Abstract
The present report examines the effects of repeated or single intrahippocampal Reelin infusions on measures of depressive-like behavior, cognition, and hippocampal neurogenesis in the repeated-corticosterone (CORT) paradigm. Rats received subcutaneous injections of CORT for 3 weeks and Reelin was infused through an inserted canula in the left hippocampus on days 7, 14, and 21, or only on day 21 of CORT injections. CORT increased immobility in the forced-swim test and impaired object-location memory. Notably, these effects were reversed by both repeated and single-Reelin infusions. CORT decreased both the number and complexity of doublecortin-labeled maturing newborn neurons in the dentate gyrus subgranular zone, and a single-Reelin infusion increased the number but not complexity of newborn neurons, while repeated Reelin infusions restored both. Injection of the AMPA antagonist CNQX blocked the rescue of the behavioral phenotype by Reelin but did completely block the effects of Reelin on hippocampal neurogenesis. Reelin is able to rescue the deficits in AMPA, NMDA, GABAA receptors, mTOR and p-mTOR induced by CORT. These novel results demonstrate that a single intrahippocampal Reelin infusion into the dorsal hippocampus has fast-acting antidepressant-like effects, and that some of these effects may be at least partially independent of Reelin actions on hippocampal neurogenesis.
Subject terms: Stress and resilience, Pharmacology, Emotion
Introduction
Depression is a debilitating psychiatric disorder that is currently estimated to be the leading cause of disability worldwide [1–4]. A recent meta-analysis has shown the effectiveness of antidepressants in relation to placebo [5, 6] although there are still issues about their real efficacy and ability to foster long-term remission of symptoms [7].
It seems clear thereby that novel efficacious and fast-acting antidepressants are required. This prompted numerous studies on the fast-antidepressant properties of ketamine [8–10] and recently resulted in the approval of esketamine by the US Food and Drug Administration for treatment-resistant depression [11].
Among the putative signal transduction pathways mediating the fast-antidepressant effects of ketamine are those implicating an upregulation of the Akt–mTOR pathway [10]. Interestingly, the extracellular matrix protein Reelin (which is downregulated in psychotic and mood disorders [12–14]) also stimulates the Akt–mTOR pathway [15, 16].
Reelin is primarily expressed by GABAergic interneuron subtypes in the adult cortex and hippocampus [17–19], and repeated administration of corticosterone (CORT) in rodents results in depressive-like behavior and decreases the number of Reelin-ir cells in the dentate gyrus subgranular zone (SGZ) of the rat hippocampus [20–22]. Immobility in the forced-swim test in CORT-treated rats is paralleled by slowed newborn granule-cell maturation and decreased Reelin expression in the SGZ [23, 24]. Conventional antidepressants like imipramine or anti-inflammatory drugs with antidepressant actions like etanercept protect against the deleterious effects of repeated CORT in the forced-swim test (FST), hippocampal Reelin expression, and dentate gyrus newborn granule cells' maturation [25–28], and cyclical corticosterone administration sensitizes depressive-like behavior in rodents [29]. Furthermore, animals expressing Reelin deficits are highly vulnerable to the behavioral effects induced by repeated CORT and show specific alterations in the expression of nitric oxide synthase in SGZ Reelin-ir cells that may perhaps relay to oxidative stress and mitochondrial dysfunctions [30–32].
It is not surprising then that genetically induced overexpression of Reelin prevents the expression of some behavioral and neurochemical alterations induced by repeated CORT [33]. Intraventricular injections of exogenous recombinant Reelin were shown to recover synaptic function and cognitive deficits in a mouse model of Angelman syndrome [34], and a single injection of exogenous Reelin was reported to increase hippocampal CA1 long-term potentiation, dendritic spine density, and spatial learning and memory in otherwise experimentally naïve mice [35].
This brought us to evaluate the hypothesis that repeated intrahippocampal infusions of exogenous recombinant Reelin will be able to prevent the development of behavioral and neurochemical alterations induced by repeated CORT, that a single-infusion of exogenous Reelin will be able to at least partially reverse the effects of repeated CORT (i.e. ketamine-like), and that these Reelin effects would be blocked by the AMPA receptor antagonist CNQX.
Materials and methods
Animals and experimental design
We used a total of 96 adult male Long-Evans rats purchased from Charles River (QC, Canada). All experimental procedures were in accordance with the guidelines of the Canadian Council and Animal Care.
For our first experiment we used a total of 60 rats assigned to the following treatment groups: 21 days of vehicle or CORT injections, while the remaining groups received either vehicle or CORT injections in addition to infusions of Reelin either: once on day 21 of injections, or three times, on days 7, 14, and 21 of injections. Half of the animals were sacrificed at time 1 while the other half were sacrificed at the end of this test at time 2 (Fig. 1aA, B).
Fig. 1. Experimental design, and evaluation of behavioral tests.
a Schematic representation of the experimental design used for the study. Stereotaxic surgery was performed and a chronic indwelling cannula was inserted into the left dorsal hippocampus. Animals were weight-matched and received 21 days of 40mg/kg of corticosterone (CORT) or vehicle injections. A subset of animals received infusions of 1 µg/µl of Reelin either on days 7, 14, and 21 of CORT/vehicle injections, or only on day 21. All rats underwent the forced-swim test (FST) on day 21, and a subset of rats were immediately sacrificed (time 1). The remaining rats then underwent the object-location memory paradigm (OBL) from days 23 to 28, and were then sacrificed (time 2). All experimental groups were evenly distributed throughout both times 1 and 2. Shown in panel C, a new group of animals underwent stereotaxic surgery and an indwelling cannula was implanted into left dorsal hippocampus. Animals were weight-matched and received 21 days of 40 mg/kg of CORT or vehicle injections. A subset of animals received infusions of 1 µg/µl Reelin on day 21, and another subset of animals received an infusion of 1 µg/µl of CNQX 30 min prior to the FST. b Mean body weight through days 1–21, collapsed across both times 1 and 2. Panel A shows that CORT-treated and CORT repeated-Reelin rats weighed less than vehicle, vehicle Reelin single, and vehicle repeated-Reelin rats on day 14 (p < 0.05) and 21 (p < 0.05) and significantly less than vehicle and vehicle Reelin single rats on day 7 (p < 0.05), and CORT Reelin single rats weighed significantly less than vehicle and vehicle Reelin single rats on days 7 and 14 (p < 0.05), and significantly less than vehicle, vehicle Reelin single, and vehicle repeated-Reelin rats on day 21 (p < 0.05). B–D: CORT had significant effects on the FST and OBL, and treatment with Reelin reversed this in a fast-acting manner. Data are collapsed across times 1 and 2. Panel B shows the effects of treatment on time spent immobile in the FST. CORT-treated rats spent significantly more time immobile than all other groups (p < 0.05). Panel C shows the effects of treatment on time spent struggling. CORT-treated rats spent significantly less time struggling than all other groups (p < 0.05). Panel D shows the effects of treatment on time spent swimming. CORT-treated rats spent significantly less time swimming than all other groups (p < 0.05). CORT had significant effects on OBL memory, and Reelin restored this. Panel E shows the discrimination ratio for the OBL variant. CORT-treated rats had a significantly lower discrimination ratio than all other groups (p < 0.05). All data are represented as means ± standard error of the mean.
