Graphical abstract
Abbreviations: ADP, adenosine diphosphate; Ala, alanine; AMP, adenosine monophosphate; Asp, aspartate; Cho, choline; Cre/pCre, creatine/phosphocreatine; GABA, γ-Aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; IMP, inosine monophosphate; Ino, inosine; Lac, lactate; Myo, myo-inositol; NAA, N-acetylaspartate; NAD+, nicotinamide adeninedinucleotide; Suc, succinate; Tau, taurine
Keywords: Diabetes, Gender, Metabolomics, Neurotransmitter, Cortex, Hippocampus
Highlights
-
•
Sex-specific metabolic differences occur in all brain regions of mice.
-
•
The hippocampal metabolism is more susceptible to T1D.
-
•
Metabolic disorders start at the cortex and hippocampus in female T1D mice.
-
•
Female mice show a hypometabolism status in the brain from normal to T1D.
-
•
T1D affects brain region-specific metabolic changes in a sex-specific manner.
Abstract
Type 1 diabetes (T1D) can cause brain region-specific metabolic disorders, but whether gender influences T1D-related brain metabolic changes is rarely reported. Therefore, here we examined metabolic changes in six different brain regions of male and female mice under normal and T1D conditions using an integrated method of NMR-based metabolomics and linear mixed-model, and aimed to explore sex-specific metabolic changes from normal to T1D. The results demonstrate that metabolic differences occurred in all brain regions between two genders, while the hippocampal metabolism is more likely to be affected by T1D. At the 4th week after streptozotocin treatment, brain metabolic disorders mainly occurred in the cortex and hippocampus in female T1D mice, but the striatum and hippocampus in male T1D mice. In addition, anaerobic glycolysis was significantly altered in male mice, mainly in the striatum, midbrain, hypothalamus and hippocampus, but not in female mice. We also found that female mice exhibited a hypometabolism status relative to male mice from normal to T1D. Collectively, this study suggests that T1D affected brain region-specific metabolic alterations in a sex-specific manner, and may provide a metabolic view on diabetic brain diseases between genders.
1. Introduction
Type 1 diabetes (T1D) is characterized by hyperglycemia and insulin deficiency due to autoimmune destruction of pancreatic β‐cells [1]. In 2017, the International Diabetes Federation (IDF) reported that more than one million children and adolescents suffered from T1D in the world [2]. Moreover, T1D can cause a series of complications invaded many organs in the body, which seriously affect human health [3]. Of note, sex differences exist in both innate and adaptive immune responses [4], resulting in different susceptibility to T1D [5], [6] between males and females. Therefore, exploring sex-specific pathological mechanisms will raise the possibility of gender-based prevention and treatment for T1D and its complications.
T1D-associated cognitive decline (DACD) is one of serious neurobehavioral complications in advanced stage diabetes [7]. In recent years, DACD has attracted increasing attention and several possible pathogeneses have also been proposed [8], [9]. For example, Wessels et al. [10] and Duinkerken et al. [11] revealed that cognitive impairment may be attributed to the reductions of white matter volume and functional connectivity in the brain of untreated T1D patients. Like Alzheimer's disease (AD), β-amyloid and tau proteins-induced neuronal death can also result in the development of DACD [12], [13]. In addition, neuroinflammation and oxidative stress could be responsible for DACD [14], [15]. In our previous study, we reported that T1D-driven gut dysbiosis may down-regulate glutamine-glutamate cycle and energy metabolism in the hippocampus and thereby cause DACD [16]. In earlier years, brain metabolic disorders have been detected in untreated T1D patients using magnetic resonance spectroscopy [17], [18]. For animal studies, using NMR-based metabolomics, our previous findings revealed that brain region-specific metabolic changes might be implicated in cognitive impairment in rats at the 8th week after streptozotocin (STZ) treatment [19], 17-week-old db/db mice [20] and 10-month-old APP/PS1 transgenic AD mice [21]. These results indicate that brain metabolic disorders also play a key role in diabetic brain diseases in an advanced stage. To our knowledge, however, little information has been made available regarding sex differences in region-specific metabolic alterations of the T1D brain especially in early and middle stages.
In this study, therefore, we examined metabolic profiles in six different brain regions (hypothalamus, cerebellum, hippocampus, striatum, midbrain and cortex) of male and female mice at the 4th week after STZ treatment. Subsequently, a linear mixed model (LMM) was used to analyze the effects of gender, diabetes and their interaction on brain region-specific metabolic changes. The purpose of this study was to uncover sex-and region-specific metabolic differences in the brain of T1D.
2. Materials and methods
2.1. Animals
Six-week-old C57BL/6 mice (male, n = 12; female, n = 12) were purchased from the SLAC Laboratory Animal Co. Ltd. (Shanghai, China). All mice were housed in the specific-pathogen-free (SPF) colony under a fully controlled condition (temperature, 22 ± 1 °C; humidity, 55 ± 5%; light/dark cycle, 12 h/12 h) at the Laboratory Animal Center of Wenzhou Medical University (Wenzhou, China). Mice were given free access to standard mouse chow and tap water. This study was conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University.
2.2. Streptozotocin (STZ)-induced diabetic mouse model
After one week of acclimation, male and female mice were weighed and randomly divided into control (CON, n = 6) and diabetic (T1D, n = 6) groups, respectively. The development of T1D mouse model was referenced to the protocol proposed by Furman [22] with minor modification. In order to avoid the high-dose STZ-induced death of mice, the multiple and low-dose STZ approach was performed in the present study. Briefly, after 12 h fasting, all mice in the T1D group were intraperitoneally (IP) received a five-day injection of STZ (Sigma-Aldrich) solution at a dose of 50 mg/kg body weight. The STZ solution was freshly prepared in 0.1 mol/L citrate buffer (pH = 4.5). Since STZ is a hazardous chemical substance, researchers must wear medical masks and gloves during STZ preparation and injection. Moreover, all materials used in this procedure must be bagged and labeled with a biohazard warning before incineration. The CON mice were intraperitoneally injected with the same volume of citrate buffer. Blood glucose level was measured after 3 days of STZ injection from a tail nick using a handheld glucometer (ACCU-CHEK Active, Mannheim, Germany), and diabetic mice were defined when their blood glucose levels were higher than 11.1 mmol/L. In this study, all male and female mice after STZ treatment were developed into diabetic mice. During the whole experiment, mice were not treated with any of drugs and their blood glucose levels and body weights were monitored once a week (see supplemental data Tables S1 and S2).
2.3. Sample collection and metabolite extraction
In this study, all mice were sacrificed at the 4th week after STZ injection by decapitation under isoflurane anaesthesia. The whole brain was collected immediately and then divided into six different brain regions, including the cortex, cerebellum, hippocampus, hypothalamus, midbrain and striatum, according to the mouse brain anatomia [23]. All brain tissues were promptly frozen in liquid nitrogen and kept at −80 °C until use. Metabolite extraction of brain tissue was referenced to a previously described method [19]. Firstly, the frozen tissue was weighted into an Eppendorf tube, and mixed with 4 mL/g ice-cold methanol and 0.85 mL/g distilled water. The mixture was homogenized completely with a handheld homogenizer (Xinzhi biotechnology co. LTD, Ningbo, China) and added with ice-cold chloroform (2 mL/g) and distilled water (2 mL/g). Then, the mixture was vortexed for 15 s, placed on ice for 15 min, and centrifuged at 10,000 g for 15 min at 4 °C. Finally, the supernatant was transferred to a new Eppendorf tube, lyophilized for 24 h, and stored at −80 °C until metabolomics analysis.
2.4. NMR-based metabolomic analysis
The 1H NMR spectra of brain tissues were measured using a Bruker AVANCE III 600 MHz NMR spectrometer (Bruker BioSpin, Rheinstetten, Germany) at 298 K. The freeze-dried extract was redissolved with 500 μL D2O (99.5%, Isotope laboratory, Cambridge, USA) containing 0.05% of sodium trimethylsilyl propionate-d4 (TSP) and then transferred to a 5 mm NMR tube for metabolomics analysis. A standard single-pulse sequence with water signal pre-saturation (ZGPR) was performed and the main acquisition parameters were set as follows: scans, 256; data points, 64 K; spectral width, 12000 Hz; acquisition time, 2.65 s/scan; relaxation delay, 6 s.
All NMR spectra were manually corrected for their phases/baselines and referenced to TSP peak at 0 ppm using Topspin software (v2.1 pl4, Bruker Biospin, Germany). The ‘icoshift’ procedure was applied to align NMR spectra using MATLAB software (R2012a, The Mathworks Inc., Natick, MA, USA) [24]. Furthermore, the spectral region from 0.5 to 9.0 ppm without residual water signals (4.5–5.5 ppm) was subdivided with a size of 0.01 ppm and integrated to binning data for multivariate analysis.
2.5. Multivariate data analysis
Prior to multivariate analysis, metabolic data were log-transformed and Pareto-scaled. First of all, principal component analysis (PCA) was employed to obtain the overview of metabolic pattern changes among different brain regions in both male and female mice from normal to diabetes using SIMCA-P+12.0 software (Umetrics AB, Umea, Sweden). Subsequently, orthogonal projections to latent structures discriminant analysis (OPLS-DA) was performed to maximize the metabolic difference between two groups using SIMCA software. Important metabolites that mainly contributed to the metabolic pattern separations were identified via the variable importance in the projection (VIP) scores.
