Institute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, Campus de Gualtar, Braga 4710-057, Portugal.
D Ribas, Centre of Molecular and Environmental Biology (CBMA), Department of Biology, University of Minho, Campus de Gualtar, Braga 4710-057, Portugal; Institute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, Campus de Gualtar, Braga 4710-057, Portugal.
M Sousa-Silva, Centre of Molecular and Environmental Biology (CBMA), Department of Biology, University of Minho, Campus de Gualtar, Braga 4710-057, Portugal; Institute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, Campus de Gualtar, Braga 4710-057, Portugal.
J Azevedo-Silva, Centre of Molecular and Environmental Biology (CBMA), Department of Biology, University of Minho, Campus de Gualtar, Braga 4710-057, Portugal; Institute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, Campus de Gualtar, Braga 4710-057, Portugal.
T Rendulić, Centre of Molecular and Environmental Biology (CBMA), Department of Biology, University of Minho, Campus de Gualtar, Braga 4710-057, Portugal; Institute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, Campus de Gualtar, Braga 4710-057, Portugal.
M Casal, Centre of Molecular and Environmental Biology (CBMA), Department of Biology, University of Minho, Campus de Gualtar, Braga 4710-057, Portugal; Institute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, Campus de Gualtar, Braga 4710-057, Portugal.
Conflicts of interest
None declared.
References
- Alonso S, Rendueles M, Díaz M. Microbial production of specialty organic acids from renewable and waste materials. Crit Rev Biotechnol. 2015;35:497–513. [DOI] [PubMed] [Google Scholar]
- Asai K, Baik S, Kasahara Y et al. . Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology. 2000;146:263–71. [DOI] [PubMed] [Google Scholar]
- Asskamp MR, Klein M, Nevoigt E. Saccharomyces cerevisiae exhibiting a modified route for uptake and catabolism of glycerol forms significant amounts of ethanol from this carbon source considered as ‘non-fermentable’. Biotechnol Biofuels. 2019;12:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augstein A, Barth K, Gentsch M et al. . Characterization, localization and functional analysis of Gpr1p, a protein affecting sensitivity to acetic acid in the yeast Yarrowia lipolytica. Microbiology. 2003;149:589–600. [DOI] [PubMed] [Google Scholar]
- Baker KE, Ditullio KP, Neuhard J et al. . Utilization of orotate as a pyrimidine source by Salmonella typhimurium and Escherichia coli requires the dicarboxylate transport protein encoded by dctA. J Bacteriol. 1996;178:7099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Ansanay V, Rachidi N et al. . Membrane potential-generating malate (MleP) and citrate (CitP) transporters of lactic acid bacteria re homologous proteins substrate specificity of the 2-hydroxycarboxylate transporter family. J Biol Chem. 1997;272:18140–6. [DOI] [PubMed] [Google Scholar]
- Bandell M, Lhotte M, Marty-Teysset C et al. . Mechanism of the citrate transporters in carbohydrate and citrate cometabolism in Lactococcus and Leuconostoc species. Appl Environ Microbiol. 1998;64:1594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Boles E. A modified Saccharomyces cerevisiae strain that consumes L-Arabinose and produces ethanol. Appl Environ Microbiol. 2003;69:4144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Wittmann C. Advanced biotechnology: metabolically engineered cells for the bio-based production of chemicals and fuels, materials, and health-care products. Angew Chem. 2015;54:3328–50. [DOI] [PubMed] [Google Scholar]
- Becker M, Borngen K, Nomura T et al. . Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta. 2013;1828:1230–40. [DOI] [PubMed] [Google Scholar]
- Biz A, Sugai-Guerios MH, Kuivanen J et al. . The introduction of the fungal D-galacturonate pathway enables the consumption of D-galacturonic acid by Saccharomyces cerevisiae. Microb Cell Fact. 2016;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese R, Canducci L, Musiani F et al. . On the role of a specific insert in acetate permeases (ActP) for tellurite uptake in bacteria: functional and structural studies. J Inorg Biochem. 2016;163:103–9. [DOI] [PubMed] [Google Scholar]