We also used 30 rats to evaluate the effect of the AMPA receptor antagonist CNQX in blocking the effects of single intrahippocampal Reelin infusions. Rats were assigned to following groups: 21 days of vehicle injections or 21 days of CORT injections, while the remaining groups received either vehicle or CORT injections in addition to infusions of Reelin on day 21 of injections, and another group of animals received infusions of Reelin on day 21 and an infusion of CNQX on day 22, just 30 min prior to undergoing the FST (Fig. 1aC).
Corticosterone injections
All CORT and vehicle injections were administered subcutaneously once per day CORT (Steraloids) was suspended in 0.9% (w/v) physiological saline with 2% (v/v) Tween-80 (Sigma-Aldrich) and given at a dose of 40 mg/kg in a volume of 1 ml/kg.
Surgery, Reelin, and CNQX infusions
Surgery was conducted as previously described [36, 37]. Rats were deeply anesthetized and secured into a stereotaxic apparatus using ear bars. At flat skull position, a single cannula (C313G/spc, Plastics 1) was chronically implanted into the left hemisphere of the dorsal hippocampus using the following coordinates relative to Bregma: −3.5 mm anteroposterior, +2.6 mm mediolateral, −3.1 mm dorsoventral [38].
Recombinant Reelin (3820-MR-025/CR; R & D Systems) was used at a concentration of 1 µg per 1 µl in 0.1 M PBS (pH = 7.4). Intrahippocampal Reelin infusions were administered in a dedicated-procedures room for the duration of the experiments. We used a 2 µl Hamilton syringe secured to an infusion pump and infused a total of 1 μl of recombinant Reelin solution directly into the left dorsal hippocampus at a rate of 0.5 µl/min [34, 39].
CNQX (Cat. No. 0190; Tocris Bioscience) was used at a working concentration of 1 µg per 1 µl in a solution containing 20% DMSO and 80% saline, in line with previously published protocols using a similar approach [40, 41]. A total of 1 μl CNQX was infused on day 22, 30 min before the FST, at a rate of 0.5 μl/min.
Behavioral paradigms
The FST was conducted on day 22, the day after the final CORT injection and intrahippocampal Reelin infusion (Fig. 1a) [23, 42]. Each rat was individually placed in a Plexiglas swim tank (25 × 25 × 60 cm, 27 ± 2 °C water, 30 cm deep) for 10 min. We measured the duration of time each rat spent immobile, struggling, and swimming.
Rats that were not sacrificed at time 1 went on to complete the object-location test (OBL) for time 2. Rats received three habituation sessions before the training session. During the first two habituation sessions, rats were brought in pairs and placed in separate arenas for 10 min. During the last habituation session, rats were brought in individually and placed in the arena for 10 min. The last habituation occurred 24–48 h before the first testing session. In both sample and test phases, objects were placed in the corners of the arena 10 cm from each of the nearest walls and subjects were placed in the center of the arena facing the wall opposite the objects [43].
The rats were allowed to explore two identical objects for 4 min during the sample phase. Twenty-four hours later, rats underwent a testing phase, during which they explored two copies of the sample objects for 4 min, but with one object moved to a corner location at the front of the box while the other maintained its original position. Data were scored by a researcher who was blind to treatment conditions, using previously published measures [43, 44].
Immunohistochemistry
Rats were sacrificed on day 22 following the FST at time 1, or upon completing the OBL on day 28 at time 2 (Fig. 1a). Following anesthesia and perfusion, brains were kept in the same formaldehyde fixative for 48 h at 4 °C, and sectioned in the coronal plane at 30 μm on a vibrating microtome (VT1200s; Leica Biosystems). Sections were collected and stored at −20 °C until use in a cryoprotectant solution.
To ensure consistent immunohistochemical processing, we processed all sections in unison with treatment groups counterbalanced across all tissue plates. Control experiments omitting the primary antibody were unable to detect any immunoreactive cells.
Doublecortin (DCX) immunohistochemistry was run as previously described [23, 45]. Sections were incubated in a rabbit anti-DCX polyclonal primary antibody (1:1000, AB-561007; Cell Signalling Technologies) diluted in blocking solution for 24 h at room temperature. Followed by incubation for 1 h in a biotinylated secondary antibody (1:500, AB-2313606; Vector Laboratories) diluted in 5% (v/v) NGS, 1% (w/v) BSA, and 0.5% (v/v) Triton X-100 in 0.1 M TBS, followed by ABC complex (1:500, Vector Laboratories) for 1 h. Sections were visualized with 0.025% (w/v) DAB, 4.167% NiSO4, and 0.002% (v/v) H2O2.
Image analysis
Immunohistochemistry quantification was conducted as previously described [23, 45]. The SGZ was traced at ×4 magnification using a computerized stereology program [23]. The total number of immunopositive cells was estimated using the following formula: Ntotal = ∑Q− × 1/ssf × A(x, y step)/a(frame) × t/h. ∑Q− represents the number of counted cells, ssf is the section sampling fraction (1 in 12), A(x, y step) is the area associated with each x, y movement, a(frame) is the area of the counting frame, t is the weighted average section thickness, and h is the height of the dissector [23, 25, 45]. To avoid counting sectioning artifacts, we used a guard zone of 2 μm.
