Abstract
Purpose
Pulmonary hypertension (PH) is an important comorbidity of lung cancer, PH in lung cancer patients is gradually gaining interest because of its apparent high prevalence, but the impact of PH on the outcomes of lung cancer remains uncertain and had rarely been discussed. We aimed to evaluate the prevalence, determinants and prognosis value of elevated pulmonary artery systolic pressure (PASP) in non-small cell lung cancer patients.
Patients and Methods
In this retrospective study, subjects with a new and pathological confirmed diagnosis of lung cancer were enrolled. All patients underwent transthoracic echocardiography before received treatment. Pulmonary artery systolic pressure was measured by transthoracic echocardiography. Lung cancer subtypes were categorized by WHO classification of lung tumors. Hazard ratios (HR) were estimated by using Cox regression models.
Results
Among 612 non-small cell lung cancer (NSCLC) patients, 19.8% coexisted with PH. After adjustment for age, symptom, coagulation disorders, lymph node metastasis, distant metastasis, histological type, clinical stage, PASP ≥35mmHg was significantly associated with the decreased overall survival (OS) of NSCLC (P= 0.028). Moreover, PASP ≥45mmHg was an independent predictor for perioperative death. Independent factors of comorbid elevated PASP were age, the presence of intrapulmonary metastasis and coagulation disorders.
Conclusion
These findings suggest that PASP is an independent prognostic risk factor for NSCLC patients. Main determinants of elevated PASP are age, the presence of intrapulmonary metastasis and coagulation disorders.
Keywords: pulmonary hypertension, non-small cell lung cancer, tumoral pulmonary hypertension, overall survival
Introduction
Lung cancer is still the most common type of cancer worldwide, and being the leading cause of cancer death, its 5-year relative survival rates is currently 18%.1 While there is a 20% reduction in lung cancer mortality and the death rate for lung cancer dropped steadily because early diagnosis through low-dose computed tomography screening and developments in the therapy,2 however, survivors of lung cancer have the worse quality of life (QoL) than other cancer survivors.3 Among the reasons, dyspnea and asthenia had a significant impact on QoL, and cardiovascular diseases (CVD) and chronic obstructive pulmonary disease are the two most prevalent comorbidities among lung cancer patients.4,5 A recent study found that 48% (250/519) of lung cancer patients had a mean PA diameter of ≥28 mm, suggestive of PH, as assessed by high-resolution computed tomography (CT) of the chest. However, few studies have discussed the prognosis in these population.
Lung cancer and PH are closely related and previous studies have discussed in autopsy specimens and animal models, in which pulmonary tumor thrombotic microangiopathy (PTTM) is increasingly recognized as an important cause in which underlying mechanism is believed to be microscopic tumor cell emboli and intimal proliferation.6 Other agents are implicated in the development of PH in cancer patients, including chronic thromboembolism pulmonary hypertension complicating venous thromboembolism. Chemotherapeutic drugs may also cause PH7 and pulmonary veno-occlusive disease (PVOD).8 Besides, histopathological evidence of lung vascular remodeling was found in human lung cancer tissue and mouse models of lung cancer suggested that lung cancer might trigger PH.9 From the perspective of metabonomics, pulmonary vascular cells of PH and extravascular tissue of PH patients exhibit a glycolytic switch similar to the Warburg effect in cancer, featuring increased aerobic glycolysis and decreased glucose oxidation,10 which provides intermediates for the synthesis of amino acids, lipids, and nucleotides, required for the demand of enhanced proliferation and apoptosis resistance in cells of PH.11
PH is defined as an elevation in the mean pulmonary artery pressure (mPAP) that is greater than 20 mmHg,12 which was measured at rest via right heart catheterization (RHC). The gold standard for the diagnosis of PH is right heart catheterization (RHC). However, RHC is invasive and costly. A good correlation has been found between RHC and echocardiography and the threshold of 35 mmHg is most commonly used to estimate PH.12,13
The aims of our study are to analyze the prevalence of elevated PASP in non-small cell lung cancer (NSCLC) patients and its adverse prognosis value for OS and to elucidate the risk factor associated with PH.