- Borghese R, Cicerano S, Zannoni D. Fructose increases the resistance of Rhodobacter capsulatus to the toxic oxyanion tellurite through repression of acetate permease (ActP). Antonie Van Leeuwenhoek. 2011;100:655. [DOI] [PubMed] [Google Scholar]
- Borghese R, Zannoni D. Acetate permease (ActP) Is responsible for tellurite (TeO32-) uptake and resistance in cells of the facultative phototroph Rhodobacter capsulatus. Appl Environ Microbiol. 2010;76:942–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarskiy S, Tullman-Ercek D. Getting pumped: membrane efflux transporters for enhanced biomolecule production. Curr Opin Chem Biol. 2015;28: 15–9. [DOI] [PubMed] [Google Scholar]
- Bracher JM, Verhoeven MD, Wisselink HW et al. . The Penicillium chrysogenum transporter PcAraT enables high-affinity, glucose-insensitive l-arabinose transport in Saccharomyces cerevisiae. Biotechnol Biofuels. 2018;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker M, Schaffer S, Mack C et al. . Citrate utilization by Corynebacterium glutamicum is controlled by the CitAB two-component system through positive regulation of the citrate transport genes citH and tctCBA. J Bacteriol. 2009;191:3869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke N, Schäfer R, Becker J et al. . Metabolic engineering of industrial platform microorganisms for biorefinery applications – Optimization of substrate spectrum and process robustness by rational and evolutive strategies. Bioresour Technol. 2013;135:544–54. [DOI] [PubMed] [Google Scholar]
- Camarasa C, Bidard F, Bony M et al. . Characterization of Schizosaccharomyces pombe malate permease by expression in Saccharomyces cerevisiae. Appl Environ Microbiol. 2001;67:4144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal M, Paiva S, Andrade RP et al. . The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. J Bacteriol. 1999;181:2620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal M, Paiva S, Queirós O et al. . Transport of carboxylic acids in yeasts. FEMS Microbiol Rev. 2008;32:974–94. [DOI] [PubMed] [Google Scholar]
- Casal M, Queiros O, Talaia G et al. . Carboxylic acids plasma membrane transporters in Saccharomyces cerevisiae. Adv Exp Med Biol. 2016;892:229–51. [DOI] [PubMed] [Google Scholar]
- Chai Y, Kolter R, Losick R. A widely conserved gene cluster required for lactate utilization in Bacillus subtilis and its involvement in biofilm formation. J Bacteriol. 2009;191:2423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhu X, Tan Z et al. . Activating C4-dicarboxylate transporters DcuB and DcuC for improving succinate production. Appl Microbiol Biotechnol. 2014;98:2197–205. [DOI] [PubMed] [Google Scholar]
- Chen X, Dong X, Wang Y et al. . Mitochondrial engineering of the TCA cycle for fumarate production. Metab Eng. 2015;31:62–73. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou L, Tian K et al. . Metabolic engineering of Escherichia coli: a sustainable industrial platform for bio-based chemical production. Biotechnol Adv. 2013;31:1200–23. [DOI] [PubMed] [Google Scholar]
- Darbani B, Stovicek V, van der Hoek SA et al. . Engineering energetically efficient transport of dicarboxylic acids in yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2019, DOI: 10.1073/pnas.1900287116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok S, Nijkamp JF, Oud B et al. . Laboratory evolution of new lactate transporter genes in a jen1 delta mutant of Saccharomyces cerevisiae and their identification as ADY2 alleles by whole-genome resequencing and transcriptome analysis. FEMS Yeast Res. 2012;12:359–74. [DOI] [PubMed] [Google Scholar]
- Deng W, Wang Y, Yan N. Production of organic acids from biomass resources. Curr Opin Green Sustain Chem. 2016;2:54–8. [Google Scholar]
- Domingues L, Dantas MM, Lima N et al. . Continuous ethanol fermentation of lactose by a recombinant flocculating Saccharomyces cerevisiae strain. Biotechnol Bioeng. 1999;64:692–7. [DOI] [PubMed] [Google Scholar]
- Ebbighausen H, Weil B, Krämer R. Carrier-mediated acetate uptake in Corynebacterium glutamicum. Arch Microbiol. 1991;155:505–10. [Google Scholar]
