Abstract
Background
The hepatocellular carcinoma up-regulated EZH2-associated long non-coding RNA (HEIH) has been identified to act as an oncogene to promote cell tumorigenesis in hepatocellular carcinoma (HCC); however, the roles of HEIH in sorafenib resistance in HCC cells remain elusive.
Materials and Methods
The expression of HEIH and microRNA (miR)-98-5p was detected using quantitative real-time polymerase chain reaction. Cell viability, apoptosis, migration and invasion were analyzed using cell counting kit-8 assay, flow cytometry and transwell assay. Western blot was used to measure the levels of apoptosis-related protein and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway-related protein. The interaction between HEIH and miR-98-5p was confirmed by dual-luciferase reporter and RNA immunoprecipitation assay. In vivo experiments were performed using murine xenograft models.
Results
HEIH was up-regulated in sorafenib-resistant HCC tissues and cell lines, and HEIH silence weakened sorafenib resistance by suppressing cell viability, invasion and migration, decreasing the IC50 values to sorafenib, and increasing apoptosis in sorafenib-resistant HCC cells in vitro and reinforced the anti-tumor effects of sorafenib in vivo. HEIH was a sponge of miR-98-5p, and miR-98-5p inhibition reversed the sorafenib sensitivity induced by HEIH deletion in sorafenib-resistant HCC cells. MiR-98-5p inhibition could activate PI3K/AKT pathway, and enhanced sorafenib resistance by regulating the activation of PI3K/AKT pathway in sorafenib-resistant HCC cells. Besides, HEIH also activated PI3K/AKT pathway through regulating miR-98-5p in sorafenib-resistant HCC cells.
Conclusion
HEIH conferred an advantage to sorafenib resistance in HCC by the activation of PI3K/AKT pathway through miR-98-5p, indicating a potential therapeutic strategy for HCC chemotherapy.
Keywords: HEIH, miR-98-5p, PI3K/AKT, HCC, chemoresistance
Highlights
HEIH is up-regulated in sorafenib-resistant HCC tissues and cell lines.
HEIH knockdown mitigates sorafenib resistance in HCC in vivo and in vitro.
HEIH is a sponge of miR-98-5p.
HEIH activates PI3K/AKT pathway through regulating miR-98-5p in sorafenib-resistant cells in HCC.
HEIH deletion sensitizes the sorafenib-resistant cell to sorafenib by regulating miR-98-5p/PI3K/AKT pathway in HCC.
Introduction
As one of the most common leading causes of cancer-related mortality worldwide, hepatocellular carcinoma (HCC) is still a severe threat to human health.1 Currently, surgical resection is the most effective therapy for early-stage HCC, while most HCC patients are diagnosed in the advanced stage and surgical treatment has little effects.2,3 Sorafenib is currently the only systemic agent permitted for clinical therapy in advanced HCC.4 However, the extension of the median survival time of advanced HCC cases response to sorafenib is only 3–5 months, and serious adverse side effects and the development of sorafenib resistance have hampered its use.5 Thus, further investigations on the mechanisms underlying sorafenib resistance are of great importance for developing novel therapeutic targets to overcome drug resistance.
Long noncoding RNAs (lncRNAs) are a kind of RNA molecules with longer than 200 nucleotides in length and cannot encode the protein. Growing evidence has documented that lncRNAs act as critical regulators in the physiological and pathological cellular processes, including proliferation, apoptosis, metastasis and chemoresistance, to affect cancer cell progression and development.6–8 The hepatocellular carcinoma up-regulated EZH2-associated long non-coding RNA (HEIH) is a functional lncRNA, whose gene mapped on chromosome 5q35.3, and has been recognized to act as an oncogene to promote tumor progression in many types of cancers, including HCC. For example, HEIH induced cell tumorigenesis through targeting miR-200b/a/429 in melanoma.9 HEIH promoted cell proliferation and decreased apoptosis in colorectal cancer through up-regulating Bcl-xL by miR-939.10 HEIH interacted with miR-199a-3p to block mTOR to inhibit cell growth and metastasis in liver cancer.11 However, the roles of the HEIH in sorafenib resistance in HCC remain unraveled.
In the present work, we elaborated the effects of HEIH on the resistance of HCC cells to sorafenib, as well as explored the underlying mechanism of how HEIH participated in the regulation of sorafenib resistance in HCC.
Materials and Methods
Patients and Specimens
HCC tissues were collected from a total of 48 patients who received surgical resection at Putuo Hospital Affiliated to Shanghai University of Traditional Chinese Medicine and were immediately stored at −80°C until used. All subjects were diagnosed by histopathological examination and underwent sorafenib-based chemotherapy prior to surgery. The detailed clinical information is shown in Table S1. We divided these patients into the sorafenib-resistant group (HCC/Sor, N=27, tumor stabilization or progression after 6 cycles of chemotherapy) and the sorafenib-sensitive group (HCC, N=21, tumor remission after 6 cycles of chemotherapy) depending on the sensitivity of HCC patients to sorafenib. This study was permitted by the Ethics Committee of Putuo Hospital Affiliated to Shanghai University of Traditional Chinese Medicine and we had obtained the written informed consent from all patients.