Materials and Methods
Patients
We performed a retrospective cohort study including newly diagnosed NSCLC patients underwent transthoracic echocardiography within 1 month before the therapy from January 2009 to January 2015 at a single center in Beijing. Inclusion criteria were age ≥ 18 years, cytology or histology confirmed NSCLC, clinical stability (defined as Zubrod performance status of 2 or less). The exclusion criteria were as follows: patients with clinical instability, inability to understand the study or rejection to personal data record and processing, patients with poor echocardiography quality or pulmonary artery systolic pressure (PASP) not correctly reported, the pregnancy, patients with a definite history of idiopathic PAH, chronic obstructive pulmonary disease, obstructive sleep apnea, other pulmonary diseases with mixed restrictive or obstructive patterns, left heart diseases, chronic thromboembolism, HIV infection, portal hypertension, drug and toxic exposure, connective tissue disease, or any other obvious identified cause of PH, patients less than 18 years old. The final eligible cohort encompassed 612 NSCLC patients divided into clinical subgroups (stages IA–IIB, IIIA and IIIB–IV). The study was undertaken in accordance with international guidelines on Good Clinical Practice and the Declaration of Helsinki. It was approved by the ethics committee of Peking University First Hospital. Informed written consent was obtained from all participating individuals.
Echocardiographic Measurements
PASP was measured by transthoracic echocardiography (TTE) that was performed by two experienced cardiologists. All the reading cardiologist was blinded to the clinical characteristics of the study participants. The estimation of PASP by echocardiograph derived from the application of the modified Bernoulli equation: 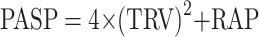 ,14 TRV is the maximum tricuspid regurgitation jet speed and RAP is the right atrium pressure, which is estimated by inferior vena cava diameter.15 Elevated PASP is defined as a PASP value above or equal to 35 mm Hg.14 We considered moderate to severe PH when PASP was above or equal to 45 mm Hg.
,14 TRV is the maximum tricuspid regurgitation jet speed and RAP is the right atrium pressure, which is estimated by inferior vena cava diameter.15 Elevated PASP is defined as a PASP value above or equal to 35 mm Hg.14 We considered moderate to severe PH when PASP was above or equal to 45 mm Hg.
Laboratory Tests
Laboratory parameters were routine tests, and coagulation test including thrombin time (TT), activated partial thromboplastin time (APTT), prothrombin time (PT), fibrinogen (FIB), fibrinogen-degradation products (FDP), D-Dimer. All measurements were performed according to standard methods.
Follow-Up and Outcomes Definitions
All patients were followed up to determine their survival status. Follow-up was conducted by telephone to obtain information on survival (date and cause of death). To evaluate the burden in relation to mortality, the main end point was overall survival (time until death from any cause or last follow-up).
Statistical Methods
All statistical analyses were performed using SPSS version 23. Continuous variables were expressed as means and standard deviation if the data was normally distributed, and vice versa by median and interquartile range. Comparison between the groups was done using Student’s t-test or a Mann–Whitney U-test. Categorical variables were compared using the chi-squared test. Univariate analysis was performed using logistic regression to assess factors associated with PH. The prognosis value of PH for predicting the study outcomes was assessed by the Kaplan–Meier analysis (survival curves were compared using a Log-rank test) and Cox regression. In multivariate Cox models, we included all variables that showed significant association with the study outcomes at univariate Cox analyses. The models also included those considered confounders factors. Differences were considered statistically significant when p value was <0.05. Survival time was defined as the interval between the date of lung cancer diagnosis and the date of death, or end of follow-up (1 December 2019).
Results
Elevated PASP and Baseline Data
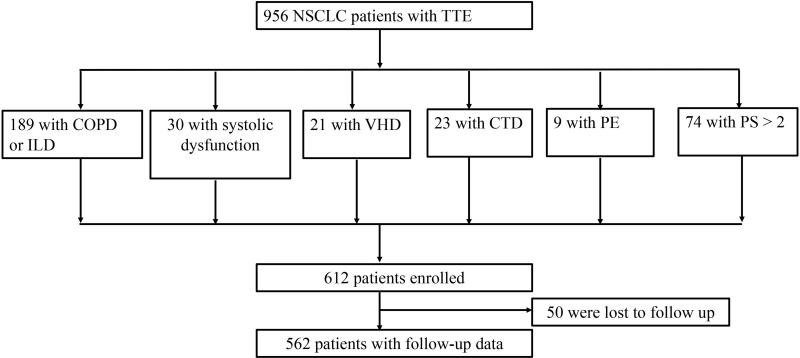
A total of 956 patients with newly diagnosed lung cancer were enrolled in this study. Among these patients, 346 were not included: 189 patients with COPD or Interstitial lung disease, 74 patients with PS score greater than 2, 30 patients with systolic dysfunction, 23 patients with connective tissue disease, 21 patients with valvular disease, 9 patients were diagnosed with pulmonary embolism. A flow chart of patient selection is shown in Figure 1. The characteristics of the patients are provided in Table 1. The age of lung cancer patients was 66 (59,73) years. Among 612 enrolled patients, 381 (62.3%) were male and 303 (49.5%) were current smokers or ex-smokers. Two hundred and two (33.0%) were squamous carcinoma, 379 (61.9%) adenocarcinoma and 31 (5.1%) other histologic types (including large cell lung cancer, carcinoid, sarcomatoid carcinoma, adenosquamous carcinoma, lymphoepithelial carcinoma). Moreover, 270 (44.1%) lung cancers were at an early stage (stage IA-IIB) and 520 (85.0%) of all lung cancers had eventually undergone surgery.