- Elías A, Díaz-Vásquez W, Abarca-Lagunas MJ et al. . The ActP acetate transporter acts prior to the PitA phosphate carrier in tellurite uptake by Escherichia coli. Microbiol Res. 2015;177:15–21. [DOI] [PubMed] [Google Scholar]
- Farwick A, Bruder S, Schadeweg V et al. . Engineering of yeast hexose transporters to transport d-xylose without inhibition by d-glucose. Proc Natl Acad Sci USA. 2014;111:5159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forward JA, Behrendt MC, Wyborn NR et al. . TRAP transporters: a new family of periplasmic solute transport systems encoded by the dctPQM genes of Rhodobacter capsulatus and by homologs in diverse gram-negative bacteria. J Bacteriol. 1997;179:5482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K, Koseki C, Yamamoto Y et al. . Identification of succinate exporter in Corynebacterium glutamicum and its physiological roles under anaerobic conditions. J Biotechnol. 2011;154:25–34. [DOI] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Cuomo C et al. . Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–15. [DOI] [PubMed] [Google Scholar]
- Geiser E, Przybilla SK, Friedrich A et al. . Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb Biotechnol. 2016;9:116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez R, Nuñez MF, Badia J et al. . The gene yjcG, cotranscribed with the gene acs, encodes an acetate permease in Escherichia coli. J Bacteriol. 2003;185:6448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobler J, Bauer F, Subden RE et al. . The mae1 gene of Schizosaccharomyces pombe encodes a permease for malate and other C4 dicarboxylic acids. Yeast. 1995;11:1485–91. [DOI] [PubMed] [Google Scholar]
- Groeneveld M, Detert Oude Weme R, Duurkens R et al. . Biochemical characterization of the C4-dicarboxylate transporter DctA from Bacillus subtilis. J Bacteriol. 2010;192:2900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Pajor AM. Functional characterization of a Na+-coupled dicarboxylate carrier protein from Staphylococcus aureus. J Bacteriol. 2005;187:5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Pajor AM. Functional reconstitution of SdcS, a Na+-coupled dicarboxylate carrier protein from Staphylococcus aureus. J Bacteriol. 2007;189:880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain AH, Li A, Brickwedde A et al. . Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger. Microb Cell Fact. 2016;15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Lu X, Li Y et al. . Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain. Microb Cell Fact. 2014;13:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkver E, Emer D, Ballan S et al. . Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J Bacteriol. 2009;191:940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karinou E, Compton ELR, Morel M et al. . The Escherichia coli SLC26 homologue YchM (DauA) is a C4-dicarboxylic acid transporter. Mol Microbiol. 2013;87:623–40. [DOI] [PubMed] [Google Scholar]
- Kay WW, Kornberg HL. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur J Biochem. 1971;18:274–81. [DOI] [PubMed] [Google Scholar]
- Kebbel F, Kurz M, Arheit M et al. . Structure and substrate-induced conformational changes of the secondary citrate/sodium symporter CitS revealed by electron crystallography. Structure. 2013;21:1243–50. [DOI] [PubMed] [Google Scholar]
- Kell DB, Swainston N, Pir P et al. . Membrane transporter engineering in industrial biotechnology and whole cell biocatalysis. Trends Biotechnol. 2015;33:237–46. [DOI] [PubMed] [Google Scholar]
- Khunnonkwao P, Jantama SS, Kanchanatawee S et al. . Re-engineering Escherichia coli KJ122 to enhance the utilization of xylose and xylose/glucose mixture for efficient succinate production in mineral salt medium. Appl Microbiol Biotechnol. 2018;102:127–41. [DOI] [PubMed] [Google Scholar]