2.6. Metabolite identification and pathway analysis
Metabolite signals in NMR spectra were assigned according to Chenomx NMR suite 7.5 software (Chenomx Inc., Alberta, Canada), reported data [19], [20] and the HMDB 4.0 [25]. Moreover, a 13C–1H heteronuclear single quantum coherence (HSQC) experiment was used to confirm uncertain identifications. The relative concentration of metabolite was calculated via its peak area by reference to the TSP concentration and expressed as μmol/g fresh weight tissue. Metabolic pathway was constructed according to KEGG database [26] and manually drawn in Adobe Photoshop CS6 (Adobe Systems Inc., San Jose, CA).
2.7. Statistical analysis
In the present study, all mice were randomly assigned to the experimental procedures including housing and feeding, STZ injection, sample collection, metabolite extraction and NMR analysis. Changes in blood glucose level and body weight of mice after STZ treatment were analyzed by a repeated measure analysis of variance (ANOVA) in SPSS 22 software (IBM Corp, USA). To estimate the effects of gender, diabetes and their interaction on the change in specific metabolite, a linear mixed-model (LMM) ANOVA was employed by the MIXED procedure in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). In this model, gender (G), diabetes (D) and their interaction (GD) were set as fixed effects, and the individual and model intercept were set as random effects, as shown in Eq. (1):
| (1) |
where M is the relative concentration of metabolite, parameters α, β and γ are model coefficients, and ε is the random effect.
Metabolite data were calculated by a least-square-means (LSM) procedure with SAS software and presented as LSM and standard error (LSM ± SE). Pair-wise multiple comparisons were analyzed using Student’s t test with Bonferroni adjustment. A statistically significant difference was defined when P value <0.05.
3. Results
3.1. Sex-specific changes in body weight and blood glucose in T1D mice
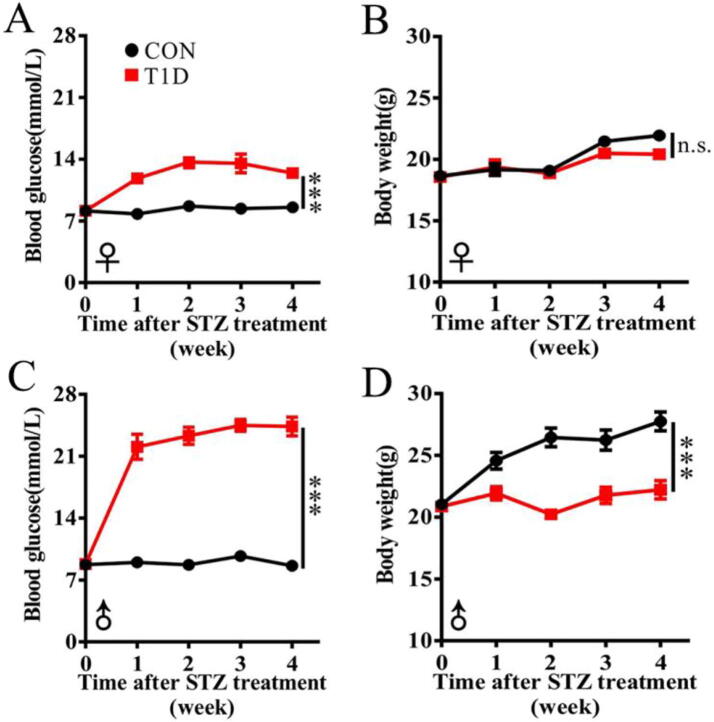
In this study, we examined sex differences in body weight and blood glucose level of STZ-induced type 1 diabetic (T1D) and age-matched control (CON) mice, as shown in Fig. 1. Female mice had a similar level of blood glucose relative to male mice (8.13 ± 0.28 mmol/L vs. 8.73 ± 0.41 mmol/L), but body weight was relatively higher in male mice than that in female mice (21.02 ± 0.19 g vs. 18.67 ± 0.27 g). After STZ treatment, as expected, blood glucose level was significantly increased in both female (Fig. 1A) and male (Fig. 1C) mice. However, of note, male mice exhibited a 2-fold increase in blood glucose level after STZ injection, while female mice only had a 1-fold increase. More interestingly, we observed that body weight was significantly reduced in male T1D mice (Fig. 1D), but not in female T1D mice (Fig. 1B). Together, our results suggest that male mice showed more typical diabetic symptoms relative to female mice after STZ induction.
Fig. 1.
Sex-specific changes in blood glucose and body weight of mice after streptozotocin treatment. Changes in blood glucose level (A) and body weight (B) in female mice treated with streptozotocin; Changes in blood glucose level (C) and body weight (D) in male mice treated with streptozotocin. Group: CON, normal control mice; T1D, streptozotocin-induced type 1 diabetic mice. Gender: ♀, female; ♂, male. Data are presented as mean ± SEM (n = 6 for each group). The differences in blood glucose level and body weight between CON and T1D mice were analyzed by repeat-measurement ANOVA. Significant level: ***P < 0.001; n.s., no significant.
3.2. Sex-specific changes in metabolic patterns in the brain of T1D mice
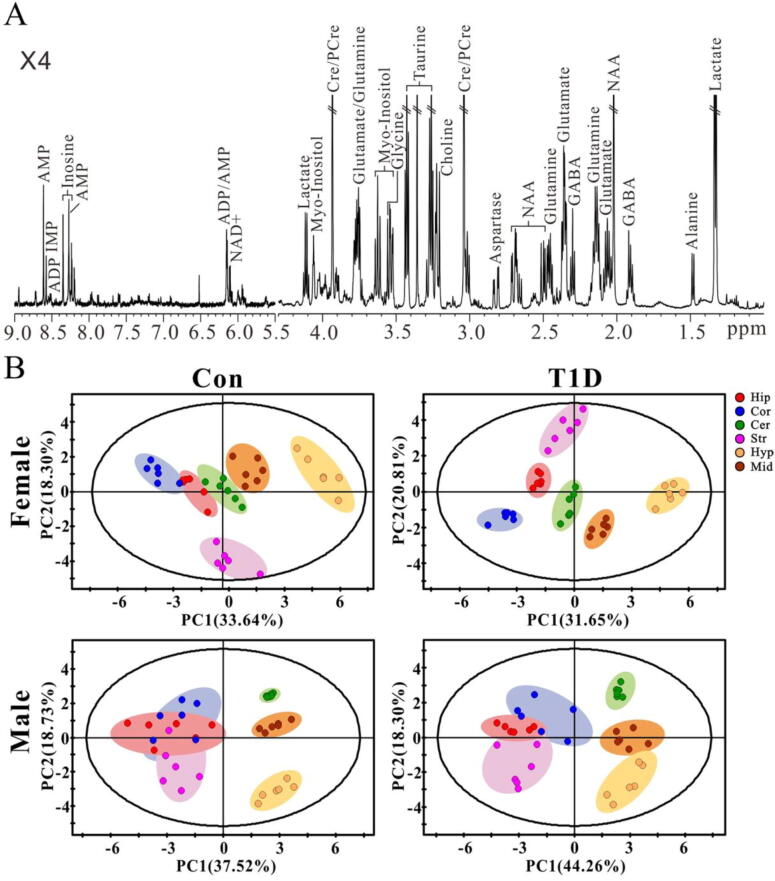
To examine sex differences in brain metabolic changes during T1D development, the metabolic profiles of six different brain regions in CON and T1D mice were measured by using a 1H NMR-based metabolomics approach. We found that all brain regions exhibited an identical metabolic profile in both male and female mice, and the typical NMR spectrum of the cortex extract in healthy male mice was illustrated in Fig. 2A. Here we identified a total of 19 metabolites mainly involving energy metabolism (ADP, adenosine diphosphate; Ala, alanine; AMP, adenosine monophosphate; Cre/PCre, creatine/phosphocreatine; IMP, inosine monophosphate; Ino, inosine; Lac, lactate; NAD+, nicotinamide adenine uiiydinucleotide; Suc, succinate), neurotransmitter metabolism (Asp, aspartate; GABA, γ-Aminobutyric acid; Glu, glutamate; Gln, glutamine; Gly, glycine), choline metabolism (Cho, choline) and astrocyte-neuron metabolism (Myo, myo-inositol; NAA, N-acetylaspartate; Tau, taurine).
Fig. 2.
NMR-based metabolomic analysis. (A) Typical 1H NMR spectrum obtained from the cortex in normal control mice. Metabolite abbreviation: ADP, adenosine diphosphate; AMP, adenosine monophosphate; Cre/pCre, creatine/phosphocreatine; GABA, γ-Aminobutyric acid; IMP, inosine monophosphate; NAA, N-acetylaspartate; NAD+, nicotinamide adeninedinucleotide. × 4, 4 times magnification. (B) Differences of metabolic patterns among different brain regions in female and male mice under normal control (CON) and type 1 diabetic (T1D) states analyzed by principal component analysis. Brain regions: Hip, hippocampus; Cor, cortex; Cer, cerebellum; Str, Striatum; Hyp, hypothalamus; Mid, midbrain.