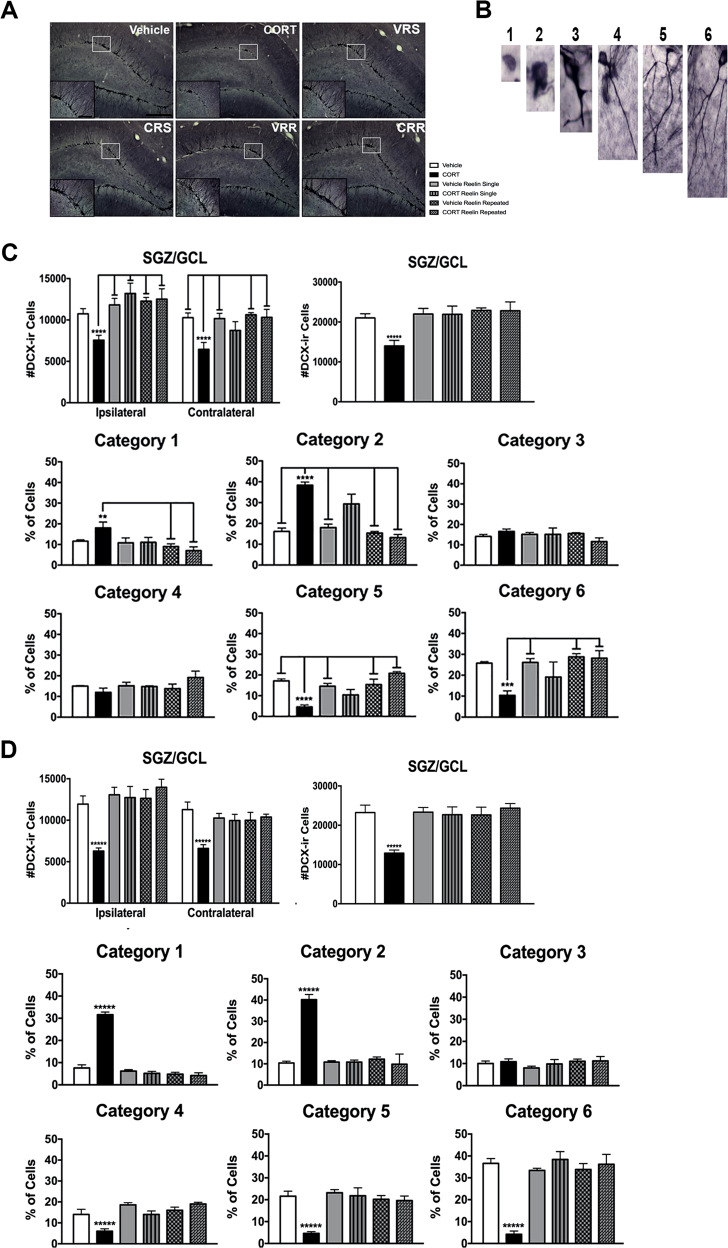
We used a dendritic categorization method that our laboratory has used in the past [23, 45]. A meander scan was used to randomly select 100 DCX-ir cells from each rat. A researcher blind to experimental conditions then assigned the cell to one of six complexity categories based on both the presence and extent of apical dendrites (see Fig. 2b). Data are presented as the percentage of DCX-ir cells for each of the six categories.
Fig. 2. Effects of CORT and Reelin on DCX-ir cells.
Effects of Reelin on hippocampal neurogenesis at times 1 (a–c) and 2 (d). CORT had significant effects on hippocampal neurogenesis. a Representative photomicrographs of doublecortin expression in the granule cell/subgranular zone. b Representative images of DCX-ir cell maturation that have been used in our categorization studies. c, d Effects of treatment on the number of doublecortin-ir cells in the granule cell/subgranular zone at times 1 and 2, respectively (see Fig. 1). CORT-treated rats had significantly fewer doublecortin-ir cells in the ipsilateral hemisphere than vehicle Reelin single, CORT Reelin single, vehicle repeated-Reelin, and CORT repeated-Reelin animals. In the contralateral hemisphere, CORT-treated rats had significantly fewer doublecortin-ir cells than vehicle, vehicle Reelin single, vehicle repeated-Reelin, and CORT repeated-Reelin animals (these effects are more evident, i.e. stronger, at time 2). Similarly, the reversal of CORT-induced alterations in the quantified categorization of dendritic complexity induced by Reelin infusions is evident already at time 1 and stronger at time 2. All data are represented as means ± standard error of the mean. VRS vehicle Reelin single, CRS CORT Reelin single, VRR vehicle repeated-Reelin, CRR CORT repeated-Reelin.
Synaptosomes and incubations
Six rats (three vehicle and three CORT-treated) were anesthetized with 5% isofluorane and killed by decapitation on day 22. Immediately after sacrifice, the hippocampus and cortex were dissected on ice, the hemispheres pooled together, and snap frozen in liquid nitrogen then stored until use at −80 °C.
Synaptosomes were obtained through homogenization of the collected tissue in ice-cold modified Krebs–Henseleit buffer (mKRBS) supplemented with a protease inhibitor cocktail (1860932; Thermoscientific). The homogenate was drawn into a 1 cc Luer lock syringe and passed through sequential filtrations [100 μm pore nylon filters (NY1H02500; EMD Millipore); 5 μm nitrocellulose Durapore membrane filters (SBLP01300; Millipore)], and was centrifuged at 1000 × g for 15 min at 4 °C, then the pellet (containing the synaptosomes) was resuspended in 350 μl pre-warmed (32 °C) ACSF. Synaptosomes were incubated for 30 min at 32 °C with 5% circling CO2 with either Reelin at a concentration of 5, 10, or 50 nM, or ACSF alone. Reactions were terminated with centrifugation at 1000 × g for 15 min at 4 °C in ice-cold mKRBS and the pellets were resuspended in 50 μl mKRBS supplemented with protease and phosphatase inhibitor cocktails (78428; Thermoscientific).
Western blotting
For western blot analysis, 10 μg of protein was electrophoretically resolved in 10% SDS-polyacrylamide gel at 200 V for 60 min, and then transferred onto PVDF membranes via wet transfer (100 V on ice for 90 min). Membranes were blocked using 5% (w/v) BSA for 1 h at room temperature. To evaluate SNP enrichment, PSD-95 (ab2723; Abcam) was used to compare against whole homogenate. Over all groups of SNPs, PSD-95, total mTOR (2983T; Cell Signaling Technology), and phosphorylated mTOR (p-mTOR) (2971S; Cell Signaling Technology) were measured. Antibodies were diluted in 10 ml of the blocking buffer and applied to the blots overnight at 4 °C. Blots were washed in TBST following incubation, and then incubated with the appropriate secondary antibody diluted at 1:5000 in blocking buffer for 1 h at room temperature. Luminata Crescendo (for p-mTOR and mTOR) or Classico (PSD-95) was use for chemiluminescent detection. All images were captured using a SynGene imaging system. Western blot bands were quantified using GeneTools companion program.