- Kim OB, Unden G. The L-tartrate/succinate antiporter TtdT (YgjE) of L-tartrate fermentation in Escherichia coli. J Bacteriol. 2007;189:1597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Islam ZU, Knudsen PB et al. . The expression of glycerol facilitators from various yeast species improves growth on glycerol of Saccharomyces cerevisiae. Metab Eng Commun. 2016;3:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koita K, Rao CV. Identification and analysis of the putative pentose sugar efflux transporters in Escherichia coli. PLoS One. 2012;7:e43700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krom BP, Aardema R, Lolkema JS. Bacillus subtilis YxkJ is a secondary transporter of the 2-hydroxycarboxylate transporter family that transports L-malate and citrate. J Bacteriol. 2001;183:5862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krom BP, Warner JB, Konings WN et al. . Complementary metal Ion specificity of the metal-citrate transporters CitM and CitH of Bacillus subtilis. J Bacteriol. 2000;182:6374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruckeberg AL. The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol. 1996;166:283–92. [DOI] [PubMed] [Google Scholar]
- Li A, Pfelzer N, Zuijderwijk R et al. . Enhanced itaconic acid production in Aspergillus niger using genetic modification and medium optimization. BMC Biotechnol. 2012;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Pfelzer N, Zuijderwijk R et al. . Reduced by-product formation and modified oxygen availability improve itaconic acid production in Aspergillus niger. Appl Microbiol Biotechnol. 2013;97:3901–11. [DOI] [PubMed] [Google Scholar]
- Li A, van Luijk N, ter Beek M et al. . A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet Biol. 2011;48:602–11. [DOI] [PubMed] [Google Scholar]
- Li H, Pajor AJ. Functional characterization of CitM, the Mg2+-citrate transporter. J Membr Biol. 2002;185:9. [DOI] [PubMed] [Google Scholar]
- Li J, Xu J, Cai P et al. . Functional analysis of two l-arabinose transporters from filamentous fungi reveals promising characteristics for improved pentose utilization in Saccharomyces cerevisiae. Appl Environ Microbiol. 2015;81:4062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Li YF, Huang MC et al. . Intracellular expression of Vitreoscilla hemoglobin in Aspergillus terreus to alleviate the effect of a short break in aeration during culture. Biotechnol Lett. 2004;26:1067–72. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen J, Du G et al. . Method for reconstructing Aspergillus niger to increase citrate productionIn: Application USP. (ed). China, 2018. [Google Scholar]
- Lodi T, Fontanesi F, Ferrero I et al. . Carboxylic acids permeases in yeast: two genes in Kluyveromyces lactis. Gene. 2004;339:111–9. [DOI] [PubMed] [Google Scholar]
- Lu M. Structure and mechanism of the divalent anion/Na+ symporter. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü W, Du J, Schwarzer NJ et al. . The formate channel FocA exports the products of mixed-acid fermentation. Proc Natl Acad Sci USA. 2012;109:13254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti RV, Lehane AM, Shafik SH et al. . A lactate and formate transporter in the intraerythrocytic malaria parasite, Plasmodium falciparum Nat Commun. 2015;6:6721. [DOI] [PubMed] [Google Scholar]
- Marty-Teysset C, Lolkema JS, Schmitt P et al. . The citrate metabolic pathway in Leuconostoc mesenteroides: expression, amino acid synthesis, and alpha-ketocarboxylate transport. J Bacteriol. 1996;178:6209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JR, Rosen BP, Liu Z. Jen1p: a high affinity selenite transporter in yeast. Mol Biol Cell. 2010;21:3934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moysés DN, Reis VCB, de Almeida JRM et al. . Xylose fermentation by Saccharomyces cerevisiae: challenges and prospects. Int J Mol Sci. 2016;17:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na D, Park JH, Jang Y-S et al. . Systems metabolic engineering of Escherichia coli for chemicals, materials, biofuels, and pharmaceuticals. : Wittmann C, Lee SY (eds). Systems Metabolic Engineering, DOI: 10.1007/978-94-007-4534-6_5 Dordrecht, Netherlands: Springer, 2012, 117–49. [Google Scholar]