Principal components analysis (PCA) was performed to obtain the overview of metabolic pattern changes among different brain regions in both male and female mice under normal and diabetic states (Fig. 2B). The results demonstrate that the metabolic patterns of the hippocampus, cortex and cerebellum were close to each other in female CON mice, but clearly separated at the 4th week after STZ treatment (Fig. 2B). Additionally, the metabolic patterns of the striatum, hypothalamus and midbrain in female mice were clearly separated from each other under both normal (Con) and diabetic (T1D) conditions (Fig. 2B). In male mice, from normal to diabetes, the metabolic patterns of the hippocampus, cortex and striatum were gradually separated, while the hypothalamus and midbrain were progressively close to each other (Fig. 2B).
Subsequently, orthogonal projection to latent structures discriminant analysis (OPLS-DA) was used to maximize the metabolic difference between two groups and identify key metabolites via the VIP scores. Fig. S1 illustrates the score and VIP plots of OPLS-DA between normal male and normal female mice, and the corresponding model parameters are listed in Table S3. We observed apparent separations of metabolic patterns between two genders in the hippocampus (Fig. S1A, R2 = 0.916, Q2 = 0.678), cortex (Fig. S1B, R2 = 0.955, Q2 = 0.832), cerebellum (Fig. S1C, R2 = 0.985, Q2 = 0.960), striatum (Fig. S1D, R2 = 0.875, Q2 = 0.702), hypothalamus (Fig. S1E, R2 = 0.818, Q2 = 0.584) and midbrain (Fig. S1F, R2 = 0.972, Q2 = 0.934). The corresponding VIP plots show that most metabolites had a VIP value >1.0, involving energy metabolism, choline metabolism, neurotransmitter metabolism and astrocytic metabolism.
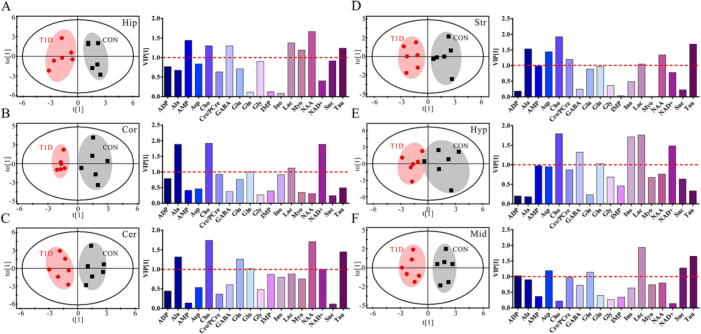
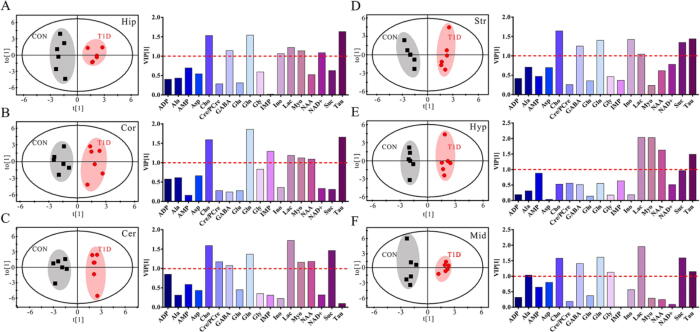
Metabolic alterations were also examined in different brain regions between CON and T1D mice in both genders using OPLS-DA, as shown in Fig. 3 for female mice and Fig. 4 for male mice. The results reveal that metabolic patterns in all brain regions of both male and female mice were clearly separated between normal and T1D states, but interestingly male mice had a better model performance than female mice (Table S4, R2: 0.908–0.984 vs. 0.745–0.912; Q2: 0.712–0.920 vs. 0.210–0.626). This finding indicates that T1D-driven brain metabolic changes were relatively more significant in male mice relative to female mice. Moreover, according to the VIP scores, important metabolites were varied in different brain regions of male and female mice for contributing to metabolic separations between CON and T1D states. In female mice, a series of metabolites with a VIP value over 1.0 were selected, such as AMP, Cho, GABA, Lac, Myo, NAA, Suc and Tau in the hippocampus (Fig. 3A), Ala, Cho, Gln, Lac and NAD+ in the cortex (Fig. 3B), Ala, Cho, Glu, Gln, NAA, NAD+ and Tau in the cerebellum (Fig. 3C), Ala, AMP, Asp, Cho, Cre/PCre, Lac, NAA and Tau in the striatum (Fig. 3D), Cho, GABA, Gln, Ino, Lac and NAD+ in the hypothalamus (Fig. 3E), and ADP, Asp, Glu, Lac, Suc and Tau in the midbrain (Fig. 3F). However, in male mice, we detected a series of metabolites that mainly contributed to separate metabolic patterns between CON and T1D mice, including Cho, GABA, Gln, Ino, Lac, Myo, NAD+ and Tau in the hippocampus (Fig. 4A), Cho, Gln, IMP, Lac, Myo, NAA and Tau in the cortex (Fig. 4B), Cho, Cre/PCre, GABA, Gln, Lac, Myo, NAA and Suc in the cerebellum (Fig. 4C), Cho, GABA, Gln, Ino, Lac, Suc and Tau in the striatum (Fig. 4D), Lac, Myo, NAA and Tau in the hypothalamus (Fig. 4E), and Ala, Cho, GABA, Gln, Gly, Lac, Suc and Tau in the midbrain (Fig. 4F).
Fig. 3.
Diabetes-driven shifts in metabolic patterns in different brain regions of female mice. OPLS-DA score plots and VIP values based on the (A) hippocampus (R2 = 0.848, Q2 = 0.428), (B) cortex (R2 = 0.895, Q2 = 0.486), (C) cerebellum (R2 = 0.848, Q2 = 0.544), (D) striatum (R2 = 0.869, Q2 = 0.552), (E) hypothalamus (R2 = 0.745, Q2 = 0.210) and (F) midbrain (R2 = 0.912, Q2 = 0.626) metabolomes between normal control (CON) and type 1 diabetic (T1D) mice. Metabolite abbreviation: ADP, adenosine diphosphate; Ala, alanine; AMP, adenosine monophosphate; Asp, aspartate; Cho, choline; Cre/pCre, creatine/phosphocreatine; GABA, γ-Aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; IMP, inosine monophosphate; Ino, inosine; Lac, lactate; Myo, myo-inositol; NAA, N-acetylaspartate; NAD+, nicotinamide adeninedinucleotide; Suc, succinate; Tau, taurine.
Fig. 4.
Diabetes-driven shifts in metabolic patterns in different brain regions of male mice. OPLS-DA score plots and VIP values based on the (A) hippocampus (R2 = 0.946, Q2 = 0.831), (B) cortex (R2 = 0.908, Q2 = 0.712), (C) cerebellum (R2 = 0.955, Q2 = 0.882), (D) striatum (R2 = 0.966, Q2 = 0.873), (E) hypothalamus (R2 = 0.984, Q2 = 0.920) and (F) midbrain (R2 = 0.967, Q2 = 0.863) metabolomes between normal control (CON) and type 1 diabetic (T1D) mice. Metabolite abbreviation: ADP, adenosine diphosphate; Ala, alanine; AMP, adenosine monophosphate; Asp, aspartate; Cho, choline; Cre/pCre, creatine/phosphocreatine; GABA, γ-Aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; IMP, inosine monophosphate; Ino, inosine; Lac, lactate; Myo, myo-inositol; NAA, N-acetylaspartate; NAD+, nicotinamide adeninedinucleotide; Suc, succinate; Tau, taurine.
3.3. The effects of gender, diabetes and their interaction on brain region-specific metabolic changes in mice
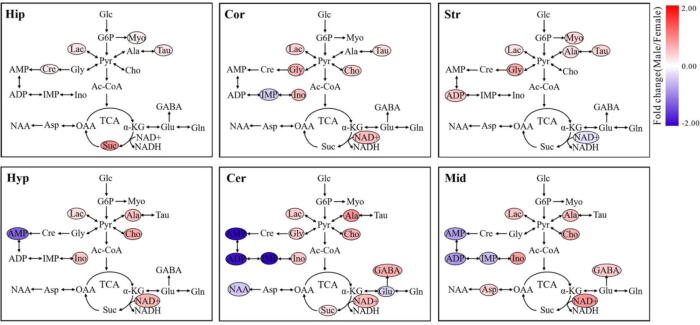
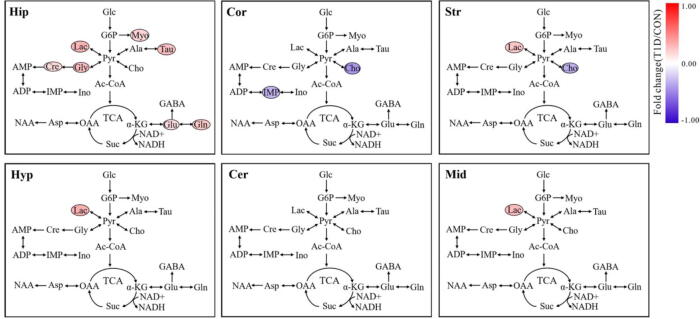
In this study, a linear mixed-model (LMM) was used to evaluate the effects of gender and diabetes on the change of specific metabolite in different brain regions of mice, and the corresponding results are listed in Table S5 for the hippocampus, Table S6 for the cortex, Table S7 for the striatum, Table S8 for the hypothalamus, Table S9 for the cerebellum and Table S10 for the midbrain. Then, metabolites showing statistically significant gender and diabetes effects were highlighted in metabolic pathways and colored according to their fold changes, as illustrated in Fig. 5, Fig. 6, respectively.
