Summary
Age-related clonal hematopoiesis is a major risk factor for myeloid malignancy and myeloid skewing is a hallmark of aging. However, while it is known that non-cell-autonomous components of the microenvironment can also influence this risk, there have been few studies of how the spatial architecture of human bone marrow (BM) changes with aging. Here, we show that BM adiposity increases with age, which correlates with increased density of maturing myeloid cells and CD34+ hematopoietic stem/progenitor cells (HSPCs) and an increased proportion of HSPCs adjacent to adipocytes. However, NGFR+ bone marrow stromal cell (NGFR+ BMSC) density and distance to HSPCs and vessels remained stable. Interestingly, we found that, upon aging, maturing myeloid cell density increases in hematopoietic areas surrounding adipocytes. We propose that increased adjacency to adipocytes in the BM microenvironment may influence myeloid skewing of aging HSPCs, contributing to age-related risk of myeloid malignancies.
Keywords: aging, adipocytes, myeloid, BMSCs, spatial, CD34+, stem cells, microenvironment, niche, stroma
Highlights
-
•
Aging increases adipose, myeloid, and CD34+ HSPC density in the human bone marrow
-
•
Human CD34+ HSPC niche is reticular, perivascular, and periadipocytic in aging
-
•
Aging increases maturing myeloid cell density surrounding adipocytes
In this article, Dick, Flores-Figueroa, and colleagues show that aging alters the spatial organization between CD34+ hematopoietic stem/progenitor cells and adipocytes within human bone marrow. An increased mature myeloid output correlates with these findings, raising the idea that these age-related changes may influence myeloid skewing and the accompanying risk of myeloid malignancy.
Introduction
The frequency of somatic mutations and clonal hematopoiesis, known risk factors of hematological cancer, increase dramatically with aging (Jaiswal and Ebert, 2019; Steensma and Ebert, 2020). Numerous mouse studies have shown that hematopoietic stem cells (HSCs) lose their repopulation capacity while increasing their quiescence and frequency with aging (de Haan et al., 1997; Rossi et al., 2005). Aging also favors myeloid, megakaryocytic, and erythroid over lymphoid lineages (Cho et al., 2008; Rundberg Nilsson et al., 2016), which has been linked to the influence of intrinsic (Beerman et al., 2010; Chambers et al., 2007; Rossi et al., 2005) and microenvironmental factors (Ergen et al., 2012; Lee et al., 2019; Vas et al., 2012). In mouse models, bone marrow (BM) microenvironmental populations that have been reported to be essential for hematopoietic regulation include osteoblasts, endothelial cells, bone marrow stromal cells (BMSCs), and adipocytes (Acar et al., 2015; Ding et al., 2012; Ding and Morrison, 2013; Zhou et al., 2017). Aging-associated changes in these populations result in changes in BM architecture, including vascular remodeling (Maryanovich et al., 2018; Saçma et al., 2019), decreases in trabecular area, and increases in adipocyte area (Singh et al., 2016). In contrast, BMSC frequency has been reported to remain stable in mouse BM with aging (Maryanovich et al., 2018; Saçma et al., 2019).
In humans, aging studies are limited by sample availability of benign BM. Furthermore, many studies compared older adult BM with pediatric groups obscuring possible differences between middle-aged and older adults; a key age range when the incidence of myeloid neoplasia markedly increases. Samples from oncologic patients (staging marrows) are often included that may not reflect normal individuals (Ogawa et al., 2000). Functional studies have found epigenetic and genetic changes of human aged HSCs (Pang et al., 2011; Rundberg Nilsson et al., 2016; Sun et al., 2014). However, these reports on the effect of aging on myeloid-lymphoid lineage skewing are few and showed contradictory findings. Although, there is an evident decline of lymphoid lineages (Kuranda et al., 2011; Pang et al., 2011; Rundberg Nilsson et al., 2016), changes within the myeloid lineage with age are less clear, as a decline or no effect has been reported (Kuranda et al., 2011; Pang et al., 2011). While older individuals show an increase in BM adipocytes (Justesen et al., 2001), the reports on BMSCs are not consistent (Maijenburg et al., 2012; Zhou et al., 2008).
To date, it remains unknown whether age-related changes occur in human CD34+ hematopoietic stem and progenitor cells (HSPCs) and their myeloid progeny in the BM microenvironment. Here, we report a systematic comparison of the frequency and location of maturing myeloid and CD34+ HSPCs with respect to the marrow microenvironment composed of adipocytes, vessels, and BMSCs of hematologically normal BM from individuals aged from 50 to 92 years.