Statistical analyses
All statistical analyses were carried out using SPSS v24. We determined statistical significance using one-way analysis of variance (ANOVA), and post hoc comparisons were made using Tukey HSD when appropriate. The criterion for statistical significance was set at p < 0.05. All graphs depict the mean ± standard error of the mean.
Results
Significant group differences for body weight were observed already after 7 days of CORT and thereon, and were not rescued by Reelin infusions (Fig. 1bA).
CORT increases immobility behavior in the FST and intrahippocampal Reelin infusions rapidly reverse this deficit
We first evaluated if repeated-Reelin infusions could reverse the deficits induced by CORT in the FST (Fig. 1bB–D). We found significant group differences for immobility (F(3, 39) = 25.507, p < 0.001), struggling (F(3, 39) = 5.797, p < 0.01), and swimming (F(3, 39) = 14.707, p < 0.001) in the FST. Post hoc analyses revealed that CORT-treated animals spent more time immobile and less time swimming than all other groups (all p values <0.001). We then evaluated a second paradigm with a single-Reelin infusion given just 24 h before the FST (Fig. 1bB). We found significant group differences for immobility (F(5,59) = 18.01, p < 0.001), struggling (F(5,59) = 3.94, p < 0.01), and swimming (F(5,59) = 9.94, p < 0.001) (Fig. 1bB–D). Post hoc analyses revealed that CORT-treated rats spent significantly more time immobile and less time swimming compared to all other groups (all p values <0.5).
CORT impairs hippocampal-dependent memory and intrahippocampal Reelin infusions restores this deficit
We observed a significant main effect of repeated-Reelin treatment on OBL memory performance (F(3, 19) = 9.433, p < 0.001) (Fig. 1bE). Post hoc analyses revealed that CORT-treated animals had a significantly lower discrimination ratio than all other groups (all p values <0.01). We also observed a significant main effect of single-Reelin treatment on object-location performance (F(5, 29) = 7.74, p < 0.001). Post hoc analyses revealed that CORT-treated rats had a significantly lower discrimination ratio than all other groups (all p values <0.01).
Intrahippocampal Reelin infusions protect against the deleterious effects of CORT on hippocampal neurogenesis
At time 1 (Fig. 2a–c) stereological analyses revealed significant group differences in both the ipsilateral (F(5, 29) = 5.329, p < 0.01) and contralateral (F(5, 29) = 4.391, p < 0.01) dentate gyrus (Fig. 2a, b). Post hoc analyses revealed that CORT-treated rats had significantly fewer DCX-ir cells within the SGZ/GCL in the ipsilateral hemisphere than all other groups (p values <0.05) except for vehicle rats (p = 0.148). Interestingly, post hoc analyses revealed that in the contralateral hemisphere, CORT Reelin single-treated rats were not significantly different from CORT-treated rats (p = 0.317). We next examined dendritic complexity. We found significant group differences for categories 1 (F(5, 29) = 3.966, p < 0.01), 2 (F(5, 29) = 18.377, p < 0.001), 5 (F(5, 29) = 10.711, p < 0.001), and 6 (F(5, 29) = 4.043, p < 0.01).
We next analyzed changes at time 2 (Fig. 2d). Stereological analyses revealed significant group differences in both the ipsilateral (F(5, 29) = 7.958, p < 0.001) and contralateral (F(5, 29) = 5.266, p < 0.01) SGZ/GCL. Post hoc analyses revealed that CORT-treated rats had significantly fewer DCX-ir cells within the SGZ/GCL across both hemispheres than all other groups (all p values <0.05). We also found significant group differences for categories 1 (F(5, 29) = 98.072, p < 0.001), 2 (F(5, 29) = 64.019, p < 0.001), 4 (F(5, 29) = 9.359, p < 0.001), 5 (F(5, 29) = 10.172, p < 0.001), and 6 (F(5, 29) = 20.731, p < 0.001).
CNQX blocks the fast-acting effects of Reelin in reversing the depressive-like behavior induced by repeated CORT on the FST
We found significant group differences for immobility (F(5, 29) = 12.433, p < 0.001), struggling (F(5, 29) = 2.652, p < 0.05), and swimming (F(5, 29) = 9.494, p < 0.001) (Fig. 3). Post hoc analyses revealed that CORT and CORT Reelin single CNQX-treated rats spent significantly longer immobile than all other groups (p values <0.05), and CORT-treated rats spent significantly less time swimming than vehicle, vehicle Reelin single, and CORT Reelin single rats (p values <0.05), Lastly, CORT Reelin single CNQX-treated rats spent significantly less time swimming than vehicle, vehicle Reelin single, and CORT Reelin single-treated rats (p values <0.05).
Fig. 3. CORT had significant effects on depressive-like behavior, treatment with Reelin restored this, and CNQX abolished Reelin’s antidepressant effect.
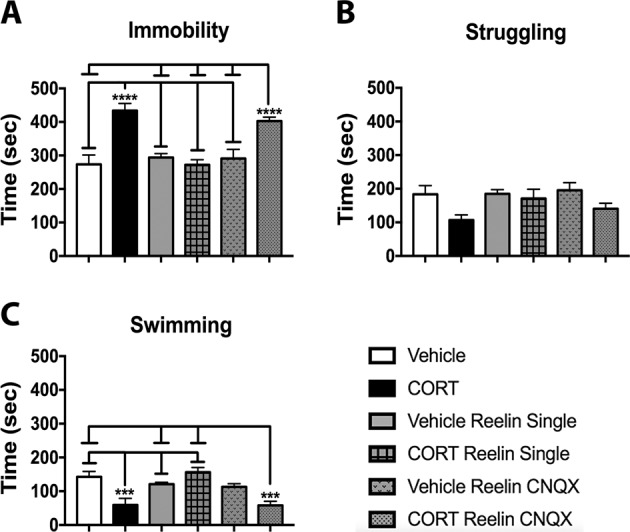
CORT and CORT Reelin CNQX-treated rats spent significantly more time immobile than all other groups (a) (p values <0.05). b Effect of treatment on time spent struggling. c Effect of treatment on time spent swimming. CORT-treated rats spent significantly less time swimming than vehicle, vehicle Reelin single, and CORT Reelin single-treated rats (p values <0.05), and CORT Reelin CNQX-treated rats spent significantly less time swimming than vehicle, vehicle Reelin single, and CORT Reelin single-treated rats (p values <0.05). All data are represented as means ± standard error of the mean.