Fig. 5.
The effect of gender on metabolic pathway changes in different brain regions of mice. Metabolites showing a statistically significant gender effect (P < 0.05) were highlighted in metabolic pathways and colored according to their fold changes (male/female). Metabolite abbreviation: ADP, adenosine diphosphate; Ala, alanine; AMP, adenosine monophosphate; Asp, aspartate; Cho, choline; Cre/pCre, creatine/phosphocreatine; GABA, γ-Aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; IMP, inosine monophosphate; Ino, inosine; Lac, lactate; Myo, myo-inositol; NAA, N-acetylaspartate; NAD+, nicotinamide adeninedinucleotide; Suc, succinate; Tau, taurine. Brain regions: Hip, hippocampus; Cor, cortex; Cer, cerebellum; Str, Striatum; Hyp, hypothalamus; Mid, midbrain. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
The effect of diabetes on metabolic pathway changes in different brain regions of mice. Metabolites showing a statistically significant diabetic effect (P < 0.05) were highlighted in metabolic pathways and colored according to their fold changes (T1D/CON). Metabolite abbreviation: ADP, adenosine diphosphate; Ala, alanine; AMP, adenosine monophosphate; Asp, aspartate; Cho, choline; Cre/pCre, creatine/phosphocreatine; GABA, γ-Aminobutyric acid; Gln, glutamine; Glu, glutamate; Gly, glycine; IMP, inosine monophosphate; Ino, inosine; Lac, lactate; Myo, myo-inositol; NAA, N-acetylaspartate; NAD+, nicotinamide adeninedinucleotide; Suc, succinate; Tau, taurine. Brain regions: Hip, hippocampus; Cor, cortex; Cer, cerebellum; Str, Striatum; Hyp, hypothalamus; Mid, midbrain. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The LMM results show that sex-specific metabolic changes exist in all brain regions studied herein (Fig. 5). In the hippocampus, male mice had significantly higher levels of Lac, Tau, Cre, Myo and Suc than female mice. We observed that the levels of Lac, Tau, Gly, Cho, Ino and NAD+ were significantly increased and the level of IMP were decreased in the cortex of male mice relative to female mice. In the striatum, compared with female mice, significant increases in Lac, Ala, Tau, Gly, Myo and ADP as well as a significant reduction of NAD+ were detected in male mice. In the hypothalamus, male mice exhibited higher Lac, Ala, Cho, Ino and NAD+ levels but lower AMP level than female mice. In addition, the levels of Lac, Ala, Cho, Ino, GABA and NAD+ were significantly higher in both cerebellum and midbrain of male mice, while decreased levels of AMP, ADP and IMP were detected in male mice, when compared with female mice. Besides, we also found higher Gly and Suc levels and lower NAA and Glu levels in the cerebellum of male mice relative to female mice. The impact of diabetes on brain metabolic changes might be region-specific (Fig. 6). In the hippocampus, we found significantly higher levels of Lac, Tau, Cre, Gly, Myo, Glu and Gln in T1D mice compared with CON mice. A reduction of IMP was only detected in the cortex of T1D mice. The level of Cho was significantly decreased in the cortex and striatum of T1D mice relative to CON mice. Increased Lac level was also observed in the striatum, hypothalamus and midbrain of T1D mice. However, there were no significant differences in cerebellum metabolic changes between T1D and CON mice.
The interaction effect of gender and diabetes on the change of specific metabolite in different brain regions of mice was also analyzed by the LMM and the corresponding results are listed in Table S11 for the hippocampus, Table S12 for the cortex, Table S13 for the striatum, Table S14 for the hypothalamus, Table S15 for the cerebellum and Table S16 for the midbrain. Interestingly, the significant interaction effect of gender and diabetes on brain metabolic changes was only found in the cortex (Fig. 7). Moreover, female mice exhibited a hypometabolism status from normal to T1D, as indicated by decreases in Lac, Tau, Myo and Gln. In contrary, the levels of these metabolites were increased in the cortex of male mice during T1D development.
Fig. 7.
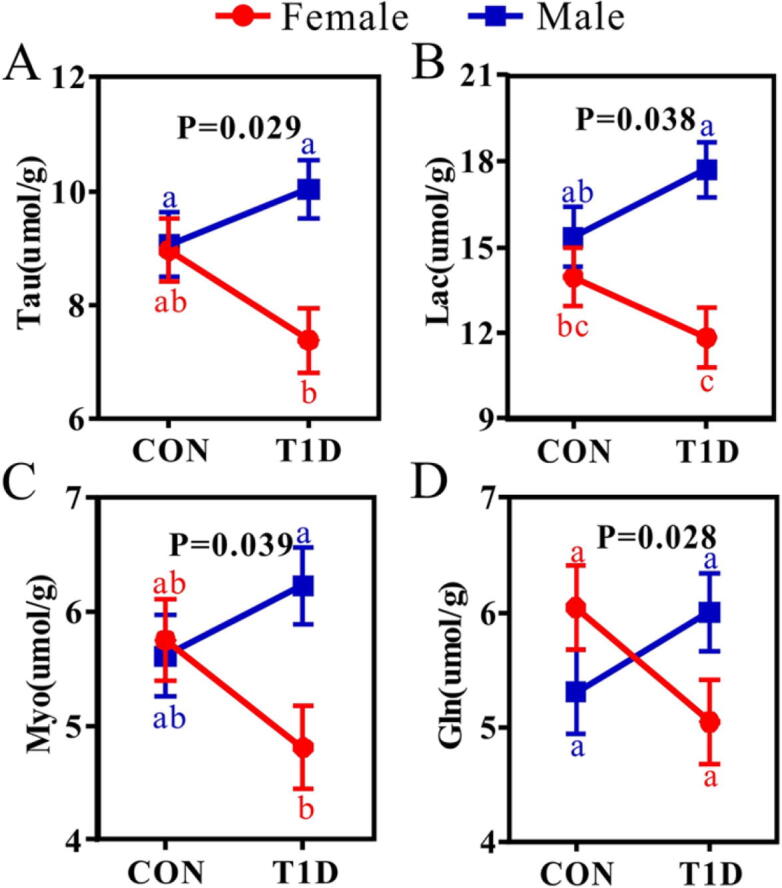
The interaction effect of gender and diabetes on metabolic changes in the cortex of mice. (A) Tau, taurine; (B) Lac, lactate; (C) Myo, myo-inositol; (D) Gln, glutamine. Metabolites described herein indicate a significant interaction effect between gender and diabetes based on the linear mixed model analysis (P < 0.05). Data are obtained from mixed-model analysis and presented as least-square-mean ± SEM (n = 6 for each group). Different lowercase letters represent significant differences (P < 0.05). Gender: Red line, female mice; Blue, male mice. Group: CON, normal control mice; T1D, type 1 diabetic mice. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
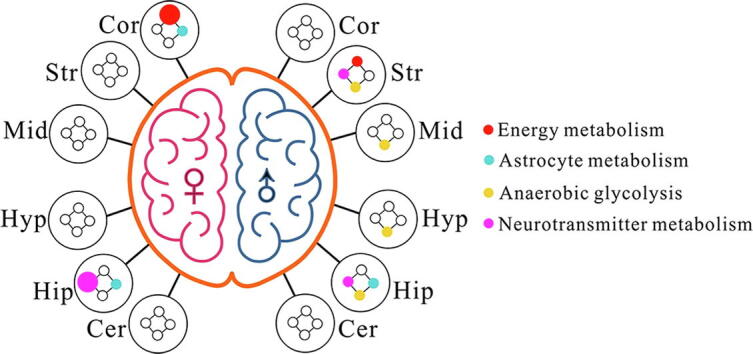
Fig. 8 illustrates an overview of the significantly altered metabolic pathways in six different brain regions between male and female mice from normal to T1D. At the 4th week after STZ treatment, brain metabolic disorders mainly occurred in the cortex and hippocampus in female T1D mice, but the striatum and hippocampus in male T1D mice. In addition, Fig. 8 also shows that T1D significantly altered anaerobic glycolysis in the striatum, midbrain, hypothalamus and hippocampus of male mice, but not in female mice.
Fig. 8.