Results and Discussion
Aging Alters BM Architecture by Increasing Adipocytes, CD34+ HSPCs, and Maturing Myeloid Cells
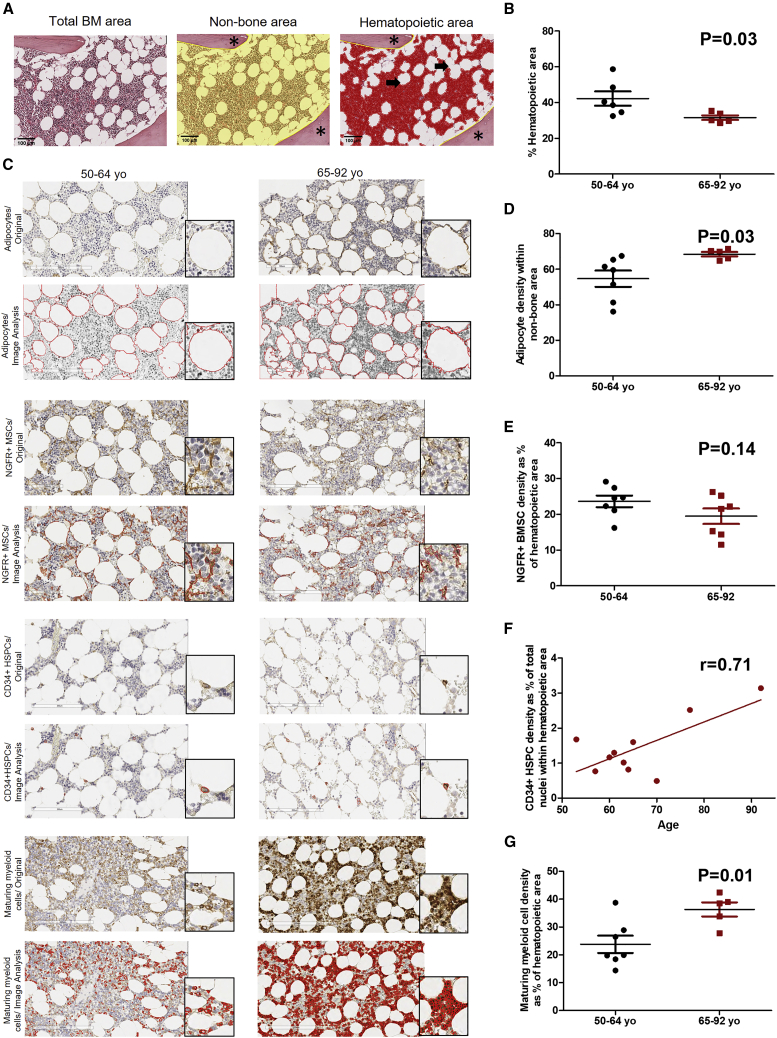
Two BM regions were defined to quantify and compare the different BM cell populations between middle-aged (50–64 years) and older adults (65–92 years). These regions were non-bone area, which excludes artifacts and trabeculae; and hematopoietic area (parenchyma), corresponding to the non-bone/non-adipocytic region, which is recognized by hematoxylin stain (Figure 1A). Non-bone area was used to quantify adipocytes and the hematopoietic area was used to quantify the rest of the BM cell populations. Cell markers were selected according to their specificity and sensitivity. Myeloperoxidase (MPO) was used for maturing myeloid cells (Tsuruta et al., 1999); CD34 was selected for HSPC detection, as it identifies all HSPCs, including HSCs and myeloid and lymphoid progenitors (Bhatia et al., 1997). Adipocytes were identified using perilipin (Boyd et al., 2017); and, for BMSCs, low-affinity nerve growth factor receptor (NGFR) was used. NGFR was chosen due to strong in vitro and in situ evidence that this marker highlights BMSCs (Quirici et al., 2002; Tormin et al., 2011). Systematic scoring and image analysis of middle-aged and older adult BM showed a statistically significant decrease in hematopoietic area (Figure 1B) and an increase in adipocytic content in older adult BM (Figures 1C and 1D). Both age groups have the same density of NGFR+ BMSCs (Figures 1C and 1E). Middle-aged and older adult BM showed no difference on the percentage of CD34+ HSPCs, measured as the percentage of CD34+ cells over total nuclei within the hematopoietic area (p = 0.14, data not shown). However, a strong positive correlation was found between CD34+ HSPCs and age (Figures 1C and 1F). Moreover, an increase in maturing myeloid cells, was observed in the hematopoietic area of BM of older adults (Figures 1C and 1G). To determine if the increase in maturing myeloid cells in the BM of older adults, is also seen in peripheral blood, peripheral blood counts from both groups were compared. We found an increase in total myeloid cells and a decrease in lymphoid cells in the older adult group (Table 1). Our findings confirm previous studies that reported an increase in HSPCs (Kuranda et al., 2011; Pang et al., 2011; Rundberg Nilsson et al., 2016). However, in contrast to our results, these studies did not find an increase in myeloid cells. While previous reports used flow cytometry to detect committed progenitors, our study measured maturing myeloid cells in situ by IHC.