CNQX fails to block the effects of Reelin in reversing the lower DCX-ir cell counts induced by repeated CORT
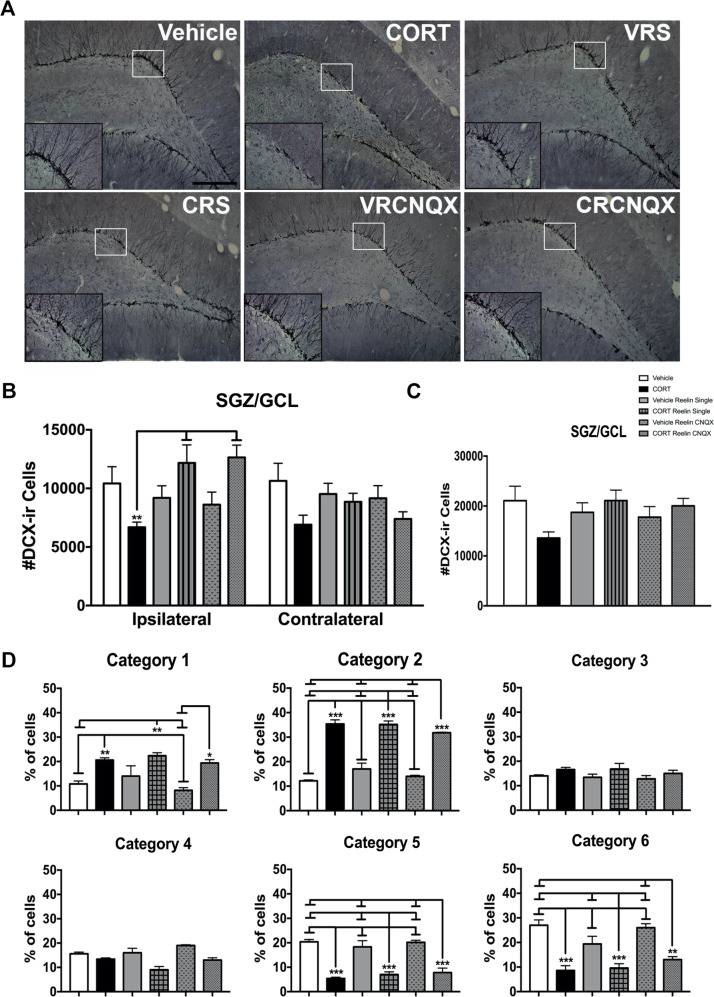
We found a significant main effect of treatment on the total number of DCX-ir cells (F(5, 29) = 3.859, p < 0.05) (Fig. 4). Post hoc analyses revealed that CORT-treated rats had significantly fewer DCX-ir cells in the ipsilateral hemisphere than CORT Reelin single and CORT Reelin single CNQX-treated rats (p values <0.05), but did not differ from vehicle (p = 0.231), vehicle Reelin single (p = 0.637), or vehicle Reelin single CNQX-treated rats (p = 0.839). We also found significant group differences for our dendritic complexity analyses: significant group differences emerged for the % of cells in categories 1 (F(5, 29) = 8.054, p < 0.001), 2 (F(5, 29) = 67.242, p < 0.001), 4 (F(5, 29) = 9.649, p < 0.001), 5 (F(5, 29) = 23.314, p < 0.001), and 6 (F(5, 29) = 15.193, p < 0.001).
Fig. 4. Effects of intrahippocampal Reelin and CNQX-treatment on hippocampal neurogenesis.
CORT had significant effects on hippocampal neurogenesis. a Representative photomicrographs of doublecortin expression in the granule cell/subgranular zone (scale bar = 200 μm). A higher magnification image is shown in the insets (scale bar = 50 μm). b Effects of treatment on the number of doublecortin-ir cells in the granule cell/subgranular zone. Overall, CORT-treated rats had significantly fewer doublecortin-ir cells than CORT Reelin single and CORT Reelin single CNQX-treated rats in the ipsilateral hemisphere (p values <0.05). c Quantified categorization of dendritic complexity using doublecortin staining. All data are represented as means ± standard error of the mean. VRS vehicle Reelin single, CRS CORT Reelin single, VRCNQX vehicle Reelin single CNQX, CRCNQX CORT Reelin single CNQX.
Reelin rescues repeated-CORT effects in mTOR and p-mTOR in hippocampal synaptosomes
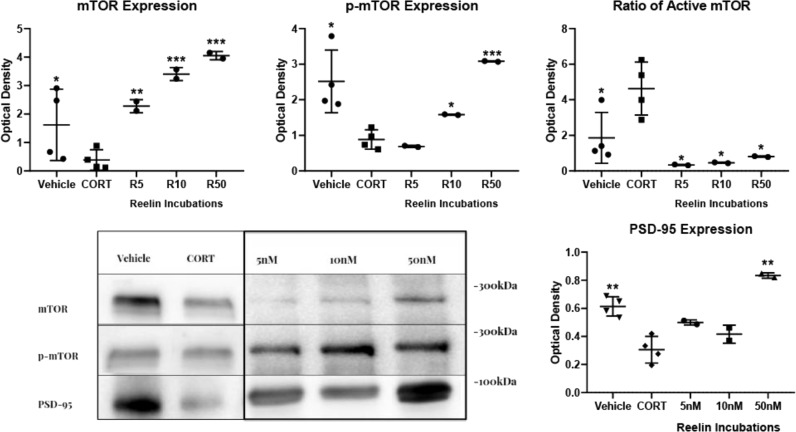
PSD-95 expression was significantly decreased in CORT-administered animals [t(5.269), p < 0.01], as well as expression of total mTOR [t(2.666), p < 0.05] and p-mTOR [t(0.0123), p < 0.05] (Fig. 5). Interestingly, CORT activity increased the ratio of mTOR activity over the vehicles [t(2.681), p < 0.05]. Significant differences were found between CORT and all experimental subgroups for PSD-95 [F(3,6) = 23.86, p < 0.001], mTOR [F(3,6) = 89.75, p < 0.0001], p-mTOR [F(3,6) = 69.6, p < 0.0001], and the ratio of active mTOR [F(3,6) = 12.17, p < 0.01]. PSD-95 was significantly increased at 50 nM Reelin (p < 0.01), but not at 5 and 10 nM. All concentrations of Reelin increased mTOR expression, with higher concentrations yielding higher expression (5 nM, p < 0.01; 10 nM, p < 0.001; 50 nM, p < 0.001). This pattern followed with p-mTOR, increasing with 10 nM (p < 0.05) and 50 nM (p < 0.001), though it was not increased at 5 nM.