- Nakamura J, Hirano S, Ito H et al. . Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce L-glutamic acid production. Appl Environ Microbiol. 2007;73:4491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Hashimoto KI, Kawasaki H et al. . “Force-From-Lipids” mechanosensation in Corynebacterium glutamicum. Biophys Rev. 2019;11:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez MaF, Kwon O, Wilson TH et al. . Transport of l-Lactate, d-Lactate, and glycolate by the LldP and GlcA membrane carriers of Escherichia coli. Biochem Biophys Res Commun. 2002;290:824–9. [DOI] [PubMed] [Google Scholar]
- Núñez MF, Teresa Pellicer M, Badía J et al. . The gene yghK linked to the glc operon of Escherichia coli encodes a permease for glycolate that is structurally and functionally similar to L-lactate permease. Microbiology. 2001;147:1069–77. [DOI] [PubMed] [Google Scholar]
- Odoni DI, Vazquez-Vilar M, van Gaal MP et al. . Aspergillus niger citrate exporter revealed by comparison of two alternative citrate producing conditions. FEMS Microbiol Lett. 2019;366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H, Matsumoto H. Cytotoxic thio-malate is transported by both an aluminum-responsive malate efflux pathway in wheat and the MAE1 malate permease in Schizosaccharomyces pombe. Planta. 2006;224:462. [DOI] [PubMed] [Google Scholar]
- Pacheco A, Talaia G, Sa-Pessoa J et al. . Lactic acid production in Saccharomyces cerevisiae is modulated by expression of the monocarboxylate transporters Jen1 and Ady2. FEMS Yeast Res. 2012;12:375–81. [DOI] [PubMed] [Google Scholar]
- Paiva S, Devaux F, Barbosa S et al. . Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast. 2004;21:201–10. [DOI] [PubMed] [Google Scholar]
- Poolman B, Molenaar D, Smid EJ et al. . Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J Bacteriol. 1991;173:6030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pos KM, Dimroth P, Bott M. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J Bacteriol. 1998;180:4160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzko RJ, Latimer LN, Martinho Z et al. . Engineering Saccharomyces cerevisiae for co-utilization of d-galacturonic acid and d-glucose from citrus peel waste. Nat Commun. 2018;9:5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudlik AM, Lolkema JS. Citrate uptake in exchange with intermediates in the citrate metabolic pathway in Lactococcus lactis IL1403. J Bacteriol. 2011;193:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudlik AM, Lolkema JS. Substrate specificity of the citrate transporter CitP of Lactococcus lactis. J Bacteriol. 2012;194:3627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirós O, Pereira L, Paiva S et al. . Functional analysis of Kluyveromyces lactis carboxylic acids permeases: heterologous expression of KlJEN1 and KlJEN2 genes. Curr Genet. 2007;51:161–9. [DOI] [PubMed] [Google Scholar]
- Rhie MN, Yoon HE, Oh HY et al. . A Na+-coupled C4-dicarboxylate transporter (Asuc_0304) and aerobic growth of Actinobacillus succinogenes on C4-dicarboxylates. Microbiology. 2014;160:1533–44. [DOI] [PubMed] [Google Scholar]
- Ribas D, Soares-Silva I, Vieira D et al. . The acetate uptake transporter family motif “NPAPLGL(M/S)” is essential for substrate uptake. Fungal Genet Biol. 2019;122:1–10. [DOI] [PubMed] [Google Scholar]
- Ribas D, Sá-Pessoa J, Soares-Silva I et al. . Yeast as a tool to express sugar acid transporters with biotechnological interest. FEMS Yeast Res. 2017;17. [DOI] [PubMed] [Google Scholar]
- Roberts SK, Milnes J, Caddick M. Characterisation of AnBEST1, a functional anion channel in the plasma membrane of the filamentous fungus, Aspergillus nidulans Fungal Genet Biol. 2011;48:928–38. [DOI] [PubMed] [Google Scholar]
- Sahu Y. Organic acids market by type (Acetic Acid, Citric Acid, Formic Acid, Lactic Acid, Itaconic Acid, Succinic Acid, Gluconic Acid, Ascorbic Acid, Fumaric Acid, and Propionic Acid), Source (Biomass, Molasses, Starch, Chemical Synthesis, Agro-Industrial Residue), End-User (Food & Beverage, Animal Feed, Chemicals & Industrial, Pharmaceuticals, Personal Care, Agriculture) - Global Opportunity Analysis and Industry Forecast, 2017–2023, 2017, 260 Available in https://www.alliedmarketresearch.com/organic-acids-market.