Figure 1.
Aged Human BM Shows an Increased Density of Adipocytes, CD34+ HSPCs, and Maturing Myeloid Cells
(A) Representative images of an H&E stain of a middle-aged adult BM (total bone marrow area) and quantification of non-bone and hematopoietic area. Non-bone area (yellow area) excluding trabeculae (asterisk). Hematopoietic area (red area) excluding trabeculae (asterisk) and adipocytes (arrow). Scale bar, 100 μm. IHC staining, DAB; counterstain, hematoxylin. 20× magnification.
(B) Percentage of BM hematopoietic area in both age groups: 50–64 years (n = 7) versus 65–92 years (n = 5), p = 0.03.
(C) Representative IHC images (original) of adipocytes, NGFR+ BMSCs, CD34+ HSPCs, and maturing myeloid cells in BM biopsies from hematologically healthy patients aged 50–64 and 65–92 years. Each image is accompanied by its objects’ identification outline image produced by CellProfiler (Image Analysis). Scale bar, 200 μm.
(D) Adipocyte density in patients aged 50–64 years (n = 7) and 65–92 years (n = 5). p = 0.03.
(E) NGFR+ BMSC density: 50–64 years (n = 7) versus 65–92 years (n = 7), p = 0.14.
(F) Association between CD34+ HSPCs and age. Pearson correlation test, r = 0.71, n = 10.
(G) Maturing myeloid cell density: 50–64 (n = 7) and 65–92 years (n = 5), p = 0.01.
Table 1.
Peripheral Blood Parameters from Hematologically Healthy Patients Aged 50–92 Years
| Age Groups | Leukocytes (103/μL) | Myeloid Cells (103/μL) | Neutrophils (103/μL) | Monocytes (103/μL) | Lymphocytes (103/μL) | Erythrocytes (103/μL) | Hematocrit (%) | Platelets (103/μL) |
|---|---|---|---|---|---|---|---|---|
| 50–64 years (n = 28) | 7.15 ± .59 | 4.92 ± .39 | 4.21 ± 0.38 | 0.52 ± 0.04 | 2.26 ± 0.14 | 4.62 ± 0.08 | 40.67 ± 0.74 | 254.1 ± 11.09 |
| 65–92 years (n = 30) | 8.36 ± .73 | 6.54 ± .55 | 5.70 ± 0.53 | 0.64 ± 0.04 | 1.78 ± 0.13 | 4.55 ± 0.09 | 41.16 ± 0.80 | 274.6 ± 15.37 |
| p values | 0.21 | 0.02 | 0.02 | 0.04 | 0.01 | 0.58 | 0.66 | 0.28 |
CD34+ HSPCs Are Immediately Adjacent to Adipocytes Maintaining Perivascular Location in Middle-Aged and Older Adult Human BM
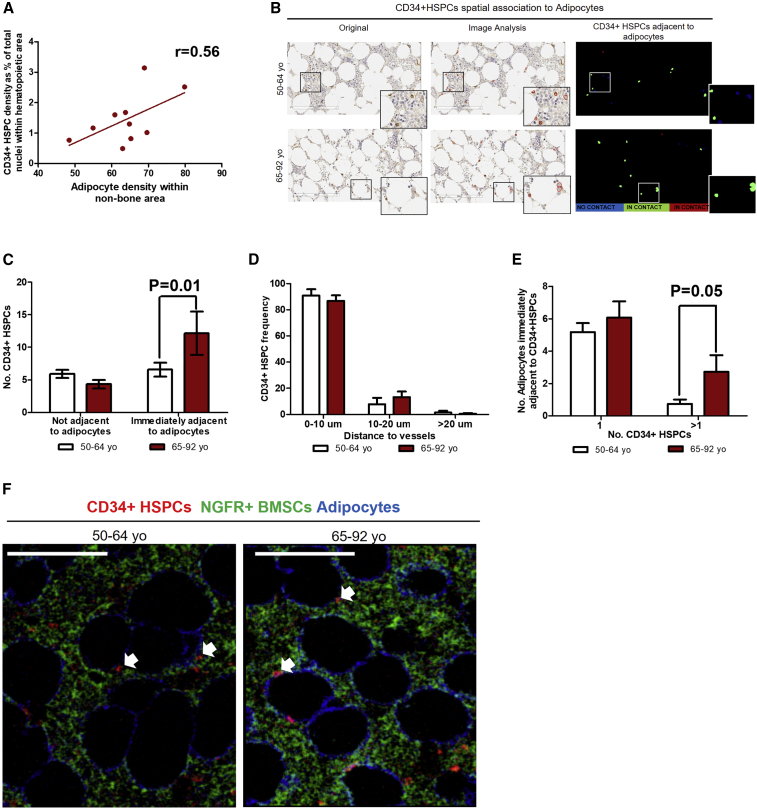
Given the increased number of CD34+ HSPCs and adipocytes in the BM of older adults, we determined if there was an association between these two populations. We confirmed a positive correlation between the density of adipocytes and CD34+ HSPCs in samples from both groups (Figure 2A). Furthermore, we observed CD34+ HSPCs located immediately adjacent to adipocytes (Figure 2B). Interestingly, we found a statistically significant increase in the percentage of CD34+ HSPCs immediately adjacent to adipocytes in the older adult group (Figures 2B and 2C). Given the increased adipocyte content in the BM of older adults, we also tested whether a single adipocyte could make contact with more than one CD34+ HSPC. Interestingly, we found a higher number of adipocytes immediately adjacent to more than one CD34+ HSPC in older individuals (Figure 2D).