Figure 1.
Flow chart of patients inclusion and diagnosis.
Abbreviations: NSCLC, non-small cell lung cancer; TTE, transthoracic echocardiography; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; VHD, valvular heart diseases; CTD, connective tissue disease; PE, pulmonary embolism; PS, Zubrod performance status.
Table 1.
Characteristics of the Included Patients
| Characteristics | All Participants (n= 612) | PASP < 35mmHg (n= 491, 80.2%) | PASP ≥ 35mmHg (n= 121, 19.8%) | P value |
|---|---|---|---|---|
| Age, y (median, range) | 66 (59, 73) | 65 (59, 71) | 72 (63, 77) | < 0.001 |
| Sex, n (%) | 0.328 | |||
| Male | 381 (62.3) | 301 (61.3) | 80 (66.1) | |
| Female | 231 (37.7) | 190 (38.7) | 41 (33.9) | |
| BMI, kg/m2 (mean ± SD) | 24.1 ± 3.5 | 24.2 ± 3.4 | 23.8 ± 3.6 | 0.324 |
| Symptom, n (%) | 0.257 | |||
| Negative | 240 (39.2) | 198 (40.3) | 42 (34.7) | |
| Positive | 372 (60.8) | 293 (59.7) | 79 (65.3) | |
| CCI, n (%) | 0.434 | |||
| ≤ 2 | 457 (75.2) | 370 (75.4%) | 87 (71.9) | |
| > 2 | 155 (25.3) | 121 (24.6%) | 34 (28.1) | |
| History of other cancer, n (%) | 20 (3.3) | 15 (3.1) | 5 (4.1) | 0.551 |
| Smoking history, n (%) | 0.065 | |||
| No | 309 (50.5) | 257 (52.3) | 52 (43.0) | |
| Yes | 303 (49.5) | 234 (47.7) | 69 (57.0) | |
| Alcohol history, n (%) | 0.781 | |||
| No | 501 (81.9) | 403 (82.1) | 98 (81.0) | |
| Yes | 111 (18.1) | 88 (17.9) | 23 (19.0) | |
| Coagulation function, n (%) | < 0.001 | |||
| Normal | 499 (81.5) | 424 (86.4) | 75 (62.0) | |
| Abnormala | 113 (18.5) | 67 (13.6) | 46 (38.0) | |
| Hb, g/L (median, range) | 135.8 (127.0, 146.0) | 136 (128.0, 146.0) | 134 (122.0, 144.0) | 0.077 |
| IBIL, μmol/L (median, range) | 9.0 (7.1, 11.4) | 9.1 (7.3, 11.4) | 8.8 (6.8, 10.8) | 0.119 |
| Platelets, x10^10/L (median, range) | 212.0 (176.0, 257.8) | 212 (176.0, 257.0) | 209 (171.5, 264.5) | 0.930 |
| GGOb, n (%) | 94 (15.4) | 75 (15.3) | 19 (15.7) | 0.907 |
| Clinical stage, n (%) | 0.132 | |||
| I | 222 (36.3) | 186 (37.9) | 36 (29.8) | |
| II | 48 (7.8) | 40 (8.1) | 8 (6.6) | |
| III–IV | 342 (55.9) | 265 (54.0) | 77 (63.6) | |
| Echocardiographic parameter | ||||
| PASP, mmHg (median, range) | 28.3 (24.0, 33.0) | 26.3 (23.0, 30.0) | 39.0 (36.9, 42.8) | < 0.001 |
| LVEF, % (median, range) | 71.0 (65.0, 76.0) | 71.0 (65.8, 76.0) | 71.0 (63.5, 75.9) | 0.403 |
| Histological type, n (%) | 0.160 | |||
| Adenocarcinoma | 379 (61.9) | 302 (61.5) | 77 (63.6) | |
| Squamous-cell carcinoma | 202 (33.0) | 160 (32.6) | 42 (34.7) | |
| Other typesc | 31 (5.1) | 29 (5.9) | 2 (1.7) | |
| Intrapulmonary metastasis, n (%) | 43 (7.0) | 24 (4.9) | 19 (15.7) | < 0.001 |
| Lymph node metastasis, n (%) | 241 (39.4) | 185 (37.7) | 56 (46.3) | 0.083 |
| Distant metastasis, n (%) | 96 (15.9) | 69 (14.1) | 27 (22.3) | 0.025 |
| Treatment types, n (%) | < 0.001 | |||
| Surgery | 520 (85.0) | 432 (88.0) | 88 (72.7) | |
| Chemotherapy | 62 (10.1) | 41 (8.4) | 21 (17.4) | |
| Radiotherapy | 5 (0.8) | 5 (1.0) | 0 | |
| Other | 25 (4.1) | 13 (2.6) | 12 (9.9) | |
| Perioperative death, n (%) | 12 (2.5) | 4 (4.8) | 8 (2.0) | |
| Surgical types, n (%) | 0.988 | |||
| Thoracotomy | 219 (42.1) | 182 (42.1) | 37 (42.0) | |
| VATS | 301 (57.9) | 250 (57.9) | 51 (58.0) |
Notes: aAbnormal coagulation function was defined as the presence of D-dimer >0.24mg/L or FDP > 5mg/L. bChest scan shows ground-glass opacity, cOther subtypes include large cell lung cancer, carcinoid, sarcomatoid carcinoma, adenosquamous carcinoma, lymphoepithelial carcinoma.