- Sauer M, Porro D, Mattanovich D et al. . 16 years research on lactic acid production with yeast - ready for the market? Biotechnol Genet Eng Rev. 2010;27:229–56. [DOI] [PubMed] [Google Scholar]
- Sauer M, Porro D, Mattanovich D et al. . Microbial production of organic acids: expanding the markets. Trends Biotechnol. 2008;26:100–8. [DOI] [PubMed] [Google Scholar]
- Shah M. Dicarboxylic acids transport, metabolism and production in aerobic Saccharomyces cerevisiae. Department of Biotechnology, Faculty of Applied Sciences, DOI: 10.4233/uuid:0f74bf4d-8919-4b5f-b1f1-75b731230468 Delft University of Technology, 2016. [DOI]
- Six S, Andrews SC, Unden G et al. . Escherichia coli possesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct). J Bacteriol. 1994;176:6470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Silva I, Paiva S, Diallinas G et al. . The conserved sequence NXX[S/T]HX[S/T]QDXXXT of the lactate/pyruvate:H+ symporter subfamily defines the function of the substrate translocation pathway. Mol Membr Biol. 2007;24:464–74. [DOI] [PubMed] [Google Scholar]
- Soares-Silva I, Paiva S, Kötter P et al. . The disruption of JEN1 from Candida albicans impairs the transport of lactate. Mol Membr Biol. 2004;21:403–11. [DOI] [PubMed] [Google Scholar]
- Soares-Silva I, Ribas D, Foskolou IP et al. . The Debaryomyces hansenii carboxylate transporters Jen1 homologues are functional in Saccharomyces cerevisiae. FEMS Yeast Res. 2015;15. [DOI] [PubMed] [Google Scholar]
- Soares-Silva I, Sa-Pessoa J, Myrianthopoulos V et al. . A substrate translocation trajectory in a cytoplasm-facing topological model of the monocarboxylate/H+ symporter Jen1p. Mol Microbiol. 2011;81:805–17. [DOI] [PubMed] [Google Scholar]
- Soares-Silva I, Schuller D, Andrade RP et al. . Functional expression of the lactate permease Jen1p of Saccharomyces cerevisiae in Pichia pastoris. Biochem J. 2003;376:781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak I, Lolkema JS. The 2-hydroxycarboxylate transporter family: physiology, structure, and mechanism. Microbiol Mol Biol Rev. 2005;69:665–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger MG, Punt PJ, Ram AFJ et al. . Characterizing MttA as a mitochondrial cis-aconitic acid transporter by metabolic engineering. Metab Eng. 2016;35:95–104. [DOI] [PubMed] [Google Scholar]
- Steiger MG, Rassinger A, Mattanovich D et al. . Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger. Metab Eng. 2019;52:224–31. [DOI] [PubMed] [Google Scholar]
- Subtil T, Boles E. Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol Biofuels. 2011a;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil T, Boles E. Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol Biofuels. 2011b;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Li R, Kong K-F et al. . Transport of haloacids across biological membranes. Biochim Biophys Acta. 2016;1858:3061–70. [DOI] [PubMed] [Google Scholar]
- Su X, Tsang JSH. Existence of a robust haloacid transport system in a Burkholderia species bacterium. Biochim Biophys Acta. 2013;1828:187–92. [DOI] [PubMed] [Google Scholar]
- Sá-Pessoa J, Paiva S, Ribas D et al. . SATP (YaaH), a succinate–acetate transporter protein in Escherichia coli. Biochem J. 2013;454:585–95. [DOI] [PubMed] [Google Scholar]
- Takkellapati S, Li T, Gonzalez MA. An overview of biorefinery derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol Environ Policy. 2018;20:1615–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kobayashi K, Ogasawara N. The Bacillus subtilis YufLM two-component system regulates the expression of the malate transporters MaeN (YufR) and YflS, and is essential for utilization of malate in minimal medium. Microbiology. 2003;149:2317–29. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Shirai T, Inui M et al. . Identification of a gene encoding a transporter essential for utilization of C4 dicarboxylates in Corynebacterium glutamicum. Appl Environ Microbiol. 2008;74:5290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Zheng X, Tong Y et al. . Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era. Microb Cell Fact. 2019;18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TL, Kim E, Hwang C et al. . Short communication: conversion of lactose and whey into lactic acid by engineered yeast. J Dairy Sci. 2017;100:124–8. [DOI] [PubMed] [Google Scholar]
- Van Dyk TK. Bacterial efflux transport in Biotechnology. Adv Appl Microbiol. 2008;63:231–47. [DOI] [PubMed] [Google Scholar]