Cell Culture and Transfection
HCC cell lines (HCCLM3 and Huh7) were purchased from Shanghai Academy of Life Science (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) harboring with 10% foetal bovine serum (FBS), 0.1 mg/mL of streptomycin and 100 U/mL of penicillin at 37°C with 5% CO2. Sorafenib-resistant HCCLM3 (HCCLM3/Sor) and -resistant Huh7 (Huh7/Sor) cells were generated by treating HCCLM3 and Huh7 cells with increasing concentrations of the cytostatic sorafenib over several months. Sorafenib-resistant cells were grown in the same media supplemented with 5 μM sorafenib.
Small interfering RNA (siRNA) targeting HEIH (si-HEIH), siRNA negative control (si-NC), the short hairpin RNA (shRNA) targeting HEIH (sh-HEIH) and shRNA scramble control (sh-NC) were generated by Invitrogen (Carlsbad, CA, USA). The miR-98-5p mimic, mimic negative control (miR-NC mimic), miR-98-5p inhibitor (anti-miR-98-5p) and inhibitor negative control (anti-miR-NC) were obtained from RIBOBIO (Guangzhou, China). The transfection was conducted using LipofectamineTM 2000 transfection reagent (Invitrogen).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from tissues and cells using TRIzol reagent (Invitrogen). Then complementary DNAs (cDNAs) were synthesized by using All-in-One™ Kit (GeneCopoeia, Rockville, MD, USA). After that, quantitative PCR was conducted using SYBR Green methods on the ABI7500 system. The fold changes were qualified using 2−ΔΔCt method and normalized with glyceraldehyde 3-phosphate dehydrogenase (GADPH) and U6 small nuclear B noncoding RNA (U6). All experiments were conducted at least three times, and the average was taken. The primers sequences were listed: HEIH, F 5ʹ-AAGAACTCTTCGCTCCAGCC-3ʹ and R 5ʹ- ACAAAAGCAGACTAGGGCGG-3ʹ; miR-98-5p, F 5ʹ- TGAGGTAGTAGTTTGTGCTGTT-3ʹ, and R 5ʹ- GCGAGCACAGAATTAATACGAC-3ʹ; U6, F 5ʹ-ACCCTGAGAAATACCCTCACAT-3ʹ, and R 5ʹ-GACGACTGAGCCCCTGATG-3ʹ; GAPDH, F 5ʹ-AAGAAGGTGGTGAAGCAGGC-3ʹ, and R 5ʹ-GTCAAAGGTGGAGGAGTGGG-3ʹ.
Cell Viability Assay
Transfected resistant cells were seed into 96-well plates (5 × 103 cell/well) and treated with increasing concentrations of sorafenib (0.75, 1.5, 3.0, 6.0, 12, 24 and 48 μM/L) for 48 h. After that, per well was incubated with CCK-8 solution (10 μL/well) (Beyotime, Shanghai, China) for about 2 h. Subsequently, the absorbance was determined by a microplate reader at 450 nm. Additionally, the half maximal inhibitory concentration (IC50 values) of drugs were calculated on the basis of the relative survival curve. All experiments were repeated three times independently.
Flow Cytometry
Transfected resistant cells were plated on 24-well plates overnight and treated with 5 μM sorafenib for 48 h. After that, cells were collected and resuspended with binding buffer, and then double-stained with fluorescein isothiocyanate (FITC) annexin V and propidium iodide (PI) (BD Biosciences, Shanghai, China). Finally, apoptotic cells were analyzed by flow cytometry. Three replicate wells were set in each group, and the experiment was repeated three times.
Western Blot
Transfected cells were lysed by RIPA lysis buffer (Beyotime). Then the lysate was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membranes. Subsequently, the membranes were incubated with primary antibody against B-cell lymphoma-2 (Bcl-2) (1:1000, ab692, Abcam, Cambridge, MA, USA), BCL2-associated X protein (Bax) (1:1000, ab32503, Abcam), phosphorylated (p)-phosphoinositide 3-kinase (p-PI3K) (1:1000, ab182651, Abcam), PI3K (1:1000, ab40776, Abcam), p-protein kinase B (p-AKT) (1:1,000, 9271, Cell Signaling Technology, Boston, MA, USA), AKT (1:1,000, 9272, Cell Signaling Technology), as well as β-Actin (1:1,000, 4967, Cell Signaling Technology). After interaction with secondary HRP-conjugated antibody (1:1000, ab9482, Abcam). Experiments were performed three times, and immunoreactive bands were visualized using electrochemiluminescence.
Cells Migration and Invasion Assays
For migrated cells detection, transfected cells were seeded on the transwell upper chamber filled with serum-free DMEM, and DMEM fixed with 10% FBS was added into the lower well. 24 h later, cells on the lower face of the membranes were fixed and stained. Finally, migrated cells were recorded by a microscope. As to the detection of invaded cells, the upper transwell chamber membranes were pre-coated with Matrigel (BD Biosciences), and other measurement procedure was similar to cell migration. All experiments were repeated three times independently.
Dual-Luciferase Reporter Assay
The wild-type (WT) or mutant (MUT) binding sequences of miR-98-5p in HEIH were separately cloned into pmirGLO dual-luciferase vectors (Promega, Shanghai, China). Afterwards, cells were co-transfected with the constructed dual luciferase reporter plasmids and miR-98-5p mimics or miR-NC mimics using LipofectamineTM 2000 (Invitrogen). Finally, the luciferase activity was analyzed by a dual luciferase assay kit (Promega).
RNA Immunoprecipitation (RIP) Assay
HCCLM3/Sor and Hun7/Sor cells transfected with miR-98-5p mimics or miR-NC mimics were lysed by RIP buffer (Millipore, Billerica, MA, USA), and then cell extracts were co-immunoprecipitated with magnetic beads containing anti-Ago2 or IgG antibody. Finally, the immunoprecipitated RNA was isolated and subjected to qRT-PCR analysis.