Abbreviations: PASP, pulmonary artery systolic pressure; BMI, body mass index; CCI, Charlson comorbidity index; Hb, hemoglobin; IBIL, indirect bilirubin; GGO, ground gross opacity; PASP, pulmonary artery systolic pressure; LVEF, left ventricular ejection fraction; VATS, video-assisted thoracic surgery.
The prevalence of elevated PASP was 19.8% and 3.1% of the population presented PASP ≥ 45mmHg. The PASP in the whole group was 28.3 (24.0, 33.0) mmHg, while in the elevated PASP group it was 39.0 (36.9, 42.8) mmHg. Data analysis according to the presence or absence of PH showed that patients with elevated PASP were significantly older, with coagulation disorders and were more likely to have intrapulmonary as well as distant metastasis.
Elevated PASP and Risk of Death
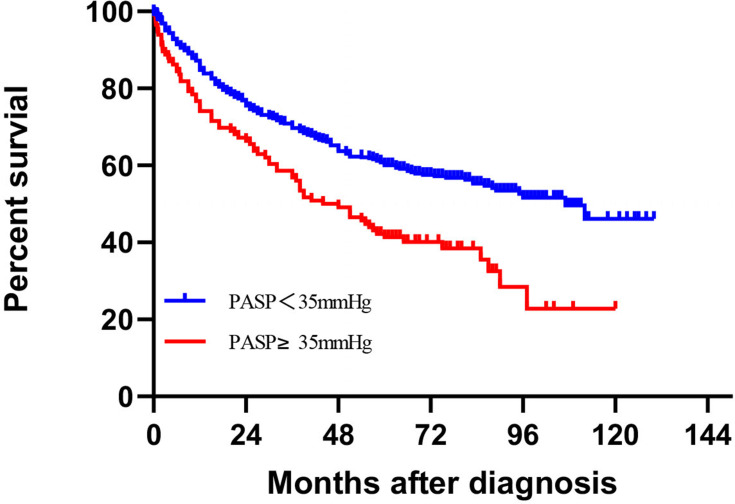
The median follow-up period of 77 months, with a follow-up rate of 91.8% (562 of 612 NSCLC patients), and 273 patients died (48.6%), 199 in the non-elevated PASP group (44.6%) and 74 in the elevated PASP group (63.8%). The 3-/5-year OS rates of NSCLC were 57.1%/49.5% in elevated PASP patients and were 91.3%/84.4% in non-elevated PASP patients. The main causes of death were cancer (80.2%). The Kaplan–Meier survival curves indicated that elevated PASP was significantly associated with the worse OS of lung cancer (Figure 2).
Figure 2.
Kaplan–Meier curves of overall survival rate of NSCLC cancer. Elevated PASP is associated with all-cause mortality in NSCLC patients. P value are shown for the Log-rank test.
The HRs of factors related to lung cancer prognosis are shown in Table 2. In univariate analysis, the presence of elevated PASP and PASP values was both associated with the decreased OS of lung cancer (hazard ratio [HR] 1.705, 95% CI 1.296–2.243; 1.039, 95% CI 1.023–1.054). Multivariate Cox regression analysis showed that, after adjustment for age, sex, respiratory symptoms, smoking status, GGO, Charlson comorbidity index (CCI), coagulation dysfunction, lymph node metastasis, distant metastasis, pathological type, elevated PASP and PASP value remained as independent predictors. Moreover, perioperative death rates were 2.5% in the whole group and PASP ≥ 45mmHg was an independent predictor of perioperative death, as shown in Table 3. Further, we found the independent predictors of PH were age, coagulation disorder and intrapulmonary metastasis as shown in Table 4.