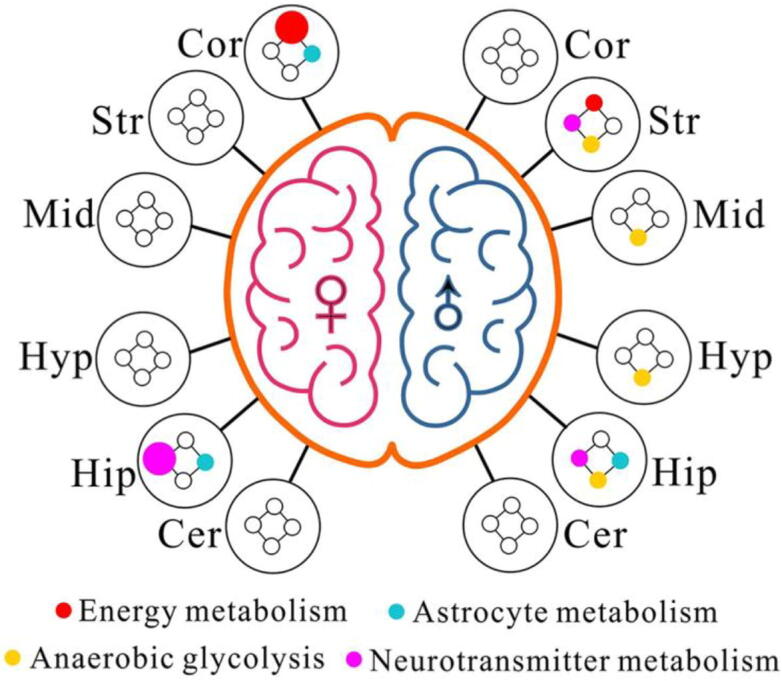
Sex-specific metabolic pathway changes in different brain regions of mice after streptozotocin treatment. The colored dots represent metabolic pathways that include one or more metabolites with a significant difference (P < 0.05) between normal control and diabetic mice, and the dot size is in proportion to the number of metabolites. Brain region: Hip, hippocampus; Cor cortex; Str, striatum; Cer, cerebellum; Hyp, hypothalamus; Mid, midbrain. Gender: ♀, female; ♂, male. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Streptozotocin (STZ), a toxic glucose analogue, can enter pancreatic β-cells via GLUT2, cause β-cells destruction and thereby induce diabetes [27]. Interestingly, here we found that male mice exhibited more typical diabetic symptoms than female mice after STZ exposure, suggesting that the sensitivity to STZ was affected by gender. This finding may be attributed to the protective effect of estrogen on β-cells and insulin sensitivity [28], [29], [30]. In clinical and basic researches, therefore, more attention needs to be paid to sex differences in the occurrence and development of diabetes and its complications [31], [32]. In our previous study, we reported that cognitive decline happened to both T1D rats and T2D mice at advanced stage (>8 weeks after STZ injection), and brain region-specific metabolic disorders might be potential pathogenic factors [19], [20]. Yet, sex differences in diabetic brain metabolism are rarely reported. In this study, we examined sex-specific metabolic alterations in six different brain regions between T1D mice at the 4th week after STZ treatment and age-matched controls. A linear mixed-model (LMM) was used to analyze the effects of gender, diabetes and their interaction on region-specific metabolic changes in the brain of mice. The LMM results demonstrate that metabolic differences existed in all brain regions between two genders, while the hippocampal metabolism may be more vulnerable to T1D. Moreover, the interaction effect of gender and diabetes on brain metabolism was mainly presented in the cortex of mice. A series of brain metabolic changes were identified mainly involving energy metabolism, choline metabolism, neurotransmitter metabolism and astrocytic metabolism.
4.1. Sex-specific changes in energy metabolism in the brain of T1D mice
The brain only holds 2% of the total body weight, but approximately 25% of glucose is utilized for energy production to maintain cerebral functions [33]. Glucose can be metabolized to pyruvate and then oxidized by the tricarboxylic acid (TCA) cycle for energy generation or transformed into lactate by anaerobic glycolysis. In this study, we found a significantly higher lactate level in all brain regions of male mice relative to female mice, indicating an increased anaerobic glycolysis in the male brain. Compared with female mice, the level of succinate, a key intermediate of the TCA cycle, was significantly increased in the hippocampus and cerebellum of male mice. Previously, we also detected a higher succinate level in the kidney of male mice relative to female mice [34]. Moreover, several energy-related biomolecules such as inosine and NAD+ were also increased in the cortex, hypothalamus, cerebellum and midbrain of male mice. However, in the cerebellum and midbrain, male mice had significantly lower levels of AMP, ADP and IMP than female mice. These results suggest that, overall, male mice exhibited a higher brain glucose metabolism than female mice, which had a brain region-specific difference. In human studies, sex-and region-specific differences in cerebral glucose metabolism have been also reported. For example, Gur et al. [35] observed that men had a higher glucose metabolism than women in the cerebellum and temporal-limbic regions by using positron emission tomography (PET). Relative to females, another PET study showed that glucose metabolism was significantly higher in the right insula, middle temporal gyrus and medial frontal lobe but lower in the hypothalamus of males [36]. Males were also found to show a higher brain glucose metabolism than females in the bilateral visual cortices and cerebellum by Hsieh et al. [37]. In the brain, glucose uptake into the cells is dependent on glucose transporters (GLUTs), such as GLUT1 in astrocytes and oligodentrocytes and GLUT3 in neurons and microglia [38], [39]. Of note, Nagai et al. [40] found that male mice had a lower GLUT2 and a higher GLUT3 level in the brain than female mice. We speculate that sex-specific expressions of GLUTs may be also responsible for the differences in brain glucose metabolism between genders.
Our results also reveal that T1D induced a significant increase in lactate level in the hippocampus, striatum, hypothalamus and midbrain of mice. However, in our previous studies, high lactate level was found in all brain regions in T1D rats at the 8th week after STZ treatment [19]. The results presented herein suggest that increased cerebral lactate level has already occurred in T1D mice at the 4th week after STZ injection and long-term high lactate in the brain would cause diabetic cognitive impairment. Previously, we reported that excess lactate level in the brain resulted into cognitive decline via GPR81-dependent PKA/CREB signaling pathway in diabetic rats [41]. More interestingly, we identified a significant interaction effect of gender and diabetes on the change of lactate in the cortex, as indicated by increased lactate level in male mice but decreased lactate level in female mice from normal to diabetes. A similar result was also obtained for the change of lactate in the kidney of mice during T1D development [34]. One possible explanation is that estrogens increase mitochondrial function and aerobic glycolysis [42], [43], resulting in the reduction of lactate production from anaerobic glycolysis in female mice. In addition, of note, lactate has two sides on brain functions. It can be utilized as a neuronal energy substance [44], but excess lactate can cause cognitive decline in both human [45], [46] and animal models [41]. Thus, it is of great importance to monitor the change of brain lactate for diabetic patients, and the sex-dependent effect on brain energy metabolism also needs to be taken into account in clinical practice.
4.2. Sex-specific changes in choline metabolism in the brain of T1D mice
Choline is an important nutrient for brain development and functions due to its roles in the syntheses of cell membranes and acetylcholine [47], [48]. Here, we found that male mice had a significantly higher level of choline in the cortex, hypothalamus, cerebellum and midbrain than female mice. This finding is in agreement with the earlier MRS results in human brain, where they also observed a relatively higher choline in males relative to females [49]. However, data regarding sex-difference in brain choline is still scarce. Choline has a positive effect on cognitive functions via multiple mechanisms. For example, Tabassum et al. [50] revealed that choline can improve cognitive performance of rats by suppressing oxidative stress and increasing cholinergic neurotransmission. Besides, choline has been reported to attenuate microglia activation [51] and enhance mitochondrial function [52] and thereby ameliorate cognitive decline. In our previous study, the region-specific reduction in brain choline level was linked with cognitive deficits in T1D rats at the 8th week after STZ treatment [19]. However, here we found that choline deficiency may already occur in the cortex and striatum of T1D mice at the 4th week after STZ injection. A decrease in choline was also detected in the kidney of mice from normal to diabetes, especially in male mice [34]. These results suggest that dietary choline supplementation may require to be considered for T1D patients.
4.3. Sex-specific changes in neurotransmitter metabolism in the brain of T1D mice
Neurotransmitter metabolism, especially the glutamine/glutamate/γ-aminobutyric acid (Gln/Glu/GABA) cycle, plays an important role in regulating neurotransmitter homeostasis between astrocytes and neurons in the brain [53]. In this cycle, glutamine can be directly metabolized to glutamate and indirectly transformed into GABA. Glutamate and GABA belong to excitatory and inhibitory neurotransmitters, respectively, which are closely related to cognitive functions [54]. In this study, we found that male mice had a significantly higher GABA in the cerebellum and midbrain but lower glutamate in the cerebellum than female mice. Sex-and region-specific differences in glutamate and glutamine were reported in the human brain using MRS analysis [55]. In addition, using multi-tissue metabolomics analysis, we previously revealed that the levels of glutamate and glutamine were significantly reduced in the kidney of male mice during T1D development, but not in female mice [34]. Aspartate and glycine are two major amino acid neurotransmitters in multiple brain regions [56] and serve as a selective agonist for N-methyl-d-aspartate (NMDA) receptors, which has also been associated with cognitive functions [57]. Relative to female mice, we detected a higher level of aspartate in the midbrain and higher glycine in the cortex, striatum and cerebellum in male mice. Therefore, neurotransmitter metabolism in the brain is dependent on sex. Additionally, Zhang et al. [34] found higher levels of hepatic glycine and renal aspartate in male mice relative to female mice.