In vivo Chemosensitivity Assay
BALB/c nude mice (aged 4–6 weeks, N=12) were bought from Vital River Laboratory Animal Technology (Beijing, China) and manipulated in line with the guidelines of the Animal Research Committee of Putuo Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. The protocol was approved by the Animal Care and Ethics Committee of Shanghai University of Traditional Chinese Medicine. The mice were randomly divided into four groups of three mice each. HCCLM3/Sor cells (5 × 106) transfected with the lentivirus-(lenti)-sh-HEIH or lenti-sh-NC were subcutaneously injected into the posterior flank of each mouse. When the size of xenografts reached approximately 100 mm3, all mice were intraperitoneally injected with sorafenib (100 mg/kg) or the same volume of PBS every 10 days. Tumor size was examined every 7 days, and tumor volume was calculated. At day 28, mice were killed and tumor masses were weighed and used for further molecular analysis.
Statistical Analysis
Data were expressed as the mean ± standard deviation (SD). Group comparison was analyzed using one-way analysis of variance (ANOVA) or Student’s t-test on GraphPad Prism 7 software (GraphPad Inc., San Diego, CA, USA). The correlation analysis was performed using Pearson correlation analysis. P values < 0.05 indicated statistically significant.
Results
HEIH Expression Is Up-Regulated in Sorafenib-Resistant HCC Tissues and Cell Lines
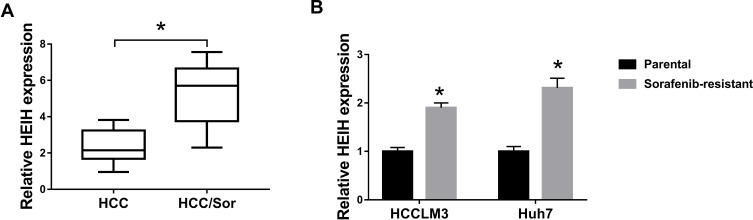
To investigate the influence of HEIH on HCC sorafenib resistance, we detected the expression of HEIH in HCC. QRT-PCR analysis showed HEIH expression was higher in sorafenib-resistant HCC tissues than that in the sensitivity group (Figure 1A). Subsequently, sorafenib-resistant HCCLM3 and -resistant Huh7 cells, named HCCLM3/Sor and Huh7/Sor, were generated, HCCLM3/Sor and Huh7/Sor cells exhibited increased sorafenib resistance (Figure S1, IC50 values: 5.7 vs 13.7 μM in HCCLM3 and HCCLM3/Sor cells, 7.3 vs 17.4 μM in Huh7 and Huh7/Sor cells). After that, we also observed that compared with the parental HCC cells, HEIH expression was elevated in sorafenib-resistant HCC cell lines (Figure 1B). These data indicated HEIH expression might be associated with sorafenib resistance in HCC.
Figure 1.
The expression of HEIH in sorafenib-resistant HCC tissues and cell lines. (A and B) qRT-PCR analysis of HEIH expression in HCC sorafenib-sensitive tumor tissues and sorafenib-resistant tumor tissues, as well as in sorafenib-resistant HCC cell lines and corresponding parental HCC cell lines. *P<0.05.
HEIH Knockdown Mitigates Sorafenib Resistance in Sorafenib-Resistant Cells in HCC
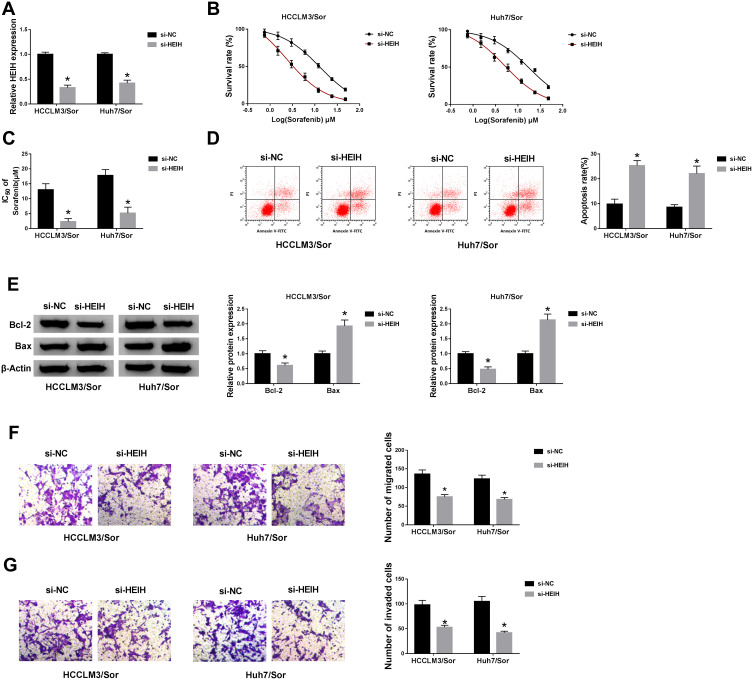
In order to evaluate the detailed function of HEIH in sorafenib resistance of HCC, sorafenib-resistant HCC cell lines (HCCLM3/Sor and Huh7/Sor cells) were established. Immediately, HEIH was knocked down through transfecting si-HEIH into HCCLM3/Sor and Huh7/Sor cells, as confirmed by the down-regulation of HEIH expression in si-HEIH groups (Figure 2A). Subsequently, CCK-8 assay showed HEIH knockdown combined with increasing doses of sorafenib (0.75, 1.5, 3.0, 6.0, 12, 24 and 48 μM/L) gradually decreased the survival rate of sorafenib-resistant cells (Figure 2B) and the IC50 values of HCCLM3/Sor and Huh7/Sor cells to sorafenib were also decreased in the si-HEIH group compared with that in controls (Figure 2C), indicating HEIH silence sensitized HCCLM3/Sor and Huh7/Sor cells to sorafenib. Conversely, HEIH knockdown improved the apoptosis of HCCLM3/Sor and Huh7/Sor cells by decreasing the level of Bcl-2 and increasing the level of Bax (Figure 2D and E). Moreover, HEIH deletion also suppressed the migration and invasion capabilities of sorafenib-resistant HCC cells (Figure 2F and G). Taken together, HEIH knockdown sensitized sorafenib-resistant cells to sorafenib by regulating cell viability, apoptosis, migration and invasion in HCC.