Table 2.
HRs for Overall Survival Among NSCLC Patients
| Univariate Analysis | Multivariate Cox Regressiona | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| PASP (increase) | 1.039 (1.023–1.054) | <0.001 | 1.018 (1.003–1.034) | 0.020b |
| PASP ≥ 35mmHg (present) | 1.705 (1.296–2.243) | <0.001 | 1.386 (1.036–1.853) | 0.028c |
| Age (increase) | 1.029 (1.015–1.042) | <0.001 | 1.030(1.017–1.044) | < 0.001c |
| Sex (female) | 0.622 (0.478–0.810) | <0.001 | 0.179c | |
| Symptom (yes) | 2.852 (2.147–3.788) | <0.001 | 1.720(1.270–2.329) | < 0.001c |
| Smoke (yes) | 1.412 (1.148–1.735) | 0.001 | 0.540c | |
| GGO (yes) | 0.679 (0.467–0.987) | 0.042 | 0.692c | |
| CCI (>2) | 1.514 (1.169–1.960) | 0.002 | 0.598c | |
| Coagulopathy (yes) | 2.857 (2.204–3.703) | <0.001 | 1.363(1.030–1.804) | < 0.001c |
| Intrapulmonary metastasis (yes) | 2.892 (2.024–4.133) | <0.001 | 0.148c | |
| Lymph node metastasis (yes) | 2.848 (2.237–3.626) | <0.001 | 1.698(1.272–2.266) | < 0.001c |
| Distant metastasis (yes) | 3.696 (2.825–4.836) | <0.001 | 2.077(1.469–2.936) | < 0.001c |
| Histological type | <0.001 | 0.004c | ||
| Adenocarcinoma | 0.400 (0.253–0.633) | <0.001 | 0.447(0.279–0.718) | 0.001c |
| Squamous-cell carcinoma | 0.638 (0.400–1.019) | 0.06 | 0.476(0.292–0.776) | 0.003c |
| LVEF (increase) | 0.979 (0.966–0.993) | 0.003 | 0.123c | |
Notes: aThere were two Cox regression models. PASP (continuous, increase), PASP ≥ 35mmHg (dichotomous, referent: PASP < 35mmHg) were, respectively, distributed in models 1, 2. Age (continuous, increase), sex (dichotomous, referent: male), Symptom (dichotomous, referent: without symptom), Smoke (dichotomous, referent: non-smoking), GGO (dichotomous, referent: without-GGO), CCI (dichotomous, referent: CCI ≤ 2), coagulopathy (dichotomous, referent: without coagulation disorder), Intrapulmonary metastasis (dichotomous, referent: without Intrapulmonary metastasis), Lymph node metastasis (dichotomous, referent: without lymph node metastasis). Distant metastasis (dichotomous, referent: without distant metastasis), Clinical stage (dichotomous, referent: IA-IIB), LVEF (continuous, increase). bModel 1; cModel 2.
Table 3.
Logistic Regression Analyses for Variables Associated with Perioperative Death
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Coagulopathy | 4.313 (1.342–13.859) | 0.014 | 3.504 (1.006–12.202) | 0.115d |
| PASP range | 0.030 | 0.102d | ||
| PASP 35–45mmHgb | 1.368 (0.284–6.577) | 0.696 | 0.899d | |
| PASP ≥ 45mmHg c | 9.575 (1.799–50.958) | 0.008 | 7.849 (1.260–48.878) | 0.042d |
| PASP | 1.064 (1.013–1.116) | 0.013 | 0.088e | |
| Age | 1.088 (1.010–1.171) | 0.025 | 1.090 (1.005–1.181) | 0.038 d |
| CCI | 5.170 (0.594–44.976) | 0.137 | ||
| Symptom | 4.043 (0.876–18.656) | 0.073 | ||
| Sex | 0.995 | |||
| BMI | 0.841 (0.702–1.008) | 0.061 | ||
| Alcohol | 1.363 (0.362–5.135) | 0.648 | ||
| GGO | 1.051 (0.226–4.896) | 0.949 | ||
| Smoke | 2.607 (1.062–6.400) | 0.036 | 3.213 (1.149–8.986) | 0.026 d |
| Intrapulmonary metastasis | 2.247 (0.275–18.365) | 0.450 | ||
| Lymph node metastasis | 2.552 (0.797–8.169) | 0.114 | ||
| Distant metastasis | 6.682 (1.689–26.427) | 0.007 | 10.287 (2.230–47.445) | 0.003 d |
| Histological type | 0.058 | |||
| Adenocarcinoma | 0.250 (0.025–2.496) | 0.238 | ||
| Squamous-cell carcinoma | 1.272 (0.152–10.625) | 0.687 | ||
| Clinical stage | 1.974 (0.586–6.647) | 0.272 | ||
| Surgery types | 3.614 (0.966–13.522) | 0.056 | ||
Notes: aThere were two logistic models, PASP range (dichotomous, referent: PASP 35–44mmHg), PASP (continuous, increase) were, respectively, distributed in models 1, 2. bPulmonary artery systolic pressure between 35–44mmHg. cPulmonary artery systolic pressure above or equal to 45mmHg. dModel 1; eModel 2.