Figure 2.
Human Aging Is Associated with Increased Adjacency between CD34+ HSPCs and Adipocytes
(A) Positive correlation between adipocytes and CD34+ HSPCs. Pearson correlation test, r = 0.56, n = 10.
(B) Representative images of CD34+ HSPCs spatially associated with adipocytes in BM biopsies from hematologically healthy patients aged 50–64 and 65–92 years. From left to right, CD34+ HSPCs IHC image, CD34+ objects’ detection image and digitalized image showing cells not adjacent (in blue) and immediately adjacent to adipocytes (in green and red). Scale bar, 200 μm.
(C) Bar graph showing number of CD34+ HSPCs not adjacent and immediately adjacent to adipocytes: 50–64 years (n = 6) versus 65–92 years (n = 4), two-way ANOVA, p = 0.01, unpaired t test, immediately adjacent to adipocytes, p = 0.01.
(D) Bar graph showing number of adipocytes immediately adjacent to 1 or >1 CD34+ HSPCs: 50–64 years (n = 6) versus 65–92 years (n = 4), two-way ANOVA p = 0.052, unpaired t test, >1 in both age groups, p = 0.05.
(E) Bar graph showing CD34+ HSPC distance to vessels. Graph representing percentage of CD34+ HSPCs at a distance of 0–10, 10–20, and >20 μm from vessels: 50–64 years (n = 3) versus 65–92 years (n = 3), two-way ANOVA p = 0.27.
(F) Representative IMC images showing an overlay of CD34+ HSPCs (red channel), NGFR+ BMSCs (green channel), and adipocytes (blue channel) in BM biopsies from hematologically healthy patients aged 50–64 (n = 2) and 65–92 years (n = 3). Scale bar, 100 μm. Adjacency between CD34+ HSPCs, NGFR+ BMSCs, and adipocytes are indicated by white arrows.
Functional studies using mouse models have shown that BMSCs are key components of the hematopoietic stem cell niche (Ding et al., 2012); and in humans it has been reported that more than 80% of CD34+ HSPCs are in close contact to NGFR+ BMSCs in perivascular areas (Flores-Figueroa et al., 2012). Therefore, we assessed whether the increased association of CD34+ HSPCs to adipocytes in older adults had an impact on their perivascular distribution and contact with NGFR+ BMSCs. We found that CD34+ HSPCs in human BM, from both groups, are located in perivascular areas, with >80% of HSPCs located <10 μm from the closest blood vessel, with no differences between age groups (Figure 2E). Using imaging mass cytometry (IMC) technology, we found that, in all samples analyzed from both age groups, CD34+ HSPCs remained in close contact to NGFR+ BMSCs (Figure 2F).