Fig. 5. Western blot evaluation of PSD-95 and mTOR expression upon incubation with Reelin of hippocampal synaptosomes from CORT-treated animals.
Optical density analysis demonstrates that CORT decreases expression of mTOR, p-mTOR, active mTOR ratio (p-mTOR/mTOR), and PSD-95, and that Reelin incubations are able to rescue these deficits in a concentration-dependent manner.
We also observed that Reelin is able to rescue deficits in glutamatergic and GABAergic markers induced by CORT (Supplementary Data and Figs. 1–3).
Discussion
We have previously demonstrated that in the repeated-CORT paradigm both conventional tricyclic antidepressants like imipramine or anti-inflammatory drugs that have shown an antidepressant effect like etanercept are able to rescue the increases in immobility in the FST induced by repeated CORT [25, 26]. One should consider that although recent reports have partially questioned the validity of the FST to evaluate depression-like behavior [46], this test has been widely used to predict antidepressant efficacy [47], which was our rationale to use it to ascertain the putative antidepressant-like effects of exogenous Reelin in this study. We have also evaluated a hippocampal-dependent memory test (OBL) because cognitive disturbances in major depression related to memory impairments have been shown as prominent in major depression [48–50]. However, an extensive analysis of multiple depression-like behavioral tasks would be necessary to further evaluate the effects of exogenous Reelin.
Understanding the limitations and advantages of the use of animal models and behavioral tests in preclinical psychiatric research is essential for fostering translation of research data [51, 52], thereby we also believe as important the evaluation of Reelin effects on other models (and strains/species) for the study of depression-like deficits such as that using the chronic unpredictable stress paradigm.
The repeated-CORT paradigm results in similar behavioral alterations in the FST in male and female rats [53]. Although this provides a rationale for a single sex study, we recognize that further studies need to be done to evaluate putative sex differences that may underlie the antidepressant-like effects of exogenous Reelin.
Previous reports indicated that genetic overexpression of Reelin prevents the development of an increase in immobility in the FST after repeated CORT [33] prompting us to investigate if intrahippocampal infusions of exogenous Reelin administered weekly during CORT treatment would result in a preventive effect of the increase in immobility in the FST induced by CORT. Our results indicate that this is the case as repeated Reelin completely solves the increase in immobility and decrease in swimming time in the FST, and parallels the effects of imipramine [25] or the TNF-α antagonist etanercept [26] using the same paradigm.
We furthered our study by examining if a single intrahippocampal infusion of exogenous Reelin administered on the final day of CORT treatment would prevent the increase in immobility induced by CORT. Our data have shown that Reelin infusions have a fast-effect in reversing the alterations in the FST induced by repeated CORT that resemble those of ketamine. In fact, our studies also provide some additional data towards that as synaptosomes from CORT-treated animals show downregulation in levels of PSD-95, mTOR, and p-mTOR, that are rescued by in vitro incubations with Reelin in a concentration-dependent manner, similarly to the effects of ketamine [54, 55].
Chronic stress also impairs hippocampal-dependent memory as assayed by the OBL [26, 56, 57]. Reelin is critically involved in cognition, particularly spatial memory [35]. Furthermore, Reelin supplementation in a mouse model of Angelman syndrome recovers deficits in spatial learning and memory [34]. Our findings are in line with previous research, as we report that both repeated and single intrahippocampal Reelin infusions restore CORT-induced deficits in OBL memory.
The hippocampus requires NMDA receptor synaptic plasticity [58] and hippocampal Reelin regulates the composition and trafficking of NMDA receptor subunits [59], and one can surmise that intrahippocampal Reelin infusions may have played a role in the normalization of cognitive behavior in CORT-treated animals, and most likely require a contribution of glutamate. Our data demonstrate that repeated CORT brings about alterations in hippocampal glutamatergic and GABAergic receptors that probably underlie some of the excitatory/inhibitory imbalance brought about by chronic stress [28, 33], and that Reelin infusions are able to reverse these effects.
The role of hippocampal neurogenesis in depression is currently unclear, as antidepressant effects can be achieved without increases in neurogenesis [60] and studies of post-mortem depressed tissue have generally failed to find reductions in neurogenesis [61]. However, increasing neurogenesis through a transgenic mouse line rescued a CORT-induced depressive phenotype [62]. It seems that deficits in neurogenesis may be associated more with a lack of response to antidepressant treatment than with the development of a depressive phenotype [63]. Interestingly, Pujadas et al. [64] found that mice overexpressing Reelin have a significant increase in DCX-ir granule cells, accompanied by increased complexity in their dendrites. We have shown that CORT decreases hippocampal neurogenesis, and the tricyclic antidepressant imipramine restores this [23, 25]. Repeated infusions of exogenous Reelin result in a rescuing of both the number and maturation of DCX-ir cells in the dentate SGZ in both the ipsilateral and contralateral sides to the Reelin infusion when animals are sacrificed at times 1 or 2. Interestingly, single infusions of exogenous Reelin that are able to completely reverse CORT effects in the FST only have a partial effect in rescuing DCX-ir SGZ neurons. This may indicate that a complete restoration of DCX-ir neurons numbers and maturation is not a fast event and might not be necessary for rescuing the phenotype in the FST.
Intrahippocampal infusion of CNQX thirty minutes before the FST was enough to block the rescuing effects of Reelin on immobility induced by repeated CORT but failed to block the effects of exogenous Reelin in hippocampal neurogenesis. As some fast-antidepressant actions of ketamine appear to be mediated by its actions on AMPA receptors [65] it seemed logic to develop an experimental design to evaluate if an antagonist of AMPA receptors could block Reelin actions in rescuing the effects of repeated CORT. While we have shown this to be the case for the behavioral effects in the FST, that does not happen when analyzing hippocampal neurogenesis by evaluation of DCX-ir neurons in the dentate SGZ. Considering the data available implicating hippocampal neurogenesis in antidepressant effects [66], our data could indicate that perhaps some behavioral components may rely on Reelin effects on modulating glutamatergic synaptic strength while other behaviors could relate to Reelin effects on hippocampal neurogenesis and newborn cells maturation.