Table 4.
Logistic Regression Analyses for Variables Associated with Elevated PASP
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.065 (1.039–1.090) | <0.001 | 1.060 (1.034–1.087) | < 0.001 |
| Sex | 0.812 (0.535–1.233) | 0.329 | ||
| BMI | 0.969 (0.914–1.027) | 0.291 | ||
| Symptom | 1.271 (0.839–1.926) | 0.258 | ||
| Alcohol history | 1.075 (0.646–1.789) | 0.781 | ||
| Smoking history | 1.457 (0.976–2.177) | 0.066 | ||
| GGO (yes) | 1.033 (0.597–1.787) | 0.907 | ||
| CCI (>2) | 1.195 (0.765–1.867) | 0.434 | ||
| Coagulopathyb | 3.867 (2.468–6.057) | <0.001 | 3.380 (2.053–5.566) | < 0.001 |
| Intrapulmonary metastasis (yes) | 3.625 (1.913–6.866) | <0.001 | 3.329 (1.556–7.125) | 0.002 |
| Lymph node metastasis (yes) | 1.425 (0.954–2.129) | 0.084 | ||
| Distant metastasis (yes) | 1.757 (1.068–2.890) | 0.027 | 0.857 (0.455–1.614) | 0.633 |
| Histological type | 0.148 | |||
| Adenocarcinoma | 3.697 (0.863–15.833) | 0.078 | ||
| Squamous-cell carcinoma | 3.806 (0.873–16.598) | 0.075 | ||
| Clinical stagec | 1.492 (0.990–2.250) | 0.056 | ||
Notes: aMultivariate model included all variables that were associated with elevated PASP at univariate analyses. bCoagulopathy (dichotomous). cClinical stage (dichotomous).
Discussion
In an analysis of 562 participants of lung cancer with long-term follow-up, we found that elevated PASP was common in an unselected cohort of lung cancer patients (19.8%), whereas its prevalence varies from 11 to 26 cases per million adults.16 Our study explored insight into the association between elevated PASP and the prognosis of lung cancer. These associations persisted after adjusting for several covariates, including pathological type, clinical stages. Moreover, PASP≥45mmHg, smoking history, distant metastasis and coagulopathy are independent predictors of perioperative mortality in NSCLC patients. Further, we found the main predictors of elevated PASP in NSCLC lung cancer patients are age, coagulation disorder and intra-pulmonary metastasis.
In recent decades, the treatment of lung cancer has achieved great medical advances, but the survival rate of lung cancer is still very low.17 It is therefore important to identify factors related to the prognosis of lung cancer. Our study supported the results of previous investigators who found that the prevalence of PH among lung cancer patients was high.9 Several studies have reported that approximately 1.4% of cancer coexist with pulmonary tumor thrombotic microangiopathy (PTTM) which eventually results in pulmonary hypertension, among this lung cancer accounts for 16.7% and the most frequent histological type was adenocarcinoma (93.3%).18 However, our study found no difference in tumor histological typing between the two groups. Moreover, some studies have reported that PH was closely related to the occurrence of lung cancer. A prospective multicenter study has revealed that the cancer incidence in pre-capillary PH was 14.45%, and lung cancer was the most common frequent.19 The risk of cancer may be increased in patients with chronic lung diseases.