Aging Increases Maturing Myeloid Cell Density Surrounding Adipocytes
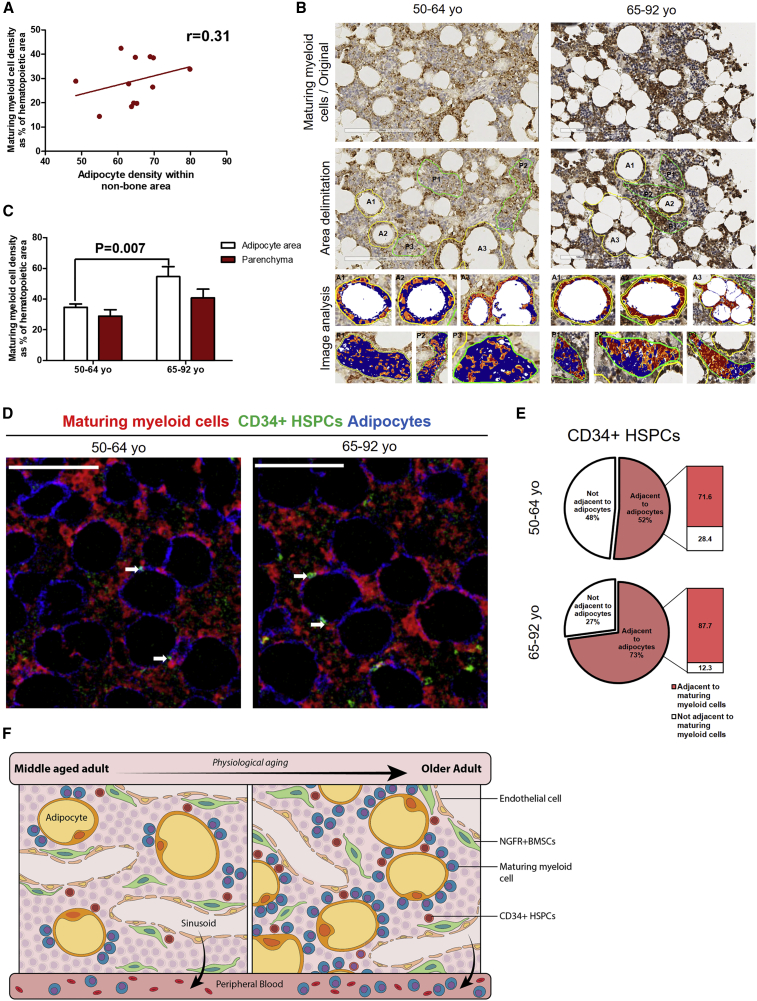
We next assessed whether there was an association between the density of maturing myeloid cells and adipocytes and found only a weak positive association (Figure 3A). However, given that both cell populations are increased with aging, we studied whether maturing myeloid cells preferentially locate closer to adipocytes. We analyzed maturing myeloid cell density in the immediate hematopoietic area surrounding adipocytes (within one cell distance from adipocyte membrane) and in the parenchyma (at least two-cell distance from adipocyte membrane) (Figure 3B, middle panel). Using image analysis software, we quantified maturing myeloid cell density in the above-mentioned areas of BM from middle-aged and older adult groups (Figure 3B, lower panel). Maturing myeloid cells distribute equally in both hematopoietic areas in both age groups (Figure 3C). However, we found a higher density of maturing myeloid cells surrounding adipocytes in the older adult group in comparison with the middle-aged group (Figure 3C). Such difference was not observed when comparing parenchyma areas of both age groups, suggesting that increases in maturing myeloid cell density associated to aging occur in the hematopoietic areas close to adipocytes. Our results support recent findings suggesting that adipocytes are the niche cells for myelopoiesis (Boyd et al., 2017).
Figure 3.
Human Aging Increases Maturing Myeloid Cell Density in Hematopoietic Areas Surrounding Adipocytes
(A) Weak correlation between adipocytes and maturing myeloid cell density. Pearson correlation test, r = 0.31, n = 11.
(B) Representative IHC images of maturing myeloid cells (MPO) (original) in BM biopsies from hematologically healthy patients aged 50–64 and 65–92 years (upper panel). Adipocyte area (A) and parenchyma (P) delimitation for image analysis quantification (middle panel). Image analysis of A and P, showing positive (yellow to red) and negative (blue) areas (lower panel). Scale bar, 200 μm.
(C) Bar graph showing the density of maturing myeloid cells in adipocyte area and parenchyma of the 50–64-year (n = 7) and 65–92-year (n = 5) groups. Two-way ANOVA, p = 0.002, unpaired t test, adipocyte area, p = 0.007.
(D) Representative IMC images showing an overlay of maturing myeloid cells (red channel), CD34+ HSPCs (green channel), and adipocytes (blue channel) in BM biopsies from hematologically healthy patients aged 50–64 years (n = 2) and 65–92 years (n = 3). Scale bar, 100 μm. Adjacency between maturing myeloid cells, CD34+ HSPCs, and adipocytes are indicated by white arrows.
(E) Diagram showing the percentage of CD34+ HSPCs adjacent to maturing myeloid cells out of percentage of CD34+ HSPCs adjacent to adipocytes in both age groups. Percentage of CD34+ HSPCs adjacent to adipocytes was calculated from IHC analysis and the percentage of CD34+ HSPCs adjacent to both adipocytes and maturing myeloid cells was calculated from IMC analysis.
(F) Proposed model of physiological aging-related changes in human BM spatial organization. Increasing marrow adiposity correlates with an increased CD34+ HSPC and maturing myeloid density and adjacency with adipocytes, while contact with MSCs and perivascular localization remains unchanged across age groups.
Based on the increases that we found in CD34+ HSPCs and maturing myeloid cells surrounding adipocytes with aging, we next assessed whether CD34+ HSPCs adjacent to adipocytes were also close to maturing myeloid cells. Using IMC technology, we identified CD34+ HSPCs that were both adjacent to adipocytes and maturing myeloid cells in BM samples from both age groups (Figure 3D). Consequently, we calculated the percentage of CD34+ HSPCs adjacent to adipocytes that were also adjacent to maturing myeloid cells in both age groups (50–64 years, n = 2; 65–92 years, n = 3). In all samples analyzed, >70% of CD34+ HSPCs adjacent to adipocytes were adjacent to maturing myeloid cells (Figure 3E). The increased association of CD34+ HSPCs to adipocytes with aging and the spatial association of myeloid cells with adipocytes suggests that adipocytes may be favoring myeloid differentiation (Figure 3E).