Figure 2.
HEIH knockdown mitigates sorafenib resistance in sorafenib-resistant cells in HCC. HCCLM3/Sor and Huh7/Sor cells were transfected with si-HEIH or si-NC. (A) The interference efficiency was detected using qRT-PCR. (B) The survival rates of resistant cells in combination with increasing concentrations of sorafenib were detected by CCK-8 assay. (C) The IC50 values of resistant cells to sorafenib were assessed by CCK-8 assay. (D) The apoptosis of resistant cells was analyzed using flow cytometric analysis. (E) The expressions of Bcl-2 and Bax in resistant cells were examined by Western blot assay. (F and G) Transwell assay was used to determine migration and invasion abilities of resistant cells. *P<0.05.
HEIH Is a Sponge of miR-98-5p
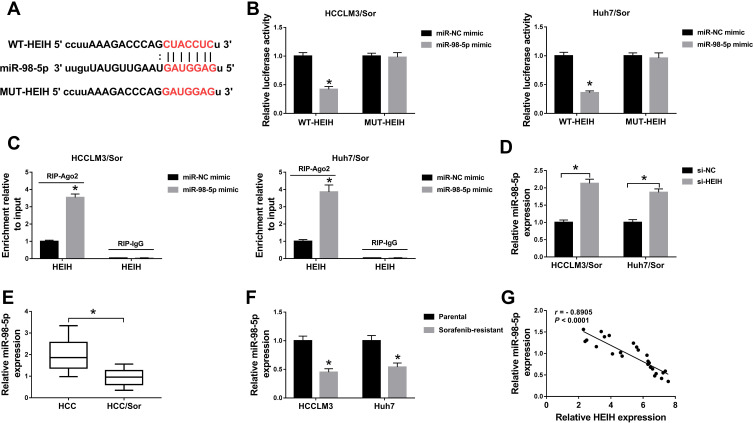
Through using the online software program StarBase, miRNAs with complementary base pairing with HEIH were searched. MiR-98-5p contained the putative binding sites of HEIH (Figure 3A). After that, the reduction of luciferase activity in HCCLM3/Sor and Huh7/Sor cells co-transfected with WT-HEIH and miR-98-5p mimic confirmed the direct interaction between HEIH and miR-98-5p (Figure 3B). Furthermore, RIP assay showed HEIH expression were significantly enriched in Ago2 immunoprecipitates compared with control IgG immunoprecipitates in the presence of abundant miR-98-5p mimic (Figure 3C), further indicating the interaction between HEIH and miR-98-5p. Besides, we also found HEIH silence promoted miR-98-5p expression in HCCLM3/Sor and Huh7/Sor cells (Figure 3D). Meanwhile, the qRT-PCR analysis showed miR-98-5p expression was down-regulated in sorafenib-resistant HCC tissues and cell lines compared with the sensitivity groups (Figure 3E and F), and a negative correlation between HEIH and miR-98-5p expression in sorafenib-resistant HCC tissues was observed (r=−0.8905, P<0.0001) (Figure 3G). Thus, we concluded HEIH targetedly inhibited miR-98-5p expression.
Figure 3.
HEIH is a sponge of miR-98-5p. (A) The putative binding site between HEIH and miR-98-5p was predicted. (B and C) The interaction between HEIH and miR-98-5p was verified by the dual-luciferase reporter assay and RIP assay in sorafenib-resistant cells, respectively. (D) The level of miR-98-5p in HCCLM3/Sor and Huh7/Sor cells transfected with si-HEIH or si-NC was detected using qRT-PCR. (E and F) qRT-PCR analysis of miR-98-5p expression in HCC sorafenib-sensitive tumor tissues and sorafenib-resistant tumor tissues, as well as in sorafenib-resistant HCC cell lines and corresponding parental HCC cell lines. (G) The correlation between HEIH and miR-98-5p was analyzed using Pearson correlation analysis. *P<0.05.