T1D-induced shifts in neurotransmitters were only observed in the hippocampus, as indicated by increased levels of glutamate, glutamine and glycine in T1D mice compared with CON mice. In our previous study, we have reported that disrupted neurotransmitter metabolism was implicated in cognitive decline of T1D rats at the 8th week after STZ treatment [19] and db/db mice [20]. However, here we observed that the disturbances of neurotransmitters may already occur in the hippocampus of T1D mice at the 4th week after STZ injection. Moreover, a significant interaction effect of gender and diabetes was identified on the change of glutamine in the cortex. From normal to diabetes, the level of glutamine was reduced in female mice, but increased in male mice. Sex-specific changes in neurotransmitter metabolism can be regulated by many factors, such as hormones [58] and gut flora [59], so exploring their potential relationships would advance a better understanding of sex differences in brain functions during the development of T1D.
4.4. Sex-specific changes in astrocytic metabolism in the brain of T1D mice
Taurine and myo-inositol are regarded as markers of astrocytes and exert an essential role in controlling astrocytic volume in the brain [60], [61]. In this study, we found that the levels of taurine in the hippocampus, cortex and striatum and myo-inositol in the hippocampus and striatum were significantly higher in male mice when compared with female mice. These findings may indicate a higher astrocytic number and activity in males than in females. Sex differences in astrocytes have been reported, but the results might be complicated. For example, in the hippocampus, a higher number of astrocytes were detected in the CA3 area of male rats [62], but females had more astrocytes in the CA1 area of both mice [63] and rats [62]. In addition, compared with CON mice, significantly increased levels of taurine and myo-inositol were observed in the hippocampus of mice at the 4th week after STZ treatment, but not in other brain regions, suggesting a proliferation of hippocampal astrocytes induced by diabetes [64]. In our previous studies, the region-specific increases in taurine and myo-inositol have been found in the brain of T1D rats at the 8th week after STZ treatment [19] and db/db mice [20]. However, in this study, we suggest that hippocampal astrocytic changes may already occur in T1D at the 4th week after STZ injection. More interestingly, changes in these two metabolites exhibited a significant interaction effect of gender and diabetes. To be specific, from normal to diabetes, their levels were reduced in female mice, but increased in male mice. This phenomenon implies a sex-specific change in astrocytic metabolism during T1D development.
5. Conclusions and perspectives
In this study, we characterized sex-and region-specific changes in brain metabolism of mice from normal to diabetes using an integrated method of metabolomics and linear mixed-model. The results show that sex-specific metabolic alterations occurred in all brain regions, but the hippocampus might be a major brain region of metabolic disorders affected by T1D. In addition, the interaction effect of gender and diabetes on brain metabolic changes was mainly presented in the cortex of mice. Of note, the cortex and hippocampus as the pivotal brain regions are involved in cognitive control [65], [66]. Davis et al. [67] reported that the cortex can deliver sensory information to the hippocampus for learning and memory. Thus, metabolic disorders in these two brain regions need to be paid more attention to the changes in diabetic cognitive functions in clinical practice. Another interesting finding is that male mice but not female mice had a notably altered anaerobic glycolysis in the striatum, midbrain, hypothalamus and hippocampus from normal to diabetes. Therefore, we suggested that developing MRS techniques to detect sex-and region-specific metabolic changes in the brain will contribute to monitor the development of diabetic brain diseases and establish gender-based prevention and treatment for T1D in clinic.
It should be noted that estrogens can affect neurotransmitter metabolism, such as serotonin, dopamine, GABA and glutamate, and sequentially alter brain structure and functions [68]. Moreover, estrogens have been also reported to regulate energy homeostasis in the hypothalamus via estrogen receptors [69]. Tawfik et al. [70] found that diabetic female rats after ovariectomy exhibited more serious metabolic disorders as characterized by disrupted lipid metabolism, hyperinsulinemia, higher leptin and lower adiponectin levels. These metabolic changes may promote the development of cardiovascular disease in females after menopause [70]. Altunkaynak et al. [71] revealed that ovariectomy aggravated disturbances of lipid profiles and oxidative stress in the hippocampus of diabetic female rats. Collectively, the menopausal status of diabetic females is an important influence factor for metabolomics analysis. In the current study, all mice were at puberty; therefore, T1D-induced brain metabolic changes may also be influenced synchronously by sex hormones. In order to explore the specific metabolic changes induced by T1D, in our next study, we will analyze metabolic differences in the brain of mice under non-ovariectomized and ovariectomized conditions.
Systemic inflammation has been associated with the pathogenesis of diabetes and its complications [72], [73], [74]. Furthermore, of note, inflammation can affect brain metabolism, such as glucose metabolism [75], energy metabolism [76], [77] and tryptophan metabolism [78]. The limitation of this study is that inflammation-induced metabolic alterations were not investigated. Therefore, we suggested that brain metabolic changes need to be studied in a mouse model of systemic inflammation induced by lipopolysaccharides (LPS).
Another noteworthy fact is that STZ as a cytotoxic agent of insulin-producing cells can also influence brain metabolic characteristics [79]. Müller et al. [80] revealed that the levels of free fatty acids and phospholipids were disordered in the temporal cortex and hippocampus of rats after intracerebroventricular (ICV) injection of STZ. In addition, the disturbances of gene expression in the brain were detected in rats following ICV-STZ injection [81], [82]. The adverse effects of ICV-STZ were also reported on insulin signaling [83], oxidative stress [84] and neuroinflammation [85]. Interestingly, sex differences in the cognitive and hippocampal changes were found in rats treated with ICV-STZ [86], [87]. Here we found that IP-STZ induced region-specific metabolic changes in the brain of mice in a sex-specific manner. To eliminate the STZ effect, however, our results still need to be confirmed from other diabetic models, such as non-obese diabetic (NOD) or AKITA mouse model.
In addition, several other limitations or further works can be considered: (1) This study did not include a brain function analysis, so the causal relationship between metabolic changes and brain dysfunction (e.g. cognitive decline) needs to be further established; (2) A multi-analytical platform is recommended to obtain a more detailed metabolic pathway analysis; (3) An integrated analysis of gene and protein expression involved in metabolic pathways (e.g. multi-omics) will provide a comprehensive view for studying the association between metabolic changes and brain functions during T1D development.
CRediT authorship contribution statement
Qiaoying Jiang: Methodology, Investigation, Formal analysis, Writing - original draft. Hangying Xu: Investigation, Formal analysis, Writing - original draft. Junjie Yan: Methodology, Investigation. Qingqing Xu: Methodology, Investigation. Yafei Zheng: Investigation, Formal analysis, Writing - review & editing. Chen Li: Investigation, Formal analysis, Writing - review & editing. Liangcai Zhao: Formal analysis, Writing - review & editing. Hongchang Gao: Conceptualization, Supervision, Project administration, Funding acquisition, Writing - review & editing. Hong Zheng: Conceptualization, Supervision, Formal analysis, Project administration, Funding acquisition, Writing - original draft, Writing - review & editing.
Acknowledgments
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos.: 81771386 and 21974096) and the Qianjiang Talent Project of Zhejiang Province (No. QJD1802023).
Author contributions
HZ and HCG contributed to the experimental design. QYJ, HYX, JJY and QQX contributed to animal experiments. QYJ, HYX, YFZ, CL and LCZ contributed to the sample collection and NMR metabolomic analysis. HZ and HCG contributed to the data analysis, result interpretation and writing. All authors have read, revised and approved the final manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2020.07.019.
Contributor Information
Hongchang Gao, Email: gaohc27@wmu.edu.cn.