Our study refines the location of CD34+ HSPCs in humans across the spectrum of middle-aged to elderly BM. We showed that CD34+ HSPCs are located in a perivascular area and in close contact to NGFR+ BMSCs across all age groups, but there was increased association with adipocytes in older age groups. To our knowledge, this is the first report that locates CD34+ HSPCs immediately adjacent to adipocytes and highlights that aging correlates with changes in the location of CD34+ HSPCs in humans. This result highlights the importance of human studies, since adipocytes are underrepresented in mouse models (Flores-Figueroa and Gratzinger, 2016), which tend to rely on nearly adipocyte-free mouse femurs. While some previous studies suggested a negative role for adipocytes as regulators of the hematopoietic microenvironment (Naveiras et al., 2009), there is now accumulating evidence pointing to adipocytes as positive regulators by promoting the regeneration of HSCs (Zhou et al., 2017) and playing an important role in HSPC mobilization (Wilson et al., 2018). Our findings refine a periadipocytic/perivascular location of CD34+ HSPCs in humans that increases with aging (Figure 3F). The increased mature myeloid output that correlates with these findings raises the idea that these age-related changes in the interplay of HSPCs to their microenvironment may influence myeloid skewing and the attendant risk of myeloid malignancy.
Experimental Procedures
BM Samples
Femoral heads were collected from non-hematological, non-oncological patients aged 50 to 92 years (n = 58), undergoing hip replacement surgery at the Traumatology and Orthopedics Hospital Lomas Verdes (IMSS), Mexico. Ethical approval was obtained from IMSS institutional review board (R-2012-785-092). Clinical data from patients were retrieved.
Histology and Immunohistochemistry
Femoral heads were fixed in 10% formaldehyde. BM pieces of 2–3 cm in length were cut from the femoral neck and decalcified in 12% EDTA/PBS (pH 8; Sigma-Aldrich) solution for 10 days, then dehydrated and paraffin-embedded (Paraplast, Leica Biosystems) using a Multifunctional Tissue Processor KOS (Milestone). All samples were evaluated by two independent hematopathologists. A total of 75% of samples were excluded due to identified dysplasia of any of the hematopoietic lineages or lack of hematopoietic tissue present. IHC staining was performed as described previously (Flores-Figueroa et al., 2012). Antigen retrieval was performed using citrate buffer solution (pH 6; Sigma-Aldrich) with 0.05% Tween 20 (Sigma-Aldrich) at 110°C for 5 min using KOS (Milestone). Primary antibodies used were the following: MPO, rabbit, 1:250 (polyclonal/Abcam); Perilipin, rabbit, 1:1,000 (polyclonal/Abcam); NGFR, rabbit, 1:50 (clone EP1039Y/Biogenex), and CD34, rabbit, 1:5,000 (clone EP373Y/Genetex). Secondary antibody kit used was anti-rabbit (Vector Laboratories).
Image Analysis
Slides were scanned using Aperio CS2 (Leica Biosystems). Three 20× field images per sample were analyzed. For NGFR and adipocytes previous published pipelines were used (Berry et al., 2014; Johnson et al., 2014). For CD34 and MPO, pipelines were created and are available in https://github.com/Alicia-AguilarN/Cell-Profiler-pipelines. For CD34+ HSPC and adipocyte spatial association analysis, a pipeline with neighbor analysis module was created, which is also available in https://github.com/Alicia-AguilarN/Cell-Profiler-pipelines. ImageJ software was used to measure distance between CD34+ HSPCs and vessels and for manual quantification of CD34+ HSPCs adjacent to both adipocytes and NGFR+ BMSCs and maturing myeloid cells and adipocytes. For quantification of positive area of maturing myeloid cells surrounding adipocytes and parenchyma, positive pixel count algorithm was used in Image Scope software (Leica Biosystems).
IMC
IMC staining and ablation was carried out as reported previously (Chang et al., 2017). Antibodies used for IMC staining were CD34-158Gd (1:100), Perilipin-142Nd (1:100), NGFR-160Gd (1:50), and MPO-146Nd (1:2,000). Overlaid images were obtained using Wolfram Mathematica v.10.3 software (Fluidigm). Images were contrast-enhanced by Adobe photoshop only for visualization purposes.
Statistics
Statistics were performed using GraphPad Prism version 5.01 for Windows (GraphPad software, La Jolla, CA, USA, www.graphpad.com). For two-group comparisons, unpaired t test was used. For multiple comparisons, two-way ANOVA followed by Bonferroni post-test were performed. Results are presented as the mean ± SEM. Correlation analysis were performed using Pearson correlation test.