It is also important to reflect that in un-lesioned animals, psychotropic drugs administration also results in alterations in Reelin expression levels as well as in molecules mediating the Reelin signal transduction pathways [67], providing further indication that Reelin levels may relate to some pathological/therapeutic events in mood and psychotic disorders.
In conclusion, our data indicate a putative fast-action of Reelin that may result in some antidepressant-like actions. However, there is a necessity to provide additional probes of a possible fast-antidepressant-like actions of Reelin mostly when considering possible limitations associated with preclinical models and behavioral tests.
Funding and disclosure
The authors declare no competing financial interests. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to LK and HC. KB is supported by an NSERC Doctoral Canada Graduate Scholarship.
Supplementary information
Authors contributions
KJB, JJ, JB, RR-T, MAM, and JA: acquired data, interpreted results, wrote first draft (KJB) or revised the manuscript, approved the final version. GP, HC, and LK: conceived experiments and designed work, interpreted data, revised manuscript, approved the final version.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hector J. Caruncho, Lisa E. Kalynchuk
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-0609-z).
References
- 1.Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization) Depression and other common mental disorders: global health estimates. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.IHME (Institute for Health Metrics and Evaluation IHME) Findings for the global burden of disease study 2017. Seattle, WA: IHME; 2018. [Google Scholar]
- 4.Mahli GS, Mann JJ. Depression. Lancet. 2018;392:2299–312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 5.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinohara K, Efthimiou O, Ostinelli EG, Tomlinson A, Geddes JR, Nierenberg AA, et al. Comparative efficacy and acceptability of antidepressants in the long-term treatment of major depression: protocol for a systematic review and network meta-analysis. BMJ Open. 2019;9:e027574. doi: 10.1136/bmjopen-2018-027574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack J, Korownyk C. Effectiveness of antidepressants. BMJ. 2018;360:k1073. doi: 10.1136/bmj.k1073. [DOI] [PubMed] [Google Scholar]
- 8.Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharm Rev. 2018;70:621–60. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould TD, Zarate CA, Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharm Toxicol. 2019;59:213–36. doi: 10.1146/annurev-pharmtox-010617-052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadriu B, Mussazi L, Henter ID, Graves M, Poppoli M, Zarate CA., Jr Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol. 2019;22:119–35. doi: 10.1093/ijnp/pyy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression. First FDA-approved antidepressant in a new class. N. Engl J Med. 2019;381:1–4. doi: 10.1056/NEJMp1903305. [DOI] [PubMed] [Google Scholar]
- 12.Impagnatiello F, Guidotti A, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A decrease of Reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA. 1998;95:15718–23. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti A, Auta J, Davis JM, DiGiorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in Reelin and glutamic-acid decarboxylase 67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–9. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 14.Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;2000:654–63. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 15.Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E, et al. A Reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci USA. 2003;100:5479–84. doi: 10.1073/pnas.1031602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee GH, D’Arcangelo G. New insights into Reelin-mediated signaling pathways. Front Cell Neurosci. 2016;10:122. doi: 10.3389/fncel.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, et al. Regional and cellular patterns of Reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–99. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, et al. Reelin in preferentially expressed in neurons synthesizing γ-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci USA. 1998;95:3221–6. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ. Cortical bitufted, horizontal and Martinotti cells preferentially express and secrete Reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci USA. 1999;96:3217–22. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168:280–8. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged corticosterone exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:777–90. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Lussier AL, Caruncho HJ, Kalynchuk LE. Repeated exposure to corticosterone, but not restraint, decreases the number of Reelin-positive cells in the adult rat hippocampus. Neurosci Lett. 2009;460:170–4. doi: 10.1016/j.neulet.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 23.Lussier AL, Lebedeva K, Fenton EY, Guskjolen A, Caruncho HJ, Kalynchuk LE. The progressive development of depression-like behavior in corticosterone-treated rats is paralleled by slowed granule cell maturation and decreased Reelin expression in the adult dentate gyrus. Neuropharmacology. 2013;71:174–83. doi: 10.1016/j.neuropharm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Caruncho HJ, Brymer K, Romay-Tallon R, Mitchell MA, Rivera-Baltanas T, Botterill J, et al. Reelin-related disturbances in depression: implication for translational studies. Front Cell Neurosci. 2016;10:48. doi: 10.3389/fncel.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenton EY, Fournier NM, Lussier A, Romay-Tallon R, Caruncho HJ, Kalynchuk LE. Imipramine protects against the deleterious effects of chronic corticosterone on depression-like behavior, hippocampal Reelin expression and neuronal maturation. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:52–9. doi: 10.1016/j.pnpbp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Brymer KJ, Fenton EY, Kalynchuk LE, Caruncho HJ. Peripheral etanercept administration normalizes behavior, hippocampal neurogenesis, and hippocampal Reelin and GABAA expression in a preclinical model of depression. Front Pharmacol. 2018;9:121. doi: 10.3389/fphar.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brymer KJ, Romay-Tallon R, Allen J, Caruncho HJ, Kalynchuk LE. Exploring the potential antidepressant mechanisms of TNFα antagonists. Front Neurosci. 2019;13:98. doi: 10.3389/fnins.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lussier AL, Romay-Tallon R, Caruncho HJ, Kalynchuk LE. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience. 2013;231:38–48. doi: 10.1016/j.neuroscience.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 29.Lebedeva KA, Caruncho HJ, Kalynchuk LE. Cyclical corticosterone administration sensitizes depression-like behavior in rats. Neurosci Lett. 2017;650:45–51. doi: 10.1016/j.neulet.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Lussier AL, Romay-Tallon R, Kalynchuk LE, Caruncho HJ. Reelin as a putative vulnerability factor for depression: examining the depressiogenic effects of repeated corticosterone in heterozygous reeler mice. Neuropharmacology. 