MiR-98-5p Mediates the Action of HEIH on Sorafenib Resistance in HCC Cells
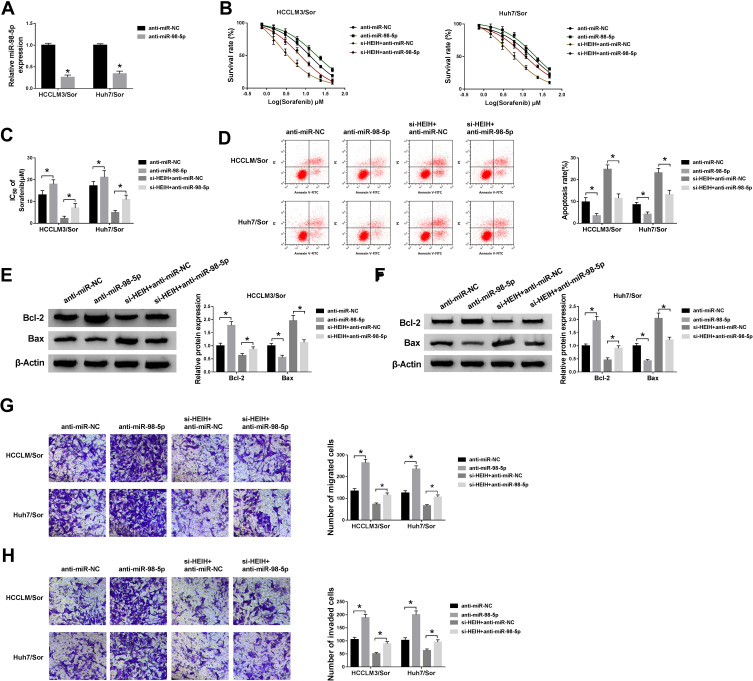
To explore whether the effects of HEIH on sorafenib resistance was mediated by miR-98-5p, HCCLM3/Sor and Huh7/Sor cells were transfected with anti-miR-NC, anti-miR-98-5p. As expected, miR-98-5p expression was remarkably decreased (Figure 4A). After that, we found miR-98-5p inhibition increased the survival rates of sorafenib-resistant cells with increasing doses of sorafenib (Figure 4B) and the IC50 values of HCCLM3/Sor and Huh7/Sor cells to sorafenib (Figure 4C). Besides that, miR-98-5p inhibition reduced the apoptosis of HCCLM3/Sor and Huh7/Sor cells through up-regulating the level of Bcl-2 and down-regulating the level of Bax (Figure 4D–F). Meanwhile, the migrated and invaded HCCLM3/Sor and Huh7/Sor cells were markedly increased by miR-98-5p inhibition (Figure 4G and H). These data suggested miR-98-5p decrease enhanced sorafenib resistance in sorafenib-resistant cells in HCC. Afterwards, HCCLM3/Sor and Huh7/Sor cells were transfected with si-HEIH + anti-miR-NC, or si-HEIH + anti-miR-98-5p to conduct rescue assay, and results exhibited that miR-98-5p inhibition reversed HEIH knockdown-mediated inhibition on CCLM3/Sor and Huh7/Sor cell viability (Figure 4B and C), migration and invasion (Figure 4G and H) as well as promotion on apoptosis (Figure 4D–F) in HCC. Altogether, HEIH knockdown restored the sensitivity of sorafenib-resistant HCC cells to sorafenib by regulating miR-98-5p.
Figure 4.
MiR-98-5p mediates the sorafenib resistance of HEIH on sorafenib-resistant cells in HCC. HCCLM3/Sor and Huh7/Sor cells were transfected with anti-miR-NC, anti-miR-98-5p, si-HEIH + anti-miR-NC, si-HEIH + anti-miR-NC. (A) The expression of miR-98-5p was detected to confirm transfection efficiency. (B) CCK-8 assay analysis for the survival rates of resistant cells in combination with increasing concentrations of sorafenib was performed. (C) CCK-8 assay was used to measure the IC50 values of resistant cells to sorafenib. (D) Flow cytometric analysis for resistant cell apoptosis was conducted. (E and F) The expressions of Bcl-2 and Bax in resistant cells were determined by Western blot assay. (G and H) The migration and invasion abilities of resistant cells were analyzed by transwell. *P<0.05.