Author Contributions
Conceptualization, A.G.A.-N., D.G., J.E.D., and E.F.-F.; Investigation, A.G.A.-N., B.M.-L., D.G., F.G.J.-A., and Q.C.; Clinical Investigation, R.E.-G. and A.H.-R.; Data Analysis, A.G.A.-N., D.G., Q.C., O.O., H.T., and S.Z.X.; Writing – Original Draft, A.G.A.-N. and E.F.-F.; Writing – Review & Editing, A.G.A.-N., D.G., S.Z.X., O.O., J.E.D., and E.F.-F.; Review and Final Approval, A.G.A.-N., J.E.D., D.G., S.Z.X., E.F.-F., B.M.-L., D.G., F.G.J.-A., Q.C., R.E.-G., and A.H.-R.; Funding Acquisition, Resources, and Supervision, J.E.D. and E.F.-F.
Conflicts of Interest
Q.C. and O.O. are employees of Fluidigm Inc. The authors declare no competing financial interests.
Acknowledgments
A.G.A.-N. is a doctoral student from Programa de Doctorado de Ciencias Biomédicas, Universidad Nacional Autónoma de México, UNAM, and received fellowship 246604 from CONACyT, México, and Instituto Mexicano del Seguro Social (IMSS) (99096800). A.G.A.-N. and E.F.-F. received a scholarship from Programa de Cooperación Internacional del Instituto Mexicano del Seguro Social. This work was supported by funds to J.E.D. from the Princess Margaret Cancer Centre Foundation, Ontario Institute for Cancer Research with funding from the Province of Ontario, Canadian Institutes of Health Research, Joint Canada Israel Health Research Program of the IDRC, Canadian Cancer Society Research Institute, Terry Fox Research Institute PPG, and a Canada Research Chair and with funding to E.F.-F. from CONACYT (CB-2012-179417) and Canadian Institutes of Health Research, Joint Canada Israel Health Research Program of the IDRC. This work was carried out with the aid of a grant from the International Development Research Centre, Ottawa, Canada; the views expressed herein do not necessarily represent those of IDRC or its Board of Governors. We thank Monica Reynoso, Victor Pérez, Jorge Anaya, and Fernando Valverde for their technical support. We acknowledge Dr. Patricia Piña and Laboratorio de Microscopía Avanzada IMSS for the use of slide scanners. We thank Queralt Vallmajo-Martin and the Dick laboratory members for the discussion on the paper, especially to Kerstin Kaufman.
Published: July 9, 2020
Contributor Information
John E. Dick, Email: jdick@uhnresearch.ca.
Eugenia Flores-Figueroa, Email: eugenia.flores-figueroa@uhnresearch.ca.
References
- Acar M., Kocherlakota K.S., Murphy M.M., Peyer J.G., Oguro H., Inra C.N., Jaiyeola C., Zhao Z., Luby-Phelps K., Morrison S.J. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., Rossi D.J. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R., Church C.D., Gericke M.T., Jeffery E., Colman L., Rodeheffer M.S. Imaging of adipose tissue. Methods Enzymol. 2014;537:47–73. doi: 10.1016/B978-0-12-411619-1.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M., Wang J.C.Y., Kapp U., Bonnet D., Dick J.E. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A.L., Reid J.C., Salci K.R., Aslostovar L., Benoit Y.D., Shapovalova Z., Nakanishi M., Porras D.P., Almakadi M., Campbell C.J.V. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat. Cell Biol. 2017;19:1336–1347. doi: 10.1038/ncb3625. [DOI] [PubMed] [Google Scholar]
- Chambers S.M., Shaw C.A., Gatza C., Fisk C.J., Donehower L.A., Goodell M.A. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Ornatsky O., Hedley D. Staining of frozen and formalin-fixed, paraffin-embedded tissues with metal-labeled antibodies for imaging mass cytometry analysis. Curr. Protoc. Cytometry. 2017;82:12.47.1–12.47.8. doi: 10.1002/cpcy.29. [DOI] [PubMed] [Google Scholar]
- Cho R.H., Sieburg H.B., Muller-Sieburg C.E. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Morrison S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Saunders T.L., Enikolopov G., Morrison S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergen A.V., Boles N.C., Goodell M.A. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119:2500–2509. doi: 10.1182/blood-2011-11-391730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Figueroa E., Gratzinger D. Beyond the niche: myelodysplastic syndrome topobiology in the laboratory and in the clinic. IJMS. 2016;17:553. doi: 10.3390/ijms17040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Figueroa E., Varma S., Montgomery K., Greenberg P.L., Gratzinger D. Distinctive contact between CD34+ hematopoietic progenitors and CXCL12+ CD271+ mesenchymal stromal cells in benign and myelodysplastic bone marrow. Lab. Invest. 2012;92:1330–1341. doi: 10.1038/labinvest.2012.93. [DOI] [PubMed] [Google Scholar]
- de Haan G., Nijhof W., Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–1550. [PubMed] [Google Scholar]