Hong Zheng, Email: 123zhenghong321@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Katsarou A., Gudbjörnsdottir S., Rawshani A., Dabelea D., Bonifacio E., Anderson B.J. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:1–17. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation . 8th ed. International Diabetes Federation; Brussels, Belgium: 2017. IDF diabetes atlas. [Google Scholar]
- 3.Papatheodorou K., Banach M., Bekiari E., Rizzo M., Edmonds M. Complications of diabetes. J Diabetes Res. 2018;2018:3086167. doi: 10.1155/2018/3086167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 5.Mauvais-Jarvis F. Sex and gender factors affecting metabolic homeostasis, diabetes and obesity. Springer; Cham: 2017. Epidemiology of gender differences in diabetes and obesity; pp. 3–8. [DOI] [PubMed] [Google Scholar]
- 6.Samuelsson U., Anderzén J., Gudbjörnsdottir S., Steineck I., Åkesson K., Hanberger L. Teenage girls with type 1 diabetes have poorer metabolic control than boys and face more complications in early adulthood. J Diabetes Complicat. 2016;30:917–922. doi: 10.1016/j.jdiacomp.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Mijnhout G.S., Scheltens P., Diamant M., Biessels G.J., Wessels A.M., Simsek S. Diabetic encephalopathy: a concept in need of a definition. Diabetologia. 2006;49:1447. doi: 10.1007/s00125-006-0221-8. [DOI] [PubMed] [Google Scholar]
- 8.Chaytor N.S., Barbosa-Leiker C., Ryan C.M., Germine L.T., Hirsch I.B., Weinstock R.S. Clinically significant cognitive impairment in older adults with type 1 diabetes. J Diabetes Complicat. 2019;33:91–97. doi: 10.1016/j.jdiacomp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Shalimova A., Graff B., Gąsecki D., Wolf J., Sabisz A., Szurowska E. Cognitive dysfunction in type 1 diabetes mellitus. J Clin Endocrinol Metab. 2019;104:2239–2249. doi: 10.1210/jc.2018-01315. [DOI] [PubMed] [Google Scholar]
- 10.Wessels A.M., Rombouts S.A.R.B., Remijnse P.L., Boom Y., Scheltens P., Barkhof F. Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia. 2007;50:1763–1769. doi: 10.1007/s00125-007-0714-0. [DOI] [PubMed] [Google Scholar]
- 11.Van Duinkerken E., Schoonheim M.M., Sanz-Arigita E.J., IJzerman R.G., Moll A.C., Snoek F.J. Resting-state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes. 2012;61:1814–1821. doi: 10.2337/db11-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbondante S., Baglietto-Vargas D., Rodriguez-Ortiz C.J., Estrada-Hernandez T., Medeiros R., LaFerla F.M. Genetic ablation of tau mitigates cognitive impairment induced by type 1 diabetes. Am J Pathol. 2014;184:819–826. doi: 10.1016/j.ajpath.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao B., Pan B.S., Shen S.W., Sun X., Hou Z.Z., Yan R. Diabetes-induced central neuritic dystrophy and cognitive deficits are associated with the formation of oligomeric reticulon-3 via oxidative stress. J Biol Chem. 2013;288:15590–15599. doi: 10.1074/jbc.M112.440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeilly A.D., Gallagher J.R., Dinkova-Kostova A.T., Hayes J.D., Sharkey J., Ashford M.L. Nrf2-mediated neuroprotection against recurrent hypoglycemia is insufficient to prevent cognitive impairment in a rodent model of type 1 diabetes. Diabetes. 2016;65:3151–3160. doi: 10.2337/db15-1653. [DOI] [PubMed] [Google Scholar]
- 15.Zeinivand M., Nahavandi A., Zare M. Deferoxamine regulates neuroinflammation and oxidative stress in rats with diabetes-induced cognitive dysfunction. Inflammopharmacology. 2020;28:575–583. doi: 10.1007/s10787-019-00665-7. [DOI] [PubMed] [Google Scholar]
- 16.Gao H.C., Jiang Q.Y., Ji H., Ning J., Li C., Zheng H. Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. BBA-Mol Basis Dis. 2019;1865 doi: 10.1016/j.bbadis.2019.165541. [DOI] [PubMed] [Google Scholar]
- 17.Mäkimattila S., Malmberg-Cèder K., Häkkinen A.M., Vuori K., Salonen O., Summanen P. Brain metabolic alterations in patients with type 1 diabetes-hyperglycemia-induced injury. J Cereb Blood Flow Metab. 2004;24:1393–1399. doi: 10.1097/01.WCB.0000143700.15489.B2. [DOI] [PubMed] [Google Scholar]
- 18.Sarac K., Akinci A., Alkan A., Aslan M., Baysal T., Özcan C. Brain metabolite changes on proton magnetic resonance spectroscopy in children with poorly controlled type 1 diabetes mellitus. Neuroradiology. 2005;47:562–565. doi: 10.1007/s00234-005-1387-3. [DOI] [PubMed] [Google Scholar]
- 19.Zheng H., Lin Q., Wang D., Xu P., Zhao L., Hu W. NMR-based metabolomics reveals brain region-specific metabolic alterations in streptozotocin-induced diabetic rats with cognitive dysfunction. Metab Brain Dis. 2017;32:585–593. doi: 10.1007/s11011-016-9949-0. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H., Zheng Y., Zhao L., Chen M., Bai G., Hu Y. Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. BBA-Mol Basis Dis. 2017;1863:266–273. doi: 10.1016/j.bbadis.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H., Zhou Q., Du Y., Li C., Xu P., Lin L. The hypothalamus as the primary brain region of metabolic abnormalities in APP/PS1 transgenic mouse model of Alzheimer's disease. BBA-Mol Basis Dis. 2018;1864:263–273. doi: 10.1016/j.bbadis.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5–47. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G., Franklin K.B. Academic Press; 2019. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. [Google Scholar]
- 24.Savorani F., Tomasi G., Engelsen S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J Magn Reson. 2010;202:190–202. doi: 10.1016/j.jmr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Wishart D.S., Feunang Y.D., Marcu A., Guo A.C., Liang K., Vázquez-Fresno R. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda S., Yamada T., Hamajima M., Itoh M., Katayama T., Bork P. KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 2008;36:W423–W426. doi: 10.1093/nar/gkn282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 28.Tiano J.P., Delghingaro-Augusto V., Le May C., Liu S., Kaw M.K., Khuder S.S. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest. 2011;121:3331–3342. doi: 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel H., Mirhashemi F., Liehl B., Taugner F., Kluth O., Kluge R. Estrogen deficiency aggravates insulin resistance and induces β-cell loss and diabetes in female New Zealand obese mice. Hormone Metab Res. 2013;45:430–435. doi: 10.1055/s-0032-1331700. [DOI] [PubMed] [Google Scholar]
- 30.Yan H., Yang W., Zhou F., Li X., Pan Q., Shen Z. Estrogen improves insulin sensitivity and suppresses gluconeogenesis via the transcription factor Foxo1. Diabetes. 2019;68:291–304. doi: 10.2337/db18-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20–23. doi: 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seghieri G., Policardo L., Anichini R., Franconi F., Campesi I., Cherchi S. The effect of sex and gender on diabetic complications. Curr Diabetes Rev. 2017;13:148–160. doi: 10.2174/1573399812666160517115756. [DOI] [PubMed] [Google Scholar]
- 33.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X., Xu H., Ning J., Ji H., Yan J., Zheng Y. Sex-specific metabolic changes in peripheral organs of diabetic mice. J Proteome Res. 2020 doi: 10.1021/acs.jproteome.0c00049. [DOI] [PubMed] [Google Scholar]
- 35.Gur R.C., Mozley L.H., Mozley P.D., Resnick S.M., Karp J.S., Alavi A. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267:528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- 36.Kawachi T., Ishii K., Sakamoto S., Matsui M., Mori T., Sasaki M. Gender differences in cerebral glucose metabolism: a PET study. J Neurol Sci. 2002;199:79–83. doi: 10.1016/s0022-510x(02)00112-0. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh T.C., Lin W.Y., Ding H.J., Sun S.S., Wu Y.C., Yen K.Y. Sex-and age-related differences in brain FDG metabolism of healthy adults: an SPM analysis. J Neuroimaging. 2012;22:21–27. doi: 10.1111/j.1552-6569.2010.00543.x. [DOI] [PubMed] [Google Scholar]
- 38.Souza D.G., Almeida R.F., Souza D.O., Zimmer E.R. Seminars in cell & developmental biology. Academic Press; 2019. The astrocyte biochemistry; pp. 142–150. [DOI] [PubMed] [Google Scholar]
- 39.Wang L., Pavlou S., Du X., Bhuckory M., Xu H., Chen M. Glucose transporter 1 critically controls microglial activation through facilitating glycolysis. Mol Neurodegener. 2019;14:2. doi: 10.1186/s13024-019-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai K., Yoshida S., Konishi H. Gender differences in the gene expression profiles of glucose transporter GLUT class I and SGLT in mouse tissues. Pharmazie. 2014;69:856–859. [PubMed] [Google Scholar]
- 41.Zhao L., Dong M., Ren M., Li C., Zheng H., Gao H.C. Metabolomic analysis identifies lactate as an important pathogenic factor in diabetes-associated cognitive decline rats. Mol Cell Prote. 2018;17:2335–2346. doi: 10.1074/mcp.RA118.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guevara R., Gianotti M., Roca P., Oliver J. Age and sex-related changes in rat brain mitochondrial function. Cell Physiol Biochem. 2011;27:201–206. doi: 10.1159/000327945. [DOI] [PubMed] [Google Scholar]
- 43.Gaignard P., Liere P., Thérond P., Schumacher M., Slama A., Guennoun R. Role of sex hormones on brain mitochondrial function, with special reference to aging and neurodegenerative diseases. Front Aging Neurosci. 2017;9:406. doi: 10.3389/fnagi.2017.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyss M.T., Jolivet R., Buck A., Magistretti P.J., Weber B. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31:7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liguori C., Stefani A., Sancesario G., Sancesario G.M., Marciani M.G., Pierantozzi M. CSF lactate levels, τ proteins, cognitive decline: a dynamic relationship in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2015;86:655–659. doi: 10.1136/jnnp-2014-308577. [DOI] [PubMed] [Google Scholar]