PI3K/AKT Pathway Mediates the Sorafenib Resistance of miR-98-5p on Sorafenib-Resistant Cells in HCC
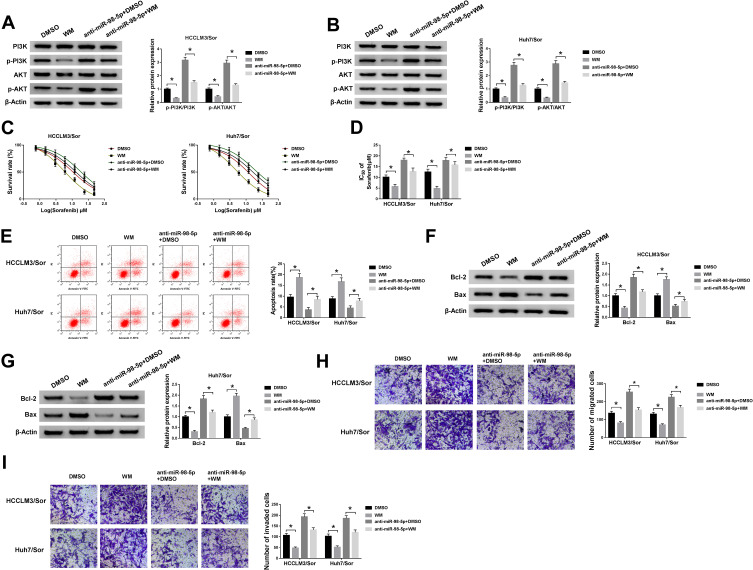
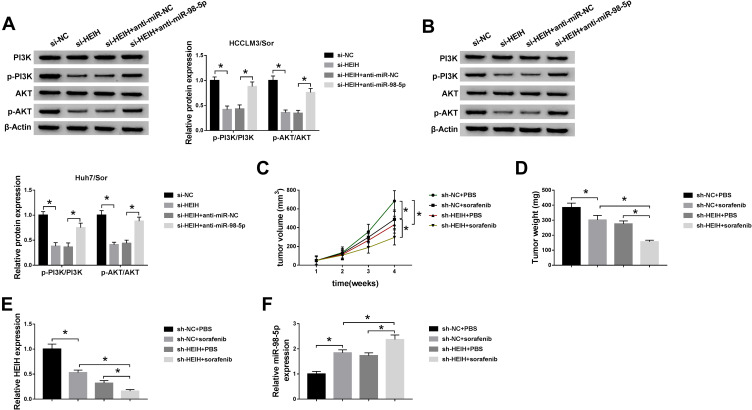
PI3K/AKT pathway is a significant intracellular signaling pathway, in which activation has been demonstrated to associate with acquired resistance to sorafenib in HCC.12 Thus, we further verified whether the effects of miR-98-5p on sorafenib-resistant were mediated by PI3K/AKT pathway. HCCLM3/Sor and Huh7/Sor cells were separately treated with DMSO, PI3K inhibitor wortmannin (WM), anti-miR-98-5p + DMSO, or anti-miR-98-5p + WM. Then Western blot analysis showed WM treatment reduced the level of p-PI3K and p-AKT; besides, WM treatment also abated miR-98-5p inhibition-induced increase of p-PI3K and p-AKT levels in sorafenib-resistant cells in HCC (Figure 5A and B), indicating miR-98-5p inhibition activated PI3K/AKT pathway in sorafenib-resistant cells. After that, functional experiments were performed and we found WM treatment inhibited the viability (Figure 5C), invasion and migration (Figure 5H and I) of sorafenib-resistant cells, reduced the IC50 values of sorafenib-resistant cells to sorafenib (Figure 5D), and increased sorafenib-resistant cell apoptosis (Figure 5E–G) through the inactivation of PI3K/AKT pathway. However, we also discovered that miR-98-5p inhibition promoted cell sorafenib resistance in HCC by activating PI3K/AKT pathway, while these promotions could be reversed by WM treatment (Figure 5C–I). Therefore, we demonstrated that miR-98-5p inhibition enhanced sorafenib resistance in sorafenib-resistant HCC cell lines by regulating the activation of PI3K/AKT pathway. Additionally, Western blot analysis also indicated that HEIH deletion decreased the expression of p-PI3K and p-AKT in HCCLM3/Sor and Huh7/Sor cells, while this decrease was attenuated by following miR-98-5p inhibition (Figure 6A and B), suggesting HEIH activated PI3K/AKT pathway through regulating miR-98-5p in sorafenib-resistant cells in HCC.
Figure 5.
PI3K/AKT pathway mediates the sorafenib resistance of miR-98-5p on sorafenib-resistant cells in HCC. HCCLM3/Sor and Huh7/Sor cells were separately treated with DMSO, WM, anti-miR-98-5p + DMSO, or anti-miR-98-5p + WM. (A and B) The expression of p-PI3K, PI3K, p-AKT, and AKT were detected using Western blot. (C and D) The survival rates of resistant cells in combination with increasing concentrations of sorafenib and the IC50 values to sorafenib were detected by CCK-8 assay. (E) Apoptotic resistant cells were counted using Flow cytometric analysis. (F and G) Western blot was applied to measure the expressions of Bcl-2 and Bax in resistant cells. (H and I) Transwell assay analysis for the migration and invasion abilities of resistant cells was carried out. *P<0.05.
Figure 6.
HEIH deletion contributes to sorafenib-mediated inhibition on HCC tumor growth in vivo. (A and B) The expression of p-PI3K, PI3K, p-AKT, and AKT were detected in HCCLM3/Sor and Huh7/Sor cells transfected with si-NC, si-HEIH, si-HEIH + anti-miR-NC, si-HEIH + anti-miR-98-5p using Western blot. (C) Tumor volumes were calculated every 7 days. (D) Mice were killed on day 28 and tumor masses were excised and weighed. (E and F) The expression of HEIH and miR-98-5p in each excised tumor masses was analyzed using qRT-PCR. *P<0.05.
HEIH Deletion Contributes to Sorafenib-Mediated Inhibition on HCC Tumor Growth in vivo
We further investigated the effects of HEIH on sorafenib-mediated tumor growth in vivo. As shown in Figure 6C and D, knockdown of HEIH significantly reinforced sorafenib-mediated suppression on the tumor growth, reflected by the decrease of tumor volume and lowered tumor weight in si-HEIH plus sorafenib group. In addition, we also found HEIH deletion combined with sorafenib significantly decreased the expression of HEIH, whereas increased the expression of miR-98-5p (Figure 6E and F). Therefore, HEIH deletion enhanced sorafenib-mediated inhibition on HCC tumor growth through miR-98-5p in vivo.
Discussion
Sorafenib is currently regarded as a first-line systemic drug for chemotherapeutic therapy in advanced HCC patients in clinical, while only approximately 30% of patients obtain a significant survival benefit from the sorafenib treatment.13,14 A great majority of HCC patients are refractory to this chemotherapy and develop acquired resistance, which limit the use of sorafenib.8,15 Hence, it is imperative to uncover the precise molecular mechanism underlying sorafenib resistance in HCC cells.