- Jaiswal S., Ebert B.L. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673. doi: 10.1126/science.aan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.C., Kurzer J.H., Greenberg P.L., Gratzinger D. Mesenchymal stromal cell density is increased in higher grade myelodysplastic syndromes and independently predicts survival. Am. J. Clin. Pathol. 2014;142:795–802. doi: 10.1309/AJCP71OPHKOTLSUG. [DOI] [PubMed] [Google Scholar]
- Justesen J., Stenderup K., Ebbesen E.N., Mosekilde L., Steiniche T., Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Kuranda K., Vargaftig J., de la Rochere P., Dosquet C., Charron D., Bardin F., Tonnelle C., Bonnet D., Goodhardt M. Age-related changes in human hematopoietic stem/progenitor cells: aging of human HSC. Aging Cell. 2011;10:542–546. doi: 10.1111/j.1474-9726.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- Lee G.-Y., Jeong S.-Y., Lee H.-R., Oh I.-H. Age-related differences in the bone marrow stem cell niche generate specialized microenvironments for the distinct regulation of normal hematopoietic and leukemia stem cells. Sci. Rep. 2019;9:1007. doi: 10.1038/s41598-018-36999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maijenburg M.W., Kleijer M., Vermeul K., Mul E.P.J., van Alphen F.P.J., van der Schoot C.E., Voermans C. The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica. 2012;97:179–183. doi: 10.3324/haematol.2011.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanovich M., Zahalka A.H., Pierce H., Pinho S., Nakahara F., Asada N., Wei Q., Wang X., Ciero P., Xu J. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 2018;24:782–791. doi: 10.1038/s41591-018-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O., Nardi V., Wenzel P.L., Hauschka P.V., Fahey F., Daley G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kitagawa M., Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech. Ageing Dev. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Pang W.W., Price E.A., Sahoo D., Beerman I., Maloney W.J., Rossi D.J., Schrier S.L., Weissman I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirici N., Soligo D., Bossolasco P., Servida F., Lumini C., Deliliers G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- Rossi D.J., Bryder D., Zahn J.M., Ahlenius H., Sonu R., Wagers A.J., Weissman I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundberg Nilsson A., Soneji S., Adolfsson S., Bryder D., Pronk C.J. Human and murine hematopoietic stem cell aging is associated with functional impairments and intrinsic megakaryocytic/erythroid bias. PLoS One. 2016;11:e0158369. doi: 10.1371/journal.pone.0158369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saçma M., Pospiech J., Bogeska R., de Back W., Mallm J.-P., Sakk V., Soller K., Marka G., Vollmer A., Karns R. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat. Cell Biol. 2019;21:1309–1320. doi: 10.1038/s41556-019-0418-y. [DOI] [PubMed] [Google Scholar]
- Singh L., Brennan T.A., Russell E., Kim J.-H., Chen Q., Brad Johnson F., Pignolo R.J. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016;85:29–36. doi: 10.1016/j.bone.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensma D.P., Ebert B.L. Clonal hematopoiesis as a model for premalignant changes during aging. Exp. Hematol. 2020;83:48–56. doi: 10.1016/j.exphem.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Luo M., Jeong M., Rodriguez B., Xia Z., Hannah R., Wang H., Le T., Faull K.F., Chen R. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14:673–688. doi: 10.1016/j.stem.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormin A., Li O., Brune J.C., Walsh S., Schutz B., Ehinger M., Ditzel N., Kassem M., Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta T., Tani K., Hoshika A., Asano S. Myeloperoxidase gene expression and regulation by myeloid cell growth factors in normal and leukemic cells. Leuk. Lymphoma. 1999;32:257–267. doi: 10.3109/10428199909167386. [DOI] [PubMed] [Google Scholar]
- Vas V., Senger K., Dörr K., Niebel A., Geiger H. Aging of the microenvironment influences clonality in hematopoiesis. PLoS One. 2012;7:e42080. doi: 10.1371/journal.pone.0042080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Fu H., Schiffrin M., Winkler C., Koufany M., Jouzeau J.-Y., Bonnet N., Gilardi F., Renevey F., Luther S.A. Lack of adipocytes alters hematopoiesis in lipodystrophic mice. Front. Immunol. 2018;9:2573. doi: 10.3389/fimmu.2018.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B.O., Yu H., Yue R., Zhao Z., Rios J.J., Naveiras O., Morrison S.J. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 2017;19:891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Greenberger J.S., Epperly M.W., Goff J.P., Adler C., LeBoff M.S., Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]