2011;60:1064–74. doi: 10.1016/j.neuropharm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Romay-Tallon R, Rivera-Baltanas T, Kalynchuk LE, Caruncho HJ. Differential effects of corticosterone on the colocalization of Reelin and neuronal nitric oxide synthase in the adult hippocampus in wild type and heterozygous reeler mice. Brain Res. 2015;1594:274–83. doi: 10.1016/j.brainres.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 32.Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, Kalynchuk LE. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front Neurosci. 2018;12:386. doi: 10.3389/fnins.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixeira CM, Martin ED, Sahun I, Masachs N, Pujadas L, Corvelo A, et al. Overexpression of Reelin prevents the manifestation of behavioural phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology. 2011;36:2395–405. doi: 10.1038/npp.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hethorn W, Ciarlone S, Filonova I, Rogers JT, Aguirre D, Ramirez RA, et al. Reelin supplementation recovers synaptic plasticity and cognitive deficits in a mouse model for Angelman syndrome. Eur J Neurosci. 2015;41:1372–80. doi: 10.1111/ejn.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers JT, Rusiana I, Trotter J, Zhao L, Donaldson E, Pak DT, et al. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem. 2011;18:558–64. doi: 10.1101/lm.2153511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botterill JJ, Brymer KJ, Caruncho HJ, Kalynchuk LE. Abberrant hippocampal neurogenesis after limbic kindling: relationship to BDNF and hippocampal-dependent memory. Epilepsy Behav. 2015;47:83–92. doi: 10.1016/j.yebeh.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 37.Botterill JJ, Nogovitsyn N, Caruncho HJ, Kalynchuk LE. Selective plasticity of hippocampal GABAergic interneuron populations following kindling of different brain regions. J Comp Neurol. 2016;525:389–406. doi: 10.1002/cne.24071. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academic Press; 1998. [Google Scholar]
- 39.Rogers JT, Zhao L, Trotter JH, Rusiana I, Peters MM, Li Q, et al. Reelin supplementation recovers sensorimotor gating, synaptic plasticity and associative learning deficits in the heterozygous reeler mouse. J Psychopharmacol. 2013;27:386–95. doi: 10.1177/0269881112463468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies DA, Greba Q, Howland JG. GluN2B-containing NMDA receptors and AMPA receptors in medial prefrontal cortex are necessary for odor span in rats. Front Behav Neurosci. 2013;7:183. doi: 10.3389/fnbeh.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izquierdo I, Bianchin M, Silva MB, Zanatta MS, Walz R, Ruschel AC, et al. CNQX infused into rat hippocampus or amygdala disrupts the expression of memory of two different tasks. Behav Neural Biol. 1993;59:1–4. doi: 10.1016/0163-1047(93)91061-Q. [DOI] [PubMed] [Google Scholar]
- 42.Gregus A, Wintink AJ, Davi AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156:105–14. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Howland JG, Cazakoff BN, Zhang Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience. 2012;201:184–98. doi: 10.1016/j.neuroscience.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howland JG, Cazakoff BN. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object-place recognition memory. Neurobiol Learn Mem. 2010;93:261–7. doi: 10.1016/j.nlm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Botterill JJ, Guskjolen AJ, Marks WM, Caruncho HJ, Kalynchuk LE. Limbic but not non-limbic kindling impairs conditioned fear and promotes plasticity of NPY and its Y2 receptor. Brain Struct Funct. 2015;220:3641–55. doi: 10.1007/s00429-014-0880-z. [DOI] [PubMed] [Google Scholar]
- 46.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017;8:955–60. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuen E, Swanson S, Witkin JM. Prediction of human efficacious antidepressant doses using the mouse forced swim test. Pharm Biochem Behav. 2017;161:22–9. doi: 10.1016/j.pbb.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:851–61. doi: 10.1016/S2215-0366(19)30291-3. [DOI] [PubMed] [Google Scholar]
- 49.Kube T, Schwarting R, Rozenkrantz L, Glombiewski JA, Rief W. Distorted cognitive processes in major depression: a predictive processing perspective. Biol Psychiatry. 2019;pii: S006-3223(19):31550–1. doi: 10.1016/j.biopsych.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2019. 10.1038/s41380-019-0615-x. [DOI] [PMC free article] [PubMed]
- 51.Herzog DP, Beckmann H, Lieb K, Ryu S, Muller MB. Understanding and predicting antidepressant response: using animal models to move toward precision psychiatry. Front Psychiatry. 2018;9:512. doi: 10.3389/fpsyt.2018.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bale TL, Abel T, Akil H, Carlezon WA, Jr, Moghaddam D, Nestler EJ, et al. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019;44:1349–53. doi: 10.1038/s41386-019-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalynchuk LE, Gregus A, Boudreau D, Perrot-Sinai TS. Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behav Neurosci. 2004;118:1365–77. doi: 10.1037/0735-7044.118.6.1365. [DOI] [PubMed] [Google Scholar]
- 54.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou W, Wang N, Yang C, Li X-M, Zhou Z-Q, Yang J-J. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29:419–23. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK. Object location and object recognition memory impairments, motivation deficits and depression in a model of gulf war illness. Front Behav Neurosci. 2014;8:78. doi: 10.3389/fnbeh.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffman AN, Lorson NG, Sanabria F, Olive MF, Conrad CD. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal fos expression in an animal model of post-traumatic stress disorder. Neurobiol Learn Mem. 2014;112:139–47. doi: 10.1016/j.nlm.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barker GR, Warburton EC. NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci. 2008;28:2837–44. doi: 10.1523/JNEUROSCI.4447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bosch C, Masachs N, Exposito-Alonso D, Martinez A, Teixeira CM, Fernaud I, et al. Reelin regulates the maturation of dendritic spines, synaptogenesis and glial ensheathment of newborn granule cells. Cereb Cortex. 2016;2016:4282–98. doi: 10.1093/cercor/bhw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589–602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–22. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 62.Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–78. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park S-C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019;377:95–106. doi: 10.1007/s00441-019-03043-5. [DOI] [PubMed] [Google Scholar]
- 64.Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, et al. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30:4636–49. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanos P, Moaddel R, Morris PJ, Georgiou P, FIschell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Planchez B, Surget A, Belzung C. Adult hippocampal neurogenesis and antidepressants effects. Cur Opin Pharm. 2020;50:17–24. doi: 10.1016/j.coph.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Fatemi SH, Reutiman TJ, Folson TD. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats. Schizophrenia Res. 2009;111:138–52. doi: 10.1016/j.schres.2009.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.