- Verhoeven MD, Bracher JM, Nijland JG et al. . Laboratory evolution of a glucose-phosphorylation-deficient, arabinose-fermenting S. cerevisiae strain reveals mutations in GAL2 that enable glucose-insensitive l-arabinose uptake. FEMS Yeast Res. 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira N, Casal M, Johansson B et al. . Functional specialization and differential regulation of short-chain carboxylic acid transporters in the pathogen Candida albicans. Mol Microbiol. 2010;75:1337–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li Y, Qiu C et al. . Identification of important amino acids in Gal2p for improving the L-arabinose transport and metabolism in Saccharomyces cerevisiae. Front Microbiol. 2017;8:1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shen Y, Zhang Y et al. . Improvement of L-arabinose fermentation by modifying the metabolic pathway and transport in Saccharomyces cerevisiae. Biomed Res Int. 2013;2013:461204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang Y, Wang J et al. . Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel. Nature. 2009;462:467. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang C, Liu G et al. . Elucidating the role and regulation of a lactate permease as lactate transporter in Bacillus coagulans DSM1. Appl Environ Microbiol. 2019;85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Shiwa Y, Itaya M et al. . Complete sequence of the first chimera genome constructed by cloning the whole genome of Synechocystis Strain PCC6803 into the Bacillus subtilis 168 genome. J Bacteriol. 2012;194:7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Guffanti AA, Ito M et al. . Bacillus subtilis YqkI Is a novel malic/Na+-lactate antiporter that enhances growth on malate at low protonmotive force. J Biol Chem. 2000;275:30287–92. [DOI] [PubMed] [Google Scholar]
- Wu B, Rambow J, Bock S et al. . Identity of a Plasmodium lactate/H+ symporter structurally unrelated to human transporters. Nat Commun. 2015;6:6284. [DOI] [PubMed] [Google Scholar]
- Wu W, Liu F, Singh S. Toward engineering E. coli with an autoregulatory system for lignin valorization. Proc Natl Acad Sci USA. 2018;115:2970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Zou W, Chen X et al. . Fumaric acid production in Saccharomyces cerevisiae by in silico aided metabolic engineering. PLoS One. 2012;7:e52086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Park SY, Park YS et al. . Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020, DOI: 10.1016/j.tibtech.2019.11.007. [DOI] [PubMed] [Google Scholar]
- Yang L, Christakou E, Vang J et al. . Overexpression of a C4-dicarboxylate transporter is the key for rerouting citric acid to C4-dicarboxylic acid production in Aspergillus carbonarius. Microb Cell Fact. 2017;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E, Poucher A, Comer A et al. . Functional survey for heterologous sugar transport proteins, using Saccharomyces cerevisiae as a host. Appl Environ Microbiol. 2011;77:3311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J-W, Jolkver E, Krämer R et al. . Identification and characterization of the dicarboxylate uptake system DccT in Corynebacterium glutamicum. J Bacteriol. 2008;190:6458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambanini T, Hosseinpour Tehrani H, Geiser E et al. . Metabolic engineering of Ustilago trichophora TZ1 for improved malic acid production. Metab Eng Commun. 2017;4:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelle RM, de Hulster E, van Winden WA et al. . Malic acid production by Saccharomyces cerevisiae: engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl Environ Microbiol. 2008;74:2766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang K, Mehmood M et al. . Deletion of acetate transporter gene ADY2 improved tolerance of Saccharomyces cerevisiae against multiple stresses and enhanced ethanol production in the presence of acetic acid. Bioresour Technol. 2017a;245:1461–8. [DOI] [PubMed] [Google Scholar]
- Zhang T, Song R, Wang M et al. . Regulating C4-dicarboxylate transporters for improving fumaric acid production. RSC Advances. 2017b;7:2897–904. [Google Scholar]
- Zhu N, Xia H, Yang J et al. . Improved succinate production in Corynebacterium glutamicum by engineering glyoxylate pathway and succinate export system. Biotechnol Lett. 2014;36:553–60. [DOI] [PubMed] [Google Scholar]
- Zientz E, Janausch I, Six S et al. . Functioning of DcuC as the C4-Dicarboxylate Carrier during Glucose Fermentation by Escherichia coli. J Bacteriol. 1999;181:3716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zientz E, Six S, Unden G. Identification of a third secondary carrier (DcuC) for anaerobic C4-dicarboxylate transport in Escherichia coli: roles of the three Dcu carriers in uptake and exchange. J Bacteriol. 1996;178:7241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]