Recently, a growing body of studies have demonstrated the essential roles of lncRNA in sorafenib resistance. For instance, lncRNA-SRLR increased sorafenib resistance to responsive renal cell carcinoma cells by inducing the activation of evoking IL-6/STAT3 axis.16 SNHG1 deletion sensitized resistant HCC cells to sorafenib through inducing cell apoptosis and autophagy via the inhibition of Akt pathway.17 LncRNA SNHG3 regulated the miR-128/CD151 pathway to facilitate sorafenib resistance in HCC cells.18 FOXD2-AS1 contributed to the sensitivity of HCC cells to sorafenib by modulating the miR-150-5p/TMEM9 axis.8 Therefore, lncRNAs are important regulators for sorafenib resistance. In this research, we demonstrated that HEIH was highly expressed, and depletion of HEIH sensitized sorafenib-resistant HCC cells to sorafenib through decreasing the viability, invasion and migration, and increasing apoptosis of resistant cells in HCC. What’s more, in vivo experiments also confirmed HEIH knockdown enhanced sorafenib-mediated inhibition on tumor growth. Thus, HEIH may represent a promising method for sorafenib resistance of HCC.
It is well-recognized that lncRNAs can function as molecular sponges for miRNAs, thereby abolishing the endogenous inhibition of these miRNAs on their targets.19 MicroRNAs (miRNAs) are small non-protein coding RNAs, which have been reported to involve in the regulation of various cell possesses through post-transcriptional gene silencing.20 Additionally, numerous miRNAs have been investigated to implicate in regulating sorafenib resistance.21 For example, miR-137 sensitized sorafenib-resistant HCC cells to sorafenib via inhibiting ANT2 expression.22 MiR-21 contributed to sorafenib resistance in HCC cells through suppressing autophagy by the Akt/PTEN pathway.23 In this study, we demonstrated HEIH directly bound to miR-98-5p. MiR-98-5p is a functional miRNA, which has been confirmed to serve as an oncogene or tumor suppressor in the tumorigenesis of many cancers to affect cancer development.24,25 In HCC, Jiang et al revealed miR-98-5p acted as a tumor suppressor to repress cell viability and induced apoptosis and cell cycle arrest, thus hindering HCC progression.26 Besides that, it also was demonstrated that aberrant miR-98-5p expression was connected with cisplatin resistance in ovarian cancer27 and lung cancer.28 Thus, we assumed miR-98-5p might regulate sorafenib resistance in HCC. Subsequently, further investigates demonstrated miR-98-5p was decreased, and miR-98-5p inhibition increased sorafenib resistance in nonresponsive HCC cells. More importantly, miR-98-5p inhibition reversed HEIH-mediated response to sorafenib treatment in HCC cells. Emerging evidence has documented that PI3K/AKT pathway is an important underlying mechanism of acquired resistance to sorafenib.12,29 In this review, we found miR-98-5p inhibition could activate PI3K/AKT pathway, and inactivating the PI3K/AKT pathway abrogated miR-98-5p inhibition-mediated acquired resistance of sorafenib. Furthermore, we further revealed HEIH also activated PI3K/AKT pathway by suppressing miR-98-5p in sorafenib-resistant HCC cells.
In recent years, it has been identified that regorafenib clinically improves the overall survival of patients with HCC progressing during sorafenib treatment, and regorafenib have survival benefit for sorafenib-resistant HCC.30 Besides, Teufel et al exhibited that expression patterns of plasma proteins and miRNAs was associated with increased overall survival times of patients with HCC following regorafenib treatment.31 Thus, we assume that molecular therapy based on HEIH/miR-98-5p/PI3K/AKT axis combine with regorafenib may provide a novel insight into the improvement in outcome of sorafenib-resistant HCC patients. Lenvatinib is a novel first-line drug in the treatment of advanced HCC, and cabozantinib can act as a second-line option to the first-line drugs sorafenib and lenvatinib and the second-line drug regorafenib in advanced HCC.32 Thus, whether lenvatinib or cabozantinib combines with RAN-based molecular therapy to overcome sorafenib resistance in HCC still needs further study.
Conclusion
In conclusion, this study demonstrated that HEIH impaired the sensitivity to sorafenib in nonresponsive HCC cells through miR-98-5p/PI3K/AKT pathway, providing new insight into the pathogenesis of sorafenib resistance and new therapeutic strategies to improve responses to sorafenib in HCC patients.