- 46.Kaufmann P., Shungu D.C., Sano M.C., Jhung S., Engelstad K., Mitsis E. Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology. 2004;62:1297–1302. doi: 10.1212/01.wnl.0000120557.83907.a8. [DOI] [PubMed] [Google Scholar]
- 47.Michel V., Yuan Z., Ramsubir S., Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med. 2006;231:490–504. doi: 10.1177/153537020623100503. [DOI] [PubMed] [Google Scholar]
- 48.Tayebati S.K., Tomassoni D., Di Stefano A., Sozio P., Cerasa L.S., Amenta F. Effect of choline-containing phospholipids on brain cholinergic transporters in the rat. J Neurol Sci. 2011;302:49–57. doi: 10.1016/j.jns.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 49.Komoroski R.A., Heimberg C., Cardwell D., Karson C.N. Effects of gender and region on proton MRS of normal human brain. Magn Reson Imaging. 1999;17:427–433. doi: 10.1016/s0730-725x(98)00186-6. [DOI] [PubMed] [Google Scholar]
- 50.Tabassum S., Haider S., Ahmad S., Madiha S., Parveen T. Chronic choline supplementation improves cognitive and motor performance via modulating oxidative and neurochemical status in rats. Pharmacol Biochem Behavior. 2017;159:90–99. doi: 10.1016/j.pbb.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Velazquez R., Ferreira E., Knowles S., Fux C., Rodin A., Winslow W. Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell. 2019;18 doi: 10.1111/acel.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pacelli C., Coluccia A., Grattagliano I., Cocco T., Petrosillo G., Paradies G. Dietary choline deprivation impairs rat brain mitochondrial function and behavioral phenotype. J Nutr. 2010;140:1072–1079. doi: 10.3945/jn.109.116673. [DOI] [PubMed] [Google Scholar]
- 53.Bak L.K., Schousboe A., Waagepetersen H.S. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 54.Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Rev. 2003;41:268–287. doi: 10.1016/s0165-0173(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 55.Hädel S., Wirth C., Rapp M., Gallinat J., Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J Magn Reson Imaging. 2013;38:1480–1487. doi: 10.1002/jmri.24123. [DOI] [PubMed] [Google Scholar]
- 56.Kölker S. Metabolism of amino acid neurotransmitters: the synaptic disorder underlying inherited metabolic diseases. J Inherit Metab Dis. 2018;41:1055–1063. doi: 10.1007/s10545-018-0201-4. [DOI] [PubMed] [Google Scholar]
- 57.Gilmour G., Dix S., Fellini L., Gastambide F., Plath N., Steckler T. NMDA receptors, cognition and schizophrenia–testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012;62:1401–1412. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Uddin M.M., Mahmood A.H., Ibrahim M.M., Briski K.P. Sex-dimorphic estrogen receptor regulation of ventromedial hypothalamic nucleus glucoregulatory neuron adrenergic receptor expression in hypoglycemic male and female rats. Brain Res. 2019;1720 doi: 10.1016/j.brainres.2019.146311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jameson K.G., Hsiao E.Y. Linking the gut microbiota to a brain neurotransmitter. Trends Neurosci. 2018;41:413–414. doi: 10.1016/j.tins.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isaacks R.E., Bender A.S., Kim C.Y., Prieto N.M., Norenberg M.D. Osmotic regulation ofmyo-inositol uptake in primary astrocyte cultures. Neurochem Res. 1994;19:331–338. doi: 10.1007/BF00971582. [DOI] [PubMed] [Google Scholar]
- 61.Pasantes-Morales H. Taurine homeostasis and volume control. Adv Neurobiol. 2017;16:33–53. doi: 10.1007/978-3-319-55769-4_3. [DOI] [PubMed] [Google Scholar]
- 62.Conejo N.M., González-Pardo H., Pedraza C., Navarro F.F., Vallejo G., Arias J.L. Evidence for sexual difference in astrocytes of adult rat hippocampus. Neurosci Lett. 2003;339:119–122. doi: 10.1016/s0304-3940(02)01484-2. [DOI] [PubMed] [Google Scholar]
- 63.Mouton P.R., Long J.M., Lei D.L., Howard V., Jucker M., Calhoun M.E. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- 64.Nagayach A., Patro N., Patro I. Astrocytic and microglial response in experimentally induced diabetic rat brain. Metab Brain Dis. 2014;29:747–761. doi: 10.1007/s11011-014-9562-z. [DOI] [PubMed] [Google Scholar]
- 65.Treves A., Rolls E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- 66.Wu T., Wang X., Wu Q., Spagna A., Yang J., Yuan C. Anterior insular cortex is a bottleneck of cognitive control. Neuroimage. 2019;195:490–504. doi: 10.1016/j.neuroimage.2019.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis A.E., Gimenez A.M., Therrien B. Effects of entorhinal cortex lesions on sensory integration and spatial learning. Nurs Res. 2001;50:77–85. doi: 10.1097/00006199-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Barth C., Villringer A., Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X., Shi H. Regulation of estrogen receptor α expression in the hypothalamus by sex steroids: implication in the regulation of energy homeostasis. Int J Endocrinol. 2015;2015 doi: 10.1155/2015/949085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tawfik S.H., Mahmoud B.F., Saad M.I., Shehata M., Kamel M.A., Helmy M.H. Similar and additive effects of ovariectomy and diabetes on insulin resistance and lipid metabolism. Biochem Res Int. 2015;2015 doi: 10.1155/2015/567945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Altunkaynak B.Z., Unal D., Altunkaynak M.E., Halici Z., Kalkan Y., Keles O.N. Effects of diabetes and ovariectomy on rat hippocampus (a biochemical and stereological study) Gynecol Endocrinol. 2012;28:228–233. doi: 10.3109/09513590.2011.593662. [DOI] [PubMed] [Google Scholar]
- 72.Jin Y., Sharma A., Carey C., Hopkins D., Wang X., Robertson D.G. The expression of inflammatory genes is upregulated in peripheral blood of patients with type 1 diabetes. Diabetes Care. 2013;36:2794–2802. doi: 10.2337/dc12-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sochett E., Noone D., Grattan M., Slorach C., Moineddin R., Elia Y. Relationship between serum inflammatory markers and vascular function in a cohort of adolescents with type 1 diabetes. Cytokine. 2017;99:233–239. doi: 10.1016/j.cyto.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Aulich J., Cho Y.H., Januszewski A.S., Craig M.E., Selvadurai H., Wiegand S. Associations between circulating inflammatory markers, diabetes type and complications in youth. Pediatric Diabetes. 2019;20:1118–1127. doi: 10.1111/pedi.12913. [DOI] [PubMed] [Google Scholar]
- 75.Hannestad J., Subramanyam K., DellaGioia N., Planeta-Wilson B., Weinzimmer D., Pittman B. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med. 2012;53:601–607. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin F., Sancheti H., Patil I., Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radical Biol Med. 2016;100:108–122. doi: 10.1016/j.freeradbiomed.2016.04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kealy J., Murray C., Griffin E.W., Lopez-Rodriguez A.B., Healy D., Tortorelli L.S. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J Neurosci. 2020;40:5681–5696. doi: 10.1523/JNEUROSCI.2876-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parrott J.M., Redus L., Santana-Coelho D., Morales J., Gao X., O'connor J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiat. 2016;6:e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Labak M., Foniok T., Kirk D., Rushforth D., Tomanek B., Jasiński A., Grieb P. Brain Edema XIV. Springer; Vienna: 2010. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer’s disease; pp. 177–181. [DOI] [PubMed] [Google Scholar]
- 80.Müller D., Nitsch R.M., Wurtman R.J., Hoyer S. Streptozotocin increases free fatty acids and decreases phospholipids in rat brain. J Neural Transm. 1998;105:1271–1281. doi: 10.1007/s007020050130. [DOI] [PubMed] [Google Scholar]
- 81.Grünblatt E., Koutsilieri E., Hoyer S., Riederer P. Gene expression alterations in brain areas of intracerebroventricular streptozotocin treated rat. J Alzheimer's Dis. 2006;9:261–271. doi: 10.3233/jad-2006-9305. [DOI] [PubMed] [Google Scholar]
- 82.Pfutzenreuter G., Nieradka K., Pincerati M.R., da Silva I.S. Intracerebroventricular streptozotocin induces behavioral impairments and increases short–term C3 gene expression in the hippocampus of Wistar rats. Acta Neurobiol Exp. 2020;80:160–171. [PubMed] [Google Scholar]
- 83.Kamat P.K., Kalani A., Rai S., Tota S.K., Kumar A., Ahmad A.S. Streptozotocin intracerebroventricular-induced neurotoxicity and brain insulin resistance: A therapeutic intervention for treatment of sporadic Alzheimer’s disease (sAD)-like pathology. Mol Neurobiol. 2016;53:4548–4562. doi: 10.1007/s12035-015-9384-y. [DOI] [PubMed] [Google Scholar]
- 84.Sharma M., Gupta Y.K. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–1029. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 85.Mishra S.K., Singh S., Shukla S., Shukla R. Intracerebroventricular streptozotocin impairs adult neurogenesis and cognitive functions via regulating neuroinflammation and insulin signaling in adult rats. Neurochem Int. 2018;113:56–68. doi: 10.1016/j.neuint.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 86.Biasibetti R., dos Santos J.P.A., Rodrigues L., Wartchow K.M., Suardi L.Z., Nardin P. Hippocampal changes in STZ-model of Alzheimer’s disease are dependent on sex. Behav Brain Res. 2017;316:205–214. doi: 10.1016/j.bbr.2016.08.057. [DOI] [PubMed] [Google Scholar]
- 87.Bao J., Mahaman Y.A., Liu R., Wang J.Z., Zhang Z., Zhang B. Sex differences in the cognitive and hippocampal effects of streptozotocin in an animal model of sporadic AD. Front Aging Neurosci. 2017;9:347. doi: 10.3389/fnagi.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.