Tumoral PH currently lies within group 5 of the classification of PH according to the 6th World Symposium on Pulmonary Hypertension,20 and the pathophysiological correlates of lung cancer with PH are numerous and complex. (i) Cohesive tumor cells occlude the small pulmonary arteries as has been defined as pulmonary tumor microembolism (PTE),20 the interaction between tumor cells and endothelial cell then initiates clot formation, and releases cytokines which initiate macrophage recruitment and intimal proliferation and ultimately PH, which is the feature of pulmonary tumor thrombotic microangiopathy (PTTM).21–23 Pulmonary microvascular disease comprises PTE and PTTM, the tumor emboli increase pulmonary vascular resistance through mechanical obstruction and may relate to vascular remodeling.21–23 Half of the reported PTTM cases have features of microangiopathic haemolytic anaemia (MHAH) or disseminated intravascular coagulation, while no significant difference was found between the two groups with respect to hemoglobin, platelets, indirect bilirubin. (ii) Malignancy is a risk factor for chronic thromboembolism (CTEPH) and large tumor metastasis can mimic pulmonary embolism; however, our study did not present the evidence of such condition and the latter is more often reported in cases of breast cancer,24 hepatocellular carcinoma.25 (iii) Cancer therapies may induce PAH. The tyrosine kinase inhibitors (TKIs) include dasatinib which are reported to cause direct pulmonary artery endothelial cell toxicity through the production of mitochondrial reactive oxygen species.26 Besides, cytotoxic chemotherapy including gemcitabine,27 mitomycin-C and irradiation, occupation organic solvent exposure is likely to relate to venous endothelial injury, which is the common mechanism of cancer treatment-related PVOD.28 Nevertheless, our patients enrolled were all first diagnosed and untreated before undergoing the echocardiogram. (iv) COPD is an important comorbidity of lung cancer, and PH-COPD is classified as group III of PH.20 However, patients with a definite history of COPD history are excluded from the study. (v) In vitro and in vivo experiments suggested that tumor cells interact with inflammatory cells and release of several cytokines in the tumor microenvironment promoted lung vascular cell proliferation and migration. Thus, anti-inflammatory therapies present potential in lung cancer-associated PH.
Current treatment of PH still focusing on vasodilators failed to reverse the vascular remodeling which may be related to the overlap mechanisms with lung cancer, featured by sustained proliferation and escape from apoptosis. Thus, antitumor drugs show therapeutic potential in the management of PH.29 Besides, several cases have reported that imatinib was effective in patients with PTTM associated PH and remarkably improves acute right heart failure caused by PTTM, whereas it was weaker in the case of colon and breast cancer and the mechanism may be associated with the production of growth factors varies between different tumors.29 Subsequently, we can speculate that imatinib may be an effective therapeutic option for PH in some cases of PTTM.30
The strengths of this study include the larger sample size and long-term follow-up. To the best of our knowledge, our work represents the first study evaluating the prognosis of lung cancers with elevated PASP. Although this cohort is the largest ever reported, our work has two main limitations. (i) We have only estimated the PASP by echocardiography instead of using right heart catheterization (RHC) which remains the gold standard for PH diagnosis. However, most of studies use the estimate PASP because of its noninvasive and economic nature, besides, it has a good relation with RHC. (ii) Another limitation in our study was a retrospective study and echocardiography was not a routine investigation in lung cancer patients at our hospital because of the unawareness of the presence of PH in this population, thus may lead to selective bias, further, well-designed prospective studies are required to solve these problems.
Conclusion
Elevated PASP was an independent prognostic risk factor for NSCLC and PASP ≥45mmHg is related to perioperative death. Moreover, advanced age, with coagulation disorder and intra-pulmonary metastasis at the time of diagnosis are independent risk factors for elevated PASP in NSCLC patients. Result of this study provides important implications on the research of the mechanism between lung cancer and PH and contributed to the clinical identification of lung cancer patients with poor prognosis.
Funding Statement
The present study was supported by the National Natural Science Foundation of China (grant no. 81670043).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quintanal-Villalonga Á, Molina-Pinelo S. Epigenetics of lung cancer: a translational perspective. Cell Oncol. 2019;42(6):739–756. doi: 10.1007/s13402-019-00465-9 [DOI] [PubMed] [Google Scholar]
- 3.Sugimura H, Yang P. Long-term survivorship in lung cancer: a review. Chest. 2006;129(4):1088–1097. doi: 10.1378/chest.129.4.1088 [DOI] [PubMed] [Google Scholar]