Data Sharing Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the ethical review committee of Putuo Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. Written informed consent was obtained from all enrolled patients.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061 [DOI] [PubMed] [Google Scholar]
- 3.Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res. 2016;35(1):159. doi: 10.1186/s13046-016-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keating GM. Sorafenib: a review in hepatocellular carcinoma. Target Oncol. 2017;12(2):243–253. doi: 10.1007/s11523-017-0484-7 [DOI] [PubMed] [Google Scholar]
- 5.Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38(5):614–622. doi: 10.1038/aps.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz SU, Grote P, Herrmann BG. Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci. 2016;73(13):2491–2509. doi: 10.1007/s00018-016-2174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. doi: 10.4161/rna.20481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui C, Dong Z, Yang C, et al. LncRNA FOXD2-AS1 as a competitive endogenous RNA against miR-150-5p reverses resistance to sorafenib in hepatocellular carcinoma. J Cell Mol Med. 2019;23(9):6024–6033. doi: 10.1111/jcmm.14465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Xing G, Wang Y, Luo Z, Liu G, Meng H. Long noncoding RNA HEIH promotes melanoma cell proliferation, migration and invasion via inhibition of miR-200b/a/429. Biosci Rep. 2017;37(3). doi: 10.1042/BSR20170682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui C, Zhai D, Cai L, Duan Q, Xie L, Yu J. Long noncoding RNA HEIH promotes colorectal cancer tumorigenesis via counteracting miR-939mediated transcriptional repression of Bcl-xL. Cancer Res Treat. 2018;50(3):992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Cao LG, Hu J, Liu X, Liu J. Silence of lncRNA HEIH suppressed liver cancer cell growth and metastasis through miR-199a-3p/mTOR axis. J Cell Biochem. 2019;120(10):17757–17766. doi: 10.1002/jcb.29041 [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Wang Q, Liu J, Cao H. Inhibition of the PI3K/Akt signaling pathway reverses sorafenib-derived chemo-resistance in hepatocellular carcinoma. Oncol Lett. 2018;15(6):9377–9384. doi: 10.3892/ol.2018.8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu L, Liu L, Yang S, Ren J, Lai PBS, Chen GG. New insights into sorafenib resistance in hepatocellular carcinoma: responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer. 2017;1868(2):564–570. doi: 10.1016/j.bbcan.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 14.Sun T, Liu H, Ming L. Multiple roles of autophagy in the sorafenib resistance of hepatocellular carcinoma. Cell Physiol Biochem. 2017;44(2):716–727. doi: 10.1159/000485285 [DOI] [PubMed] [Google Scholar]
- 15.Gao C, Peng FH, Peng LK. MiR-200c sensitizes clear-cell renal cell carcinoma cells to sorafenib and imatinib by targeting heme oxygenase-1. Neoplasma. 2014;61(6):680–689. doi: 10.4149/neo_2014_083 [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Yang F, Wei D, et al. Long noncoding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene. 2017;36(14):1965–1977. doi: 10.1038/onc.2016.356 [DOI] [PubMed] [Google Scholar]
- 17.Li W, Dong X, He C, et al. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR-21 in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2019;38(1):183. doi: 10.1186/s13046-019-1177-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang PF, Wang F, Wu J, et al. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma. J Cell Physiol. 2019;234(3):2788–2794. doi: 10.1002/jcp.27095 [DOI] [PubMed] [Google Scholar]
- 19.Juan L, Wang G, Radovich M, et al. Potential roles of microRNAs in regulating long intergenic noncoding RNAs. BMC Med Genomics. 2013;6(Suppl 1):S7. doi: 10.1186/1755-8794-6-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang S, Tan G, Jiang X, et al. An artificial lncRNA targeting multiple miRNAs overcomes sorafenib resistance in hepatocellular carcinoma cells. Oncotarget. 2016;7(45):73257–73269. doi: 10.18632/oncotarget.12304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu AQ, Lv B, Qiu F, Wang XY, Cao XH. Upregulation of miR-137 reverses sorafenib resistance and cancer-initiating cell phenotypes by degrading ANT2 in hepatocellular carcinoma. Oncol Rep. 2017;37(4):2071–2078. doi: 10.3892/or.2017.5498 [DOI] [PubMed] [Google Scholar]
- 23.He C, Dong X, Zhai B, et al. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget. 2015;6(30):28867–28881. doi: 10.18632/oncotarget.4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Wei M, Wang G. [miR-98-5p promotes apoptosis and inhibits migration by reducing the level of STAT3 in A549 cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018;34(6):522–527.Chinese. PMID: 30236205. [PubMed] [Google Scholar]
- 25.Fu Y, Liu X, Chen Q, et al. Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4. J Exp Clin Cancer Res. 2018;37(1):130. doi: 10.1186/s13046-018-0807-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang T, Li M, Li Q, et al. MicroRNA-98-5p inhibits cell proliferation and induces cell apoptosis in hepatocellular carcinoma via targeting IGF2BP1. Oncol Res. 2017;25(7):1117–1127. doi: 10.3727/096504016X14821952695683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Bao W, Liu Y, et al. miR-98-5p contributes to cisplatin resistance in epithelial ovarian cancer by suppressing miR-152 biogenesis via targeting Dicer1. Cell Death Dis. 2018;9(5):447. doi: 10.1038/s41419-018-0390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang P, Wu X, Wang X, Huang W, Feng Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016;7(28):43337–43351. doi: 10.18632/oncotarget.9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg L, Yoon CH, Sharma G, Bertagnolli MM, Cho NL. Sorafenib inhibits proliferation and invasion in desmoid-derived cells by targeting Ras/MEK/ERK and PI3K/Akt/mTOR pathways. Carcinogenesis. 2018;39(5):681–688. doi: 10.1093/carcin/bgy038 [DOI] [PubMed] [Google Scholar]
- 30.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 31.Teufel M, Seidel H, Köchert K, et al. Biomarkers associated with response to regorafenib in patients with hepatocellular carcinoma. Gastroenterology. 2019;156(6):1731–1741. doi: 10.1053/j.gastro.2019.01.261 [DOI] [PubMed] [Google Scholar]
- 32.Kudo M. Cabozantinib as a second-line agent in advanced hepatocellular carcinoma. Liver Cancer. 2018;7(2):123–133. doi: 10.1159/000488542 [DOI] [PMC free article] [PubMed] [Google Scholar]