- 4.Lam CSP, Borlaug BA, Kane GC, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119(20):2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strange G, Playford D, Stewart S, et al. Pulmonary hypertension: prevalence and mortality in the armadale echocardiography cohort. Heart. 2012;98(24):1805–1811. doi: 10.1136/heartjnl-2012-301992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price LC, Wells AU, Wort SJ. Pulmonary tumour thrombotic microangiopathy. Curr Opin Pulm Med. 2016;22:421–428. [DOI] [PubMed] [Google Scholar]
- 7.Tabbò F, D’Aveni A, Tota D, et al. Pulmonary arterial hypertension in ALK receptor tyrosine kinase–positive lung cancer patient: adverse event or disease spread? J Thorac Oncol. 2019;14(2):e38–e40. doi: 10.1016/j.jtho.2018.10.154 [DOI] [PubMed] [Google Scholar]
- 8.McGee M, Whitehead N, Martin J, Collins N. Drug-associated pulmonary arterial hypertension. Clin Toxicol. 2018;56(9):801–809. doi: 10.1080/15563650.2018.1447119 [DOI] [PubMed] [Google Scholar]
- 9.Pullamsetti SS, Kojonazarov B, Storn S. Lung cancer-associated pulmonary hypertension: role of microenvironmental inflammation based on tumor cell-immune cell cross-talk. Sci Transl Med. 2017;9(416):eaai9048. doi: 10.1126/scitranslmed.aai9048 [DOI] [PubMed] [Google Scholar]
- 10.Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest. 2018;128(9):3704–3715. doi: 10.1172/JCI120847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115(1):148–164. doi: 10.1161/CIRCRESAHA.115.301130 [DOI] [PubMed] [Google Scholar]
- 12.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemla D, Humbert M, Sitbon O, et al. Systolic and mean pulmonary artery pressures: are they interchangeable in patients with pulmonary hypertension? Chest. 2015;147(4):943–950. doi: 10.1378/chest.14-1755 [DOI] [PubMed] [Google Scholar]
- 14.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–662. doi: 10.1161/01.CIR.70.4.657 [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–788. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 16.Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;14(360):j5492. doi: 10.1136/bmj.j5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allemani C, Matsuda T, Di Carlo V, et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uruga H, Fujii T, Kurosaki A. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med. 2013;52(12):1317–1323. doi: 10.2169/internalmedicine.52.9472 [DOI] [PubMed] [Google Scholar]
- 19.Bravos E, Cottin V, Dauphin C. Cancer incidence in patients with pre-capillary pulmonary hypertension. J Heart Lung Transplant. 2019;38(7):778–780. doi: 10.1016/j.healun.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Galiè N, McLaughlin VV 2, Rubin LJ, Simonneau G. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J. 2019;53(1):1802148. doi: 10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts KE, Hamele-Bena D, Saqi A, et al. Pulmonary tumor embolism: a review of the literature. Am J Med. 2003;115(3):228–232. doi: 10.1016/S0002-9343(03)00305-X [DOI] [PubMed] [Google Scholar]
- 22.Kumar N, Price LC, Montero MA, et al. Pulmonary tumour thrombotic microangiopathy: unclassifiable pulmonary hypertension? Eur Respir J. 2015;46(4):1214–1217. doi: 10.1183/13993003.00052-2015 [DOI] [PubMed] [Google Scholar]
- 23.Price LC, Seckl MJ, Dorfmüller P, et al. Tumoral pulmonary hypertension. Eur Respir Rev. 2019;28(151):180065. doi: 10.1183/16000617.0065-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geschwind J-FH, Dagli MS, Vogel-Claussen J, et al. Metastatic breast carcinoma presenting as a large pulmonary embolus: case report and review of the literature. Am J Clin Oncol. 2003;26(1):89–91. doi: 10.1097/00000421-200302000-00017 [DOI] [PubMed] [Google Scholar]
- 25.Jakel J, Ramaswamy A, Kohler U, et al. Massive pulmonary tumor microembolism from a hepatocellular carcinoma. Pathol Res Pract. 2006;202(5):395–399. doi: 10.1016/j.prp.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Weatherald J, Chaumais MC, Montani D. Pulmonary arterial hypertension induced by tyrosine kinase inhibitors. Curr Opin Pulm Med. 2017;23:392–397. doi: 10.1097/MCP.0000000000000412 [DOI] [PubMed] [Google Scholar]
- 27.Turco C, Jary M, Kim S, et al. Gemcitabine-induced pulmonary toxicity: a case report of pulmonary veno-occlusive disease. Clin Med Insights Oncol. 2015;9:75–79. doi: 10.4137/CMO.S26537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer MR 1, Estenne M, Berkman N. Radiation-induced pulmonary veno-occlusive disease. Chest. 1993;104(4):1282–1284. doi: 10.1378/chest.104.4.1282 [DOI] [PubMed] [Google Scholar]
- 29.Boucherat O, Vitry G, Trinh I, Paulin R, Provencher S, Bonnet S. The cancer theory of pulmonary arterial hypertension. Pulm Circ. 2017;7(2):285–299. doi: 10.1177/2045893217701438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubota K, Shinozaki T, Imai Y, Kario K. Imatinib dramatically alleviates pulmonary tumour thrombotic microangiopathy induced by gastric cancer. BMJ Case Rep. 2017;2017:bcr-2017-221032. doi: 10.1136/bcr-2017-221032 [DOI] [PMC free article] [PubMed] [Google Scholar]